Abstract
Purpose
Although there are several prospective clinical studies comparing radiofrequency ablation (RFA) and hepatic resection (HR) for the treatment of hepatocellular carcinoma, there are few trials that have been performed in strictly homogeneous patients.
Methods
Patients who were newly diagnosed with a solitary hepatocellular carcinoma were randomized to the HR or RFA group. Inclusion criteria were as follows: age ≥ 20 years but ≤ 70 years, Child-Pugh class A, maximal diameter of the tumor ≥ 2 cm but ≤ 4 cm, no previous treatment history, and platelet count > 80,000/mm3.
Results
Although the study was early terminated, 29 and 34 patients were enrolled in the HR and RFA groups, respectively, and prospectively followed on an intention-to-treat basis. The 5-year overall survival rates were 83.4% and 86.2% in the HR and RFA groups, respectively, which were not significantly different (P = 0.812 by log-rank, P = 0.990 by Breslow). The 3- and 5-year disease-free survival rates in the HR group were significantly superior to those in the RFA group (66.7%, 44.4% vs. 44.1%, 31.2%, P = 0.071 by log-rank, P = 0.023 by Breslow). Intrahepatic local recurrence tended to develop more frequently in the RFA group (P = 0.042), while the frequency of intrahepatic distant and extrahepatic recurrence was similar bet ween the 2 groups. There were no significant differences in the frequency and severity of complications between the 2 groups.
Conclusion
HR was significantly superior to RFA in terms of disease-free survival; however, the overall survival was excellent in both groups.
Keywords: Hepatocellular carcinoma, Hepatectomy, Catheter ablation, Randomized controlled trial, Disease-free survival
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most frequently developed primary malignant tumor of the liver and the sixth most common cancer of all malignancies [1]. It is endemic in Asia and Africa, and its incidence is also increasing in Western countries [2,3]. Liver transplantation is theoretically the best treatment option for early HCCs with the Milan criteria because both the tumors and the underlying liver disease are simultaneously treated; however, the application of this attractive treatment is mostly limited by a serious donor organ shortage. Hence, hepatic resection (HR) is still considered to be a useful and effective first treatment option [4,5], although it is only suitable for less than 30% of all HCC patients [6,7]. The underlying cirrhosis and decompensated liver function that frequently accompany HCC are major obstacles to HR in many patients. In addition, the recurrence of tumors within the liver remnant is common, even after curative HR.
Today, small HCCs can be easily detected due to the wide use of screening tests. Local ablative therapies are safe and effective for treating small HCCs, and of these therapies, radiofrequency ablation (RFA) is considered the most effective one [5,8]. RFA provides encouraging results in treating small HCCs, with less invasiveness, shorter hospitalization, and lower expense [5]. In some cohort studies, the tumor control by RFA was shown to be excellent. The complete tumor ablation rate of RFA was more than 90%, and the local recurrence rate was less than 10% [9,10,11,12]. In addition, the 3-year survival rate was as good as 62% to 68% [9,12]. On the other hand, the results of HR have been recently improved by experienced surgeons. The treatment-related mortality of HR was only less than 2%, and the 5-year survival after HR for HCC was 40 to 70% [5]. Therefore, it is unclear which modality is superior for treating small HCCs.
There are some recent reports comparing RFA and HR for treating small HCCs. Chen et al. [7] claimed that percutaneous local ablative therapy might be as effective as surgical resection in the treatment of solitary and small HCCs. Four year later, however, Huang et al. [5] showed that surgical resection provided better survival and lower recurrence rates than RFA for patients with HCC conforming to the Milan criteria. Although both these studies were prospective randomized trials, they drew quite opposite conclusions. This may be because patients enrolled in these studies were too heterogeneous to allow a direct comparison between these 2 treatment modalities. Therefore, to compare the efficacy and safety of RFA and HR, we performed a prospective randomized study in HCC patients with very homogeneous underlying conditions.
METHODS
Diagnostic criteria and inclusion and exclusion criteria
Among the patients who were newly diagnosed with a solitary HCC, those who met the inclusion and exclusion criteria were enrolled in this clinical study. The diagnosis of HCC was made clinically based on the presence of risk factors, serum α-FP level, and typical imaging appearance on dynamic imaging studies, including CT, MRI, or hepatic artery angiography. Patients were diagnosed with HCC when they met the following criteria: (1) patients with risk factors for HCC, such as HBV, HCV, or cirrhosis; (2) serum α-FP ≥ 400 ng/mL + typical appearance on one dynamic imaging study or serum α-FP < 400 ng/mL + typical appearance on 2 dynamic imaging studies.
Inclusion criteria were as follows: (1) age between 20 and 70 years, (2) Child-Pugh class A (score 5–6), (3) single HCC, (4) maximal diameter of the tumor from 2 to 4 cm when measured on MRI or CT (The diameter on MRI was measured prior to that on CT), (5) no previous treatment, (6) platelet count > 80,000/mm3.
Patients with HCC expected to be poorly controlled by RFA, as described below, were excluded: (1) HCC abutting main hepatic veins or the first branches of the main portal vein, (2) HCC abutting vessels ≥ 0.5 cm.
Study design
The purpose of this study was to test the null hypothesis that the effect of RFA was not different from that of HR for the treatment of small HCCs. The patient's death was the primary end point. Thus, the primary goal of this study was to compare the 5-year overall survival between the 2 treatment groups. In addition, HCC recurrence was considered as the secondary end point. The 5-year disease-free survival in the two groups was also investigated. Based on the Clavien-Dindo classification system for grading complications [13], the frequency and severity of adverse events after primary treatment were compared. Because of the nature of the interventions, the double-blind technique was not used. This clinical study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of each center participating in this study. This study was registered in ClinicalTrials.gov (https://clinicaltrials.gov, Registration number: NCT02482909).
Sample size
A 5-year overall survival rate after treatment was used as the outcome measurement to estimate the sample size. According to the previous data [14,15], the 5-year overall survival rate was expected to be 60% for HR and 40% for RFA. By the Freedman Equation, a sample size of at least 217 patients was calculated to be needed to detect a difference at a 5% type-I error and 80% power for a 2-tailed test with 10% of patients estimated to be lost to follow-up.
Enrollment and assignment
Two hospitals participated in this clinical study: Seoul National University Hospital and the National Cancer Center. All patients diagnosed with a single HCC were eligible for enrollment. Once a patient met the inclusion criteria, information about this study was provided by the physician to the patient. Written informed consent was obtained before the patient was recruited into the study. Patients were recruited and assigned to 2 groups (the RFA and HR groups) by a stratified randomization method beforehand, which was developed by the Medical Research Collaborating Center (MRCC) of Seoul National University Hospital. The registry number for patients was printed on envelopes in order, and the corresponding group name was mentioned in each sealed envelope. Research nurses opened the envelope for each patient in the registry sequence after informed consent was obtained. Patients were then informed about the specific intervention. Patients could freely withdraw consent after they were informed about the assigned treatment modality and even after the treatment was performed.
Radiofrequency ablation
Patients assigned to the RFA group were treated by using a commercially available radiofrequency generator (Radionics, Burlington, MA, USA) and a single needle electrode (Cool-tip) (tumor <3 cm) or a clustered electrode composed of 2 needles (tumor ≥3 cm), ground pads, a pump for internal cooling, and ultrasound equipment. The percutaneous RFA procedure was performed as follows: Grounding was achieved by attaching 2 pads to the patient's thighs. The electrode was inserted into the tumor under ultrasound or CT guidance after local anesthesia was given. When the electrode was in position, the system was switched to the impedance mode. After measurement of the baseline impedance, generator output power was gradually increased with a pump infusing cold saline into the electrode lumen to cool the tip temperature. The target area was heated to 90℃–100℃ and maintained at that level for 10–30 minutes. When the HCC was located on the surface and close to adjacent organs, such as the duodenum, colon, or diaphragm, saline was injected between the liver and the adjacent organs before ablation to prevent thermal injuries. During the treatment, a hyperechoic, necrotic area was observed around the electrode tip on ultrasonic monitoring. The aim of the treatment was to have this necrotic area covering a larger area than the tumor. The total ablation area was required to be 0.5–1 cm over the tumor edge. Occasionally, it was necessary to insert the electrode at different sites, and ablation was performed repeatedly to achieve a satisfactory ablation area. An enhanced CT was performed 24 hours after treatment. When any possible undestroyed lesions remained, the RFA was repeated. If there were no serious complications, patients with satisfactory ablation were discharged 24–48 hours after treatment.
Hepatic resection
Surgical resection was carried out under general anesthesia. Systematic anatomical resection (segmentectomy, sectionectomy, or hemihepatectomy) was performed after intraoperative ultra sound examination. Intraoperative ultrasonography was routinely performed to estimate the location and feeding vessels of the tumor, as well as to give an accurate vascular map of the liver anatomy. The Cavitron Ultrasonic Aspirator (CUSA, Valleylab Corp., Boulder, CO, USA) was used to dissect the hepatic parenchyma. Pringle's maneuver was occasionally used with a clamp/unclamp time of 15 minutes/5 minutes to control the bleeding during hepatic dissection. Hemostasis was achieved by using a bipolar electric coagulator, argon beam coagulator, titanium clips, tie or suturing, and some commercially manufactured hemostats. Patients remained hospitalized until liver functions approached normal and serious complications had resolved.
Follow-up
Basic patient information was collected after enrollment, including age, sex, past medical history, physical examination, vital signs, the results of radiological and laboratory tests, and Child-Pugh score. All posttreatment complications were also investigated. Patients were required to visit the outpatient clinic 1 month after treatment and then every 3 months for 24 months. They were followed up every 4 months after 2-year post treatment. Additional visits were allowed when there were certain problems. At every visit, serum α-FP, PIVKA-II, complete blood cell count, liver function test, prothrombin time, and CT or MRI were performed, and the results were recorded. Although MRI was usually performed as an alternative imaging tool when CT was intolerable because of contrast media-related hyper sensitivity, it was also used as an additional workup when intrahepatic recurrence was difficult to ascertain. The end of follow-up was originally designed for up to 5 years after treatment; however, additional follow-up was made to evaluate long-term outcomes only through the patients' medical records after getting the approval of the Institutional Review Board.
Recurrence and subsequent treatment
When recurrence was suspected, an individualized additional workup, such as a chest/brain CT, bone scintigraphy, or positron emission tomography, was performed. All confirmed recurrences were classified into three categories: intrahepatic local recurrence, intrahepatic distant recurrence, and extrahepatic recurrence. Intrahepatic local recurrence was defined as a recurrence that developed within 2 cm of the primary treatment margin.
Once recurrence was confirmed, the subsequent treatment plan was proposed based on the decision of a multidisciplinary team of doctors, including surgeons, internists, and radiologists. HR, RFA, percutaneous ethanol injection therapy (PEIT), transarterial chemoembolization (TACE), chemotherapy, radiotherapy, or transplantation was selected as the subsequent treatment.
Statistical analysis
Case report forms were delivered to the MRCC of Seoul National University Hospital after every visit. Data collection and statistical analysis, as well as patient assignment, were independently performed by the MRCC.
In principle, comparison of the 2 treatment groups was performed on an intention-to-treat (ITT) basis, i.e., according to the initially assigned treatment methods; however, 2 additional analysis methods were also used. One method was per-protocol (PP) analysis, and the other method was per-treatment (PT) analysis. Cases with protocol violations were excluded from the PP analysis, and patients were regrouped according to the actual treatment methods instead of the initially assigned methods for the PT analysis.
Differences in demographic and medical data between the 2 groups were analyzed by Student t-test for continuous variables and by the chi-square test or Fisher exact test for categorical variables. Overall survival curves and recurrence-free survival curves were generated by the Kaplan-Meier method and compared by the log-rank and Breslow tests. All statistical tests were 2-sided, and the null hypothesis was rejected when P < 0.05. The statistical analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient groups
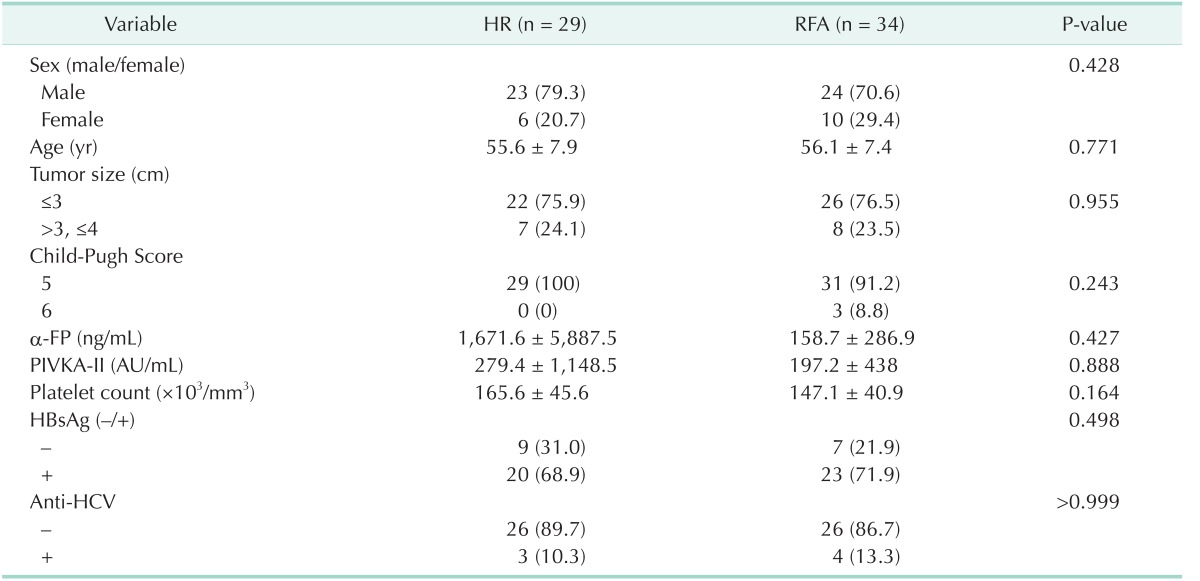
The recruitment of participants was discontinued in September 2009. The study could be biased due to prolonged study duration. In addition, the posttreatment recurrence was expected to be higher in the RFA groups. Thus, the study was early terminated even though the sample size had not achieved the calculated goal. Between July 2005 and September 2009, 68 patients met the criteria and were randomly assigned to each group. Five patients in the HR group withdrew their consent after randomization and were excluded from the study. Thus, 29 and 34 patients in the HR and RFA groups, respectively, were included in the ITT analysis (Fig. 1). The pretreatment data of the patients are presented in Table 1. All variables were similar between the 2 groups. Median follow-up duration in all patients was 1,948 days (range, 28–2,807 days).
Fig. 1. The number of patients included in the ITT, PP, and PT an alyses. Excluding protocol violations, 26 and 29 patients of the HR and RFA groups, respectively, were included in the PP an alysis. The final PT analysis was performed in 31 and 32 pa tients of the HR and RFA groups, respectively, according to actual treatment modalities and not the assigned modalities. HR, hepatic resection; RFA, radiofrequency ablation; ITT, intention-to-treat; PP, per-protocol; PT, per-treatment.
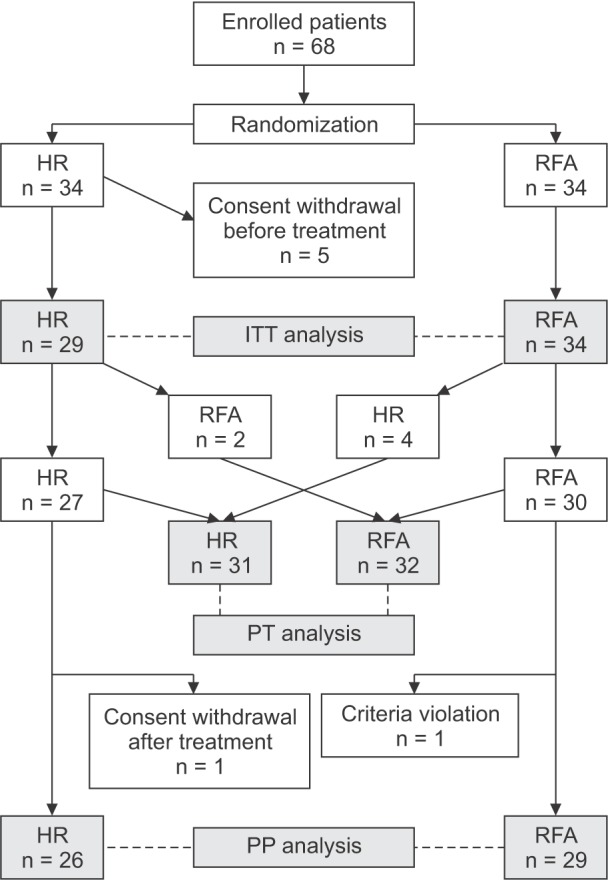
Table 1. Pretreatment data of the patients.
Values are presented as number (%) or mean ± standard deviation.
HR, hepatic resection; RFA, radiofrequency ablation; PIVKA-II, protein induced by vitamin K absence/antagonist-II.
A total of 8 patients had protocol violations after randomization. There were 3 protocol violations in the HR group. Two patients requested a change in the treatment modality to RFA and actually underwent RFA as per their desire. One patient withdrew his consent after treatment. In the RFA group, 5 patients had protocol violations. Treatment modality was actually changed to HR in 4 patients, and a criteria violation was detected in 1 patient. Therefore, PP analysis was performed in the 26 and 29 patients in the HR and RFA groups, respectively, who followed the study protocol without violations (Fig. 1).
After randomization, 31 patients actually received HR for HCC. Twenty-seven patients were originally randomized to HR, and 4 patients changed their treatment modality from RFA to HR. In the same manner, there were 32 patients who actually received RFA. Thirty patients were primarily assigned to RFA, and 2 patients selected RFA over HR. PT analysis was performed according to the actual treatment methods (Fig. 1).
Overall and disease-free survival
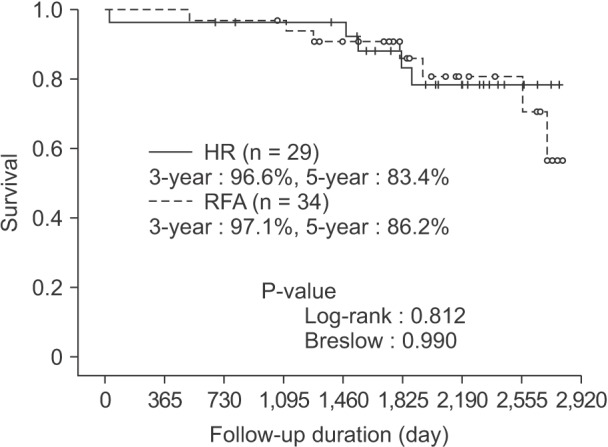
During the follow-up, 12 patients died: 5 patients in the HR group and 7 patients in the RFA group. The 3- and 5-year overall survival rates were 96.6% and 83.4%, respectively, in the HR group and 97.1% and 86.2%, respectively, in the RFA group (Fig. 2), which was not a statistically significant difference (P = 0.812 by log-rank test, P = 0.990 by Breslow test). In the HR group, one patient died of serious pulmonary complication on postoperative day 28, and the other patients died of progressive hepatic insufficiency during the management of HCC recurrence. In the RFA group, 2 deaths occurred due to intracranial or intestinal lymphoma, and 5 deaths were directly related to HCC. Also in the PP and PT analysis, the 3- and 5-year overall survival rates were not different between the 2 groups. On the PP basis, the 3- and 5-year survival rates were 96.2% and 85.6%, and 100% and 87.7%, respectively (P = 0.862 by log-rank teat, P = 0.933 by Breslow test). On the PT basis, the 3- and 5-year survival rates were 96.8% and 83.3%, and 96.9% and 85.9%, respectively (P = 0.903 by log-rank test, P = 0.990 Breslow test).
Fig. 2. Overall survival based on the intention-to-treat analysis. The 5-year overall survival rate was 83.4% in the HR group and 86.2% in the RFA group; however, this difference was not statistically significant. HR, hepatic resection; RFA, radiofrequency ablation.
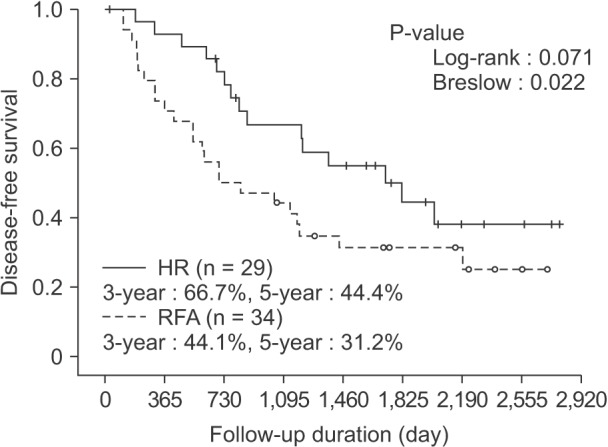
HCC recurrence developed in 39 patients during follow-up: in 15 and 24 patients in the HR and RFA groups, respectively (51.7% vs. 70.6%). On the ITT basis, the disease-free survival in the HR group was significantly superior to that in the RFA group. The disease-free survival rate was 66.7% at 3 years and 44.4% at 5 years after treatment in the HR group, while it was 44.1% at 3 years and 31.2% at 5 years in the RFA group (P = 0.071 by log-rank test, P = 0.023 by Breslow test) (Fig. 3). Also in the PP analysis, the 3- and 5-year disease-free survival rates were superior in the HR group (62.5% and 37.4% vs. 41.4% and 26.3% in the RFA group, P = 0.073 by log-rank test, P = 0.033 by Breslow test). On the PT basis, the 3- and 5-year disease-free survival rates were 65.6% and 43.3% in the HR group and 43.8% and 30.3% in the RFA group, respectively. Therefore, HR was superior to RFA in terms of disease-free survival, even in the PT analysis (P = 0.044 by log-rank test, P = 0.027 by Breslow test).
Fig. 3. Disease-free survival based on the intention-to-treat analysis. On the ITT basis, the 5-year disease-free survival rate was 42.9% in the HR group and 31.2% in the RFA group (P = 0.084 by log-rank test, 0.030 by Breslow test). HR, hepatic resection; RFA, radiofrequency ablation.
Recurrence pattern and risk factors of HCC recurrence
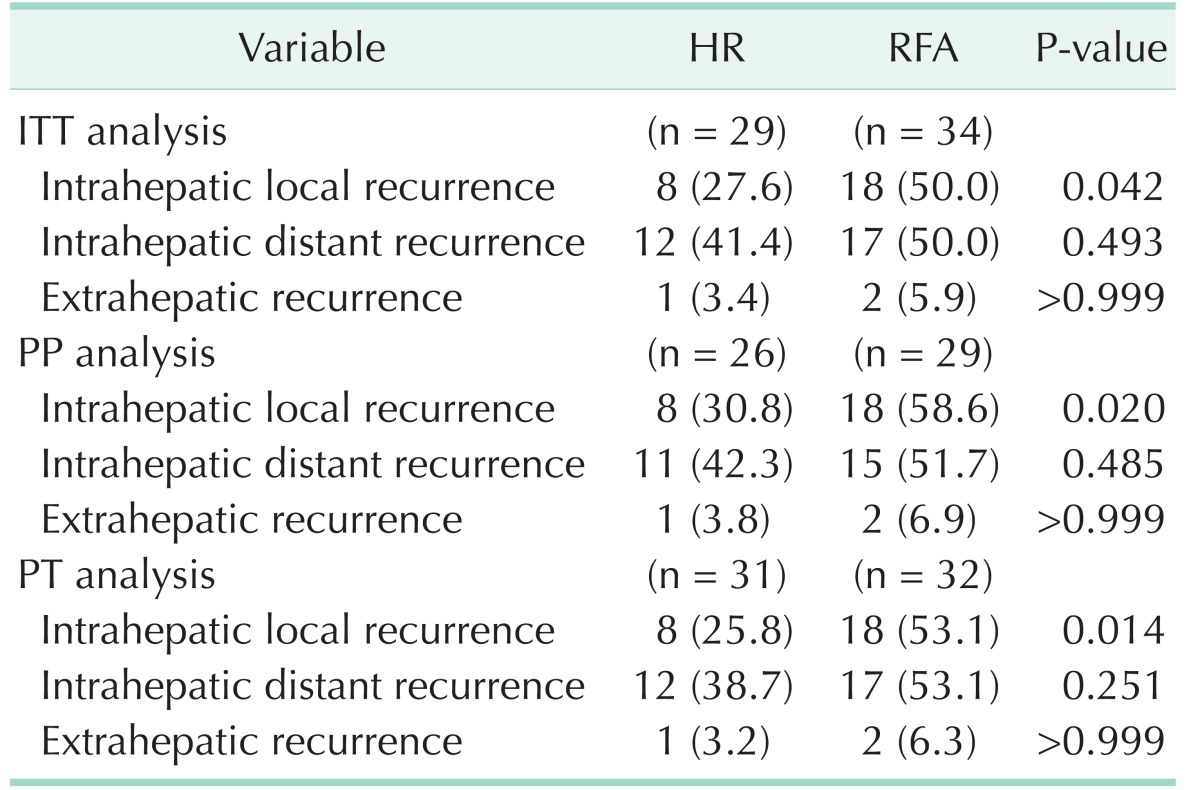
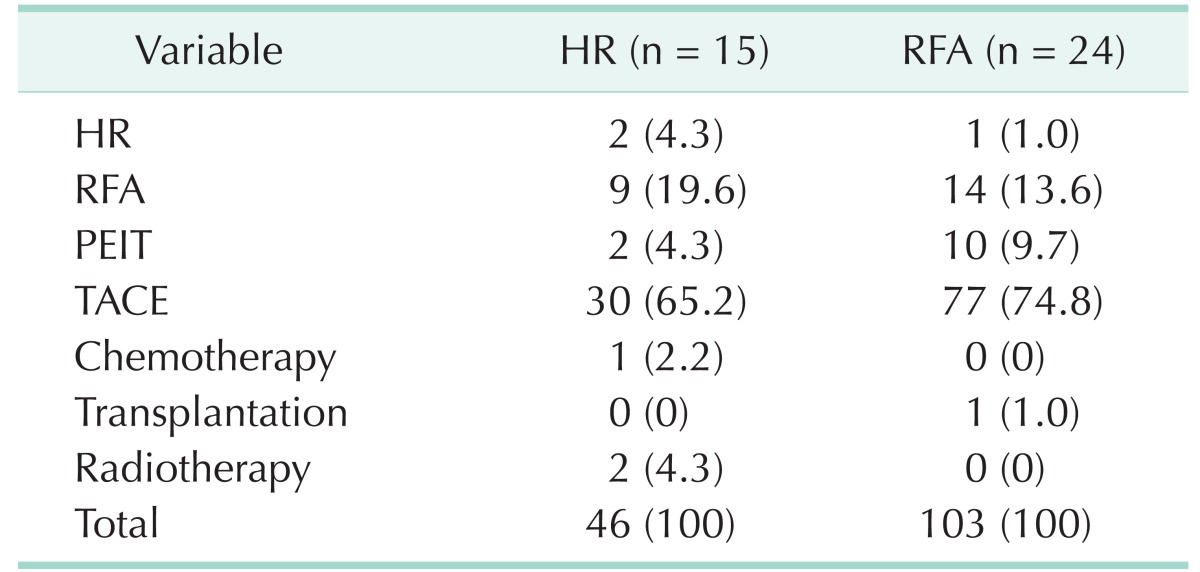
Intrahepatic local recurrence was more frequent in the RFA group. On the other hand, the frequency of intrahepatic distant recurrence and extrahepatic recurrence was similar between the 2 groups (Table 2). A total of 149 subsequent treatments were performed in 39 patients with HCC recurrence during the follow-up period (Table 3). The subsequent treatments were more frequently performed in the RFA group (103 treatments in 24 patients) than in the HR group (46 treatments in 15 patients). The most common subsequent treatment was TACE, not only in the HR group (65.2%), but also but also in the RFA group (74.8%), followed by RFA.
Table 2. Recurrence pattern.
Values are presented as number (%).
HR, hepatic resection; RFA, radiofrequency ablation; ITT, intention-to-treat; PP, per-protocol; PT, per-treatment.
Table 3. Subsequent treatments after hepatocellular carcinoma recurrence.
Values are presented as number (%).
HR, hepatic resection; RFA, radiofrequency ablation; PEIT, percutaneous ethanol injection therapy; TACE, transarterial chemoembolization.
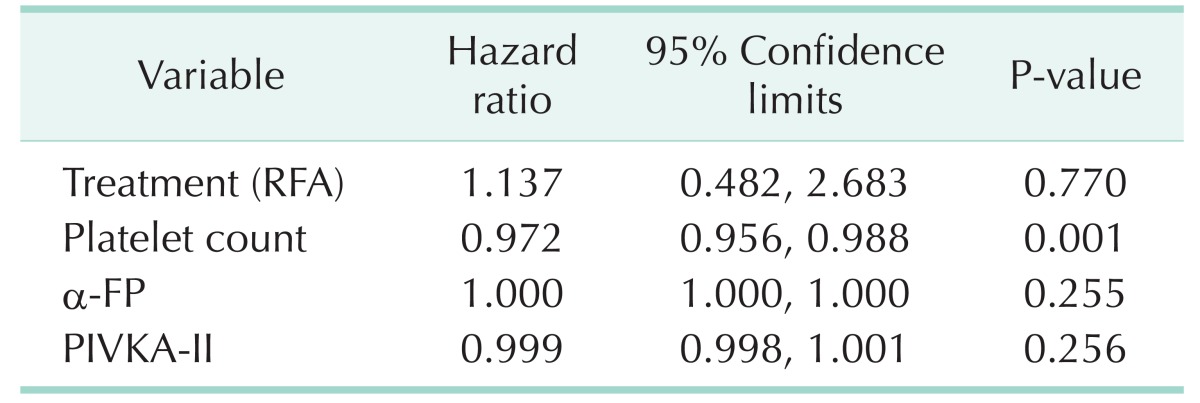
In the multivariate analysis, only the platelet count was associated with recurrence. The platelet count was significantly lower in patients with recurrence, although its effect was minimal (hazard ratio, 0.972; P = 0.001). RFA was also associated with a high risk of HCC recurrence; however, it was not statistically significant (Table 4).
Table 4. Risk factors of recurrence.
RFA, radiofrequency ablation; PIVKA-II, protein induced by vitamin K absence/antagonist-II.
Complications after treatment
A total of 28 complications developed in 20 patients (31.7%) after primary treatment: 11 patients in the HR group and 9 patients in the RFA group (37.9% vs. 26.5%, P = 0.330). There was no significant difference in the number of patients with complications between the 2 groups. The most common complications were pleural effusion in the HR group (24.1%) and pain in the RFA group (8.8%). According to the Clavien-Dindo classification system, all complications except for one were grade I. Only one grade IV complication developed in the HR group, which was postoperative death. Therefore, there was no statistically significant difference in the severity of complications between the 2 groups.
DISCUSSION
Surgical HR is still considered the first treatment option with curative intent in patients with resectable HCCs [7]. Recent developments in surgical skills and accumulated experiences in perioperative management have improved the results of HR [7]. The postoperative mortality after HR is approaching 0% in noncirrhotic patients, and it is less than 5% even in cirrhotic patients [7,16]. Recent advances in RFA, however, have brought its results close to those of HR. Because nonrandomized and randomized comparative studies have shown that outcomes of RFA were similar to those of HR [14,15,17], the necessity of a prospective, randomized study comparing the efficacy of RFA and HR in patients with resectable small HCCs has been suggested [8].
The first prospective randomized trial comparing local ablative therapy and partial hepatectomy for HCC was performed by Chen et al. [7]. In their study, the 4-year overall survival rate was 65.9% for the local ablative therapy group and 64.0% for the surgical resection group. The 4-year disease-free survival rates for the 2 groups were 48.2 and 51.6%, respectively, and they were not significantly different. These results are not identical to those of the present study. The most remarkable difference is the disease-free survival rate in the local ablative therapy group. On the ITT basis, the 3- and 5-year disease-free survival rates in the RFA group were only 44.1% and 31.2% in the present study, whereas they were much higher in the earlier study. With respect to the recurrence pattern, intrahepatic local recurrence was remarkably more frequent in the RFA group compared to the HR group (Table 3). Therefore, the low recurrence rates in their study might be due to effective local control of the tumor, possibly by addition of PEIT or TACE to RFA. Hence, their study does not directly compare RFA to HR as a single treatment modality. In addition, the overall survival rates in the resection group were lower in their study compared to the present study. The 3- and 5-year overall survival rates in the HR group were 96.6% and 83.4% in the present study, while the 4-year overall survival rate was only 64.0% in their study. This low survival was likely to be due to hepatic insufficiency and HCC recurrence. Although only patients with good liver function belonging to Child-Pugh class A were enrolled in their study, patients with a low platelet count (reduced up to 40,000/mm3) or high indocyanine green retention rate at 15 minutes (ICG-R15) (up to 30%) were allowed to participate in the study. Some patients with moderate to severe portal hypertension and cirrhosis, which are the well-known prognostic factors of HCC, might have been enrolled in their study and could be responsible for the low overall survival rate. This may be due to a high complication rate after HR in their study.
In the present study, the overall survival rate was not different between the 2 treatment groups. Although the disease-free survival was superior in the HR group, patient survival was not improved. This might be due to the excellent liver function and effective subsequent treatments. It is well known that the prognosis of HCC depends not only on tumor staging but also on liver function. In addition, various salvage or palliative treatment modalities have been developed for treating HCC. Therefore, the survival expectancy can be more than 3–5 years, even after HCC recurrence with good liver function and effective subsequent treatments. As only those patients with small HCC and excellent liver function were enrolled in the present study, the difference in overall survival was not noticeable during follow-up.
Early recurrence, however, leads to more salvage treatments and hospitalization. In the present study, the most frequent subsequent treatment after recurrence was TACE, which was performed repeatedly in many cases. Because recurrence developed earlier in the RFA group, more salvage treatments and hospitalization were required during follow-up, in spite of similar survival. In addition, it is questionable that the earlier and more frequent recurrences do not have any effect on the final outcomes. Recent prospective studies and meta-analyses have reported on the superiority of HR for the treatment of HCC [5,18,19,20]. According to these studies, the HCC recurrence rate was higher and the overall survival rate was lower in the RFA group. HCC recurrence was the main cause of death in both the HR and RFA groups. Therefore, more frequent recurrence is likely to eventually result in poorer survival. Additionally, in the present study, the survival curve shows that the patient survival may be superior in the HR group after long-term follow-up of more than 5 years (Fig. 2).
Huang et al. [5] mentioned that the difference in recurrence rates could be explained by the difference in tumor clearance between the 2 treatments. HCC tumor cells are mainly spread by the portal venous flow. Thus, HCC frequently disseminates along the portal vein branches in the same segment of the liver [5,21,22,23,24]. Segment-based anatomical HR systematically resects the liver, not keeping a simple safety margin from the tumor but completely removing tributaries of the segment portal vein. Therefore, anatomical HR can eradicate potential tumor emboli in the same segment, as well as the original tumor [5,25]. Repeated electrode insertion and ablation are usually needed in treating HCC by RFA. In those situations, it is essential to fully overlap ablations and to not leave nonablated tissue in the 3-dimensional target area; however, it is difficult under 2-dimensional imaging guidance [5]. Actually, according to 1 previous report, which histologically evaluated hepatic specimens after sequential RFA and HR, viable tumor cells can be found within extensively necrotized specimens by RFA [5,26]. In the present study, intrahepatic local recurrence developed more frequently in the RFA group, although intrahepatic distant and extrahepatic recurrences were similar between the 2 groups. This result corresponds with the findings of the study by Huang et al. [5].
In the present study, the frequency and severity of complications were not different between the 2 groups. In general, the superiority of RFA with respect to adverse events is obvious because of its microinvasive characteristics. The unexpected results of the present study might be due to the methods used for assessing adverse events. Many adverse subjective symptoms, such as nausea and pain, were assessed only based on the patient's complaint. Moreover, the pain was classified only as absence or presence of pain, and it was not assessed according to the pain rating scale. Therefore, this study had a limitation in comparing the safety of these 2 treatments. According to the study by Huang et al. [5], the length of hospitalization was significantly shorter and the risk of adverse events was lower in the RFA group however, it was clear that serious complications were very rare in both the HR and RFA groups.
The present study has some limitations. First, a small number of patients were recruited. Patient recruitment was difficult because of very strict inclusion criteria. In addition, we decided to stop the study when it was presumed that HCC recurrence was significantly frequent in the RFA group. Second, the effectiveness of treatment methods, especially RFA, depends on the operator's experience and skill. In the present study, treatments were performed by several surgeons and radiologists in 2 centers. Hence, the results of the present study may not be reproducible. This is a common limitation of many clinical studies.
However, the present clinical study can be considered valuable in spite of the aforementioned minor limitations. There are only a few comparable clinical studies that have been performed in strictly homogeneous patients, similar to the present study. The condition of enrolled patients was so homo geneous that the tumors could be treated safely and effectively by either HR or RFA. The comparison between these 2 treatment modalities was equitably made, thus minimizing confounding factors such as patients' liver function and tumor location. Therefore, the result of the present study can be considered highly valid and unbiased.
In conclusion, although the present study was early terminated, it showed that HR was significantly superior to RFA in terms of disease-free survival, which might be due to more frequent intrahepatic local recurrences after RFA. However, with good liver function and effective subsequent treatments, the overall survival was excellent in both groups and not significantly different up to 5 years after treatment.
ACKNOWLEDGEMENTS
This study was financially supported by the National Cancer Center of Korea (grant number: 0520090, 1510520).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979-94. Lancet. 1997;350:1142–1143. doi: 10.1016/S0140-6736(05)63789-0. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl 1):S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 6.Lai EC, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg. 1995;221:291–298. doi: 10.1097/00000658-199503000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237:171–179. doi: 10.1097/01.SLA.0000048443.71734.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi S, Buscarini E, Garbagnati F, Di Stasi M, Quaretti P, Rago M, et al. Percutaneous treat ment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998;170:1015–1022. doi: 10.2214/ajr.170.4.9530052. [DOI] [PubMed] [Google Scholar]
- 10.Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood TF, Rose DM, Chung M, Allegra DP, Foshag LJ, Bilchik AJ. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol. 2000;7:593–600. doi: 10.1007/BF02725339. [DOI] [PubMed] [Google Scholar]
- 12.Buscarini L, Buscarini E, Di Stasi M, Vallisa D, Quaretti P, Rocca A. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11:914–921. doi: 10.1007/s003300000659. [DOI] [PubMed] [Google Scholar]
- 13.Clavien PA, Camargo CA, Jr, Croxford R, Langer B, Levy GA, Greig PD. Definition and classification of negative outcomes in solid organ transplantation. Application in liver transplantation. Ann Surg. 1994;220:109–120. doi: 10.1097/00000658-199408000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montorsi M, Santambrogio R, Bianchi P, Donadon M, Moroni E, Spinelli A, et al. Survival and recurrences after hepatic resection or radiofrequency for hepatocellular carcinoma in cirrhotic patients: a multivariate analysis. J Gastrointest Surg. 2005;9:62–67. doi: 10.1016/j.gassur.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, et al. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102–107. doi: 10.1097/01.sla.0000129672.51886.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau WY. Management of hepatocellular carcinoma. J R Coll Surg Edinb. 2002;47:389–399. [PubMed] [Google Scholar]
- 17.Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol. 2005;39:247–252. doi: 10.1097/01.mcg.0000152746.72149.31. [DOI] [PubMed] [Google Scholar]
- 18.Ni JY, Xu LF, Sun HL, Zhou JX, Chen YT, Luo JH. Percutaneous ablation therapy versus surgical resection in the treatment for early-stage hepatocellular carcinoma: a meta-analysis of 21,494 patients. J Cancer Res Clin Oncol. 2013;139:2021–2033. doi: 10.1007/s00432-013-1530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PLoS One. 2014;9:e84484. doi: 10.1371/journal.pone.0084484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Q, Kobayashi S, Ye X, Meng X. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: a meta-analysis of 16,103 patients. Sci Rep. 2014;4:7252. doi: 10.1038/srep07252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252–259. doi: 10.1097/01.sla.0000171307.37401.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regimbeau JM, Kianmanesh R, Farges O, Dondero F, Sauvanet A, Belghiti J. Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surgery. 2002;131:311–317. doi: 10.1067/msy.2002.121892. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki A, Kai S, Iwashita Y, Hirano S, Ohta M, Kitano S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005;103:299–306. doi: 10.1002/cncr.20798. [DOI] [PubMed] [Google Scholar]
- 24.Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28:376–381. doi: 10.1007/s00268-003-7308-x. [DOI] [PubMed] [Google Scholar]
- 25.Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245:36–43. doi: 10.1097/01.sla.0000231758.07868.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman AL, Wu SS, Obaid AK, French SW, Lois J, McMonigle M, et al. Histologic evaluation and treatment outcome after sequential radiofrequency ablation and hepatic resection for primary and metastatic tumors. Am Surg. 2002;68:1038–1043. [PubMed] [Google Scholar]