Abstract
Complaints of stress are common in modern life. Psychological stress is a major cause of lifestyle-related issues, contributing to poor quality of life. Chronic stress impedes brain function, causing impairment of many executive functions, including working memory, decision making and attentional control. The current study sought to describe newly developed stress mitigation techniques, and their influence on autonomic and endocrine functions. The literature search revealed that the most frequently studied technique for stress mitigation was biofeedback (BFB). However, evidence suggests that neurofeedback (NFB) and noninvasive brain stimulation (NIBS) could potentially provide appropriate approaches. We found that recent studies of BFB methods have typically used measures of heart rate variability, respiration and skin conductance. In contrast, studies of NFB methods have typically utilized neurocomputation techniques employing electroencephalography, functional magnetic resonance imaging and near infrared spectroscopy. NIBS studies have typically utilized transcranial direct current stimulation methods. Mitigation of stress is a challenging but important research target for improving quality of life.
Keywords: Stress management, Biofeedback, Neurofeedback, Noninvasive brain stimulation
Introduction
Stress is the inability to cope with a perceived threat to one’s physical, emotional, or psychological well-being. Stress-related disorders can pose a serious threat to quality of life, and dysfunction in daily life can interfere with social life and physical health. Stress is reported to have a range of behavioral (Carneiro et al. 2012; Gärtner et al. 2014), cognitive (Lyle and Yaroush 2003), neurovascular (Durantin et al. 2014), cardiovascular (Healey and Picard 2005) and molecular effects (Mariotti 2015). In recent years, a number of neuroimaging studies have reported evidence of stress-related cognitive disturbance, leading to the degradation of physiological and mental health (Marin et al. 2011) and memory impairment (Roozendaal et al. 2009). The International Statistical Classification of Mental Disorders (ICD-10; WHO) identifies stress as a nonpsychotic mental disorder (code: F43) (Organization 1993). Moreover, stress-induced plasticity is reported to be associated with shrinkage of the hippocampus and prefrontal cortex (PFC) (Chattarji et al. 2015) as well as weakening of prefrontal networks (Arnsten 2015). In severe cases, chronic stress can develop into stress-related diseases with serious impacts, including burnout, depression and post-traumatic stress disorder (de Kloet et al. 2005; Marin et al. 2011). Stress-related elevation of cortisol in the blood has been found to metabolize white blood cells and weaken the immune system. This can lead to an excess of blood cholesterol, causing artery plaques that may result in hypertension and chronic cardiac disease (Mor et al. 1995).
Despite the severe effects of stress on the human body, treatment of stress has remained marginal in medical practice, due to the absence of established methods for the treatment of stress. However, with recent developments in physiology, neuroscience and bioengineering, advanced methods for the detection and identification of stress have been developed. Moreover, a number of studies have investigated the origins of stress in the body (de Kloet et al. 2005; Dimitriadis et al. 2015; Kuipers et al. 2008; Wager et al. 2009) as well as developing suitable assessment methods (Sharma and Gedeon 2012).
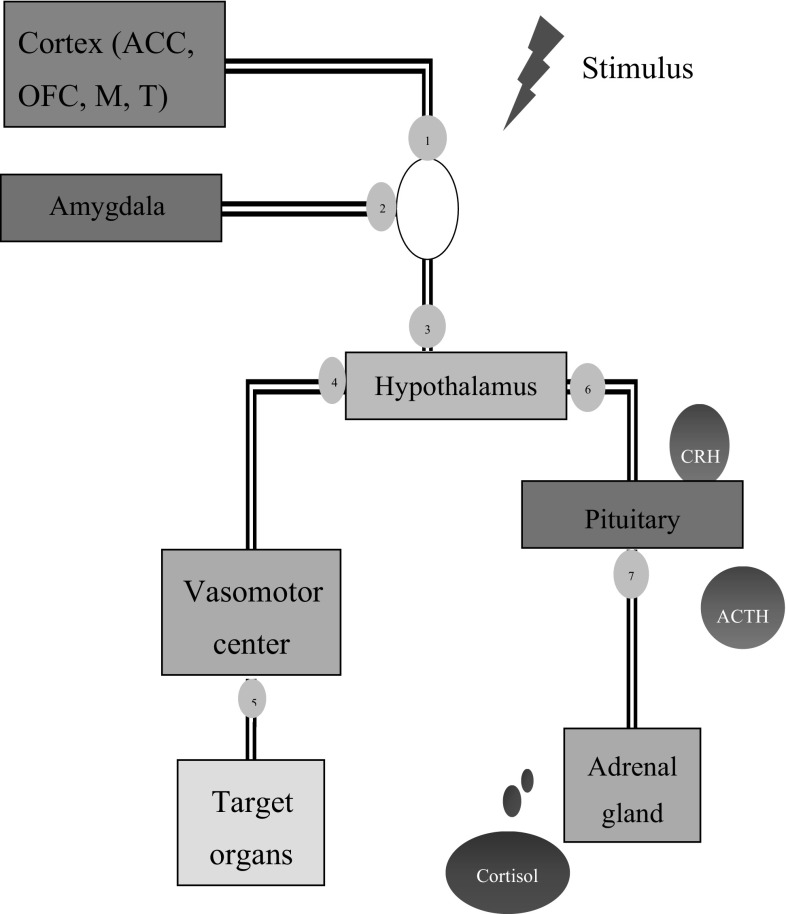
The stress response cycle typically begins with the hypothalamus, when sensory information is received from cortical areas and associated with memory-related information registered in the amygdala (McEwen et al. 2015). The neocortex processes sensory perception, language and motor commands, decoding cognition into emotion and classifying stimuli as threat- or pleasure-related. This information is transmitted to nerve endings in various parts of the body via the brain stem and reticular fibers that link the brain with the spinal cord. Responses are then transmitted via neuronal synapses to the sympathetic and parasympathetic branches of the autonomic nervous system (ANS) via the vasomotor center located in the reticular area, as a rapid recovery method (de Kloet et al. 2005). The hypothalamus then initiates the secretion of hormones as an intermediate recovery method. Cortisol is the most common hormone produced under stress, and is secreted from the adrenal glands located on top of each kidney. The secretion of cortisol is not directly activated; rather, it is an outcome of a sequential process that includes the activation of the hypothalamic–pituitary–adrenal (HPA) axis (de Kloet et al. 2005).
Perceived stress causes the hypothalamus to stimulate the production of corticotrophin-releasing hormone (CRH), which travels through blood vessels to reach pituitary gland and results in the secretion and release of adrenocorticotropic hormone (ACTH) (Holsboer and Ising 2010). Upon secretion into the blood, ACTH sequentially activates the adrenal cortex (Dedovic et al. 2009a, b; Holsboer and Ising 2010). The adrenal cortex produces and releases glucocorticoids, particularly cortisol in humans (Holsboer and Ising 2010). The glucocorticoids assist in the production of glucose by degrading proteins (amino acids) in the liver to provide essential energy required by the CNS as well skeletal muscles (Lupien et al. 2007). In addition, cortisol assists in breaking down and mobilizing of fatty acids for energy, in a process called lipolysis (Lupien et al. 2007). Moreover, cortisol metabolizes white blood cells and hence weakens the immune system (Maduka et al. 2015). Importantly, increased cortisol levels can cause an excess of cholesterol in the blood (Maduka et al. 2015), resulting in artery plaques that can lead to hypertension and chronic cardiac disease. The stress response cycle is shown in Fig. 1.
Fig. 1.
Brain areas exhibiting activation in response to stress. The sensory information is received from cortical areas ① and associated with memory-related information registered in the amygdala ② before proceeding to the hypothalamus ③. This information is transmitted to nerve endings in various parts of the body via the brain stem and reticular fibers that link the brain with the spinal cord. Responses are then transmitted via neuronal synapses to the sympathetic and parasympathetic branches of the autonomic nervous system (ANS) via the vasomotor center ④ located in the reticular area, as a rapid recovery method. The hypothalamus then initiates the secretion of hormones as an intermediate recovery method. Perceived stress causes the hypothalamus to stimulate the production of corticotrophin-releasing hormone (CRH) ⑥, which travels through blood vessels to reach pituitary gland and results in the secretion and release of adrenocorticotropic hormone (ACTH) ⑦. Upon secretion into the blood, ACTH sequentially activates the adrenal cortex. The adrenal cortex produces and releases glucocorticoids, particularly cortisol in humans
Several recent literature reviews have examined the identification of stress using different approaches, including measures and computational techniques used to examine stress (Sharma and Gedeon 2012), methods for measuring autonomic nervous system activity in response to stressors (Jarczok et al. 2013), stress detection using physiological measurement (Sioni and Chittaro 2015), automatic stress recognition in office environments (Alberdi et al. 2016) and cortisol-sensing techniques (Kaushik et al. 2014). In addition to these reviews, a number of recent studies have highlighted the potential for the identification of stress using neuroimaging techniques such as functional magnetic resonance imaging (fMRI) (K. Dedovic et al. 2009a, b; Pruessner et al. 2010), electroencephalography (EEG) and finite near-infrared spectroscopy (fNIRS) (Mandrick et al. 2013). Although the identification of assessment methods is important, practical application of these identification methods in treatment is a critical step for addressing the health impacts of stress. However, even the most recent reviews of new stress treatment methods are relatively out of date (Prinsloo et al. 2014; Sampaio et al. 2012; Schestatsky et al. 2013; Schoenberg and David 2014). The current review sought to compile new studies of stress treatment that have been conducted since the publication of previous review articles.
Measurement of stress response
Heart rate variability
Heart rate variability (HRV) has been used as a quantitative indicator of autonomic activity for several decades (Malik 1996), and is considered to provide an accurate indication of mental task difficulty and mental stress (Chanel et al. 2009; Healey and Picard 2005; Ryu and Myung 2005). HRV is typically measured using electrocardiography (ECG), which provides a visualization of heart-generated electrical activity.
HRV analysis can be performed in both time and frequency domains. For each QRS complex detected with ECG, the normal-to-normal (NN) beat interval and instantaneous HR can be determined (Malik 1996). The total power of RR variability (variance) has been used in a number of studies as a selective indicator of cardiac parasympathetic tone (Brennan et al. 2001). However, in augmented sympathetic activity, RR variability does not appropriately reflect the balance with related vagal withdrawal. The vagus nerve enables communication between the parasympathetic nervous system (PNS) and the heart. For healthy subjects under resting conditions, power spectral analysis represents the two dominant rhythmic oscillations in the arterial blood pressure heart period fluctuations (Palacios-Garcia et al. 2015). The low frequency (LF) component typically concentrates at a frequency of 0.1 Hz, while the high frequency (HF) component, synchronous with respiration, has a central frequency of 0.25 Hz. In HRV measurement, the LF/HF ratio is typically greater than 1. However, in some cases the LF/HF ratio has been reported to be elevated as high as 20 (Malliani et al. 1991). In addition to these two rhythms, some studies have examined the very low frequency (VLF) component of HRV. The power component of VLF is typically measured in absolute values of power (ms2). However, the power of LF and HF can either be measured in absolute values of power (ms2) or in normalized units (n.u.) whose value can be calculated (Palacios-Garcia et al. 2015).
Skin conductance (SC)
Varying body temperature causes changes in skin resistance due to a momentary boost in the conductance of skin that is proportional to sweat discharge (Healey and Picard 2005). The galvanic skin response (GSR) provides an electrical measure of skin resistance by measuring the difference in skin conductance between two body parts (Micoulaud-Franchi et al. 2014). There are two types of GSR measurement: endosomatic and exosomatic. The former involves the measurement of the skin’s own electrical activity, while the latter involves the application of external voltage to measure changes in the electrical properties of the skin (Schmidt and Walach 2000).
Two key components are typically considered in the measurement of SC: the skin conductance level (SCL), and the skin conductance response (SCR) (Setz et al. 2010). The SCR typically occurs in response to a distinct stimulus, causing increased SC for a particular period of time, then falling to the normal level (Healey and Picard 2005). SCR has four important characteristics: the latency of stimulus response onset (SCR lat.), the maximum rise in amplitude (SCR amp.), the response time for the wave to rise to the peak (SCR rise t.) and half of the recovery time (SCR rec. 1/2) (Setz et al. 2010).
GSR is considered to provide a reliable stress indicator, because sweat glands are under the control of sympathetic nervous system (SNS) and exhibit sympathetic changes under stress. SC has been used in several studies for inferring psychological stress (Chanel et al. 2006; Chanel et al. 2009; Haapalainen et al. 2010; Zhai and Barreto 2006).
Blood volume changes
The change in the volume of blood flow in the arteries and veins is under the autonomic control of the peripheral vascular tone (Allen 2007; Tanaka and Sawada 2003). Blood volume changes can be measured using noninvasive photoplethysmography (PPG). PPG measures blood volume in peripheral regions such as at the earlobe and fingertips, where blood vessels have close contact with the skin. The waveforms recorded at the fingertips demonstrate variations in blood volume in the finger, and are considered to reflect the sympathetic activation of the small arteries in the fingertip (Chan et al. 2005). Blood volume pulses have been examined in several studies of psychological stress (Kageyama et al. 2007; Zhai and Barreto 2006). Several features of the PPG waveform have been analyzed in previous studies, including variability in beat-to-beat PPG rise time, pulse transit time (PTT), amplitude and shape (Allen 2007; Chan et al. 2005).
Respiration
In addition to the cardiac cycle, respiration is also strongly associated with circulation (Healey and Picard 2005), and is reported to affect arterial blood pressure and heart period variability (Nemati et al. 2010). Many studies of psychological stress have also utilized respiration as a measure of sympathetic changes under stress (G. Chanel et al. 2006; Guillaume Chanel et al. 2009; Healey and Picard 2005; Wilson et al. 2000).
Respiration can be recorded using a respiration belt that measures thoracic expansion, and rhythmic ejections in phase with respiratory activity are associated with sympathetic and vagal depletion (Malliani et al. 1991).
Endocrinal changes
Endocrinal response to acute or chronic stress has also received much attention in research and development. Teixeira and colleagues recently examined the occurrence of chronic stress in business executives and its relationship with cortisol levels, as well as cognitive performance and ANS activity (Teixeira et al. 2015). The results revealed higher cortisol levels among subjects with chronic stress, with no gender-related differences. As mentioned above, the HPA axis plays a pivotal role in the generation of stress-related hormones. In one laboratory study, Foley and Kirschbaum tested HPA axis responses to acute stress (Foley and Kirschbaum 2010). Cortisol levels were selected to examine changes in the HPA axis response to stress. The results revealed different cortisol levels between healthy subjects and patients.
Brain activation during stress
The brain is sensitive to the detrimental effects of stress exposure. Recent research indicates that even acute uncontrollable stress can cause a rapid and dramatic decline in executive functions, and chronic stress exposure can cause architectural changes in brain dendrites (Arnsten 2009).
Advances in neuroimaging techniques have revealed that psychiatric conditions can cause dysregulation in neural circuits (Bonelli and Cummings 2007; Lozano and Lipsman 2013). A number of magnetic resonance imaging (MRI) studies have been conducted with humans and animals to examine the patterns of brain activation involved in various mental states and thought processes. Studies using animal models have revealed the activation of amygdala during fear conditioning (LeDoux 2000; Phelps and LeDoux 2005). Human studies have also shown the activation of the amygdala in fear processing. Moreover, a functional MRI (fMRI) study reported increased amygdala activity among city-dwellers engaged in a stressful lifestyle compared to the inhabitants of rural areas (Lederbogen et al. 2011). Another fMRI study reported that threat-related stimuli are first registered in the amygdala, followed by cortical responses (Pruessner et al. 2010).
Other neuroimaging modalities such as electroencephalography (EEG) and near infrared spectroscopy (NIRS) have also assisted to unveil brain states under various conditions. For example, features of EEG signals have been found capable of recognizing emotional changes (Atkinson and Campos 2016). Similarly, NIRS has been found to be capable of identifying mental overload (Durantin et al. 2014).
Mitigation of stress
Biofeedback
Stress causes variability in ANS function, including reduced body temperature, increased SC, increased HR, muscle tension and respiration rate (Jarczok et al. 2013). BFB methods provide a way to regulate these variations and display the information in real time. Thus, BFB enables a subject to be aware of their thoughts, emotions or actions and their impact on the body, as well as assisting in the development of a degree of control over their physiology.
Several comprehensive reviews have examined the role of BFB in mediating stress (Prinsloo et al. 2014; Schoenberg and David 2014; Van Den Broek and Westerink 2012), compiling studies published before February 2014. In this section, we focus on studies published after that date. A list of these studies is shown in Table 1. In these studies, HRV-BFB was most commonly used (five studies), followed by RSA-BFB (four studies) and SCR-BFB (two studies).
Table 1.
List of BFB studies of stress mitigation
| First author | Training | Control | Assessment | Training sessions and duration | Sample size and characteristics (male/female; training/control) | Occupation | Age of subjects | Results |
|---|---|---|---|---|---|---|---|---|
| De Jonckheere et al. (2014) | RSA-BFB | No control | HR | 1 session | 19 (10 male) | ND | 22–60 | Cardiac coherence index scores were higher during breathing exercise than random ventilation. However, further validation is required to confirm system performance |
| Whited et al. (2014) | HRV-BFB | No intervention | PSS and BSI | 4–8 sessions | 27 | Undergraduate and graduate students | 22.54 ± 3.82 | Treated subjects exhibited greater parasympathetic responses during stress in post-treatment session. However, no treatment effects were evident on self-reported measures, psychological symptoms, or affect |
| Kudo et al. (2014) | HRV-BFB | Control group | Edinburgh postnatal depression scale | Daily sessions for 4 weeks | 55 | Women in the early postpartum period | 31.9 ± 6.6 | Intervention group showed a significant increase in HRV and reduced vagal activity. Other effects included reduced anxiety and quality of sleep |
| Sarabia-Cobo (2015) | Coherent heart training | The Maslach burnout inventory and Zarit burden inventory | Weekly 1-h sessions for 3 weeks | 74 | Caregivers of patients with dementia | 50.9 | Heart coherence and positive psychology training had effective results over a long period | |
| van der Zwan et al. (2015) | HRV-BFB | MM, PA | PSS | Daily sessions for 5 weeks | 75 (20 male), PA: 23 (5 male), MM: 27 (7 male), BFB: 25 (8 male) | 26.19 ± 6.53 | HRV-BFB, MM and PA were equally effective in reducing stress and related symptoms | |
| Ratanasiripong et al. (2015) | HRV-BFB | MM and control | PSS and state anxiety score | Sessions 3 times a day for 4 weeks | 89 (all female), BFB: 29; MM: 20; control: 31 | Nursing students | 19.27 ± 0.56 | BFB significantly reduced anxiety and maintained low stress levels. Mindfulness training decreased anxiety and lowered stress levels |
| Palekar et al. (2015) | GSR-BFB | Not included | PSS, pulse rate, respiratory rate, BR | 10-min daily for 5 days, weekly sessions for 3 weeks | 43 | Physiotherapy students with PSS scores above 20 | 19.30 ± 2.98 | Pulse rate, respiratory rate, BP and PSS values significantly decreased after 3-week training |
| Dillon et al. (2016) | SCR | No intervention | TSST, perceived stress, HR and mood assessment | 30 min | 50 | 26.7 ± 5.1 | BFB group showed significant reduction in stress level and HR after training compared to control group | |
| Munafò et al. (2016) | RSA BF | Daily questionnaire diary | Pre- and post-training assessment using self-report questionnaires and physiological measures (HR, BP and SCL) | 5 weekly sessions, 45 min | 31; 15 control | Managers from public and private companies | 48.37 ± 8.71 | RSA-BF increased vagal control, decreased sympathetic arousal and reduced emotional interference |
| Kraemer et al. (2016) | Mind–body skills training (meditation, BF, guided imagery, relaxation, breathing and autogenic training) | No intervention | Self-assessment questionnaires (distress tolerance scale) | 11 weekly 2 h sessions | 52 (24 males) | Medical students | 23.45 ± 1.5 | Mind–body group showed improved distress tolerance subscales as well as reported a better capability to bear affective distress |
| Dziembowska et al. (2016) | HRV BF | No intervention | Self-assessment questionnaires (STAI) and EEG | 10 BF sessions within 3 weeks | 41 | Athletes | 18.34 ± 1.36 | |
| Meier and Welch (2016) | Pace breathing (BFB), self-paced walk (exercise) | Quietly studying | State anxiety and affect measurements, HRV | 2 crossover sessions | 32, (21 females) | College students | 21.7 ± 3.1 | BFB minimized anxiety and momentarily improved calmness. Exercise momentarily raised energy |
BFB biofeedback, BSI brief symptoms inventory, HRV heart rate variability, MM mindfulness meditation, ND not described, PA physical activity, PSS perceived stress scale, STAI state trait anxiety
In a randomized controlled trial (Munafò et al. 2016) evaluated the effectiveness of respiratory sinus arrhythmia (RSA) BFB as a stress treatment intervention among managers with high-level work responsibilities. A sample of 31 managers participated in the study, split into a training group (N = 16) and a control group (N = 15). The training group received five weekly 45-min sessions of RSA BFB, while the control group were instructed to compile a daily stress diary that was provided to the experimenters once a week. The results revealed that RSA BFB training was associated with a decrease in resting heart rate, lower anxiety levels and improved health-related quality of life in both groups (Munafò et al. 2016). Importantly, managers in the RSA-BF group exhibited increased vagal control (as indexed by increased RSA), decreased sympathetic arousal (as indexed by reduced SC and systolic blood pressure) and less emotional interference, compared with managers in the control group. The results of this study indicated that RSA-BF training was effective for improving cardiac autonomic balance at rest. Moreover, these findings highlight the effectiveness of BFB in reducing negative psychophysiological outcomes associated with stress in managers
Neurofeedback
EEG-based NFB
NFB has traditionally been considered only in the treatment of cognitive disorders such as ADHD. However, several studies have found that NFB is a successful treatment for various conditions that share common physiological mechanisms with stress.
NFB is indirectly related to stress, impacting on its symptoms and contributing factors. As stress cannot be directly measured, the development of appropriate treatments often relies on targeting indicators of stress, such as anxiety or depression.
Hardt and Kamiya conducted the first study of NFB as a treatment for mitigating anxiety in 1978 (Hardt and Kamiya 1978). In this study, EEG alpha activity was used to regulate state anxiety. Subjects were categorized into high and low trait anxiety groups, based on the Minnesota Multiphasic Personality Inventory (MMPI). Training took place over seven consecutive days. Each day, alpha enhancement and alpha reduction feedback were recorded from each subject with eyes closed, following resting baseline measurement. In each session, state anxiety was measured using the Multiple Affect Adjective Check List (MAACL) before and after the feedback recording. Feedback was provided in the form of audio tone which was in proportion with instantaneous Oz alpha voltage. The results revealed that alpha activity was negatively correlated with state anxiety in high trait anxiety individuals. The inverse relationship between increased alpha activity and reduced state anxiety became stronger when the high trait anxiety group was trained for more than 2 h. Despite these effects in the high trait anxiety group, the low trait anxiety group showed no significant relationship between alpha activity and state anxiety (Hardt and Kamiya 1978).
Fedotchev (2010) tested a combined EEG NFB approach to alleviate stress-related functional disorders. In double EEG NFB, EEG band oscillations were simultaneously used to operate two independent feedback cycles. According to this approach, narrow band EEG oscillators with particular characteristics for each patient were detected in real time and simultaneously used in two independent feedback loops: the traditional adaptive BFB loop and an additional resonance stimulation loop. The traditional feedback loop was operated through theta band EEG oscillation to control the pitch and intensity of an audio signal. In the latter loop, the feedback signals from individual alpha band EEG oscillators play a role in the automatic modulation of the parameters of light stimuli and were unknowingly observed by the subject. Twenty-five subjects participated in two to four experimental sessions and their behaviors were analyzed using the Well-being Activity and Mood (WAM) inventory. The results revealed that, following double EEG NFB, subjects were able to successfully eliminate theta power and instead produce alpha power activation, compared with traditional NFB training. However, although self-estimation measures showed significant changes in mood (P < 0.01) and well–being (P < 0.05), the proportion of subjects exhibiting elimination of theta power did not reach significance.
Symptom-focused NFB methods have many potential applications for stress research. Van Boxtel et al. (2012) conducted a double blind placebo-controlled study of relaxation among people experiencing stress, and compared alpha power training with random beta training or no training for inducing relaxation (Van Boxtel et al. 2012). Post-training EEG measurement revealed that only the alpha power training group exhibited a significant increase in alpha power at posterior regions. This effect was found to be sustained in 3-month follow-up recording, particularly in the eyes-open condition, in which an additional 10% increase was observed. On subjective feedback measures, almost twice as many participants in the alpha training group mentioned that the training was relaxing, compared to those in either the beta or no training control groups. Behavioral measures of stress and relaxation also indicated an effect of alpha activity training. However, these effects did not reach statistical significance.
Altered SCPs have been reported among patients with impaired attentional functioning, including patients with schizophrenia. A controlled study found that SCP NFB led to improved cognitive function in patients with schizophrenia, (Gruzelier et al. 1999). The researchers speculated that the impaired cognitive performance in schizophrenia was due to tension and anxiety (Gruzelier et al. 1999). Psychosocial stress has been established as an important risk factor, increasing the likelihood of relapse in schizophrenia (Stein and Nikolic 1989). It has been found that schizophrenic patients living in stressful home environments are at a substantially greater risk of relapse than those living in low-stress homes (Leff and Vaughn 1981).
Hendler (2013) conducted a longitudinal study of effects of NF training on mental resilience to stress and emotional regulation ability, seeking to develop an efficient EEG-based NFB approach that is specific to amygdala function and stress. The study had three main aims: (a) to develop an innovative EEG-NFB LMI approach for stress resilience; (b) to evaluate the developed EG-NFB approach with the conventional alpha/theta (A/T) training outcomes; and (c) to use fMRI to verify that the proposed EEG-NFB-LMI approach is more amygdala-specific than conventional A/T training. Both EEG-NFB training methods were compared against a placebo group. This research group has published another study on emotional regulation training with NFB (Cavazza et al. 2014).
NFB for improving performance anxiety
Fear of failure under stressful conditions is a common cause of performance anxiety, including test-related anxiety (Hembree 1988), and performance anxiety in sports (Tharawadeepimuk and Wongsawat 2017), music and acting (Mor et al. 1995). A number of studies examining this type of stress have revealed that NFB can improve performance. Egner and Gruzelier (2003) conducted a study of alpha/theta NFB with musicians while performing under stressful conditions (Egner and Gruzelier 2003). Performance was compared with other groups who received either different NFB protocols (SMR and beta1) or alternative interventions, such as mental skills training, sports psychotherapy and training with the widely-used Alexander Technique. Only the alpha/theta NFB group exhibited enhanced real-life musical performance under stress.
In addition, a randomized controlled study of the effects of NFB on performance anxiety reported improved performance among ballroom dancers (Raymond et al. 2005). Twenty-four dancers were randomly assigned to groups receiving NFB training, HRV BFB or no intervention. Performance was evaluated before and after training. Both BFB groups exhibited improved performance, while the control group showed no change. The NFB group showed the greatest improvement in overall performance, with a significant improvement in timing synchronization. The quantitative EEG (QEEG)-based approach has also been reported to successfully reduce performance anxiety among baseball players (Sherlin et al. 2013).
The Performance anxiety and decision making skill are two important factors that reflect on the performance of an athlete. QEEG is able to measure the cognitive anxiety and decision making level and thus can predict the performance of an athlete during the competition. This idea was executed in a study (Tharawadeepimuk and Wongsawat 2017) where 29 female professional soccer players participated to record their QEEG three time, twice before the competition and once after the competition. The QEEG markers of the cognitive anxiety and decision making were absolute power in the alpha band in the posterior brain region and coherence in the delta band in the whole brain, respectively. The study found out that the QEEG markers before competition correlated with the average performance score of the players during the competition. Players who had lower brain activity before competition had lower performance scores during the competition. QEEG markers could be an alternative evaluation approach to assist the coaching staff to select key players based on the prediction of their performance.
Neurofeedback for improving impaired concentration
Impaired concentration is commonly reported by patients with anxiety disorders, suggesting that attentional control and cognitive performance are related to anxiety and psychological stress (Eysenck and Derakshan 2011; Palacios-Garcia et al. 2015). The top-down attentional network involves the PFC, particularly the dorsolateral PFC (Arnsten 2009; Arnsten and Rubia 2012; Bishop 2008). Inducing stress has been reported to cause an immediate increase in the concentration of catecholamines noradrenaline and dopamine in the PFC, and an excessive concentration of any hormone has been found to affect the performance of the prefrontal attentional network (Arnsten 2009). Some evidence suggests that excessive glucocorticoid levels may cause additional disturbance in PFC executive functioning (Lupien et al. 2007), which can exacerbate impairments of concentration during stress (Lupien et al. 2007). Putman et al. (2014) reported that the deleterious effects of anxious stress on the control of attentional state can be mediated by theta/beta ratio. While previous studies have examined central cortical activation during NFB theta/beta ratio training in patients with ADHD (Arns et al. 2014; Barry et al. 2003), the effects of theta/beta NFB training on the PFC are yet to be examined.
Changes in autonomic activation
HRV is another well-studied physiological marker of stress. In one study, Bazanova and colleagues used alpha frequency-based NFB training and examined the effects on HRV (Bazanova et al. 2013). A group of 27 healthy males were included in the study, divided into experimental and control groups. The experimental group participated in 10 sessions of NFB alpha power training under eyes-closed conditions, while the control group was engaged in a matched number of sham NFB sessions. HRV features (LF/HF and PNN50) as well as psychometric indicators of cognitive performance were recorded before, during, and after training. The results revealed that alpha NFB training was associated with improved cognitive performance, decreased anxiety and frontal activation (measured with EMG), as well as increased resting frequency, width and power in the individual upper alpha range, exclusively in subjects with low baseline levels of alpha activity. In contrast, sham NFB enhanced resting alpha power only in participants with high baseline levels of resting alpha activity, and did not affect cognitive performance or HRV indices. NFB training helped sustain alpha power in response to an arithmetic task among subjects with high and low baseline levels of alpha activity, and this effect was observed in 1-month follow-up measurement. Sham NFB training revealed no such effect. In addition, subjects with low baseline alpha activity exhibited a positive correlation between the alpha-peak frequency and the pNN50. In contrast, subjects with high baseline alpha activity demonstrated a negative correlation between the two measures. These bidirectional NFB-related effects on HRV in low and high baseline alpha activity subjects may be related to the effective of alpha training on HRV. As such, these findings may have predictive value for developing individual approaches to NFB training that can be used in clinical treatment and the rehabilitation of psychosomatic disorders, as well as educational training.
NIRS based NFB
Near infrared spectroscopy (NIRS) is another neuroimaging modality that can be used in neurofeedback. It measures the brain activity by monitoring the variations in cerebral blood flow (CBF). The exposure of NIR light (650–1000 nm) to the scalp penetrates through the human tissues and returns back to the scalp. The exposed NIR light is partially absorbed by chromophore hemoglobin (the oxygen transport red blood cell protein) or locally absorbed and scattered in tissues before undergoing absorption in the cerebrum.
One NIRS study revealed increased frontal CBF under excessive workload (Mandrick et al. 2013). In addition, Durantin and colleagues detected mental load with NIRS and HRV simultaneously (Durantin et al. 2014).
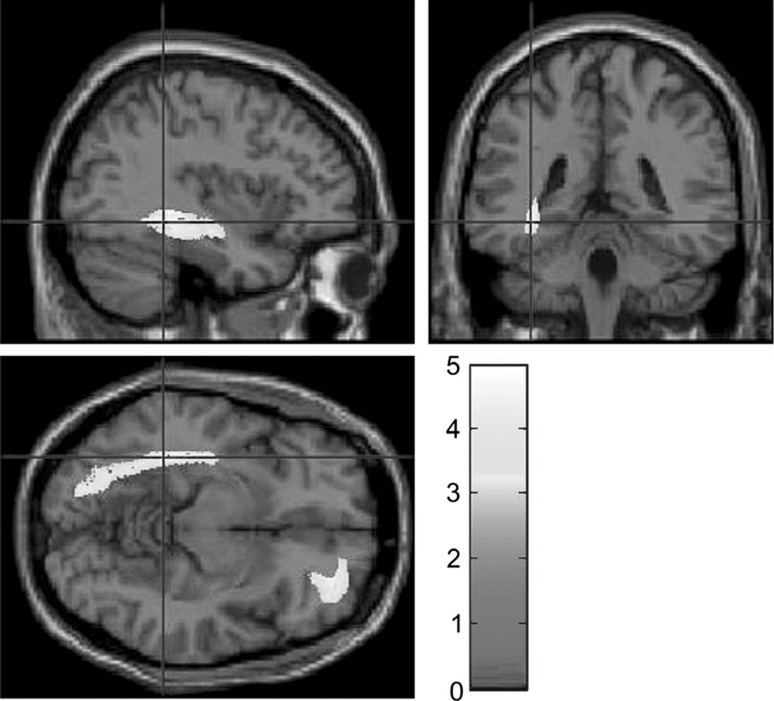
Kotozaki and colleagues tested NIRS and HR-based BFB as a stress coping intervention (Kotozaki et al. 2014). Moreover, they measured salivary cortisol levels to track the efficacy of stress-coping skills training using BFB. After 28 days of daily 5-min BFB sessions (in combination with a range of tasks to control heart rate and cerebral blood flow), the intervention group showed significantly reduced cortisol levels compared to the control group. This study also identified the anatomical correlates of the effectiveness of NFB. MRI scans revealed that the intervention group had significantly expanded regional gray matter (GM) volume in the right lateral orbitofrontal cortex compared with the control group. These brain regions are related to stress responses and a range of evidence suggests that they are sensitive to its harmful effects (Kotozaki et al. 2014), as shown in Fig. 2.
Fig. 2.
Significantly increased gray matter volume in biofeedback group compared to non-intervention group. These areas include the right lateral orbitofrontal cortex and an anatomical region including the left hippocampus. Reproduced from Kotozaki et al. (2014)
Previous studies of NFB have mostly relied on the conventional use of questionnaires to track behavioral changes related to training. However, a study by Durousseau (2013) validated the effects of NFB training by measuring cortisol changes in the blood, as well as measuring changes in brain activity patterns, mood and performance under stressful conditions using QEEG evaluation. In this study, two military veterans underwent 20 NFB sessions with pre- and post-training EEG recording. Pre- and post-training changes were evaluated using neuropsychological assessments. Additionally, blood samples were taken from one subject to evaluate changes in plasma cortisol to examine changes in stress levels during training. In total, 7 blood samples were drawn throughout the course of training (3 pre-, 2 mid- and 2 post-training samples). The results revealed that cortisol levels changed from an initial concentration of 14.07–11.45 mcg/dl after training, exhibiting a 43% training-related reduction in cortisol concentration. In addition, the reduction in cortisol levels was in line with the reported post-training behavioral changes in anxiety, impulsivity and anger, as well as improved mood and life satisfaction (Durousseau 2013).
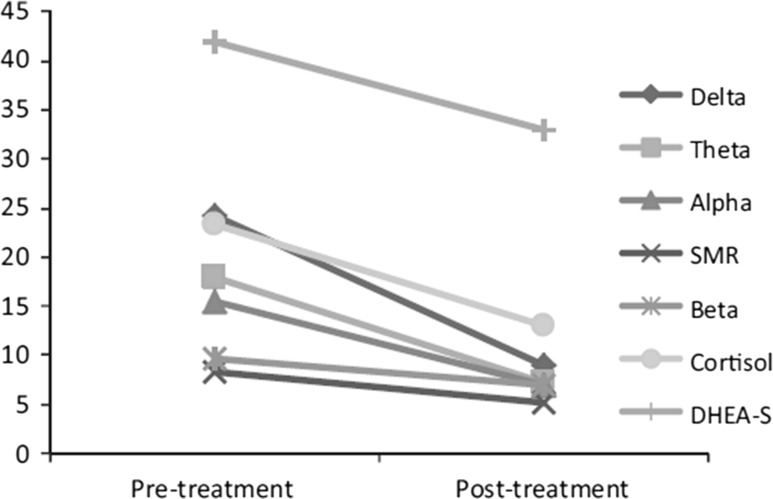
The role of NFB in altering cortisol and dehydroepiadrosterone (DHEA) was also examined in a case study by (Burns 2015) on the treatment of a patient with hereditary angioedema (HAE). The study reported that NFB training altered cortisol and DHEA levels. The onset of an HAE episode typically involves psychological and physical stress, as well as other bodily and environmental changes. A 43-year-old female patient who experienced her first HAE episode at age 8, underwent 20 half-hour NFB sessions, and cortisol and DHEAS levels were measured pre- and post-NFB training. As shown in Fig. 3, the pre-NFB cortisol level was 23.3, with a pre-NFB DHEAS level of 44 and a cortisol/DHEAS ratio of 0.55. Post-NFB measures taken 4 weeks after the last NFB session revealed a cortisol level of 13.1, a DHEAS level of 33 and a cortisol/DHEAS ratio 0.40. In addition, the results indicated a sustained effect of NFB treatment in reducing stress after 4 weeks. Because HAE is such a rare condition (affecting only 1 in 50,000 people), this study included only one subject. However, this represents pioneering research, suggesting the potential usefulness of NFB in reducing psychological and physiological stress in HAE. Moreover, reductions in the level of cortisol and the cortisol/DHEA ratio suggesting reduced stress were in line with the patient’s self-reported improved quality of life.
Fig. 3.
Pre- and post-treatment comparison of brainwaves, cortisol and DHEA-S. Reproduced from Burns (2015)
The exposed NIR light is partially absorbed by the hemoglobin while about 10 time more light is scattered in tissues before undergoing absorption in the cerebrum (Delpy and Cope 1997). In order to retrieve reliable and accurate signals from the cerebral cortex, numerous methods to compensate the scattering effect have been proposed such as multi-distance independent component analysis (MD-ICA). MC-ICA is capable of scalp signal separation but it requires time series data for statistical purposes. Due to this constraint, MD-ICA cannot be used for real-time processing as required in NFB. Alternatively, (Ung et al. 2017) examined the real-time scalp signal separating (RT-SSS) algorithm for the retrieval of reliable scalp signals. RT-SSS takes the source-detector (SD) distance into account. According to RT-SSS algorithm, when the SD distance is larger than the minimum distance for the exposed NIR light to penetrate the deep layer, the scalp signals can be considered as constant. Therefore, the scalp signal sensed at the extra detector can be discarded from the mixed NIRS signal received at standard-distance detectors to yield deep signals.
fMRI-based NFB
Tegethoff (2016) recently proposed a study of the modulation of stress reactivity using fMRI-based neurofeedback that seeks to establish a real time fMRI-based NF protocol targeting subjective, endocrine and neural reactivity to psychosocial stress (Tegethoff 2016) (ClinicalTrials.gov Identifier: NCT02560233). The proposed study has three main goals: (a) to evaluate whether RT-fMRI-NF-based training of volitional control over specific brain activity can modulate neural and subjective reactivity to stress; (b) to evaluate whether acquired neuromodulation can provide a new method for modulating peripheral hormone release; and (c) to evaluate the efficacy of acquired neuromodulation and examine the effects on activity in the central nervous system (CNS) and ANS, measured using EEG and HRV, respectively. The results of this study have not yet been published. A list of neurofeedback based studies is shown in Table 2.
Table 2.
List of EEG NFB studies
| First author | Training | Control | Assessment | Training sessions and duration | Sample size and characteristics (male/female; training/control) | Occupation/condition | Age of subjects | Results |
|---|---|---|---|---|---|---|---|---|
| Bazanova et al. (2013) | Increased alpha activity at Pz with right ear as reference | Sham | Psychometric tests, HRV, EMG | 10 sessions | 27 (14 male) | ND | 18–34 years | Individual NFB alpha training effectively altered HRV, providing predictive value. Subjects with low baseline alpha power exhibited a positive correlation between alpha peak frequency and pNN50, while a negative correlation was observed in subjects with high baseline alpha power. Increased accuracy and control of cognitive performance, decreased anxiety and frontal EMG activity, and enhanced resting frequency was caused by alpha training. Alpha training also abolished a decline in alpha power in response to an arithmetic task even after 1 month |
| Durousseau (2013) | Z-score NFB | ND | Neuropsychological assessment, plasma cortisol from one subject | 20 | 2 | Military specialists | NFB-related Z-score changes in brain waves towards normative patterns, and reduction in anxiety and stress. Improvement in management of daily behaviors | |
| Gilroy et al. (2013) | Alpha asymmetry at F3-F4 | fMRI | 2 NFB windows of 30 s during an active session of 8 min | 15 (12 male), | ND | 29.38 ± 7.6 | Prefrontal alpha asymmetry NFB training increased empathic support responses. MRI scans revealed activation in associated brain regions during the expression of support | |
| Kotozaki et al. (2014) | 1chNIRS at frontopolar cortex (double blind) | Sham | MRI, psychological measures and salivary cortisol | 4 weeks of daily 30-min training sessions | 30 males; 15 controls | ND | 23–53 | Intervention group exhibited significant improvement in psychological test scores and reduced salivary cortisol levels. Findings indicate that BFT is effective for protecting GM structures from the effects of stress |
| Burns (2015) | Increased SMR, decreased theta, decreased high beta at T4-P4 | None | Psychometric assessment (SCL-90-R and QOLI), cortisol and DHEAS | 20–30-min sessions | 1 female | HAE | 43 | The results suggest a reduction of emotional, mental, and physical triggers of stress. NFB training led to reduced cortisol levels, reduced cortisol-DHEA-S ratio, decreased use of acute medication, reduced activity on all average brainwave measures (delta, theta, beta, alpha and SMR) and improved quality of life assessment scores |
| Brühl et al. (2014) | RT-FMRI-NFB in amygdala | None | 4 sessions | 6 (2 male) | ND | 26 ± 3.8 | The results support the use of RT-fMRI-NFB training for regulating brain activity during negative emotional stimulation, and have implications for clinical treatment of emotional disorders |
HAE: Hereditary Angioedema; ND: not described; SCL-90-R: symptom checklist-90-R; QOLI: quality of life inventory; DHEA: dehydroepiandrosterone-sulfate
Non-invasive brain stimulation
Techniques involving non-invasive brain stimulation (NIBS) have recently become the focus of renewed interest following developments in fundamental and clinical neuroscience. Two main techniques of NIBS have been intensively studied: transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) (Fregni and Pascual-Leone 2007). Although research into both techniques has provided new insight into cortical excitability and the treatment of numerous clinical conditions (Fregni and Pascual-Leone 2007), their mechanisms of action remain poorly understood.
NIBS has been found to modulate stress and stress-related physiological mechanisms, and systematic reviews of research into the impact of NIBS on cortisol and ANS–vital indicators of stress—have been published (Sampaio et al. 2012; Schestatsky et al. 2013). Here we review a number of new studies on the physiological effects of NIBS on ANS, and neuroendocrine effects on cortisol, published since these previous review articles. According to a SCOPUS literature search, eight studies have been published in the last 3 years. To systematically compare the outcomes of these studies, we have examined the data using several parameters involved in the performance of NIBS, presented in Table 3. These parameters include: (1) number of subjects; (2) location of brain stimulation; (3) reference location of brain stimulation; (4) polarity of stimulation; (5) blindness of the study design; (6) current density; (7) duration of stimulation; (8) number of sessions of brain stimulation; (9) assessment parameters and (10) use of a stressor.
Table 3.
List of NIBS studies
| First author | Number of subjects | Stimulation electrode location | Reference electrode location | Polarity | Blind | Current density (mA/cm2) | Stimulation duration (min) | Number of sessions | Assesment | Task | Result |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brunoni et al. (2013) | 20, healthy subjects, 24.9 ± 3.8 | L DLPFC | R DLPFC | A/C/S | Single blind | 0.04 | 33 | 1 | HRV and salivary cortisol | Picture-induced stress | Left anodal tDCS inhibited cortisol and enhanced vagal tone |
| Antal et al. (2014) | 60, 25 ± 6 | R mPFC (F2-FPz) | O2-P4 | A/C/S | Double blind | 20 | Salivary cortisol, fMRI | Task-induced stress | Anodal stimulation inhibited cortisol and increased rCBF in the right mPFC (compared with sham stimulation) and increased rCBF in the right amygdala and PFC. Cathodal stimulation increased cortisol levels | ||
| Feeser et al. (2014) | 42, 29.8 ± 6.2 | R DLPFC | Left supraorbital region | A/S | Double blind | 0.04 | 20 | 1 | SCR and eye tracking | Picture-induced stress | Depending on the goal, tDCS can upregulate or downregulate cognitive appraisal |
| Sarkar et al. (2014) | 23.54 ± 3.11 | L DLPFC (F3) | R DLPFC (F4) | A/S | Double blind | 0.04 | 30 | 2 crossover | Salivary cortisol | Mathematics task | tDCS improved reaction times on simple arithmetic decisions and decreased cortisol concentration in subjects with high levels of mathematics-related anxiety. In contrast, tDCS increased reaction times for subjects with low levels of mathematics-related anxiety and prevented a decrease in cortisol concentration compared with sham stimulation |
| Schroeder et al. (2015) | 22, rejected: 4, 3 male, 31.3 ± 2.5 | L DLPFC (F3) | Right upper arm | A/S | Double blind | 0.02 | 20 | 2 crossover | SCR | Delayed responses in working memory task and emotional picture task | Anodal stimulation reduced SCR to emotional pictures |
| Okano et al. (2015) | 10, 33 ± 9 | T3 | Fp2 | A/S | Single blind | 0.05 | 20 | 2 crossover | HR, R–R interval | Physical workload (cycling) | Anodal stimulation improved cycling performance while reducing HR and delaying parasympathetic vagal tone |
| Austin et al. (2016) | 66 female, 21.6 ± 2.3 years | F3 | F4 | A/S | E1: single blind, E2: double blind | 0.06 | 12 | E1: 5, E2: 3 | Mood assessment questionnaire | Prefrontal stimulation successfully improved mood states in non-depressed individuals | |
| Bogdanov and Schwabe (2016) | 120, 60 female, 25.2 ± 0.31 | DLPFC (F4) | Cz | A/C/S | Double blind | 0.043 for active and 0.011 for reference electrode location | 1 | Subjective measures with BP, blood pulse and salivary cortisol | Stressor manipulation | Anodal stimulation led to improved working memory performance after stress | |
| Raimundo et al. (2012) | 50 | M1-L (C3) | Right supraorbital region (FP2) | A/S | Double blind | 0.03 | 10 | 1 | BP, BT, HR, RR, plasma cortisol | None | Both active and sham stimulation significantly changed hand skin temperature and cortisol with active stimulation having no significant difference to sham stimulation |
| Vigod et al. (2014) | 20–40 | F3 | F4 | A/S | Double blind | 0.57 | 15–30 | 15 | BR, HR | None | tDCS is predicted to result in significant improvement of depression without affecting autonomic rate, core body temperature and ventilation rate |
| Hamner et al. (2015) | 15, 21–30 years | M1-L (C3) | Right supraorbital region (FP2) | A/S | 0.57 | 40 | 2 crossover sessions with minimum 7 days to maximum 8 weeks separation | BP, HR, leg blood flow and leg vascular resistance | Cold pressor test | Anodal tDCS at C3 did not affect basal hemodynamics or ANS and had only modest effects on responses to acute pain in healthy subjects |
M male subjects, f female subjects, E1 experiment 1, E2 experiment 2
A total of 385 subjects participated in these studies, with a mean age of 26.79 ± 9 years. The active stimulation locations tested include: left dorsolateral prefrontal cortex (left DLPFC, F3; used in four studies, three times with reference to its lateral location F4, and once with the right upper arm as a reference); right DLPFC (R-DLPFC, F4; used in two studies, once with Cz and once with the left supraorbital region FP1 as a reference); and, right medial prefrontal cortex (R-mPFC, FP2-FPz; with a left temporal location, T3, actively stimulated in each study, and O2-P4 and FP2 as reference locations, respectively). Five of the studies used anodal stimulation compared with sham stimulation, while three studies also applied cathodal stimulation in addition to anodal and sham stimulation conditions. Two studies were single-blind, while five were double-blind. One study included two experiments, one of which was single blind while the other was double blind, with a total of three single blind and six double blind experiments. The minimum current density used was 0.02 mA/cm2, and the maximum was 0.06 mA/cm2. The stimulation period in all studies was between 12 and 33 min. Except for one study involving two experiments composed of five and three sessions of stimulation, all other studies were either composed of one session of parallel stimulation (active or sham in four studies) or two crossover sessions of stimulation testing the same subject in both active and sham stimulation conditions (three studies). Four studies utilized ANS markers for assessment such as SC (two studies), HR (one study), HRV (two studies), and two of the studies measured changes in cortisol levels. Of these, one study also recorded stimulation-related changes using fMRI. Most studies used a stressor in their methodology.
Several of these recent studies examined the mediation of stress responses after the application of tDCS. (Brunoni et al. 2013) investigated emotionally-triggered neuroendocrine responses using salivary cortisol levels, associated with modulation of dorsolateral PFC (DLPFC) excitability induced by tDCS. A study (Antal et al. 2014) investigated the mechanisms mediating psychosocial stress responses after the application of tDCS to the medial PFC (mPFC). Combining functional imaging, endocrinological measurement and psychophysics, the results revealed that mPFC activation was mediated by applying tDCS before performing an experimental task. This intervention was found to modify baseline regional cerebral blood flow (rCBF) under the electrode location. rCBF showed greater increases under anodal stimulation conditions, compared with sham and cathodal stimulation. Moreover, tDCS mediated cortisol release in a polarity-dependent way: cathodal stimulation increased the salivary cortisol response, while anodal stimulation decreased it. This finding of cortisol inhibition with anodal stimulation is in accord with the findings of Brunoni and colleagues (2013). However, that study reported that left frontal stimulation induced cortisol inhibition (Brunoni et al. 2013), in contrast to the significant effects of right frontal stimulation reported by Antal and colleagues (Antal et al. 2014). This difference may have been related to the different experimental methodologies used in the two studies, with the induction of negative emotion used in the former study, and the induction of psychosocial stress in the latter study. In both studies, sham stimulation did not cause any significant changes.
Some evidence suggests that tDCS may alter ANS and hypothalamic function, and several studies have reported non-specific effects (Sampaio et al. 2012; Schestatsky et al. 2013). However, it remains unclear whether conventional tDCS protocols cause acute changes in autonomic or hypothalamic functioning.
Raimundo et al. (2012) studied the effects of 10-min tDCS sessions on 50 subjects, divided into active and sham stimulation groups. Both groups exhibited significant variations in skin temperature and cortisol levels, suggesting a generic stress response to the stimulation protocol. Thus, the study found no evidence of tDCS-specific alterations in autonomic function, body temperature or ventilation rate (Raimundo et al. 2012). In addition, Hamner and colleagues reported no tDCS-related effects on autonomic functioning in a study of 15 subjects participating in cold pressor tests at 0, 7 and 14 °C followed by a single session of active and sham stimulation (Hamner et al. 2015). Although the active stimulation group showed marginal differences in heart rate, blood pressure and the perception of pain after 14 °C cold pressor tests, the effects did not reach statistical significance. In addition, tDCS had no significant effect on heart rate, blood pressure, leg blood flow, and leg vascular resistance at rest (Hamner et al. 2015).
Non-specific changes in ANS and cortisol due to brain stimulation might be due to a lack of consistent stimulation parameters and ANS and cortisol measuring techniques. So far, no uniform stimulation parameters have been identified, an issue that requires more attention in future research. However, the significant variation reported by Raimundo and colleagues in the sham stimulation condition appears likely to be caused by more than parameter selection alone, and may require a closer examination of the study design (Raimundo et al. 2012).
Vigod et al. (2014) proposed a protocol for depressed women during pregnancy. The proposed treatment is designed to minimize changes of autonomic function, body temperature and ventilation rate.
Practical methods for stress intervention
emWave is a handheld Personal Stress Relieving (PSR) technology (Institute of HeartMath, USA (“HeartMath, emWave 2016, Available: https://www.heartmath.com/,” 2016)) designed to induce heart coherence, a positive mental state associated with a particular heart rate activity pattern, stress reduction and greater emotional stability. In addition, the Stress Control Suite (Thought Technology, Canada) is a set of BFB tools designed to help a user assess and track their stress responses, using a stress test. This test uses simple BFB methodology to teach self-regulation and body awareness strategies, training users to quickly and efficiently relax and return to a baseline relaxation state. This suite utilizes SC, body temperature variation and respiration rate to provide measures of arousal and stress levels. The BrainMaster StressTherapy Solutions system (“Stress Therapy Solutions 2015, Available: http://stresstherapysolutions.com/”) utilizes QEEG, sLORETA and database-based NFB training using BrainMaster EEG equipment and Neuroguide software.
Conclusion
The mitigation of stress is an important research area with the increasingly ubiquitous demands of modern lifestyles. With the advent of physiological measures for the identification of stress, recent research into applications of these measures has opened new horizons for the objective treatment of stress. The measurement of BFB using HRV, respiration and skin conductance may be particularly useful in this regard, targeting direct quantitative markers of ANS.
With substantial developments in the field of neuroscience in recent years, neuroimaging methods have revealed the role of several important brain regions during exposure to stress. For example, the amygdala has been identified as a key brain region for the processing of fear. NFB is an emerging technique for rehabilitation and intervention that makes use of operant conditioning to alter brain and behavioral responses by self-regulating brain activity. Until recently, NFB training was limited to the clinical treatment of several conditions, including epilepsy and sleep disorders. However, the use of this technique is now expanding to applications in diverse fields, including clinical as well as non-clinical applications. Various NFB training approaches have been studied and implemented with different technical methodologies, but with a common goal to train the brain to operate optimally under given conditions.
NFB training has been found to significantly improve the conditions of stress and related dysfunctions, including anxiety and depression. Alpha enhancement training has been extensively studied as a treatment for stress-related anxiety and depression. For example, Van Boxtel et al. (2012) reported significant positive effects of alpha enhancement training compared with beta training, supported by evidence from a range of behavioral measures (Van Boxtel et al. 2012). In addition, maintaining asymmetry in alpha power across the hemispheres has been established as an effective treatment for patients with depression (Baehr et al. 2001; Harmon-Jones et al. 2010; Henriques and Davidson 1990, 1991).
In many studies, the effects of NFB training have been validated with questionnaires to confirm training-related improvements, and physiological measures can provide further objective validation methods. Cortisol is one such physiological measure of stress. However, to date there have been few studies of the effects of NFB training using cortisol measures. Additional cortisol measurement studies are required to further clarify the mechanisms of NFB training-related improvements in stress responses. Changes in HRV have also been measured in response to NFB training, serving as a useful marker of stress and an appropriate validation measure.
Although NFB has been established as a valid treatment method for many disorders, the optimal training duration remains an open question. The studies included in the present review ranged from a single training session to 35 sessions. However, in most studies, eight to ten training sessions were considered appropriate. In addition, the duration of the gap between consecutive sessions has not yet been standardized.
In studies of NFB training, particularly frequency-based training targeting specific cortical areas, the effects of training on other parts of the brain remain largely unclear. Further research is required to clarify this question, investigating whether the effects of NFB training are localized to specific brain areas or if widespread effects are involved.
NIBS has recently been the subject of renewed interest, including its potential regulatory effects on ANS and hypothalamic functioning. Despite several studies reporting the success of NIBS methods in neurorehabilitation, its mechanism of action remains largely unclear. However, a small number of studies have also highlighted the nonspecific effects of NIBS on ANS and hypothalamic functioning. Clarifying the specific effects of NIBS methods requires further investigation.
Acknowledgements
This work is partially supported by the Ministry of Education Malaysia under Higher Institution Centre of Excellence (HiCoE) Grant (0153CA-005) and Yayasan Universiti Teknologi PETRONAS Fundamental Research Grant (YUTP-FRG) (0153AA-E54).
References
- Alberdi A, Aztiria A, Basarab A. Towards an automatic early stress recognition system for office environments based on multimodal measurements: a review. J Biomed Inform. 2016;59:49–75. doi: 10.1016/j.jbi.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28(3):R1–39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- Antal A, Fischer T, Saiote C, Miller R, Chaieb L, Wang DJ, Kirschbaum C. Transcranial electrical stimulation modifies the neuronal response to psychosocial stress exposure. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arns M, Feddema I, Kenemans JL. Differential effects of theta/beta and SMR neurofeedback in ADHD on sleep onset latency. Front Hum Neurosci. 2014;8:1019. doi: 10.3389/fnhum.2014.01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten Amy F T. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci. 2015;18(10):1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51(4):356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Atkinson John, Campos Daniel. Improving BCI-based emotion recognition by combining EEG feature selection and kernel classifiers. Expert Syst Appl. 2016;47:35–41. [Google Scholar]
- Austin A, Jiga-Boy GM, Rea S, Newstead SA, Roderick S, Davis NJ, Boy F. Prefrontal electrical stimulation in non-depressed reduces levels of reported negative affects from daily stressors. Front Psychol. 2016 doi: 10.3389/fpsyg.2016.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr Elsa, Rosenfeld J Peter, Baehr Rufus. Clinical use of an alpha asymmetry neurofeedback protocol in the treatment of mood disorders. J Neurother. 2001;4(4):11–18. [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I Qualitative and quantitative electroencephalography. Clin Neurophys. 2003;114(2):171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Bazanova OM, Balioz NV, Muravleva KB, Skoraia MV. Voluntary alpha-power increasing training impact on the heart rate variability. Fiziol Cheloveka. 2013;39(1):103–116. doi: 10.7868/s0131164612060033. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neural mechanisms underlying selective attention to threat. Ann NY Acad Sci. 2008;1129:141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Bogdanov M, Schwabe L. Transcranial stimulation of the dorsolateral prefrontal cortex prevents stress-induced working memory deficits. J Neurosci. 2016;36(4):1429–1437. doi: 10.1523/JNEUROSCI.3687-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9(2):141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne LE, Jr, Yaroush RA. Stress and cognition a cognitive psychological perspective. Colorado: University of Colorado; 2003. [Google Scholar]
- Brennan M, Palaniswami M, Kamen P. Do existing measures of Poincare plot geometry reflect nonlinear features of heart rate variability? IEEE Trans Biomed Eng. 2001;48(11):1342–1347. doi: 10.1109/10.959330. [DOI] [PubMed] [Google Scholar]
- Brühl AB, Scherpiet S, Sulzer J, Stämpfli P, Seifritz E, Herwig U. Real-time neurofeedback using functional MRI could improve down-regulation of amygdala activity during emotional stimulation: a proof-of-concept study. Brain Topogr. 2014;27(1):138–148. doi: 10.1007/s10548-013-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Vanderhasselt MA, Boggio PS, Fregni F, Dantas EM, Mill JG, Benseñor IM. Polarity- and valence-dependent effects of prefrontal transcranial direct current stimulation on heart rate variability and salivary cortisol. Psychoneuroendocrinology. 2013;38(1):58–66. doi: 10.1016/j.psyneuen.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Burns ST. Neurofeedback in hereditary angioedema: a single case study of symptom reduction. Appl Psychophysiol Biofeedback. 2015 doi: 10.1007/s10484-015-9288-7. [DOI] [PubMed] [Google Scholar]
- Carneiro D, Castillo JC, Novais P, Fernández-Caballero A, Neves J. Multimodal behavioral analysis for non-invasive stress detection. Expert Syst Appl. 2012;39(18):13376–13389. [Google Scholar]
- Cavazza M, Charles F, Aranyi G, Porteous J, Gilroy SW, Raz G, Hendler T (2014) Towards emotional regulation through neurofeedback. In: Paper presented at the proceedings of the 5th augmented human international conference, Kobe, Japan
- Chan GSH, Middleton PM, Lovell NH, Celler BG (2005) Extraction of photoplethysmographic waveform variability by lowpass filtering, Shanghai [DOI] [PubMed]
- Chanel G, Kronegg J, Grandjean D, Pun T (2006) Emotion assessment: arousal evaluation using EEG’s and peripheral physiological signals. In: International workshop on multimedia content representation, classification and security, MRCS 2006: Vol. 4105 LNCS (pp. 530-537). Istanbul
- Chanel G, Kierkels JJM, Soleymani M, Pun T. Short-term emotion assessment in a recall paradigm. Int J Hum Comput Stud. 2009;67(8):607–627. [Google Scholar]
- Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci. 2015;18(10):1364–1375. doi: 10.1038/nn.4115. [DOI] [PubMed] [Google Scholar]
- De Jonckheere J, Ibarissene I, Flocteil M, Logier R (2014) A smartphone based cardiac coherence biofeedback system. In: Conference proceedings annual international conference of the IEEE Engineering in Medicine and Biology Society. Annual Conference, 2014, 4791–4794. 10.1109/EMBC.2014.6944695 [DOI] [PubMed]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Rexroth M, Wolff E, Duchesne A, Scherling C, Beaudry T, Pruessner JC. Neural correlates of processing stressful information: an event-related fMRI study. Brain Res. 2009;1293:49–60. doi: 10.1016/j.brainres.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, A J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. NeuroImage. 2009;47(3):864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Delpy DT, Cope M. Quantification in tissue near-infrared spectroscopy. Philos Trans R Soc B Biol Sci. 1997;352(1354):649–659. [Google Scholar]
- Dillon A, Kelly M, Robertson IH, Robertson DA. Smartphone applications utilizing biofeedback can aid stress reduction. Front Psychol. 2016 doi: 10.3389/fpsyg.2016.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis SI, Laskaris NA, Micheloyannis S. Transition dynamics of EEG-based network microstates during mental arithmetic and resting wakefulness reflects task-related modulations and developmental changes. Cogn Neurodyn. 2015;9(4):371–387. doi: 10.1007/s11571-015-9330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durantin G, Gagnon JF, Tremblay S, Dehais F. Using near infrared spectroscopy and heart rate variability to detect mental overload. Behav Brain Res. 2014;259:16–23. doi: 10.1016/j.bbr.2013.10.042. [DOI] [PubMed] [Google Scholar]
- Durousseau DR (2013) QEEG biomarkers: assessment and selection of special operators, and improving individual performance. vol. 8027 LNAI. Lecture Notes in Computer Science (including subseries lecture notes in artificial intelligence and lecture notes in bioinformatics) (pp 562–571)
- Dziembowska I, Izdebski P, Rasmus A, Brudny J, Grzelczak M, Cysewski P. Effects of heart rate variability biofeedback on EEG alpha asymmetry and anxiety symptoms in male athletes: a pilot study. Appl Psychophysiol Biofeedback. 2016;41(2):141–150. doi: 10.1007/s10484-015-9319-4. [DOI] [PubMed] [Google Scholar]
- Egner T, Gruzelier JH. Ecological validity of neurofeedback: modulation of slow wave EEG enhances musical performance. Neuro Rep. 2003;14(9):1221–1224. doi: 10.1097/01.wnr.0000081875.45938.d1. [DOI] [PubMed] [Google Scholar]
- Eysenck Michael W, Derakshan Nazanin. New perspectives in attentional control theory. Personal Individ Differ. 2011;50(7):955–960. [Google Scholar]
- Fedotchev AI. Efficacy of EEG biofeedback procedures in correcting stress-related functional disorders. Human Physiol. 2010;36(1):86–90. [Google Scholar]
- Feeser M, Prehn K, Kazzer P, Mungee A, Bajbouj M. Transcranial direct current stimulation enhances cognitive control during emotion regulation. Brain Stimul. 2014;7(1):105–112. doi: 10.1016/j.brs.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Foley P, Kirschbaum C. Human hypothalamus-pituitary-adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci Biobehav Rev. 2010;35(1):91–96. doi: 10.1016/j.neubiorev.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology Insight: noninvasive brain stimulation in neurology—perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3(7):383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Gärtner M, Rohde-Liebenau L, Grimm S, Bajbouj M. Working memory-related frontal theta activity is decreased under acute stress. Psychoneuroendocrinology. 2014;43:105–113. doi: 10.1016/j.psyneuen.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Gilroy SW, Porteous J, Charles F, Cavazza M, Soreq E, Raz G, Hendler T (2013) A brain-computer interface to a plan-based narrative. In: Paper presented at the IJCAI international joint conference on artificial intelligence
- Gruzelier J, Hardman E, Wild J, Zaman R. Learned control of slow potential interhemispheric asymmetry in schizophrenia. Int J Psychophysiol. 1999;34(3):341–348. doi: 10.1016/s0167-8760(99)00091-4. [DOI] [PubMed] [Google Scholar]
- Haapalainen E, Kim S, Forlizzi JF, Dey AK (2010) Psycho-physiological measures for assessing cognitive load. In: Paper presented at the UbiComp’10—Proceedings of the 2010 ACM conference on ubiquitous computing
- Hamner JW, Villamar MF, Fregni F, Taylor JA. Transcranial direct current stimulation (tDCS) and the cardiovascular responses to acute pain in humans. Clin Neurophysiol. 2015;126(5):1039–1046. doi: 10.1016/j.clinph.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Hardt JV, Kamiya J. Anxiety change through electroencephalographic alpha feedback seen only in high anxiety subjects. Science. 1978;201(4350):79–81. doi: 10.1126/science.663641. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biol Psychol. 2010;84(3):451–462. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Healey JA, Picard RW. Detecting stress during real-world driving tasks using physiological sensors. IEEE Trans Intell Transp Syst. 2005;6(2):156–166. [Google Scholar]
- HeartMath, emWave (2016) https://www.heartmath.com/
- Hembree R. Correlates, causes, effects, and treatment of test anxiety. Rev Edu Res. 1988;58(1):47–77. [Google Scholar]
- Hendler T (2013) fMRI based EEG neurofeedback as a method of enhancing emotional resilience among soldiers. Retrieved 27 June 2016, from https://clinicaltrials.gov/ct2/show/record/NCT02020265
- Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. J Abnorm Psychol. 1990;99(1):22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnorm Psychol. 1991;100(4):535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Stress hormone regulation: biological role and translation into therapy. Ann Rev Psychol. 2010;61:81–109. doi: 10.1146/annurev.psych.093008.100321. [DOI] [PubMed] [Google Scholar]
- Jarczok MN, Jarczok M, M D, Koenig J, Li J, Herr RM, Thayer JF. Autonomic nervous system activity and workplace stressors—A systematic review. Neurosci Biobehav Rev. 2013;37(8):1810–1823. doi: 10.1016/j.neubiorev.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Kageyama Y, Odagaki M, Hosaka H (2007) Wavelet analysis for quantification of mental stress stage by finger-tip photo-plethysmography. In: Paper presented at the 29th annual international conference of IEEE-EMBS, engineering in Medicine and Biology Society, EMBC’07, Lyon [DOI] [PubMed]
- Kaushik A, Vasudev A, Arya SK, Pasha SK, Bhansali S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens Bioelectron. 2014;53:499–512. doi: 10.1016/j.bios.2013.09.060. [DOI] [PubMed] [Google Scholar]
- Kotozaki Y, Takeuchi H, Sekiguchi A, Yamamoto Y, Shinada T, Araki T, Kawashima R. Biofeedback-based training for stress management in daily hassles: an intervention study. Brain Behav. 2014;4(4):566–579. doi: 10.1002/brb3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer KM, Luberto CM, O’Bryan EM, Mysinger E, Cotton S. Mind-body skills training to improve distress tolerance in medical students: a pilot study. Teach Learn Med. 2016;28(2):219–228. doi: 10.1080/10401334.2016.1146605. [DOI] [PubMed] [Google Scholar]
- Kudo N, Shinohara H, Kodama H. Heart rate variability biofeedback intervention for reduction of psychological stress during the early postpartum period. Appl Psychophysiol Biofeedback. 2014;39(3–4):203–211. doi: 10.1007/s10484-014-9259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers NT, Sauder CL, Carter JR, Ray CA. Neurovascular responses to mental stress in the supine and upright postures. J Appl Physiol. 2008;104(4):1129–1136. doi: 10.1152/japplphysiol.01285.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, Meyer-Lindenberg A. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474(7352):498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leff J, Vaughn C. The role of maintenance therapy and relatives’ expressed emotion in relapse of schizophrenia: a two-year follow up. Br J Psychiatry. 1981;139:102–104. doi: 10.1192/bjp.139.2.102. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77(3):406–424. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 2007;65(3):209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Maduka Ignatius C, Neboh Emeka E, Ufelle Silas A. The relationship between serum cortisol, adrenaline, blood glucose and lipid profile of undergraduate students under examination stress. Afr Health Sci. 2015;15(1):131–136. doi: 10.4314/ahs.v15i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84(2):482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- Mandrick K, Derosiere G, Dray G, Coulon D, Micallef J-P, Perrey S. Prefrontal cortex activity during motor tasks with additional mental load requiring attentional demand: a near-infrared spectroscopy study. Neurosci Res. 2013;76(3):156–162. doi: 10.1016/j.neures.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Marin M-F, Lord C, Andrews J, Juster R-P, Sindi S, Arsenault-Lapierre G, Lupien SJ. Chronic stress, cognitive functioning and mental health. Neurobiol Learn Mem. 2011;96(4):583–595. doi: 10.1016/j.nlm.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Mariotti A. The effects of chronic stress on health: new insights into the molecular mechanisms of brain–body communication. Future Sci OA. 2015 doi: 10.4155/fso.15.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, G JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C. Mechanisms of stress in the brain. Nat Neurosci. 2015;18(10):1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier NF, Welch AS. Walking versus biofeedback: a comparison of acute interventions for stressed students. Anxiety Stress Coping. 2016;29(5):463–478. doi: 10.1080/10615806.2015.1085514. [DOI] [PubMed] [Google Scholar]
- Micoulaud-Franchi JA, Kotwas I, Lanteaume L, Berthet C, Bastien M, Vion-Dury J, Bartolomei F. Skin conductance biofeedback training in adults with drug-resistant temporal lobe epilepsy and stress-triggered seizures: a proof-of-concept study. Epilepsy Behav. 2014;41:244–250. doi: 10.1016/j.yebeh.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Mor S, Day HI, Flett GL, Hewitt PL. Perfectionism, control, and components of performance anxiety in professional artists. Cognit Therapy Res. 1995;19(2):207–225. [Google Scholar]
- Munafò M, Patron E, Palomba D. Improving managers’ psychophysical well-being: effectiveness of respiratory sinus arrhythmia biofeedback. Appl Psychophysiol Biofeedback. 2016;41(2):129–139. doi: 10.1007/s10484-015-9320-y. [DOI] [PubMed] [Google Scholar]
- Nemati S, Malhotra A, Clifford GD. Data fusion for improved respiration rate estimation. EURASIP J Adv Signal Process. 2010;2010:1–11. doi: 10.1155/2010/926305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano AH, Fontes EB, Montenegro RA, Farinatti PD, Cyrino ES, Li LM, Noakes TD. Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. Br J Sports Med. 2015;49(18):1213–1218. doi: 10.1136/bjsports-2012-091658. [DOI] [PubMed] [Google Scholar]
- Palacios-Garcia I, Villena-Gonzalez M, Campos-Arteaga G, Artigas C, Jaramillo K, Silva J, Rodriguez E. Psychosocial stress affects attentional control and neural oscillatory activity. Psychoneuroendocrinology. 2015;61:44–45. [Google Scholar]
- Palekar TJ, Mokashi MG, Anwer S, Kakrani AL, Khandare SD, Alghadir AH. Effect of galvanic skin resistance-aided biofeedback training in reducing the pulse rate, respiratory rate, and blood pressure due to perceived stress in physiotherapy students. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi. 2015;61(2):116–119. [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Prinsloo GE, Laurie Rauch HG, Derman WE. A brief review and clinical application of heart rate variability biofeedback in sports, exercise, and rehabilitation medicine. Phys Sportsmed. 2014;42(2):88–99. doi: 10.3810/psm.2014.05.2061. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Lupien SJ. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations—2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35(1):179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Putman P, Verkuil B, Arias-Garcia E, Pantazi I, Van Schie C. EEG theta/beta ratio as a potential biomarker for attentional control and resilience against deleterious effects of stress on attention. Cognit Affect Behav Neurosci. 2014;14(2):782–791. doi: 10.3758/s13415-013-0238-7. [DOI] [PubMed] [Google Scholar]
- Raimundo RJS, Uribe CE, Brasil-Neto JP. Lack of clinically detectable acute changes on autonomic or thermoregulatory functions in healthy subjects after transcranial direct current stimulation (tDCS) Brain Stimul. 2012;5(3):196–200. doi: 10.1016/j.brs.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Ratanasiripong P, Park JF, Ratanasiripong N, Kathalae D. Stress and anxiety management in nursing students: biofeedback and mindfulness meditation. J Nurs Educ. 2015;54(9):520–524. doi: 10.3928/01484834-20150814-07. [DOI] [PubMed] [Google Scholar]
- Raymond J, Sajid I, Parkinson LA, Gruzelier JH. Biofeedback and dance performance: a preliminary investigation. Appl Psychophysiol Biofeedback. 2005;30(1):65–73. doi: 10.1007/s10484-005-2175-x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10(6):423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Ryu K, Myung R. Evaluation of mental workload with a combined measure based on physiological indices during a dual task of tracking and mental arithmetic. Int J Ind Ergon. 2005;35(11):991–1009. [Google Scholar]
- Sampaio LANPC, Fraguas R, Lotufo PA, Benseñor IM, Brunoni AR (2012) A systematic review of non-invasive brain stimulation therapies and cardiovascular risk: Implications for the treatment of major depressive disorder. Front Psychiatry. 10.3389/fpsyt.2012.00087 [DOI] [PMC free article] [PubMed]
- Sarabia-Cobo CM. Heart coherence: a new tool in the management of stress on professionals and family caregivers of patients with dementia. Appl Psychophysiol Biofeedback. 2015;40(2):75–83. doi: 10.1007/s10484-015-9276-y. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Dowker A, Kadosh RC. Cognitive enhancement or cognitive cost: trait-specific outcomes of brain stimulation in the case of mathematics anxiety. J Neurosci. 2014;34(50):16605–16610. doi: 10.1523/JNEUROSCI.3129-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schestatsky P, Simis M, Freeman R, Pascual-Leone A, Fregni F. Non-invasive brain stimulation and the autonomic nervous system. Clin Neurophysiol. 2013;124(9):1716–1728. doi: 10.1016/j.clinph.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Walach H. Electrodermal activity (EDA) - State-of-the-art measurement and techniques for parapsychological purposes. J Parapsychol. 2000;64(2):139–163. [Google Scholar]
- Schoenberg PLA, David AS. Biofeedback for psychiatric disorders: a systematic review. Appl Psychophysiol Biofeedback. 2014;39(2):109–135. doi: 10.1007/s10484-014-9246-9. [DOI] [PubMed] [Google Scholar]
- Schroeder PA, Ehlis AC, Wolkenstein L, Fallgatter AJ, Plewnia C (2015) Emotional distraction and bodily reaction: Modulation of autonomous responses by anodal tDCS to the prefrontal cortex. Front Cell Neurosci. 10.3389/fncel.2015.00482 [DOI] [PMC free article] [PubMed]
- Setz C, Arnrich B, Schumm J, La Marca R, Tröster G, Ehlert U. Discriminating stress from cognitive load using a wearable eda device. IEEE Trans Inf Technol Biomed. 2010;14(2):410–417. doi: 10.1109/TITB.2009.2036164. [DOI] [PubMed] [Google Scholar]
- Sharma N, Gedeon T. Objective measures, sensors and computational techniques for stress recognition and classification: a survey. Comput Methods Programs Biomed. 2012;108(3):1287–1301. doi: 10.1016/j.cmpb.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Sherlin LH, Larson NC, Sherlin RM. Developing a performance brain training™ approach for baseball: a process analysis with descriptive data. Appl Psychophysiol Biofeedback. 2013;38(1):29–44. doi: 10.1007/s10484-012-9205-2. [DOI] [PubMed] [Google Scholar]
- Sioni R, Chittaro L. Stress detection using physiological sensors. Computer. 2015;48(10):26–33. [Google Scholar]
- Stein F, Nikolic S. Teaching stress management techniques to a schizophrenic patient. Am J Occup Ther. 1989;43(3):162–169. doi: 10.5014/ajot.43.3.162. [DOI] [PubMed] [Google Scholar]
- Stress Therapy Solutions (2015) http://stresstherapysolutions.com/
- Tanaka G, Sawada Y. Examination of normalized pulse volume-blood volume relationship: toward a more valid estimation of the finger sympathetic tone. Int J Psychophysiol. 2003;48(3):293–306. doi: 10.1016/s0167-8760(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Tegethoff M (2016) Modulating stress-reactivity by real-time multimodal functional neuroimaging based neurofeedback. https://clinicaltrials.gov/ct2/show/NCT02560233
- Teixeira RR, Díaz MM, Da Silva Santos TV, Bernardes JTM, Peixoto LG, Bocanegra OL, Espindola FS. Chronic stress induces a hyporeactivity of the autonomic nervous system in response to acute mental stressor and impairs cognitive performance in business executives. PLoS ONE. 2015 doi: 10.1371/journal.pone.0119025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharawadeepimuk K, Wongsawat Y. Quantitative EEG evaluation for performance level analysis of professional female soccer players. Cognit Neurodyn. 2017;11(3):233–244. doi: 10.1007/s11571-017-9427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung WC, Funane T, Katura T, Sato H, Tang TB, Mohammad Hani AF, Kiguchi M. Effectiveness evaluation of real-time scalp signal separating algorithm on near-infrared spectroscopy neurofeedback. IEEE J Biomed Health Inform. 2017 doi: 10.1109/JBHI.2017.2723024. [DOI] [PubMed] [Google Scholar]
- Van Boxtel GJM, Denissen AJM, Jäger M, Vernon D, Dekker MKJ, Mihajlović V, Sitskoorn MM. A novel self-guided approach to alpha activity training. Int J Psychophysiol. 2012;83(3):282–294. doi: 10.1016/j.ijpsycho.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Van Den Broek EL, Westerink JHDM (2012) Biofeedback systems for stress reduction: Towards a bright future for a revitalized field. In: Paper presented at the HEALTHINF 2012—Proceedings of the international conference on health informatics, Vilamoura, Algarve
- van der Zwan JE, de Vente W, Huizink AC, Bögels SM, de Bruin EI. Physical activity, mindfulness meditation, or heart rate variability biofeedback for stress reduction: a randomized controlled trial. Appl Psychophysiol Biofeedback. 2015;40(4):257–268. doi: 10.1007/s10484-015-9293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigod S, Dennis CL, Daskalakis Z, Murphy K, Ray J, Oberlander T, Blumberger D. Transcranial direct current stimulation (tDCS) for treatment of major depression during pregnancy: study protocol for a pilot randomized controlled trial. Trials. 2014 doi: 10.1186/1745-6215-15-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, Part II: prefrontal-subcortical pathways and relationship with anxiety. NeuroImage. 2009;47(3):836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whited A, Larkin KT, Whited M. Effectiveness of emWave biofeedback in improving heart rate variability reactivity to and recovery from stress. Appl Psychophysiol Biofeedback. 2014;39(2):75–88. doi: 10.1007/s10484-014-9243-z. [DOI] [PubMed] [Google Scholar]
- Wilson GF, Lambert JD, Russell CA (2000) Performance enhancement with real-time physiologically controlled adaptive aiding. In: Paper presented at the proceedings of the XIVth Triennial congress of the international ergonomics association and 44th annual meeting of the Human Factors and Ergonomics Association, ‘Ergonomics for the New Millennnium’, San Diego, CA
- World Health Organization . The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva: World Health Organization; 1993. [Google Scholar]
- Zhai J, Barreto A (2006) Stress detection in computer users based on digital signal processing of noninvasive physiological variables. In: Paper presented at the 28th annual international conference of the IEEE engineering in medicine and biology society, EMBS’06, New York, NY [DOI] [PubMed]