Introduction
This paper reviews the current knowledge and understanding of Cryptosporidium spp. and Giardia spp. in humans, animals and the environment in 10 countries in the eastern part of Europe: Bosnia and Herzegovina, Croatia, Czech Republic, Estonia, Hungary, Latvia, Poland, Romania, Serbia and Slovenia. Methods: Published scientific papers and conference proceedings from the international and local literature, official national health service reports, national databases and doctoral theses in local languages were reviewed to provide an extensive overview on the epidemiology, diagnostics and research on these pathogens, as well as analyse knowledge gaps and areas for further research. Results: Cryptosporidium spp. and Giardia spp. were found to be common in eastern Europe, but the results from different countries are difficult to compare because of variations in reporting practices and detection methodologies used. Conclusion: Upgrading and making the diagnosis/detection procedures more uniform is recommended throughout the region. Public health authorities should actively work towards increasing reporting and standardising reporting practices as these prerequisites for the reported data to be valid and therefore necessary for appropriate control plans.
Keywords: One Health, cryptosporidiosis, giardiasis, zoonosis
Introduction
Cryptosporidium spp. and Giardia spp. have been ranked as the sixth and 11th most important food-borne parasites globally, respectively [1]. Both parasites are shed in the faeces of infected hosts and can infect new hosts via faecal-contaminated soil, water, feed and food [2]. Several Cryptosporidium species are clearly zoonotic, including C. parvum, while human giardiasis is caused by two genetically different groups of G. intestinalis, referred to as assemblages A and B, which can infect other mammalian hosts and thus have a zoonotic potential [3]. Control of pathogens that can be transmitted among humans, animals and the environment is best achieved with the One Health approach.
Among food-borne diseases, cryptosporidiosis and giardiasis cause a considerable burden at the global level [4], but the burden at regional and national levels is largely unknown [1,5]. Moreover, the current estimates of the burden caused by zoonotic pathogens only include a part of the potential impacts and true costs. In a One Health context, the estimates of disease burden would address that in humans and that in animals, including reduced human and animal health, economic losses, environmental contamination and the impact on biodiversity.
The most common clinical presentation of human cryptosporidiosis is profuse watery diarrhoea with abdominal pain, low-grade fever, nausea, vomiting and weight loss. It is often asymptomatic, mild or self-limiting in immunocompetent individuals and serious, even fatal in immunosuppressed individuals, such as HIV-infected persons [6,7]. A Cryptosporidium spp. infection can also be fatal in several mammalian animals and chronic in reptiles [8].
The clinical features of acute giardiasis in humans are similar to cryptosporidiosis, and include severe diarrhoea, abdominal cramps, nausea and weight loss. These symptoms may persist for a few weeks or evolve into a chronic reoccurring disease. The infection may be asymptomatic or a subclinical course [9]. Giardia spp. infection in cattle, goats and sheep can cause nutrient malabsorption that can consequently result in a reduction of weight gain. Although mortality due to giardiasis is uncommon, fatal giardiasis has been reported in chinchillas and birds [10].
Microscopic examination of stool specimens remains the cornerstone of diagnostic testing for these parasites, although molecular methods and immunological assays can effectively replace microscopic approaches. Microscopy is cheap, but requires a skilled parasitologist and the diagnostic yield is dependent on proper stool collection. The treatment options for both include antiparasitic drugs and fluid therapy.
According to 2015 data on food-borne and waterborne diseases and zoonoses in the European Centre for Disease Prevention and Control’s (ECDC) Surveillance Atlas of Infectious Diseases, 0.68% (73/10,805; 95% confidence interval (CI): 0.53–0.84) of confirmed cryptosporidiosis cases and 26.71% (4,739/17,740; 95% CI: 26.1–27.4) of confirmed giardiasis cases were reported by 10 countries of the European Union (EU) that are mostly in the eastern part of Europe: Bulgaria, Czech Republic, Estonia, Hungary, Latvia, Lithuania, Poland, Romania, Slovakia and Slovenia [11]. These countries make up 20% of the EU population [12]. Considering that Cryptosporidium spp. and Giardia spp. are transmitted via similar pathways, and that one fourth of all giardiasis cases notified in the EU were from these 10 countries, the low proportion of cryptosporidiosis cases suggests under-reporting. In general, relatively little is known about the presence of Cryptosporidium spp. and Giardia spp. in the eastern part of Europe despite their public health relevance. This review aimed to assess the significance of Cryptosporidium spp. and Giardia spp. infections in humans and animals, as well as their occurrence in the environment based on (locally) available data. While the data are challenging to compare, they provide an overall picture of the situation and main knowledge gaps.
Methods
For the purpose of this analysis, we considered the following 19 countries to comprise eastern Europe: Estonia, Latvia, Lithuania, Czech Republic, Hungary, Poland, Slovakia, Slovenia, Albania, Bulgaria, Bosnia and Herzegovina, the former Yugoslav Republic of Macedonia, Montenegro, Croatia, Serbia, Belarus, Moldova, Ukraine and Romania (Figure).
Figure.
Countries invited to review data on Cryptosporidium spp. and Giardia spp. from a One Health perspective, 2016
EU: European Union.
Administrative boundaries: EuroGeographics, UN-FAO.
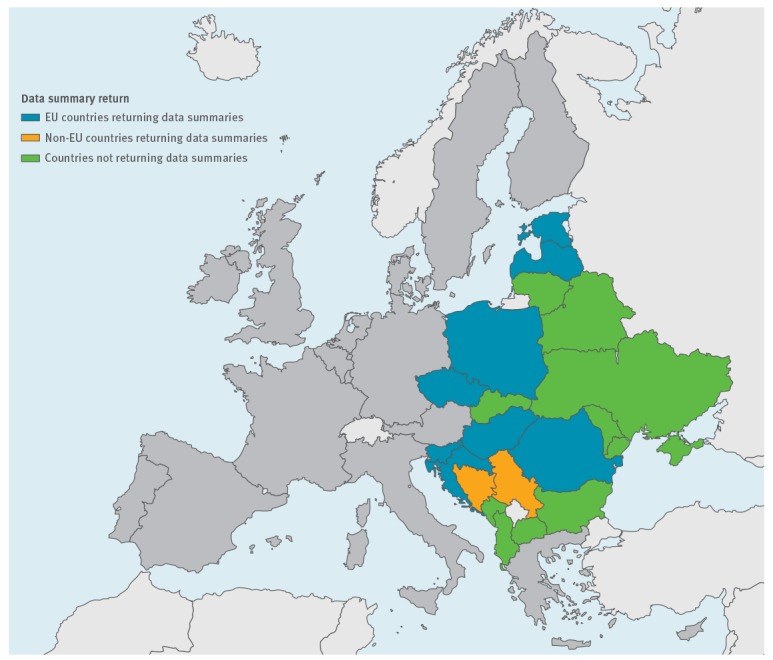
Experts including public health specialists, epidemiologists, parasitologists and other laboratory scientists working on human, animal and environmental samples from the 19 countries were invited in January 2016 to collect and review the data available for their country from a One Health perspective. Experts from 11 of 19 countries responded; those from 10 countries (Bosnia and Herzegovina, Croatia, Czech Republic, Estonia, Hungary, Latvia, Poland, Romania, Serbia, Slovenia) sent summary reviews, while those from the former Yugoslav Republic of Macedonia responded that no data were available. Eight countries (Albania, Belarus, Bulgaria, Lithuania, Moldova, Montenegro, Slovakia and Ukraine) offered no data for the review (Figure).
The contacted experts from each country gathered data from sources including national official health service reports, national databases, and international and national publications. These experts also conducted a PubMed (Medline) literature search between April and October 2016 to identify internationally published data while Google databases, using defined qualifiers for Giardia, Cryptosporidium and geographic location (e.g. Hungary), were used to identify data from grey literature. In addition, searches in local databases identified doctoral theses, journals and other publications available in the main local languages (Bosnian, Croatian, Czech, Estonian, Hungarian, Latvian, Polish, Romanian, Serbian, Slovenian) in the participating countries. Data on epidemiology, diagnostics and research of the two parasites in humans, in animals, and in the environment were extracted.
Based on the extracted data, the 95% confidence intervals (CI) of prevalence and the two-tailed p values of two-by-two table comparisons were calculated using the mid-P exact method with the OpenEpi v.3.01 programme [13]. If detailed data were not given, we report the count, percentage and CI as presented in the original publication. Data in this paper are presented on a country-by-country basis in alphabethical order.
Results
Bosnia and Herzegovina
Humans
Cryptosporidium spp. and Giardia intestinalis data available from routine human investigations are shown in Table 1. Investigations are performed by the Laboratory of Parasitology, Veterinary Faculty at the University of Sarajevo. Reporting on these parasites is not mandatory in Bosnia and Herzegovina.
Table 1. Number of laboratory-confirmed Cryptosporidium spp. and Giardia intestinalis casesa and reported incidence per 100,000 inhabitantsb, 10 countries in the eastern part of Europe, 2016.
| Country | Origin of stool samplesa | Investigation method used | Investigation period | Cryptosporidium spp.a | Giardia intestinalis a | Notifiable disease | Mean number of cases/100,000 inhabitants/yearb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n positive | n total | % (95% CI) | n positive | n total | % (95% CI) | Cry | Gia | Cry | Gia | |||||||
| European Union countries | ||||||||||||||||
| Croatia | Routine obligatory health checks of healthy people working with food and beverages | MIFC | 2006–2015 (10 years) | – | – | – | 164c | 245,321c | 0.07 (0.06–0.08)c | Yes | Yes | 0.02 | 1.42 | |||
| Patients with intestinal symptoms | MIFC | – | – | – | 41c | 17,183c | 0.24 (0.17–0.32)c | |||||||||
| Patients with bloody diarrhoea | Ziehl-Neelsen staining, microscopy | 0c | 20c | 0.00 (0.00–13.91)c | – | – | – | |||||||||
| Czech Republic | Patients with intestinal symptoms | Different concentration and staining methods | 1994–2015 (22 years) | 109 | NA | NA | 7,926 | NA | NA | Yes | Yes | 0.01 | 0.51 | |||
| Estonia | Patients with diarrhoea | NA | 2001–2015 (15 years) | 6 | NA | NA | 5,510 | NA | NA | Yes | Yes | 0.05 | 18.28 | |||
| Hungary | Not specified | Wet mount microscopy, MIFC, ELISA, ICT | 2004–2014 (11 years) | 37 | 126,947 | 0.03 (0.02–0.04) | 1,530 | 126,947 | 1.20 (1.15–1.27) | Yes | Yes | 0.16 | 0.94 | |||
| Latvia | Patients with diarrhoea | ICT | 2009–2015 (7 years) | 57 | NA | NA | – | – | – | Yes | Yes | 0.29 | 2.48 | |||
| 2000–2015 (16 years) | – | – | – | 446 | NA | NA | ||||||||||
| Poland | Not specified | NA | 2007–2016 (10 years) | 24 | NA | NA | – | – | – | Yes | Yes | 0.006 | 5.43 | |||
| 2005–2016 (12 years) | – | – | – | 27,456 | NA | NA | ||||||||||
| Romania | Not specified | NA | 2010–2015 (6 years) | – | – | – | 106,682 | 1,870,475 | 5.7 (5.60–5.81) | Yes | Yes | 0.01 | NA | |||
| 2008–2012 (5 years) | 16 | NA | NA | – | – | – | ||||||||||
| Slovenia | Patients with diarrhoea | IFT | 2002–2015 (14 years) | 78c | 5,106c | 1.53 (1.22–1.89)c | – | – | – | Yes | Yes | 0.39 | 1.27 | |||
| Patients with intestinal symptoms | Iodine wet mount microscopy, IFT | – | – | – | 237c | 24,782c | 0.96 (0.84–1.08)c | |||||||||
| Non-European Union countries | ||||||||||||||||
| Bosnia and Herzegovina | Patients with diarrhoea | Flotation, IFT | 2015–2016 (1.5 years) | 0c | 11c | 0.00 (0.00–23.84)c | 1c | 11c | 9.09 (0.45–37.34)c | No | No | NA | NA | |||
| Serbia | Routine obligatory health checks of healthy people working with food and beverages | MIFC, EIA | 2004–2008 (5 years) | – | – | – | 383c | 136,334c | 0.28 (0.25–0.30)c | No | Yes | NA | NA | |||
| Patients with diarrhoea | 2005–2014 (10 years) | – | – | – | 1,996 | NA | NA | |||||||||
–: not applicable; CI: confidence interval; Cry: cryptosporidiosis; ECDC: European Centre for Disease Prevention and Control; EIA: enzyme immunoassay; Gia: giardiasis; ICT: immunochromatographic test; IFT: immunoflourescence test; MIFC: merthiolate-iodine-formaldehyde concentration; NA: not available.
a Laboratory-confirmed cases as reported by national public health laboratories.
b Mean notification rate extracted from 2007–2016 data in the ECDC Surveillance Atlas of Infectious Diseases [11].
c Regional data, does not represent the whole country. Croatia: data from Istria region; Slovenia: data from various regions; Bosnia and Herzegovina: data from Canton of Sarajevo; Serbia: data from region of Nis.
Animals
At present, research on parasites is rare in Bosnia and Herzegovina and investigations have mainly focused on the presence of helminths. Some types of protozoa, such as the species of the genus Cryptosporidium and Giardia, were described as additional findings. Hodžić et al. provided the first written information about the occurrence and distribution of Cryptosporidium spp. and Giardia spp. in Bosnia and Herzegovina [14]. They investigated 123 faecal samples from red foxes (Vulpes vulpes) during the hunting seasons between January 2011 and March 2012. The samples were analysed for the presence of Cryptosporidium spp. oocysts and G. intestinalis cysts using sucrose flotation concentration and immunofluorescence test (IFT). Cryptosporidium spp. and G. intestinalis were detected in 3.25% (4/123; 95% CI: 1.04–7.66) and 7.32% (9/123; 95% CI: 3.63–13.00) of the samples, respectively. Co-infection with both parasites was not found. Dog faeces investigations using sucrose flotation concentration and IFT showed 5.00–6.90% positivity for Cryptosporidium spp. and 6.60–11.84% for Giardia spp. with no age-dependent differences [15,16]. However, a more recent study reported a higher prevalence (100%) of Giardia spp. in dogs ≤6 months of age compared with older dogs (p < 0.001) [17].
Croatia
Humans
There has been an obligation for clinicians to report both parasites since 2012. However, the only data available for this review for the years 2006 to 2015 were those obtained from the Department of Microbiology, Public Health Institute of the Istrian Region, which serves an area of ca 200,000 inhabitants. Of the stool samples examined for Giardia cysts, 245,321 came from the obligatory occupational health checks of healthy people working with food and beverages while 17,183 were sent by clinicians for diagnostic purposes. Routine methods are used, including merthiolate-iodine-formaldehyde concentration (MIFC), to concentrate protozoa and worm eggs from faecal samples. For patients with bloody diarrhoea, Ziehl-Neelsen staining and microscopy of bloody stool are used to determine the presence of Cryptosporidium spp. The presence of G. intestinalis and Cryptosporidium spp. in human stool samples in the region of Istria is presented in Table 1.
Animals
During the 9-year period from 2007 to 2015, a total of 5,387 stool samples from sick, but not necessarily diarrhoeic, canines and felines were examined for parasites at the Department for Parasitology and Parasitic Diseases with Clinic, Faculty of Veterinary Medicine, University of Zagreb. The canine and feline faecal samples were investigated by MERIFLUOR IFT (Meridian Bioscience, Inc. Cincinnati, United States (US)) after concentration by centrifugation-flotation with sucrose. Cryptosporidium spp. was present in 0.31% (17/5,387; 95% CI: 0.19–0.49) and G. intestinalis in 25.88% (1,394/5,387; 95% CI: 24.72–27.06) of canine and feline faecal samples.
Czech Republic
Humans
In the years 1975 to 1982, a total of 1,750 immunocompetent persons, mostly employed by agricultural enterprises, were examined for the presence of gastrointestinal parasites [18]. Of these, none were positive for Cryptosporidium spp., but 0.80% (14/1,750; 95% CI: 0.48–1.38) were positive for G. intestinalis using Breza’s, MIFC and Army Medical Service III concentration techniques and direct microscopy [19,20]. The first human cryptosporidiosis case in the Czech Republic was recorded by Ditrich et al. in an immunodeficient patient in 1991 [21]. The authors identified the Cryptosporidium isolate from that case as C. baileyi, but the identification was made without molecular analysis and therefore cannot be considered accurate. Based on the data from National Reference Laboratory for the Diagnostics of Intestinal Parasites, Department of Parasitology, Mycology and Mycobacteriology, Prague Institute of Public Health in Ústí nad Labem, 109 findings of Cryptosporidium spp. and 7,926 findings of G. intestinalis have been reported from 1994 to 2015 across the Czech Republic (Table 1). Of these, 104 (95.41%; 95% CI: 90.13–98.30) findings of Cryptosporidium spp. and 5,607 (70.74%; 95% CI: 69.73–71.74) findings of Giardia spp. were autochthonous, while the remaining Cryptosporidium and Giardia cases represent imported infections. Few Cryptosporidium genotyping/subtyping results are available. In a study mapping the occurrence of various diarrhoeal pathogens in children hospitalised with diarrhoea between 1992 and 1996, 11.32% (12/106; 95% CI: 6.28–18.45) were positive for Cryptosporidium based on aniline-methyl-violet staining of stool smears [22,23]. Nine of 106 Cryptosporidium-positive samples originated from immunocompetent children 5 months to 8 years of age and were subsequently genotyped by Hajdušek et al.; eight cases of C. parvum and one case of C. hominis were reported based on PCR amplification of partial sequences of the small subunit ribosomal ribonucleic acid (SSU rRNA) and Cryptosporidium oocyst wall protein (COWP) genes [24]. In diarrhoeal stool samples (n = 457) from 203 immunocompetent patients under 69 years of age with suspected cryptosporidiosis, five children were positive for C. parvum, one child was positive for C. hominis and one adult was positive for C. scrofarum based on PCR amplification of the SSU rRNA gene [25]. Additionally, two unusual cases of cryptosporidiosis caused by C. erinacei and a mixed infection of C. parvum and C. tyzzeri were reported by Rašková et al. and Kváč et al. [26,27].
Seroprevalence data showed that 66.83% (133/199; 95% CI: 60.07–73.11) and 71.86% (143/199; 95% CI: 65.31–77.78) of the inhabitants of the Czech Republic have antibodies and a positive response to the 15/17-kDa and 27-kDa Cryptosporidium antigen groups, respectively [28]. Pospíšilová et al. showed high titres of anti-Cryptosporidium antibodies in 10.71% of AIDS patients (15/140; 95% CI: 6.36–16.68) [29].
Animals
More than 60 studies have reported the presence of Cryptosporidium in animals, with many including data on genotyping, host and age range, pathogenicity and host–pathogen relations. The studies also resulted in the description of C. avium, C. proliferans, C. scrofarum, C. fragile, C. erinacei and C. testudinis as novel species of the genus Cryptosporidium [30-46]. In the Czech Republic, Cryptosporidium spp. was first detected in 1979 in two 14-day old emergency slaughter bulls [47]. Until the beginning of the 21st century, Cryptosporidium spp. has been found in many animal hosts (cattle, goats, sheep, pigs, poultry, wild ungulates and rodents) using microscopic techniques (flotation in Sheather's sugar or Breza's solution, native preparation, aniline-carbol-methyl violet or Giemsa staining methods); however, most studies lacked genetic characterisation of the isolates [31,48-54]. In the past decade, molecular tools have been widely used to determine the species/genotype of Cryptosporidium present in cryptosporidiosis cases in domestic, wild and companion hosts (Table 2).
Table 2. Prevalence of Cryptosporidium species and genotypes in domestic animals including pets and wild animals using RFLP or/and sequencing of PCR products, Czech Republic, 2003–2016.
| Animals | n positive | n total | % (95% CI) | Cryptosporidium spp. | Cryptosporidium subtypes | Method (and sequenced molecular markers) | Reference |
|---|---|---|---|---|---|---|---|
| Domestic | |||||||
| Cattle | 11a | NA | NA | C. parvum, C. andersoni | NA | PCR (SSU rRNA, HSP70) | [122] |
| 44 | 995 | 4.42 (3.27–5.84) | C. andersoni, C. parvum, C. bovis | IIaA15G2R1 | PCR (SSU rRNA, gp60) | [123] | |
| 56 | 309 | 18.12 (4.12–22.72) | C. andersoni, C. parvum, C. bovis | IIaA16G1R1, IIaA22G1R1, IIaA18G1R1, IIaA15G1R1 | PCR (SSU rRNA, gp60) | [119] | |
| Pigs | 1a | NA | NA | C. suis | NA | PCR (SSU rRNA, HSP70) | [122] |
| 34 | 123 | 27.64 (20.29–36.04) | C. suis, C. scrofarum, C. parvum | IIaA16G1R1 | PCR (SSU rRNA, gp60) | [124] | |
| 69 | 413 | 16.71 (13.34–20.54) | C. suis, C. scrofarum, C. muris | NA | RFLP (SSU rRNA) | [125] | |
| 177 | 477 | 37.11 (32.86–41.51) | C. suis, C. scrofarum | NA | RFLP (SSU rRNA) | [126] | |
| 353 | 1620 | 20.79 (7.95–16.67) | C. suis, C. scrofarum, C. parvum, C. muris | NA | RFLP (SSU rRNA) | [127] | |
| Horses | 12 | 352 | 3.41 ( 1.86–5.72) | C. muris, horse genotype, C. parvum, C. tyzzeri | IVaA15G4, IIaA15G2R1, IXbA22R9 | PCR (SSU rRNA, gp60) | [128] |
| Cat | 1a | NA | NA | C. felis | NA | PCR (SSU rRNA, HSP70) | [122] |
| Red-crowned parakeets | 4a | NA | NA | C. avium | NA | PCR (SSU rRNA, HSP70, actin) | [129] |
| Tortoises | 46 | 387 | 12.66 (8.66–15.11) | C. testudinis, C. ducismarci, tortoise genotype III | NA | PCR (SSU rRNA, COWP, actin) | [42] |
| Wild | |||||||
| Wild boars | 32 | 193 | 16.58 (11.83–22.33) | C. suis, C. scrofarum | NA | RFLP (SSU rRNA) | [130] |
| 61 | 460 | 13.26 (10.39–16.60) | C. suis, C. scrofarum | NA | RFLP (SSU rRNA) | [131] | |
| Giraffe | 1a | NA | NA | C. muris | NA | PCR (SSU rRNA) | [132] |
| Ungulates | 6a | NA | NA | C. ubiquitum, C parvum, C. andersoni | NA | PCR (SSU rRNA, HSP70) | [122] |
| 10 | 269 | 3.72 (1.90–6.52) | C. ubiquitum, C. muris, deer genotype | XIId | PCR (SSU rRNA, gp60) | [133] | |
| Birds | 17a | NA | NA | C. meleagridis, C. baileyi | NA | PCR (SSU rRNA, HSP70) | [122] |
| 663b | NA | NA | C. baileyi, C. meleagridis | NA | PCR (HSP70) | [134] | |
| 85b | NA | NA | C. baileyi, C. meleagridis | IIIeA16G2R1c | PCR (SSU rRNA, HSP70, gp60) | [135] | |
| Mouse | 14a | NA | NA | C. tyzzeri | IXaA6, IXaA8, IXbA6, | PCR (SSU rRNA, gp60, actin, COWP, TRAP-C1) | [136] |
| Rodents | 7 a | NA | NA | C. muris, C. andersoni | NA | PCR (SSU rRNA, HSP70) | [122] |
| Siberian chipmunks | 1 | 1 | 100 (5–100) | C. muris | NA | PCR (SSU rRNA) | [137] |
| Reptiles | 10a | NA | NA | C. serpentis, C. varanii, C. muris | NA | PCR (SSU rRNA, HSP70) | [122] |
| Rabbits | 2 | 2 | 100 (22.36–100) | C. cuniculus | NA | PCR (SSU rRNA, HSP70) | [122] |
| Hedgehogs | 12 | 15 | 80 (54.65–94.65) | C. parvum, C. erinacei | NA | PCR (SSU rRNA, gp60) | [138] |
| Bats | 3 | 263 | 1.14 (0.29–3.07) | C. parvum, bat genotype III | NA | PCR (SSU rRNA) | [139] |
CI: confidence interval; COWP: Cryptosporidium oocyst wall protein; gp60: 60 kilodalton glycoprotein; HSP70: 70 kilodalton heat shock protein; NA: not available; RFLP: restriction fragment length polymorphism; SSU rRNA: small subunit ribosomal ribonucleic acid; TRAP-C1: thrombospondin related adhesive proteins.
a Selected samples.
b Pooled samples.
A few studies have reported on the presence of Giardia in dogs, domestic animals and wild ungulates (Table 3). Unfortunately, all studies were based on microscopic examination of samples using native preparation, flotation in Sheather's sugar or Breza's solution, or staining methods. As no genotyping tools were used and information on genetic assemblages is lacking.
Table 3. Prevalence of Giardia spp. in domestic animals and wild ungulates, Czech Republic, 1993–2007.
| Animals | Category | n positive | n total | % (95% CI) | Reference |
|---|---|---|---|---|---|
| Dogs | Shelter | 26 | 243 | 10.70 (7.26–15.07) | [140] |
| Private, purebred | 2 | 83 | 2.41 (0.41–7.73) | ||
| Private, purebred, city | 37 | 3870 | 0.96 (0.68–1.30) | [141] | |
| Private purebred, rural area | 12 | 540 | 2.22 (1.21–3.75) | ||
| Sheep | Lamb 0.5–4 months | 28 | 167 | 16.77 (11.67–23.01) | [142] |
| Goats | Kids 0.5–4 months | 19 | 26 | 73.08 (53.86–87.39) | |
| Horses | Not specified | 18 | 360 | 5.00 (3.08–7.64) | [143] |
| Roe deer | Calf 7–8 months | 1 | 3 | 33.33 (1.67–86.80) | [144] |
CI: confidence interval.
Environment
Monitoring of Cryptosporidium oocysts and Giardia cysts in drinking water resources was published by Dolejš et al. in 1999 and 2000 [55-58]. Drinking water sources in the Czech Republic have been found to contain between 0 and 32,140 Cryptosporidium spp. oocysts/100 L and between 0 and 485 Giardia spp. cysts/100 L based on IFT and microscopy. Hajdušek et al. used molecular tools to identify C. parvum in an open water reservoir in 2004. This isolate was recovered from 10,000 L of water using a Super Micro-Wynd 1 µm filter (CUNO Inc., Meriden, US) [24].
Estonia
Humans
Cryptosporidiosis and giardiasis are notifiable diseases in Estonia. From 1991 until 2016, a total of 134 cases of cryptosporidiosis have been reported by the Health Board, of which only a few have been in the recent years (Table 1) [59,60]. For 1991 to 1992, the official Estonian reports mention 33 cryptosporidiosis cases (personal communication, J Epštein, February 2014). During the same years, stool samples of patients with intestinal diseases (n = 1,518) were examined at one hospital using an unspecified microscopy method and Cryptosporidium oocysts were found in 3.34% (49/1,469; 95% CI: 2.51–4.35) of the stools from patients with acute intestinal disease who were 0–14 years of age [61]. Since 1999, reports on cryptosporidiosis have originated from two of 15 counties, Harjumaa and Raplamaa, and since 2010, all the individuals diagnosed with cryptosporidiosis were children [59]. The official data thus do not appear to include known outbreaks occurring among veterinary students [62]. One such case was caused by the C. parvum subtype IIaA16G1R1, and there was evidence of the infection having originated from calf faeces [62].
According to the number of cases reported to ECDC from 2007 to 2016, Estonia has the second highest rate of laboratory-confirmed giardiasis cases with a mean of 18.3 cases per 100,000 inhabitants per year (Table 1), which is three times higher than the EU mean for the same time period [11]. In particular, the reported incidence rate among children 0–4 years of age in Estonia between 2007 to 2016 (152.22 individuals per 100,000 inhabitants) was 10 times higher than the rate in all reporting countries (15.45 individuals per 100,000 inhabitants) [11]. In a national health report of the Health Board, 46.84% (549/1,172; 95% CI: 44.00–49.71) of individuals with reported giardiasis in 2010 to 2014 were children less than 5 years of age [59]. The same report reported that 5.12–17.51% of all patients with giardiasis were hospitalised and that 70.20–80.71% of the annually reported cases in 2010 to 2014 originated from one county, Harjumaa, where the capital Tallinn is located [59].
Animals
In 2013 to 2015, 30.04% (73/243; 95% CI: 24.53–36.03) of bovine faecal samples submitted to the veterinary and food laboratory were positive for Cryptosporidium spp. oocysts [63] and (personal communication, A Kärssin, February 2016). In 2010 to 2015, 5.65% (7/124; 95% CI: 2.50–10.85) of canine faecal samples submitted to diagnostic examinations but none of the 50 feline samples tested positive for Cryptosporidium spp [63] and (personal communication, A Kärssin, February 2016). In a cross-sectional investigation, 30.28%, (281/928; 95% CI: 27.39–33.30%) of cattle tested positive for Cryptosporidium spp. oocysts using a modified Ziehl-Neelsen staining [64]. The same study found 84.44% (38/45; 95% CI: 71.64–92.93) of farms to have at least one animal shedding Cryptosporidium spp. oocysts at the time of the study. C. parvum and C. andersoni have been described in cattle less than 12 months of age [64]. The prevalence of shedding Cryptosporidium spp. oocysts was higher (p < 0.001) in animals older than 12 months of age compared with younger animals. However, evaluated with a semiquantitative scale, the younger animals appeared to shed in higher numbers [64]. Management practices that appeared to increase the magnitude of oocysts shedding included early removal of a calf from its mother [65]. Cryptosporidium spp. oocysts were detected with IFT in ovine faeces collected from 60.87% (56/92; 95% CI: 50.63–70.43) of sheep herds on the islands of Hiiumaa, Vormsi and Saaremaa [66].
In 2010 to 2015, Giardia cysts were detected in 27.08% (65/240; 95% CI: 21.75–32.97) of bovine faecal samples submitted for diagnostic investigations [63], (personal communication, A Kärssin, February 2016). In the same period, 5.65% (7/124; 95% CI: 2.50–10.85) of canine faecal samples and 14.00% (7/50; 95% CI: 6.33–25.74) of feline faecal samples were positive for Giardia cysts [64], (personal communication, A Kärssin, February 2016). Giardia shedding was detected with IFT in ovine faeces collected from in 69.57% (64/92; 95% CI: 59.61–78.31) of sheep herds on the islands of Hiiumaa, Vormsi and Saaremaa [66].
Hungary
Humans
Stool samples for both Cryptosporidium spp. oocysts and Giardia spp. cysts are routinely tested at the Department of Parasitology, National Center for Epidemiology and Regional Parasitological Laboratories in Budapest, Hungary using microscopic examination of the wet mount (saline and iodine) preparation, MIFC technique for concentration of the protozoan cysts, ELISA/immunochromatographic test (ICT) antigen detection and/or Kinyoun staining. The data are shown in Table 1.
Based on an epidemiological survey, the seroprevalence for a positive response to the 27-kDa Cryptosporidium antigen was significantly higher in communities where the drinking water originated from surface water than in the control city where riverbank filtration was used (p < 0.001). A logistic regression analysis of risk factors showed that bathing in outdoor pools was also associated with a positive response to the 15/17-kDa Cryptosporidium antigen complex (p = 0.0197) [67].
The association between the consumption of Giardia-positive drinking water and asymptomatic giardiasis was investigated in 2007. Despite this being a field investigation where only a single stool sample was examined from each participant, G. intestinalis infections were found in 4.00% (4/100; 95% CI: 1.28–9.36) of asymptomatic individuals. In both water samples and asymptomatic persons, G. intestinalis assemblage B was detected [68].
Animals
A total of 49.37% (39/79; 95% CI: 38.46–60.32) of faecal samples from calves with diarrhoea collected on 52 farms in 2006 from different Hungarian counties showed positivity using IFT. Based on sequence and phylogenetic analysis, C. ryanae was detected in one sample and the gp60 gene PCR products of 21 isolates showed that two isolates belonged to the C. parvum IId subtype group (IIdA22G1 and IIdA19G1) and the most common C. parvum subtype was IIaA16G1R1 (n = 15). Other detected subtypes were IIaA17G1R1 (n = 3) and IIaA18G1R1 (n = 1) [69].
In 2008, the combined results of a microscopic and molecular study indicated that aquatic ducks, geese, coot and cormorant may have a role in the environmental dissemination of human pathogenic assemblages of Cryptosporidium oocysts and Giardia cysts. A total of 5.82% (6/103, 95% CI: 2.39–11.72) of wild birds and 13.79% (4/29; 95% CI: 4.54–30.00) of domestic birds were C. parvum or C. baileyi positive. Additionally, 5.82% (6/103; 95% CI: 2.39–11.72) of samples from wild birds and 24.14% (7/29; 95% CI: 11.22–42.01) of samples from domestic birds were G. intestinalis positive [70].
In the past decade, the Central Veterinary Institute detected Cryptosporidium spp. in cattle, sheep and goats (Table 4).
Table 4. Prevalence of Cryptosporidium spp. in ruminants using Sheather’s sucrose flotation and direct microscopy, Hungary, 2005–2015a .
| Animal | Age category | n positive | n total | % (95% CI) |
|---|---|---|---|---|
| Cattle | Adult | 63 | 7,205 | 0.87 (0.68–1.11) |
| Post-weaned | 18 | 466 | 3.86 (2.38–5.92) | |
| Pre-weaned | 97 | 286 | 33.91 (28.60–39.55) | |
| Sheep | Adult | 1 | 517 | 0.19 (0.01–0.95) |
| Post-weaned | 3 | 175 | 1.71 (0.44–4.60) | |
| Pre-weaned | 5 | 26 | 19.23 (7.41–37.60) | |
| Goat | Adult | 0 | 117 | 0.00 (0.00–2.53) |
| Post-weaned | 1 | 78 | 1.28 (0.06–6.94) | |
| Pre-weaned | 2 | 7 | 28.57 (5.10–66.98) |
CI: confidence interval.
a Based on the database of National Food Chain Safety Office, Veterinary Diagnostic Directorate, Budapest, Hungary.
Sporadic Cryptosporidium spp. infections have been found by the same institute in piglets, puppies and kittens. Infection by Giardia spp. was detected in 27.90% of chinchillas (48/172; 95% CI: 21.59–34.96). Sporadic Giardia spp. infections were also seen in cattle, sheep, dogs, cats and laboratory rats. The presence of G. intestinalis in kennel dogs from Hungary using a specific copro-antigen ELISA test was 58.82% (110/187; 95% CI: 51.66–65.72). All sequenced SSU rRNA samples belonged to dog-specific assemblages C and D. Although canine giardiasis is highly prevalent in the studied geographical areas, it did not present zoonotic potential and the infection rate declined with increasing age of the dogs [71].
Environment
The presence of Cryptosporidium oocysts and Giardia cysts in different water sources (surface water, wastewater, raw water and drinking water) was investigated during the period 2000 to 2007 by microscopy using Method 1623 of the United States Environmental Protection Agency (US EPA). Up to three Cryptosporidium oocysts/100 L and up to 63.6 Giardia cysts/100 L were detected in drinking water [72]. The highest concentration in raw water was 50 Cryptosporidium oocysts/100 L and 1,030 Giardia cysts/100 L. A higher concentration of oocysts was found in water sources that received effluents from sewage treatment plants or originated from a forest environment. Riverbank filtrated water (n = 71) and raw water from the Danube River (n = 184) in Budapest were monitored to document the protozoan removal efficiency by riverbank filtration (RBF) during the years 2004 to 2005 [72] and (Plutzer et al. data not shown). Cryptosporidium and Giardia spp. were detected regularly in the river water but never in riverbank filtered water, suggesting the effectiveness of RBF as a method of pathogen removal. Cryptosporidium spp. were detected in 36.41% of raw river water samples (67/184; 95% CI: 29.70–43.55) and Giardia spp. were detected in 96.74% of raw river water samples (178/184; 95% CI: 93.34–98.67) [72] and (Plutzer et al. data not shown). The species and genotypes determined by molecular tools were all potentially zoonotic: C. parvum, C. meleagridis and G. intestinalis assemblages A and B [72,73].
Latvia
Humans
The epidemiological data regarding Cryptosporidium and Giardia were collected from the Centre of Disease Prevention and Control of Latvia (Table 1). Cryptosporidiosis cases in humans have only been reported since 2009, with a total of 57 cases being reported from then until 2015 (mean: 9 cases per year, range: 2–23 cases per year). The highest number of reported cases occurred in the age group of 30–39 years olds: 42.11% (24/57; 95% CI: 29.83–55.16).
From 2000 to 2015, a total of 446 cases of giardiasis were reported (mean: 30 cases per year, range: 3–124 cases per year). The highest number of reported cases, 30.94% (138/446; 95% CI: 26.78–35.35), was observed in the age group of 7–14 year olds. All diagnostics were conducted by analysing stool samples with the copro-antigen test.
Animals
During a study conducted by the Faculty of Veterinary Medicine at the Latvia University of Agriculture between 2013 and 2014, a total of 1,580 faecal samples from dairy cattle were collected from different regions in Latvia. According to the microscopy results using Ziehl-Neelsen staining, Cryptosporidium oocysts were present in 19.43% (307/1,580; 95% CI: 17.54–21.44) of the samples. A lower prevalence of Cryptosporidium spp.-positive faecal samples was found 4.64% (18/388; 95% CI: 2.86–7.09) in the Latgale region than in other regions where prevalence ranged from 20.39% (63/309; 95% CI: 16.17–25.16) to 26.38% (86/326; 95% CI: 21.81–31.37). An earlier study of 16 dairy farms and 125 animals found that 68.75% (11/16; 95% CI: 43.68–87.54) of the farms had at least one animal shedding Cryptosporidium spp. in their faeces; 40.80% (51/125; 95% CI: 32.44–49.58) of the animals tested positive using modified Ziehl–Neelsen staining of faecal smears [74]. There are no Cryptosporidium prevalence studies in other animal species. No studies have investigated the prevalence of giardiasis in animals, while sporadic G. intestinalis infections are diagnosed in dogs and cats (personal communication, G Deksne, July 2016).
Poland
Humans
In Poland, human cryptosporidiosis and giardiasis cases are notifiable diseases. In the years 2005 to 2016, the National Institute of Public Health, National Institute of Hygiene in Poland reported one to six cases of human cryptosporidiosis and 2,288 cases of giardiasis per year (Table 1). Prevalence estimates of Cryptosporidium and Giardia spp. in humans are available from research studies, but these are limited to selected population groups and regions. For example, Cryptosporidium oocysts were detected by microscopy (examination of smears of faecal samples after modified Ziehl-Neelsen and IFT) in 14.63% (36/246; 95% CI: 10.62–19.47) of stool samples collected from hospitalised patients with diarrhoea [75]. All positive samples were from children up to 4 years of age, and isolates belonged to species C. parvum and C. hominis. In 2008, Bajer et al. reported Cryptosporidium infections in persons with immunodeficiencies; C. hominis, C. meleagridis and C. parvum were found in children with primary immunodeficiencies (PID), but only C. parvum was found in children and adults with a secondary immunosuppression (i.e. after cancer treatment) [76].
A 2010 study including 232 people from the west-central region of Poland found G. intestinalis in 1.29% (3/232; 95% CI: 0.33–3.48) of the collected faecal samples by direct microscopy. Three subgenotypes of Giardia were detected: a cosmopolitan subgenotype AII and two new subgenotypes A and B [77]. Examination of the faeces of 31,504 children 7 years of age from 15 Polish provinces in 2002 to 2003 found G. intestinalis in the faeces of 0.69% (217/31,504; 95% CI: 0.60–0.78) of the children using direct microscopy and Lugol’s iodine staining method [78]. In another study from 2008 to 2009, of 120 children with watery diarrhoea resembling a parasite infection, 12.50% (15/120; 95% CI: 7.44–19.35) tested positive for Giardia antigens in the faeces using an immunochromatographic test [79].
Animals
Several prevalence studies have been performed on animals in Poland for both parasites using a wide range of detection techniques. The results are summarised in Table 5.
Table 5. Prevalence of Cryptosporidium spp. and Giardia spp. in domestic animals including pets and wild animals using different methods Poland, 1997–2014.
| Animals | n positive | n total | % (95% CI) | Cryptosporidium spp. | Giardia spp./G. intestinalis assemblage | Method (and sequenced molecular markers) | Reference |
|---|---|---|---|---|---|---|---|
| Domestic | |||||||
| Cattle | 10 | 86 | 11.63 (6.06–19.75) | NE | G. intestinalis/A, E | PCR (β-giardin) | [145,146] |
| 16 | 86 | 18.60 (11.42–27.87) | NE | Giardia spp. | IFT | ||
| 119 | 700 | 17.00 (14.35–19.92) | C. bovis, C. parvum, C. andersoni, C. ryanae | NE | PCR (SSU rRNA, COWP) | [147] | |
| Pigs | 8 | 84 | 9.52 (4.52–17.28) | NE | G. intestinalis / B, E | PCR (β-giardin) | [145,146] |
| 25 | 84 | 29.76 (20.73–40.17) | NE | Giardia spp. | IFT | ||
| 46 | 166 | 27.71 (21.31–34.89) | C. scrofarum, C. suis, C. parvum | NE | PCR (SSU rRNA, COWP) | [148] | |
| Horses | 1 | 10 | 10 (0.50–40.35) | NE | G. intestinalis / E | PCR (β-giardin) | [145,146] |
| 1 | 10 | 10 (0.50–40.35) | NE | Giardia spp. | IFT | ||
| 20 | 564 | 3.55 (2.24–5.33) | C. parvum | NE | EIA, IFT, FISH | [149] | |
| Sheep | 18 | 81 | 22.22 (14.17–32.23 | NE | G. intestinalis / A, E | PCR (β-giardin) | [145,146] |
| 17 | 81 | 20.99 (13.16–30.86) | NE | Giardia spp. | IFT | ||
| 16 | 159 | 10.06 (6.07–15.50) | C. parvum | NE | Microscopya | [150] | |
| Goats | 0 | 46 | 0.00 (0.00–6.31) | Cryptosporidium spp. | NE | Microscopya | |
| Cats | 4 | 160 | 2.50 (0.80–5.92) | NE | G. intestinalis/A, B | PCR (GDH) | [151] |
| Dogs | 3 | 60 | 5.00 (1.29–13.00) | NE | G. intestinalis/A, E | PCR (β-giardin) | [145,146] |
| 7 | 60 | 11.67 (5.25–21.72) | NE | Giardia spp. | IFT | ||
| 32 | 350 | 9.14 (6.45–12.51) | NE | G. intestinalis/A, C, D | PCR (GDH) | [152] | |
| 18 | 350 | 5.14 (3.28-7.28) | NE | G. intestinalis | Microscopya | ||
| 2 | 148 | 1.35 (0.23–4.39) | NE | G. intestinalis/C, D | PCR (β-giardin) | [153] | |
| 8 | 64 | 12.5 (5.98–22.36) | Cryptosporidium spp. | NE | IFT | [154] | |
| 23 | 64 | 35.94 (24.92–48.20) | NE | Giardia spp. | |||
| Domestic birds | 1 | 101 | 0.99 (0.50–4.79) | NE | G. intestinalis | Microscopya, FISH | [155] |
| 0 | 101 | 0.00 (0.00–2.92) | C. parvum | NE | Microscopya, EIA, FISH | ||
| Wild | |||||||
| Wild boars | 4 | 27 | 14.81 (4.89–31.97) | NE | Giardia spp. | IFT | [145,146] |
| 11 | 27 | 40.74 (23.62–59.76) | NE | G. intestinalis / B | PCR (β-giardin) | ||
| 0 | 5 | 0.00 (0.00–45.07) | Cryptosporidium spp. | NE | Microscopya, IFT, PCR (COWP) | [156] | |
| 0 | 5 | 0.00 (0.00–45.07) | NE | Giardia spp. | Microscopya, IFA | ||
| Foxes | 0 | 21 | 0.00 (0.00–13.29) | NE | G. intestinalis | PCR (β-giardin) | [145,146] |
| 4 | 21 | 19.05 (6.36–39.80) | NE | Giardia spp. | IFT | ||
| Red deer | 5 | 28 | 17.86 (6.85–35.24) | NE | G. intestinalis/B | PCR (β-giardin) | |
| 0 | 28 | 0.00 (0.00–10.15) | NE | Giardia spp. | IFT | ||
| 14 | 52 | 26.92 (16.22–40.14) | Cryptosporidium spp. | NE | Microscopya, IFT, PCR (COWP) | [156] | |
| 1 | 52 | 1.92 (0.10–9.12) | NE | Giardia spp. | Microscopya, IFT | ||
| 1 | 61 | 1.64 (0.08–7.82) | NE | Giardia spp. | Microscopya | [157] | |
| Roe deer | 11 | 48 | 22.92 (12.68–36.33) | NE | G. intestinalis/B | PCR (β-giardin) | [145,146] |
| 2 | 48 | 4.17 (0.70–13.09) | NE | Giardia spp. | IFT | ||
| 2 | 22 | 9.09 (1.55–26.92) | Cryptosporidium spp. | NE | Microscopya, IFT, PCR (COWP) | [156] | |
| 1 | 22 | 4.55 (0.23–20.44) | NE | Giardia spp. | Microscopya, IFT | ||
| 2 | 50 | 4.00 (2/50; 0.68–12.59) | NE | Giardia spp. | Microscopya | [157] | |
| Fallow deer | 0 | 65 | 0.00 (0/65; 0.00–4.50) | NE | Giardia spp. | ||
| Moose | 0 | 5 | 0.00 (0/5; 0.00–45.07) | NE | Giardia spp. | ||
| 4 | 23 | 17.39 (4/23; 5.78–36.80) | NE | G. intestinalis | PCR (β-giardin) | [145,146] | |
| 0 | 23 | 0.00 (0/23; 0.00–12.21) | NE | Giardia spp. | IFT | ||
| Wolves | 2 | 7 | 28.57 (5.10–66.98) | NE | G. intestinalis/D | PCR (β-giardin) | |
| 2 | 7 | 28.57 (5.10–66.98) | NE | Giardia spp. | IFT | ||
| 5 | 14 | 35.71 (14.44–62.40) | C. parvum, genotype 2 | NE | Microscopya, IFT, PCR (COWP) | [156] | |
| Rodents | 10b | 266 | NA | NE | G. microti, G. muris | PCR (SSU rRNA) | [158] |
| 8b | 266 | C. parvum, C. ubiquitum | NE | ||||
| 41 | 114 | 35.9 (27.74–45.54) | Cryptosporidium spp. | NE | IFT | [159] | |
| 0 | 114 | 0.00 (0.00–4.06) | NE | Giardia spp. | |||
| NA | NA | 28.1–62.3 (NA) | Cryptosporidium spp. | NE | Microscopya, IFT | [160] | |
| NA | NA | 24.4–74.2 (NA) | NE | Giardia spp. | |||
| European beaver | 7 | 22 | 31.82 (15.11–53.05) | Cryptosporidium spp. | NE | Microscopya, IFT, PCR (COWP) | [156] |
| 1 | 22 | 4.55 (0.23–20.44) | NE | Giardia spp. | Microscopya, IFT | ||
| European bison | 16 | 55 | 29.09 (18.27–42.07) | Cryptosporidium spp. | NE | Microscopya, IFT, PCR (COWP) | |
| 4 | 55 | 7.27 (2.35–16.62) | NE | Giardia spp. | Microscopya, IFT | ||
| Polish Konik (horse) | 0 | 10 | 0.00 (0.00–25.89) | Cryptosporidium spp. | NE | Microscopya, IFT, PCR (COWP) | |
| 1 | 44 | 2.27 (0.11–10.70) | Cryptosporidium spp. | NE | Microscopya | [161] | |
| Birds captive | 2 | 90 | 2.22% (0.37–7.15) | NE | G. intestinalis | Microscopya, FISH | [155] |
| 1 | 90 | 1.11% (0.06–5.36) | C. parvum | NE | Microscopya, EIA, FISH | ||
COWP: Cryptosporidium oocyst wall protein; EIA: enzyme immunoassay; IFT: immunofluorescent test; FISH: fluorescent in situ hybridisation; GDH: glutamate dehydrogenase; NA: not available; NE: not examined; SSU rRNA: small subunit ribosomal ribonucleic acid.
a Examination of smears of faecal samples or samples after Sheather’s flotation and after modified Ziehl-Neelsen or Lugol’ iodine or iron hematoxylin staining, or Willis-Schlaf or McMaster methods.
b Number of sequenced samples.
Environment
Cryptosporidium spp. contamination of tap water has been confirmed by microscopy, IFA and PCR in one of twelve examined samples from the city of Poznan [80].
Examination of surface waters
The presence of G. intestinalis assemblages A and B, and Cryptosporidium oocysts has been found in 45.57% (36/79; 95% CI: 34.84–56.61) and 32.91% (26/79; 95% CI: 23.24–43.82) of samples taken from Mazurian Lake, respectively [81]. The Vistula River (n = 21) and the Zegrzyński Lake (n = 8) were tested for the presence of Cryptosporidium oocysts and Giardia cysts using a Filta-Max filtration capsules and xpress automatic station (IDEXX Laboratories, Inc., Westbrook, US) for filter elution, immunomagnetic separation (IMS) and IFT [82]. Giardia cysts were found in all samples from the Zegrzynski Lake (range: 10–45/100 L) and in all samples from the Vistula River (range: 10–389/100 L). Cryptosporidium oocysts were present in 50.00% (4/8; 95% CI: 18.41–81.59) of samples from the Zegrzyński Lake and in 47.62% (10/21; 95% CI: 27.29–68.57) of samples from the Vistula River. Their number in both cases was similar and ranged from 5 to 25 oocyst/100 L. Cryptosporidium oocysts were also detected in 50 of 68 surface water samples collected monthly from intakes (n = 13) and recreational waters (n = 4) in the Krakow area during June to September 2012. Giardia cysts were only detected in samples taken from three sampling locations [83].
Examination of sewage waters
Cryptosporidium spp. oocysts were detected in 61.54% (8/13; 95% CI: 34.09–84.32) of wastewater treatment plants (WWTPs) and Giardia spp. cysts in 84.61% (11/13; 95% CI: 57.77–97.34) of WWTPs in eastern Poland by microscopic analyses using Method 1623 of the US EPA [84]. Cryptosporidium oocyst concentrations in raw sewage water ranged from 40 to 15,410 oocysts/100 L and Giardia cyst concentrations ranged from 70 to 66,000 cysts/100 L.
Using animals as indicators of contamination
Rotifers taken from three lakes located near the city of Poznań were used as an indicator of recreational water contamination [85]. Cryptosporidium oocysts were detected in rotifers and water from the lakes using the fluorescence in situ hybridisation (FISH) method. Mussels collected from Poznań’s municipal reservoir, Lake Malta, have been examined by direct microscopy (wet smear and smears stained with Ziehl–Neelsen and iron haematoxylin) and MERIFLUOR IFT Cryptosporidium/Giardia kit (Meridian Bioscience Inc., Cincinnati, US) [86]. Cryptosporidium oocysts were detected in 15.38% (12/78; 95% CI: 8.61–24.69) of the mussels.
Contamination of food products
Fresh vegetables and soft fruit have been investigated using IMS and molecular methods [87]. Cryptosporidium oocysts were found on 6 of 128 vegetables, and C. parvum was identified by subtyping (gp60) from celery. The authors speculated that the presence of Cryptosporidium on vegetables could be associated with products originating from regions with considerable livestock production [87].
Romania
Humans
Between 2008 and 2012, a total of 16 Cryptosporidium spp. infections were reported by the Romanian National Public Health Institute (Table 1). In a study using ELISA, a Cryptosporidium prevalence of 4.04% (17/421; 95% CI: 2.45–6.26) was reported from western Romania [88]. Molecular characterisation of five isolates indicated the presence of species C. parvum (n = 3) and C. ubiquitum (n = 2) [88]. Vieira et al. has also reported the presence of the C. parvum subtype IIdA22G1 in faecal samples of four children under 12 years of age from Timiş County in this area of Romania [89].
Data provided by the Romanian National Public Health Institute for a 6-year period (2010 to 2015) of routine investigation of patients with gastrointestinal disorders showed a cumulative Giardia infection prevalence of 5.70% (106,682/1,870,475; 95% CI: 5.60–5.81). Data from 269 hospitalised patients between 1996 and 2008 from Caraş-Severin County indicated Giardia infections in 7.81% (21/269; 95% CI: 5.03–11.49) of individuals [90]. In addition, a study conducted by Costache et al. between 2008 and 2011 in Cluj County and neighbouring areas reported a cumulative giardiasis prevalence of 0.41% (76/18,486; 95% CI: 0.33–0.51) in children and of 0.80% (141/17,645; 95% CI: 0.67–0.94) in the adult general population [91].
Animals
Over the last decade, epidemiological surveys were carried out with the aim of finding Cryptosporidium oocysts and Giardia cysts in livestock, pets and wildlife stool samples. Research focusing on livestock is limited and mostly involves the western [89,92-94], central and north-western [95] regions of the country. The methods applied included non-molecular (conventional acid-fast staining and classical microscopic examination, copro-antigen detection immunoassays) and molecular tools (PCR-restriction fragment length polymorphism (RFLP), DNA sequencing). Results are summarised in Table 6.
Table 6. Prevalence and identification of Cryptosporidium spp. subtypes and Giardia intestinalis assemblages in animals using non-molecular and molecular methods, Romania, 2005–2016.
| Animals | Non-molecular | Moleculara | References | |||||
|---|---|---|---|---|---|---|---|---|
| n positive | n total | % (95% CI) | Methods used | Species identified |
Cryptosporidium subtypes/Giardia assemblages (number of specimens) |
Method (and sequenced molecular markers) | ||
| Cryptosporidium | ||||||||
| Cattle | 65 | 258 | 25.19 (20.18–30.76) | Microscopy after mZN staining | C. parvum | IIaA15G2R1 (8), IIaA16G1R1 (7) | PCR (SSU rRNA, gp60) | [92] |
| 198 | 708 | 27.97 (24.75–31.36) | Microscopy after mZN staining | – | – | – | [95] | |
| – | – | – | – | C. parvum | IIdA27G1 (8), IIdA25G1 (5), IIdA22G1 (2), IIdA21G1a (1), IIaA16G1R1 (1) | PCR (SSU rRNA, gp60) | [89] | |
| Lambs | 24 | 175 | 13.71 (9.20–19.42) | Microscopy after mZN staining | C. parvum (83.4%), C. ubiquitum (8.3%), C. xiaoi (8.3%) | IIaA17G1R1 (2), IIaA16G1R1 (2), IIdA20G1 (2), IIdA24G1 (1), IIdA22G2R1 (1) | PCR (SSU rRNA, gp60) | [93] |
| Goat kids | 99 | 412 | 24.03 (20.09–28.33) | Microscopy after mZN staining | – | – | – | [95] |
| Pigs | – | – | – | – | C. parvum | IIdA26G1 | PCR (SSU rRNA, gp60) | [89] |
| Giardia | ||||||||
| Cattle | 239 | 621 | 38.49 (34.72–42.36) | ELISA | – | – | – | [94] |
| Lambs | 432 | 615 | 70.24 (66.54–73.76) | ELISA | – | – | – | |
| Dogs | 52 | 614 | 8.47 (6.46–10.87) | Direct microscopy after flotation | – | – | – | [162] |
| 114 | 416 | 27.40 (23.28–31.84) | ELISA | – | – | – | ||
| 102 | 215 | 47.44 (40.82–54.13) | ELISA | – | – | – | [94] | |
| – | – | – | – | G. intestinalis | D (29), C (8), E (1), C and D (1) | PCR (GDH) | [163] | |
| – | – | – | – | G. intestinalis | D (8), C (8) | PCR (SSU rRNA) | [111] | |
| Cats | 3 | 414 | 0.72 (0.18–1.96) | Direct microscopy after flotation | – | – | – | [164] |
| 51 | 183 | 27.87 (21.74–34.70) | ELISA | – | – | – | [165] | |
| 66 | 264 | 25.00 (20.06–30.49) | Direct microscopy after Lugol’s iodine staining | – | – | – | [94] | |
| – | – | – | – | G. intestinalis | D | PCR (GDH) | [163] | |
| Wolves | – | – | – | – | G. intestinalis | D | PCR (GDH) | [163] |
| Muskrat | – | – | – | – | C | |||
| Raccoon dog | – | – | – | – | D | |||
| Roe deer | – | – | – | – | E | |||
| Fallow deer | – | – | – | – | E | |||
–: not applicable; CI: confidence interval; ELISA: enzyme-linked immunosorbent assay; GDH: glutamate dehydrogenase; gp60: 60 kilodalton glycoprotein; mZN: modified Ziehl-Neelsen; SSUrRNA: small subunit ribosomal ribonucleic acid.
a For molecular studies microscopically positive samples have been used.
Environment
Investigations on the occurrence of Cryptosporidium spp. oocysts and Giardia spp. cysts in the main rivers of western Romania using the US EPA’s Method 1623 showed their presence in 7.54% (4/53; 95% CI: 2.44–17.21) and 41.50% (22/53; 95% CI: 28.87–55.06) of raw surface water samples, respectively. Genetic characterisation of the isolates demonstrated the presence of domestic/wild canid origin C. canis (n = 1) and the human/animal origin C. parvum IIaA16G1R1 subtype (n = 1), as well as G. intestinalis assemblages AII (n = 12) and E, the ruminant origin assemblage (n = 1) [96]. In another study, conducted in the same region, 27.27% (3/11; 95% CI: 7.45–57.81) of the tested wastewater samples were positive for the zoonotic C. parvum, with IIaA15G2R1 (n = 2) and IIdA18G1 subtypes. Also, the occurrence of Giardia spp. were recorded in different surface water types with a detection rate of 90.91% (10/11; 95% CI: 62.66–99.55) in wastewaters, 26.31% (5/19; 95% CI: 10.34–49.06) in brooks, 37.50% (3/8; 95% CI: 10.56–72.20) in irrigation channels, 31.25% (5/16; CI: 12.46–56.32) in lakes, and 36.36% (8/22; CI: 18.53–57.59) in ponds. The registered and successfully sequenced G. intestinalis assemblages were: assemblage E (n = 12) in all tested water bodies, assemblage AII (n = 9) in all tested water bodies except for ponds, and the domestic/wild canid specific assemblage D in a pond [97].
Serbia
Humans
In Serbia, giardiasis is a notifiable disease, while cryptosporidiosis is not. Not only that cryptosporidiosis is not reportable, it has also seldom been the subject of research. The only description of cryptosporidiosis in immunocompetent individuals is a report of a family outbreak in 2010 [98]. Conversely, a long-term analysis in immunocompromised individuals carried out between 1985 and 2008 found cryptosporidiosis in 10.50% (50/476; 95% CI: 7.98–13.50) of HIV-infected patients with gastrointestinal symptoms. This finding placed cryptosporidiosis as the second most common cause of gastrointestinal disorders, following oesophageal candidiasis, among all opportunistic diseases in this patient category [99].
On the other hand, giardiasis apparently occurs much more frequently. From 2005 to 2014, a total of 1,996 cases of giardiasis (Table 1) were reported by the Institute of Public Health of Serbia [100]. However, the number of examinations carried out, the clinical reasons for testing and the methods used in particular laboratories are not reported. Analysis of the reports showed that the number of reported cases of giardiasis decreased from 4.6 per 100,000 inhabitants in 2005 to 1.1 per 100,000 inhabitants in 2014. There was no difference (p = 0.255) in the distribution of cases between females and males (48.5% of cases were female and 51.5% were male). Infections were most often diagnosed in people aged 20–40 (45.6%), while 11.9% of all cases were reported in children up to 10 years of age. Giardiasis occurrence was associated with seasonality (p < 0.0001), with one third of the cases being diagnosed between August and October. The incidence peak coincided with increased outdoor activities and increased water consumption during hot weather periods. Giardiasis is widespread throughout Serbia, but the data seem to indicate that it is more common in northern than in central Serbia (10-year mean of 4.5 cases/100,000 inhabitants vs 2.1 cases/100,000 inhabitants). Whether the observed fluctuations reflect a real change in the infection dynamics or are merely the result of differences in the detection of cases or reporting of these remains to be explored. Official reports do not differentiate between cases and do not describe whether reported cases were symptomatic or accidental findings of possibly asymptomatic individuals, for example, during routine examinations of cooks, bakers, restaurant staff, etc. for obligatory occupational health checks. Regional investigations conducted by the Department for Parasitology at the Public Health Centre of Niš (southern Serbia) between 2004 and 2008, did report the number of investigations making it possible to estimate the prevalence of Giardia, which was 0.28% (Table 1). Miladinovic-Tasic and colleagues carried out several studies on giardiasis in different populations; the results from ones that examined healthy adults as a part of obligatory occupational health checks showed a decrease in the prevalence of giardiasis from 0.43% (64/14,833; 95% CI: 0.33–0.55) in 2002 to 0.16% (53/32,814; 95% CI: 0.12–0.21) in 2008 [101-103]. High infection rates were registered in establishments where people were in close contact, such as individuals in psychiatric institutions (6/100; 6.00%, 95% CI: 2.47–12.06) [101], specialised institutions for children with disabilities (7/106; 6.60%, 95% CI: 2.93–12.62) [101] and refugee camps (7/122; 5.74%, 95% CI: 2.54–11.02) [102]. In patients with diarrhoea, the prevalence of giardiasis was as high as 10% in adults and 4% in children under 14 years of age [101,102]. The prevalence of giardiasis has also been studied in schoolchildren. Nikolić et al conducted an extensive long-term study throughout central Serbia between 1985 and 2005 that involved a total of 6,645 asymptomatic children 7–11 years of age, representing approximately 10% of the total age-matched population (n = 69,232) [104]. The methods used included microscopy after conventional concentration techniques. Despite this being a field investigation where only a single stool sample was examined from each participant, the results showed the presence of Giardia infection in all examined regions, with infection rates ranging from 3.2 to 14.2%, and an overall prevalence of 6.10% (405/6,645; 95% CI: 5.54–6.69). This is significantly higher than the figures in the official reports. Interestingly, the prevalence of Giardia was similar in urban (7.0%) and rural (6.5%) areas. Another study had previously shown a similarly high prevalence of 8.00% (14/175; 95% CI: 4.63–12.76) in the highly urban area of the city of Belgrade [105]. Finally, a study carried out in 2004 in south-western Serbia estimated a giardiasis prevalence of 5.62% (45/800; 95% CI: 4.18–7.39) in asymptomatic schoolchildren [106].
Animals
Neither Cryptosporidium nor Giardia infections are notifiable in animals in Serbia. However, several studies have investigated such infections in cattle, swine, lambs and goats (Table 7).
Table 7. Prevalence of Cryptosporidium spp. and Giardia intestinalis and genotypes in animals using microscopya and PCR, Serbia, 2002–2015.
| Animals | n positive | n total | % (95% CI) | Cryptosporidium subtypes/Giardia assemblages (number of specimens) | Method (and sequenced molecular markers) | References |
|---|---|---|---|---|---|---|
| Cryptosporidium | ||||||
| Cattle | 72 | 160 | 45.00 (37.30–52.90) | NA | Microscopy | [107] |
| 62 | 103 | 60.19 (50.52–69.30) | C. parvum IIa (10), IIaA16G1R1b, IIaA18G1R1 IIa A20G1R1, IId (2), IId A18G1b, IIj (6), IIjA16R2, IIjA17R2 | PCR (SSU rRNA, COWP) | [108] | |
| 6 | 30 | 20.00 (8.53–37.03) | NA | Microscopy | [166] | |
| Swine | 89 | 260 | 34.23 (28.65–40.16) | NA | Microscopy | [109] |
| 14 | 34 | 41.18 (25.69–58.11) | NA | Microscopy | [166] | |
| Lambs | 53 | 126 | 42.06 (33.67–50.82) | NA | Microscopy | [167] |
| 12 | 25 | 48.00 (29.19–67.25) | NA | Microscopy | [166] | |
| Goat | 28 | 88 | 31.82 (22.74–42.08) | NA | Microscopy | [167] |
| Giardia | ||||||
| Dogs | 22 | 151 | 14.57 (9.60–20.89) | NA | Microscopy | [110] |
| 88 | 134 | 65.67 (57.33–73.34) | G. intestinalis C (8), D (6) | PCR (SSU rRNA) | [111] | |
| Cats | 18 | 81 | 22.22 (14.17–32.23) | NA | Microscopy | [112] |
| 6 | 50 | 12.00 (5.01–23.29) | NA | Microscopy | [113] | |
CI: confidence interval; COWP: Cryptosporidium oocyst wall protein; EIA: enzyme immunoassay; SSU rRNA: small subunit ribosomal ribonucleic acid; NA: not available.
a Microscopy: examination of faecal sample smears or fecal samples after Sheather’s flotation and modified Ziehl-Neelsen, Lugol’ iodine or iron hematoxylin staining.
In an examination of 160 cattle from the Belgrade area, Cryptosporidium oocysts were detected in 34.61% (9/26; 95% CI: 18.38–54.11) of weaners, 49.02% (25/51; 95% CI: 35.55–62.60) of bull calves and 47.50% (38/80; 95% CI: 36.74–58.44) of post parturient cows [107]. Another study showed a prevalence of 60.20% (62/103; 95% CI: 50.52–69.30) among dairy calves up to 1 month of age [108]. Cryptosporidium oocysts were also found in the faeces of 34.23% (89/260; 95% CI: 28.65–40.16) of swine with an observed decrease with age [109]. To expand, oocysts were detected in 45.55% (41/90; 95% CI: 35.49–55.91) of nursing, weaning and post-weaned piglets up to 3 months of age, in 32.80% (41/125; 95% CI: 25.00–41.39) of post-weaned piglets 3 to 12 months of age, and in 15.55% (7/45; 95% CI: 7.07–28.36) of sows older than 12 months of age. In all pigs older than 3 months of age, the Cryptosporidium infection was subclinical [109].
Infections with Giardia were studied in 2008 in Belgrade-area dogs and cats (Table 7). In dogs, the infection rate depended on living conditions. The lowest prevalence was detected in pet household dogs (7.41%, 6/81; 95% CI: 3.06–14.77), followed by a higher prevalence in stray (18.67%, 14/75; 95% CI: 11.04–28.68) and kennel dogs (36.36%, 4/11; 95% CI: 12.78–66.36) [110]. A 2015 study in shelter dogs, however, showed a remarkably higher prevalence of infections with Giardia of 65.67% (88/134; 95% CI: 57.33–73.34) belonging to the assemblages C and D [111]. In 2001, a study found a higher Giardia prevalence in kittens (7/23; 30.43%, 95% CI: 14.39–51.14) than in adult pet cats (11/58; 18.96%, 95% CI: 10.40–30.57), however all 95% CIs overlapped [112]. Other data from 2012 seemed to indicate a decrease in the Giardia infection rate in cats [113].
Slovenia
Humans
Between 2002 and 2015, patients with diarrhoea (n = 5,106) were examined for Cryptosporidium oocysts by IFT and patients with various gastrointestinal and/or digestive disorders and/or diseases (n = 24,782) were examined for the presence of G. intestinalis cysts by iodine wet mount microscopy and/or IFT at the Institute of Microbiology and Immunology (IMI), Faculty of Medicine at the University of Ljubljana. It was found that 78/5,106 (1.53%) and 237/24,782 (0.96%) patients were Cryptosporidium and G. intestinalis positive, respectively (personal communication, B Šoba, November 2016) (Table 1). In the same period (2002–2015), 121 cases of cryptosporidiosis and 574 cases of giardiasis were reported to the National Institute of Public Health of the Republic of Slovenia (NIJZ), with a mean cryptosporidiosis and giardiasis incidence of 0.42 and 2.02 per 100,000 inhabitants in Slovenia, respectively [114]. Cryptosporidium species and subtypes identified from human samples are summarised in Table 8.
Table 8. Cryptosporidium spp. and subtypes detected in faecal samples from humans and cattle using PCR, Slovenia, 2000–2015.
| Type of sample | Time period | Cryptosporidium spp. (number of specimens) | Cryptosporidium subtypes (number of specimens) | Method (and sequenced molecular markers) | Reference |
|---|---|---|---|---|---|
| Human | 2000–2006 | C. hominis (2) | IaA17 (1), IbA10G2 (1) | PCR (SSU rRNA, gp60) | [117,168] |
| C. parvum (31) | IIaA9G1R1 (1), IIaA11G2R1 (2), IIaA13R1 (2), IIaA14G1R1 (1), IIaA15G1R1 (4), IIaA15G2R1 (15), IIaA16G1R1 (2), IIaA17G1R1 (1), IIaA19G1R1 (1), IIcA5G3 (1), IIlA16R2 (1) | ||||
| C. ubiquitum (1) | NA | ||||
| Human | 2007–2015 | C. hominis (7) | IaA20 (1), IaA22 (1), IaA23 (1), IdA14 (1) | PCR (SSU rRNA, gp60) | (Šoba et al. data not shown) |
| C. parvum (32) | IIaA11R1 (1), IIaA13R1 (10), IIaA15G2R1 (14), IIaA15G1R1 (2), IIaA16R2 (1), IIaA16G1R1 (1), IIaA17G1R1 (1), IIaA19G1R1 (1) | ||||
| C. meleagridis (1) | NA | ||||
| Bovine | 2002–2007 | C. parvum (45) | IIaA13R1 (5), IIaA15G2R1 (27), IIaA16R1 (3), IIaA16G1R1 (6), IIlA16R2 (2), IIlA18R2 (2) | PCR (SSU rRNA, gp60) | [117] |
| C. bovis (3) | NA | ||||
| C. ryanae (3) | NA |
NA: not available: SSU rRNA: small subunit ribosomal ribonucleic acid.
From 2002 to 2013, a total of 51 G. intestinalis isolates from symptomatic human cases were genetically characterised. Assemblage A was found in 50.98% (26/51; 95% CI: 37.40–64.45) of the isolates while the remaining 49.02% (25/51; 95% CI: 35.55–62.60) of the isolates were of the assemblage B. Phylogenetic analysis showed that the successfully subtyped assemblage A isolates belonged to the sub-assemblage AII while the assemblage B isolates belonged to the sub-assemblage BIV [115].
Animals
According to genotyping studies, the transmission of Cryptosporidium between cattle and humans is of epidemiological relevance in Slovenia. The most common C. parvum subtypes in cattle were also found in humans [116,117]. The Cryptosporidium species and subtypes detected in cattle in Slovenia are presented in Table 8.
Faecal samples from cattle (n = 391), sheep (n = 35), goats (n = 9), horses (n = 14) and deer (n = 28), were examined for Giardia cysts using IFT in 2006–2007. Of the examined samples, 26.60% (104/391; 95% CI: 22.40–31.15) of cattle, 42.86% (15/35; 95% CI: 27.35–59.50) of sheep and 11.11% (1/9; 95% CI: 0.55–43.86) of goats were found to be Giardia-positive, while no cysts were found in horses and deer. In terms of cattle, only the non-zoonotic assemblage E of G. intestinalis has been found in 36 faecal samples from livestock using a real-time PCR assay [118]. Although the sample size is limited, the results of this study suggest a less important role of livestock in the transmission of Giardia to humans in Slovenia.
Discussion
Analysis of the data obtained from a total of 10 countries showed that both Cryptosporidium spp. and Giardia spp. are commonly found in animals and in the environment when investigated, while giardiasis is more commonly reported in humans than cryptosporidiosis. Based on the number of reported cases in the ECDC Surveillance Atlas of Infectious Diseases, the difference between western Europe and eastern Europe appears more striking for cryptosporidiosis than for giardiasis [11].
Both parasites are prevalent in eastern Europe, but the number of reported cases varies greatly between the investigated countries; the causes of this variation include true differences in exposure and susceptibility, variable provision and access to healthcare systems, and differences in case definition, laboratory diagnosis, recording of cases and reporting. The national health systems of the countries covered here operate differently. Eight countries are members of the EU, and in these, both cryptosporidiosis and giardiasis are notifiable. In Bosnia and Herzegovina, neither disease is notifiable, and in Serbia, only giardiasis is notifiable. The different reporting standards may lead to varied levels of underreporting and varied recognition of the diseases as a public health issue. Making a disease mandatorily notifiable is an important step for obtaining accurate data, however, the quality and representativeness of the data obtained depends strongly on which patients are tested and which diagnostic tests are used. In many countries, neither the number of samples investigated nor the methods used for testing are reported. In our opinion, more transparency and uniformity in the collection of surveillance data are needed to further improve its quality. Currently, data available from the ECDC Surveillance Atlas of Infectious Diseases does not allow for reliable inter-country comparisons as demonstrated by the discrepancy in the reported occurrence of both diseases in humans when comparing surveillance data available via in the ECDC Surveillance Atlas of Infectious Diseases with the data provided by the public health laboratories (Table 1). Some countries provided lower or higher notification rates than that reported by public health laboratories. For example, no evidence of human infections of G. intestinalis was recorded for Romania in the ECDC Surveillance Atlas of Infectious Diseases (Table 1). Primary care doctors or physicians frequently treat patients with diarrhoeal disease symptomatically, without testing faecal samples for pathogens.
Another striking observation of our analysis is the discrepancy in the number of human cases between official reports of public health authorities (Table 1) and research-derived data. Although routine investigations and research studies are never directly comparable, the studies indicate more human infections than what is reflected in the routine investigations, therefore suggesting under-reporting throughout eastern Europe. One reason why research studies report more cases than public health authorities may be the ability to use more sophisticated methodology than that available for routine purposes. Under-reporting, which leads to underestimation of the burden of infection, is further anticipated because not all infected individuals exhibit clinical symptoms and some symptomatic persons do not seek medical care.
Data on the occurrence of Cryptosporidium spp. and Giardia spp. in animals in eastern Europe differ broadly in terms of targeted animal species and depth of analysis. This review showed that both Cryptosporidium spp. and Giardia spp. are common parasites of domestic animals, including pets, in eastern Europe, and importantly, genotypes pathogenic to humans, including C. parvum and G. intestinalis assemblage A and B, are prevalent. C.parvum subtype IIaA16G1R1 is a common subtype in the region, found in both cattle and humans in the Czech Republic, Estonia, Hungary, Romania and Slovenia [62,69,89,117,119]. It has also been suggested that birds may be carriers of human pathogenic species and genotypes of Giardia and Cryptosporidium [70].
Analysis of the current status of research on Cryptosporidium spp. and Giardia spp. in the environment highlighted that to date, relatively little is known about the occurrence and genetic diversity of these parasites in natural water supplies. Reports were available from the Czech Republic, Hungary, Poland and Romania [24,55-58,72,73,80-84,96-97].
Reports on presence of Cryptosporidium spp. and Giardia spp. in food were scarce from this region. Waterborne and food-borne outbreaks are clearly important to establish the burden of disease, but it is likely that many smaller outbreaks are currently missed [120,121].
Baseline data as well as improved understanding of the epidemiology, infection sources, reservoirs and transmission of cryptosporidiosis and giardiasis in eastern Europe are needed. Surveillance studies and outbreak investigations using molecular tools at the subtype level are warranted. In addition, consensus and updated methods that are harmonised across countries are required to make the data more comparable. Reducing public health risks from zoonoses and other threats at the human-animal-ecosystem interface must consider the complexity of interactions among humans, animals and the various environments in which they live. This requires communication and collaboration among the sectors responsible for human health, animal health and the environment in a One Health approach. Although the presented results may be important for public health specialists, epidemiologists, drinking and wastewater managers, veterinarians, farmers and the public in general, further addressing the knowledge gaps in a timely manner would greatly contribute to understanding the complex picture of cryptosporidiosis and giardiasis epidemiology and thus set the stage for appropriate future control plans.
Acknowledgements
The authors received funding/support from the following sources:
- Scandinavian-Baltic Society for Parasitology funding of parasitological reviews.
- Base Financing of the Estonian University of Life Sciences (project 8P160014VLVP).
- Romanian National Authority for Scientific Research and Innovation, CNCS National University Research Council (CNCS) and Executive Agency for Higher Education, Research, Development and Innovation Funding (UEFISCDI) (project grant number PN-II-RU-TE-2014-4-1300).
- Czech Science Foundation (project number 15-01090S).
- The Serbian Ministry of Education, Science and Technological Development (project grant number III41019).
- The One Thousand Talents Plan of the Chinese Government (project grant number WQ2013630172).
- The Slovenian Research Agency (research core funding number P3-0083).
The Polish Ministry of Science and Higher Education (project grant number NN308577039).
The article is partly based upon collaboration within the framework of COST Action FA1408 (A European Network for Foodborne Parasites (Euro-FBP)), supported by COST (European Cooperation in Science and Technology)
We also acknowledge the kind help of the following individuals:
Pál Szakál for editorial work on this manuscript, Age Kärssin for helping to summarise the veterinary diagnostic data from Estonia, Eszter Mezei for retrieving the epidemiological data from Hungary, Antra Bormane and Rita Korotinska from the Latvian Disease and Control Centre for providing the epidemiological data from Latvia.
Conflict of interest: None declared.
Authors’ contributions: Judit Plutzer coordinated the data collection and wrote the manuscript.
Brian Lassen collected the data from Estonia and wrote the manuscript.
Pikka Jokelainen collected the data from Estonia and wrote the manuscript.
Olgica Djurković-Djaković collected the data from Serbia and wrote the manuscript.
István Kucsera collected the data from Hungary and prepared the summary.
Elisabeth Dorbek-Kolin collected the data from Estonia and prepared the summary.
Barbara Šoba collected the data from Slovenia and prepared the summary.
Tamás Sréter collected the data from Hungary and prepared the summary.
Kálmán Imre collected the data from Romania and prepared the summary.
Jasmin Omeragić collected the data from Bosnia and Herzegovina and prepared the summary.
Aleksandra Nikolić collected the data from Serbia and prepared the summary.
Branko Bobić collected the data from Serbia and prepared the summary.
Tatjana Živičnjak collected the data from Croatia and prepared the summary.
Snježana Lučinger collected the data from Croatia and prepared the summary.
Lorena Lazarić Stefanović collected the data from Croatia and prepared the summary.
Jasmina Kučinar collected the data from Croatia and prepared the summary.
Jacek Sroka collected the data from Poland and prepared the summary.
Gunita Deksne collected the data from Latvia and prepared the summary.
Dace Keidāne collected the data from Latvia and prepared the summary.
Martin Kváč collected the data from Czech Republic and prepared the summary.
Zuzana Hůzová collected the data from Czech Republic and prepared the summary.
Panagiotis Karanis presented the idea for this study.
All authors have edited, read and approved the manuscript.
References
- 1. Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) Multicriteria-based ranking for risk management of food-borne parasites. Microbiolological Risk Assessment Series No. 23. Rome: FAO/WHO. 2014.. Available from: http://www.fao.org/3/a-i3649e.pdf [Google Scholar]
- 2. Xiao L, Feng Y. Zoonotic cryptosporidiosis. FEMS Immunol Med Microbiol. 2008;52(3):309-23. 10.1111/j.1574-695X.2008.00377.x [DOI] [PubMed] [Google Scholar]
- 3. Sprong H, Cacciò SM, van der Giessen JW. Identification of zoonotic genotypes of Giardia duodenalis. PLoS Negl Trop Dis. 2009;3(12):e558. 10.1371/journal.pntd.0000558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO). WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007-2015. Geneva: WHO. 2015. [Accessed 15 Dec 2017]. Available from: http://apps.who.int/iris/bitstream/10665/199350/1/9789241565165_eng.pdf?ua=1
- 5. Lake RJ, Devleesschauwer B, Nasinyama G, Havelaar AH, Kuchenmüller T, Haagsma JA, et al. National Studies as a Component of the World Health Organization Initiative to Estimate the Global and Regional Burden of Foodborne Disease. PLoS One. 2015;10(12):e0140319. 10.1371/journal.pone.0140319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tallant C, Huddleston P, Alshanberi A, Misra S. Acute, Severe Cryptosporidiosis in an Immunocompetent Pediatric Patient. Clin Pract. 2016;6(2):837. 10.4081/cp.2016.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Current WL, Garcia LS. Cryptosporidiosis. Clin Lab Med. 1991;11(4):873-97. [PubMed] [Google Scholar]
- 8. Pasmans F, Blahak S, Martel A, Pantchev N. Introducing reptiles into a captive collection: the role of the veterinarian. Vet J. 2008;175(1):53-68. 10.1016/j.tvjl.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 9. Farthing MJ. Giardiasis. Gastroenterol Clin North Am. 1996;25(3):493-515. 10.1016/S0889-8553(05)70260-0 [DOI] [PubMed] [Google Scholar]
- 10. Upcroft JA, McDonnell PA, Gallagher AN, Chen N, Upcroft P. Lethal Giardia from a wild-caught sulphur-crested cockatoo (Cacatua galerita) established in vitro chronically infects mice. Parasitology. 1997;114(5):407-12. 10.1017/S0031182096008724 [DOI] [PubMed] [Google Scholar]
- 11.European Centre for Disease Prevention and Control (ECDC). Surveillance Atlas of Infectious Diseases. Years 2007-2016. Stockholm: ECDC. [Accessed 12 Dec 2017]. Available from: http://atlas.ecdc.europa.eu/public/index.aspx?Instance=GeneralAtlas
- 12.Eurostat. Population as a percentage of EU28 population, 2016. Luxembourg: Eurostat. [Accessed 12 Dec 2017]. Available from: http://ec.europa.eu/eurostat/tgm/table.do?tab=table&init=1&language=en&pcode=tps00005&plugin=1
- 13.Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health: version 3.01. Atlanta: OpenEpi. 2013. [Accessed 15 Dec 2017]. Available from: www.OpenEpi.com
- 14. Hodžić A, Alić A, Omeragić J. Occurrence of Cryptosporidium spp. and Giardia duodenalis in Red foxes (Vulpes vulpes) in Bosnia and Herzegovina. Macedonian Veterinary Review. 2014;37(2):189-92. 10.14432/j.macvetrev.2014.04.012 [DOI] [Google Scholar]
- 15.Omeragić J, Hrvat H, Crnkić Ć. Occurrence of protozoa in dogs in the area of Tuzla. Proceedings of the International Congress ‘One World - One Health - One Vision’; 2015 Oct 14-16; Sarajevo, Bosnia and Herzegovina. Available from: https://www.researchgate.net/publication/322267355
- 16.Hrvat H. Investigation of protozoa from the class of Sporozoea and Zoomastigophorea of dogs in area of northeast Bosnia. [Msc thesis]. Sarajevo: Veterinary Faculty, University of Sarajevo; 2015. [Google Scholar]
- 17.Klarić D, Radetić V, Gajić N, Cviko A, Grabovica E, Smajlović A, et al. Control of parasites in the kennel of Belgian shepherd dog. Proceedings of the International Congress ‘One World - One Health - One Vision’; 2015 Oct 14-16; Sarajevo, Bosnia and Herzegovina. Available from: https://www.researchgate.net/publication/306018973
- 18. Stĕrba J, Ditrich O, Prokopič J, Kadlcík K. Gastrointestinal parasitoses discovered in agricultural workers in South Bohemia, Czechoslovakia. Folia Parasitol (Praha). 1988;35(2):169-73. [PubMed] [Google Scholar]
- 19. Breza M. The improvement of method of coproovascopical examination of pig faeces by using a new floatative solution Mucogel Liquid. Veterinársky Cas. 1959;8:569-76. [Google Scholar]
- 20. Hunter GW, 3rd, Hodges EP, Jahnes WG, Diamond LS, Ingalis JW. Studies of Schistosomiasis: II Summary of further studies on methods of recovering eggs of S. japonicum from stools. Bull U S Army Med Dep. 1948;8(2):128-31. [PubMed] [Google Scholar]
- 21. Ditrich O, Palkovič L, Stĕrba J, Prokopič J, Loudová J, Giboda M. The first finding of Cryptosporidium baileyi in man. Parasitol Res. 1991;77(1):44-7. 10.1007/BF00934383 [DOI] [PubMed] [Google Scholar]
- 22. Milácek P, Vítovec J. Differential staining of cryptosporidia by aniline-carbol-methyl violet and tartrazine in smears from feces and scrapings of intestinal mucosa. Folia Parasitol (Praha). 1985;32(1):50. [PubMed] [Google Scholar]
- 23. Chmelík V, Ditrich O, Trnovcová R, Gutvirth J. Clinical features of diarrhoea in children caused by Cryptosporidium parvum. Folia Parasitol (Praha). 1998;45(2):170-2. [PubMed] [Google Scholar]
- 24. Hajdušek O, Ditrich O, Šlapeta J. Molecular identification of Cryptosporidium spp. in animal and human hosts from the Czech Republic. Vet Parasitol. 2004;122(3):183-92. 10.1016/j.vetpar.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 25. Kváč M, Květoňová D, Sak B, Ditrich O. Cryptosporidium pig genotype II in immunocompetent man. Emerg Infect Dis. 2009. b;15(6):982-3. 10.3201/eid1506.071621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rašková V, Květoňová D, Sak B, McEvoy J, Edwinson A, Stenger B, et al. Human cryptosporidiosis caused by Cryptosporidium tyzzeri and C. parvum isolates presumably transmitted from wild mice. J Clin Microbiol. 2013;51(1):360-2. 10.1128/JCM.02346-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kváč M, Saková K, Kvĕtoňová D, Kicia M, Wesołowska M, McEvoy J, et al. Gastroenteritis caused by the Cryptosporidium hedgehog genotype in an immunocompetent man. J Clin Microbiol. 2014. c;52(1):347-9. 10.1128/JCM.02456-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kožišek F, Craun GF, Cerovska L, Pumann P, Frost F, Muller T. Serological responses to Cryptosporidium-specific antigens in Czech populations with different water sources. Epidemiol Infect. 2008;136(2):279-86. 10.1017/S0950268807008370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pospísilová Z, Ditrich O, Stanková M, Kodym P. Parasitic opportunistic infections in Czech HIV-infected patients--a prospective study. Cent Eur J Public Health. 1997;5(4):208-13. [PubMed] [Google Scholar]
- 30. Koudela B, Vítovec J, Stĕrba J, Milácek P. An unusual localization of developmental stages of Cryptosporidium parvum Tyzzer, 1912 in the cells of small intestine of a gnotobiotic piglet. Folia Parasitol (Praha). 1989;36(3):219-22. [PubMed] [Google Scholar]
- 31. Koudela B, Modrý D, Vítovec J. Infectivity of Cryptosporidium muris isolated from cattle. Vet Parasitol. 1998;76(3):181-8. 10.1016/S0304-4017(97)00217-3 [DOI] [PubMed] [Google Scholar]
- 32. Vítovec J, Koudela B. Pathogenesis of intestinal cryptosporidiosis in conventional and gnotobiotic piglets. Vet Parasitol. 1992;43(1-2):25-36. 10.1016/0304-4017(92)90045-B [DOI] [PubMed] [Google Scholar]
- 33. Koudela B, Boková A. The effect of cotrimoxazole on experimental Cryptosporidium parvum infection in kids. Vet Res. 1997;28(4):405-12. [PubMed] [Google Scholar]
- 34. Koudela B, Jirí V. Experimental cryptosporidiosis in kids. Vet Parasitol. 1997;71(4):273-81. 10.1016/S0304-4017(97)00024-1 [DOI] [PubMed] [Google Scholar]
- 35. Vítovec J, Hamadejová K, Landová L, Kváč M, Květoňová D, Sak B. Prevalence and pathogenicity of Cryptosporidium suis in pre- and post-weaned pigs. J Vet Med B Infect Dis Vet Public Health. 2006;53(5):239-43. 10.1111/j.1439-0450.2006.00950.x [DOI] [PubMed] [Google Scholar]
- 36. Kváč M, Ditrich O, Kouba M, Sak B, Vítovec J, Květoňová D. Failed attempt of Cryptosporidium andersoni infection in lambs. Folia Parasitol (Praha). 2004;51(4):373-4. 10.14411/fp.2004.047 [DOI] [PubMed] [Google Scholar]
- 37. Kváč M, Kestřánová M, Pinková M, Květoňová D, Kalinová J, Wagnerová P, et al. Cryptosporidium scrofarum n. sp. (Apicomplexa: Cryptosporidiidae) in domestic pigs (Sus scrofa). Vet Parasitol. 2013. a;191(3-4):218-27. 10.1016/j.vetpar.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kváč M, Ondráčková Z, Květoňová D, McEvoy J, Vítovec J, Rost M, et al. The Lesser Egyptian Gerbil (Gerbillus gerbillus) is a suitable host for the long-term propagation of Cryptosporidium andersoni. Exp Parasitol. 2013. d;134(4):438-42. 10.1016/j.exppara.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 39. Kváč M, Hofmannová L, Hlásková L, Květoňová D, Vítovec J, McEvoy J, et al. Cryptosporidium erinacei n. sp. (Apicomplexa: Cryptosporidiidae) in hedgehogs. Vet Parasitol. 2014. a;201(1-2):9-17. 10.1016/j.vetpar.2014.01.014 [DOI] [PubMed] [Google Scholar]
- 40. Kváč M, Němejc K, Kestřánová M, Květoňová D, Wagnerová P, Kotková M, et al. Age related susceptibility of pigs to Cryptosporidium scrofarum infection. Vet Parasitol. 2014. b;202(3-4):330-4. 10.1016/j.vetpar.2014.02.012 [DOI] [PubMed] [Google Scholar]
- 41. Kváč M, Havrdová N, Hlásková L, Daňková T, Kanděra J, Ježková J, et al. Cryptosporidium proliferans n. sp. (Apicomplexa: Cryptosporidiidae): Molecular and Biological Evidence of Cryptic Species within Gastric Cryptosporidium of Mammals. PLoS One. 2016;11(1):e0147090. 10.1371/journal.pone.0147090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ježková J, Horčičková M, Hlásková L, Sak B, Květoňová D, Novák J, et al. Cryptosporidium testudinis sp. n., Cryptosporidium ducismarci Traversa, 2010 and Cryptosporidium tortoise genotype III (Apicomplexa: Cryptosporidiidae) in tortoises. Folia Parasitol (Praha). 2016;63:63. 10.14411/fp.2016.035 [DOI] [PubMed] [Google Scholar]
- 43. Jirků M, Valigurová A, Koudela B, Krízek J, Modrý D, Šlapeta J. New species of Cryptosporidium Tyzzer, 1907 (Apicomplexa) from amphibian host: morphology, biology and phylogeny. Folia Parasitol (Praha). 2008;55(2):81-94. 10.14411/fp.2008.011 [DOI] [PubMed] [Google Scholar]
- 44. Valigurová A, Jirků M, Koudela B, Gelnar M, Modrý D, Šlapeta J. Cryptosporidia: epicellular parasites embraced by the host cell membrane. Int J Parasitol. 2008;38(8-9):913-22. 10.1016/j.ijpara.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 45. Melicherová J, Ilgová J, Kváč M, Sak B, Koudela B, Valigurová A. Life cycle of Cryptosporidium muris in two rodents with different responses to parasitization. Parasitology. 2014;141(2):287-303. 10.1017/S0031182013001637 [DOI] [PubMed] [Google Scholar]
- 46. Holubová N, Sak B, Horčičková M, Hlásková L, Květoňová D, Menchaca S, et al. Cryptosporidium avium n. sp. (Apicomplexa: Cryptosporidiidae) in birds. Parasitol Res. 2016;115(6):2243-51. 10.1007/s00436-016-4967-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pavlásek I. First record of Cryptosporidium sp. in calves in Czechoslovakia. Folia Parasitol (Praha). 1981;28(2):187-9. [PubMed] [Google Scholar]
- 48. Pavlásek I. Racek chechtavý (Larus ridibundus L.), nový hostitel Cryptosporidium baileyi (Apicomplexa: Cryptosporidiidae). [The black-headed gull (Larus ridibundus L.), a new host for Cryptosporidium baileyi (Apicomplexa: Cryptosporidiidae)]. Vet Med (Praha). 1993;38(10):629-38. Czech. [PubMed] [Google Scholar]
- 49. Pavlásek I, Lávicka M, Tůmová E, Skřivan M. Spontánní kryptosporidiová nákaza u odstavených králícat. [Spontaneous Cryptosporidium infection in weaned rabbits]. Vet Med (Praha). 1996;41(12):361-6. Czech. [PubMed] [Google Scholar]
- 50.Pavlásek I. Nálezy kryptosporidií ve žláznatém žaludku u slepic a u volně žijících a exotických ptáků odchycených z volné přírody. [First finding of cryptosporidia in glandular stomach of hens and free living and exotic birds]. Veteriářství. 2001;51:103-8. Czech. Available from: http://vetweb.cz/nalezy-kryptosporidii-ve-zlaznatem-zaludku-u-slepic-a-u-volne-zijicich-a-exotickych-ptaku-odchycenych-z-volne-prirody/
- 51. Pavlásek I. První nález oocyst morfometricky podobných druhům Cryptosporidium muris a C. andersoni u kočky. [First finding of Cryptosporidium oocysts morphological similar to C. muris and C. andersoni in cat]. Veterinarstvi. 2005;55:480-3. Czech. Available from: http://vetweb.cz/prvni-nalez-oocyst-morfometricky-podobnych-druhum-cryptosporidium-muris-a-c-andersoni-u-kocky/ [Google Scholar]
- 52. Kváč M, Vítovec J. Prevalence and pathogenicity of Cryptosporidium andersoni in one herd of beef cattle. J Vet Med B Infect Dis Vet Public Health. 2003;50(9):451-7. 10.1046/j.0931-1793.2003.00701.x [DOI] [PubMed] [Google Scholar]
- 53. Chroust K. Parazitózy u masných plemen skotu v marginálních oblastech a jejich tlumení. [Parasitosis in beef cattle and their treatment in marginal areas]. Veterinarstvi. 2006;56:430-7. Czech. Available from: http://vetweb.cz/parazitozy-u-masnych-plemen-skotu-v-marginalnich-oblastech-a-jejich-tlumeni/ [Google Scholar]
- 54. Kváč M, Kouba M, Vítovec J. Age-related and housing-dependence of Cryptosporidium infection of calves from dairy and beef herds in South Bohemia, Czech Republic. Vet Parasitol. 2006;137(3-4):202-9. 10.1016/j.vetpar.2006.01.027 [DOI] [PubMed] [Google Scholar]
- 55.Dolejš P, Machula T, Kalousková N, Ditrich O, Půžová G. Odstraňování Cryptosporidium parvum a Giardia intestinalis při úpravě pitných vod. [Removal of Cryptosporidium parvum and Giardia intestinalis from drinking water]. SOVAK. 1999; 8(4):17-19. Czech. Available from: http://www.mzp.cz/ris/ais-ris-info-copy.nsf/da28f37425da72f7c12569e600723950/81c2400f06622697c1256a320035c346?OpenDocument
- 56.Dolejš P, Ditrich O, Machula T. Aktuální poznatky o vývoji problematiky Cryptosporidium sp. u nás a ve světě. [Cryptosporidium sp. in the Czech Republic and world: current knowledge]. Proceedings of Current Issue of Biology in Water Supply; 2000 Feb 2-3; Praha, Czech Republic. p. 134-138. Czech. [Google Scholar]
- 57.Dolejš P, Ditrich O, Machula T, Kalousková N, Půžová G. Monitoring of Cryptosporidium and Giardia in Czech drinking water sources. Schriftenr Ver Wasser Boden Lufthyg. 2000;105:147-51. PMID:10842807 [PubMed]
- 58. Dolejš P, Ditrich O, Machula T, Kalousková N, Půžová G. Occurrence and separation of Cryptosporidium oocysts in Drinking Water Treatment. Water Sci Technol. 2000;41(7):159-63. Available from: http://wst.iwaponline.com/content/41/7/159 [Google Scholar]
- 59.Epštein J, Kutsar K, Kerbo N, Aro T. Nakkushaiguste esinemine Eestis (statistikaandmed) 16. osa. [Communicable Disease Statistics in Estonia Part 16]. Tallinn: Health Board. 2016. [Accessed 15 Dec 2017]. Estonian. Available from: www.terviseamet.ee/fileadmin/dok/Kasulikku/Nakkushaigused/Stat_16_2015.pdf
- 60.Health Board. Nakkushaigused. [Infectious diseases]. Tallinn: Health Board. 2015. [Accessed 15 Dec 2017]. Estonian. Available from: http://www.terviseamet.ee/nakkushaigused/nakkushaigustesse-haigestumine.html
- 61. Šljapnikova L, Pirožkova L, Peetso R, Sudakova R, Zolotuhhina I, Zilmer K, et al. Krüptosporidioos kõhulahtisuse sündroomiga lastel.[Cryptosporidiasis in children with intestinal disorders]. Eesti Arst. 1994;3:190-3. Estonian. [Google Scholar]
- 62. Lassen B, Ståhl M, Enemark HL. Cryptosporidiosis - an occupational risk and a disregarded disease in Estonia. Acta Vet Scand. 2014. a;56(1):36. 10.1186/1751-0147-56-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Estonian Veterinary and Food Laboratory. Aastaaruanded. [Yearly reports]. Tartu: Estonian Veterinary and Food Laboratory. 2015. [Accessed 15 Dec 2017]. Estonian. Available from: http://www.vetlab.ee/?a=page&page=42f088c48f3e323aa1bbc?a=page&page=42f088c48f3e323aa1bbc&subpage=42f0a2f57776b116a280e
- 64. Lassen B, Viltrop A, Järvis T. Herd factors influencing oocyst production of Eimeria and Cryptosporidium in Estonian dairy cattle. Parasitol Res. 2009. a;105(5):1211-22. 10.1007/s00436-009-1540-8 [DOI] [PubMed] [Google Scholar]
- 65. Lassen B, Viltrop A, Raaperi K, Järvis T. Eimeria and Cryptosporidium in Estonian dairy farms in regard to age, species, and diarrhoea. Vet Parasitol. 2009. b;166(3-4):212-9. 10.1016/j.vetpar.2009.08.022 [DOI] [PubMed] [Google Scholar]
- 66.Lassen B, Järvis T, Mägi E. Gastrointestinal parasites of sheep on Estonian islands. Agraarteadus: Journal of Agricultural Science. 2013; XXIV(1):7-14. Available from: http://agrt.emu.ee/pdf/2013_1_lassen1.pdf
- 67. Farkas K, Plutzer J, Moltchanova E, Török A, Varró MJ, Domokos K, et al. Serological responses to Cryptosporidium antigens in inhabitants of Hungary using conventionally filtered surface water and riverbank filtered drinking water. Epidemiol Infect. 2015;143(13):2743-7. 10.1017/S0950268814003859 [DOI] [PubMed] [Google Scholar]
- 68. Plutzer J, Törökné A, Szénási Z, Kucsera I, Farkas K, Karanis P. Detection and genotype analysis of Giardia duodenalis from asymptomatic Hungarian inhabitants and comparative findings in three distinct locations. Acta Microbiol Immunol Hung. 2014;61(1):19-26. 10.1556/AMicr.61.2014.1.3 [DOI] [PubMed] [Google Scholar]
- 69. Plutzer J, Karanis P. Genotype and subtype analyses of Cryptosporidium isolates from cattle in Hungary. Vet Parasitol. 2007;146(3-4):357-62. 10.1016/j.vetpar.2007.02.030 [DOI] [PubMed] [Google Scholar]
- 70. Plutzer J, Tomor B. The role of aquatic birds in the environmental dissemination of human pathogenic Giardia duodenalis cysts and Cryptosporidium oocysts in Hungary. Parasitol Int. 2009;58(3):227-31. 10.1016/j.parint.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 71. Szénási Z, Marton S, Kucsera I, Tánczos B, Horváth K, Orosz E, et al. Preliminary investigation of the prevalence and genotype distribution of Giardia intestinalis in dogs in Hungary. Parasitol Res. 2007;101(S1):145-52. 10.1007/s00436-007-0622-8 17211660 [DOI] [Google Scholar]
- 72. Plutzer J, Takó MH, Márialigeti K, Törökné A, Karanis P. First investigations into the prevalence of Cryptosporidium and Giardia spp. in Hungarian drinking water. J Water Health. 2007;5(4):573-84. 10.2166/wh.2007.007 [DOI] [PubMed] [Google Scholar]
- 73. Plutzer J, Karanis P, Domokos K, Törökné A, Márialigeti K. Detection and characterisation of Giardia and Cryptosporidium in Hungarian raw, surface and sewage water samples by IFT, PCR and sequence analysis of the SSUrRNA and GDH genes. Int J Hyg Environ Health. 2008;211(5-6):524-33. 10.1016/j.ijheh.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 74.Lassen B. The prevalences of Eimeria and Cryptosporidium in large Latvian cattle herds. Vet Med Zoot. 2011;54(76):47-52. Available from: http://vetzoo.lsmuni.lt/data/vols/2011/54/pdf/lassen.pdf
- 75. Rożej W, Gołab E, Waloch M, Wąsik M, Sadkowska-Todys M, Czerwiński M, et al. Wystepowanie Cryptosporidium u dzieci i osób dorosłych z biegunka o nieustalonej etiologii. [The occurrence of Cryptosporidium in a group of children and adults with diarrhoea of undetermined earlier aetiology]. Przegl Epidemiol. 2010;64(1):35-9. Polish. [PubMed] [Google Scholar]
- 76. Bajer A, Bednarska M, Cacciò SM, Wolska-Kuśnierz B, Heropolitanska-Pliszka E, Bernatowska E, et al. Genotyping of Cryptosporidium isolates from human clinical cases in Poland. Parasitol Res. 2008;103(1):37-42. 10.1007/s00436-008-0924-5 [DOI] [PubMed] [Google Scholar]
- 77. Solarczyk P, Werner A, Majewska AC. Genotypowanie izolatów Giardia duodenalis uzyskanych od ludzi w zachodnio-centralnej Polsce. [Genotype analysis of Giardia duodenalis isolates obtained from humans in west-central Poland].Wiad Parazytol. 2010;56(2):171-7. Polish. [PubMed] [Google Scholar]
- 78. Bitkowska E, Wnukowska N, Wojtyniak B, Dzbeński TH. Analiza wystepowania pasozytów jelitowych u dzieci klas pierwszych w Polsce w roku szkolnym 2002/2003. [Occurrence of intestinal parasites among first grade students in Poland in years 2002/2003]. Przegl Epidemiol. 2004;58(2):295-302. Polish. [PubMed] [Google Scholar]
- 79. Zukiewicz M, Kaczmarski M, Topczewska M, Sidor K, Tomaszewska BM. Epidemiological and clinical picture of parasitic infections in the group of children and adolescents from north-east region of Poland. Wiad Parazytol. 2011;57(3):179-87. [PubMed] [Google Scholar]
- 80. Sulima P, Werner A, Majewska AC. Occurrence of intestinal protozoan parasite in drinking water supply in Poznań— microscopic, immunologic and molecular studies. Wiad Parazytol. 2001;47(suppl 2):46. [Google Scholar]
- 81. Sroka J, Giżejewski Z, Wójcik-Fatla A, Stojecki K, Bilska-Zając E, Dutkiewicz J, et al. Potential role of beavers (Castor fiber) in contamination of water in the Masurian Lake District (north-eastern Poland) with protozoan parasites Cryptosporidium spp. and Giardia duodenalis. Bulletin of the Veterinary Institute in Pulawy. 2015;59(2):219-28. 10.1515/bvip-2015-0033 [DOI] [Google Scholar]
- 82. Matuszewska R, Szczotko M, Krogulska B. Optymalizacja metody wykrywania pierwotniaków pasozytniczych Cryptosporidium i Giardia w środowisku wodnym przy zastosowaniu automatycznej stacji płuczacej Filta-Max xpress. [Optimization of Cryptosporidium and Giardia detection in water environment using automatic elution station Filta-Max xpress]. Rocz Panstw Zakl Hig. 2012;63(4):499-505. Polish. [PubMed] [Google Scholar]
- 83.Polus M, Kocwa-Haluch R. Occurrence of Cryptosporidium, Giardia and Toxoplasma in surface waters in the area of Cracow. Environment Protection Engineering. 2014;40(2):105-13. Available from: http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-3d80d27b-cf1b-4a8e-8220-45350d7db20c
- 84.Sroka J, Stojecki K, Zdybel J, Karamon J, Cencek T, Dutkiewicz J. Occurrence of Cryptosporidium oocysts and Giardia cysts in effluent from sewage treatment plant from eastern Poland. Ann Agric Environ Med. 2013; 20(Spec no 1):57-62. PMID:25000844 [PubMed]
- 85. Nowosad P, Kuczyńska-Kippen N, Słodkowicz-Kowalska A, Majewska AC, Graczyk TK. The use of rotifers in detecting protozoan parasite infections in recreational lakes. Aquat Ecol. 2007;41(1):47-54. 10.1007/s10452-006-9043-5 [DOI] [Google Scholar]
- 86. Słodkowicz-Kowalska A, Majewska AC, Rzymski P, Skrzypczak Ł, Werner A. Human waterborne protozoan parasites in freshwater bivalves (Anodonta anatina and Unio tumidus) as potential indicators of fecal pollution in urban reservoir. Limnologica. 2015;51:32-6. 10.1016/j.limno.2014.12.001 [DOI] [Google Scholar]
- 87. Rzezutka A, Nichols RA, Connelly L, Kaupke A, Kozyra I, Cook N, et al. Cryptosporidium oocysts on fresh produce from areas of high livestock production in Poland. Int J Food Microbiol. 2010;139(1-2):96-101. 10.1016/j.ijfoodmicro.2010.01.027 [DOI] [PubMed] [Google Scholar]
- 88.Imre K. Contribuții la cunoașterea biologiei, epidemiologiei, diagnosticului și controlului criptosporidiozei în vestul României. [Contributions in the knowledge of biology, epidemiology, diagnosis and control of cryptosporidiosis in Western Romania]. [dissertation]. Timișoara: Banat's University of Agricultural Sciences and Veterinary Medicine ‘King Michael I of Romania’ from Timişoara, Faculty of Veterinary Medicine; 2010. Romanian. Available from: http://www.usab-tm.ro/rezumat/Imre%20doc.%20teza%20pdf%20engleza%20(1).pdf
- 89.Vieira PM, Mederle N, Lobo LM, Imre K, Mederle O, Xiao L, Dărăbuş G, Matos O. Molecular characterization of Cryptosporidium (Apicomplexa) in children and cattle in Romania. Folia Parasitol (Praha). 2015;62:002. 10.14411/fp.2015.002 PMID:25960546 [DOI] [PubMed]
- 90. Neghina R, Neghina AM, Marincu I, Moldovan R, Iacobiciu I. Foodborne nematodal infections in hospitalized patients from a southwestern Romanian county. Foodborne Pathog Dis. 2010;7(8):975-80. 10.1089/fpd.2010.0533 [DOI] [PubMed] [Google Scholar]
- 91.Costache C, Colosi I, Anca L. Human giardiosis report in Romania: the principle of snowball. Proceedings of the XIth European Multicolloquium of Parasitology; 2012 Jul 25-29; Cluj-Napoca, Romania. Available from: http://www.zooparaz.net/emop11/
- 92. Imre K, Lobo LM, Matos O, Popescu C, Genchi C, Dărăbuş G. Molecular characterisation of Cryptosporidium isolates from pre-weaned calves in Romania: is there an actual risk of zoonotic infections? Vet Parasitol. 2011;181(2-4):321-4. 10.1016/j.vetpar.2011.04.042 [DOI] [PubMed] [Google Scholar]
- 93. Imre K, Luca C, Costache M, Sala C, Morar A, Morariu S, et al. Zoonotic Cryptosporidium parvum in Romanian newborn lambs (Ovis aries). Vet Parasitol. 2013;191(1-2):119-22. 10.1016/j.vetpar.2012.08.020 [DOI] [PubMed] [Google Scholar]
- 94.Sorescu DI. Studiul giardiozei la animale în zona de vest și sud-vest a României. [The study of giardiosis in Western and South-Western of Romania]. [dissertation]. Timişoara: Banat's University of Agricultural Sciences and Veterinary Medicine ‘King Michael I of Romania’ from Timişoara, Faculty of Veterinary Medicine; 2013. Romanian. [Google Scholar]
- 95.Bejan A. Criptosporidioza vițeilor și iezilor: cercetări privind diagnosticul, epidemiologia, și etiopatogeneza bolii. [Cryptosporidiosis in calves and goat kids: research concerning diagnosis, epidemiology, and etiopathogenesis]. [dissertation]. Cluj-Napoca: University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca, Faculty of Veterinary Medicine; 2009. Romanian. Available from: http://usamvcluj.ro/files/teze/bejan.pdf [Google Scholar]
- 96.Imre K, Sala C, Morar A, Ilie MS, Plutzer J, Imre M, et al. Giardia duodenalis and Cryptosporidium spp. as contaminant protozoa of the main rivers of western Romania: genetic characterization and public health potential of the isolates. Environ Sci Pollut Res Int. 2017;24(22):18672–9. 10.1007/s11356-017-9543-y PMID:28653194 [DOI] [PubMed]
- 97. Imre K, Morar A, Ilie MS, Plutzer J, Imre M, Emil T, et al. Survey of the occurrence and human infective potential of Giardia duodenalis and Cryptosporidium spp. in wastewater and different surface water sources of western Romania. Vector Borne Zoonotic Dis. 2017;17(10):685-91. 10.1089/vbz.2017.2155 [DOI] [PubMed] [Google Scholar]
- 98. Gozdenović E, Mitrović N, Dakić Z, Stojković-Švirtlih N, Dulović O. [Family outbreak of cryptosporidiosis in Serbia: case report]. Srp Arh Celok Lek. 2012;140(9-10):653-7. 10.2298/SARH1210653G [DOI] [PubMed] [Google Scholar]
- 99.Korać M. Uticaj visoko aktivne antiretrovirusne terapije na oboljenja gastrointestinalnog trakta kod bolesnika sa sindromom stečene imunodeficijencije. [The impact of highly active antiretroviral therapy on diseases of the gastrointestinal tract in patients with acquired immunodeficiency syndrome]. [dissertation]. Belgrade: School of Medicine, University of Belgrade; 2009. Serbian. [Google Scholar]
- 100.Institute of Public Health of Serbia. ‘Dr Milan Jovanovic Batut’. [Annual Reports on Infectious Diseases in Serbia 2005–2014]. Belgrade: Institute of Public Health of Serbia ‘Dr Milan Jovanovic Batut’. [Accessed 12 Dec 2017]. Available from: http://www.batut.org.rs/index.php?category_id=140
- 101.Miladinović Tasić N. Dijagnostički I epidemiološki aspekti prisustva protozoa Giardia lamblia u digestivnom traktu. [Diagnostic and epidemiological aspects of Giardia lamblia presence in the digestive tract]. [dissertation]. Nis: School of Medicine, University of Nis; 2006. Serbian. [Google Scholar]
- 102. Miladinović Tasić N, Tasić S, Kranjčić-Zec I, Tasić G, Tasić A. Asymptomatic giardiasis - more prevalent in refugees than in native inhabitants of the city of Nis, Serbia. Cent Eur J Med. 2008;3(2):203-6. https://doi.org/10.2478/s11536-008-0013-2 [Google Scholar]
- 103. Miladinović Tasić N, Tasić S, Tasić A, Zdravković D, Đorđević J, Micić T. Advantages of enzyme immunoassay in diagnosing lambliosis of population under sanitary supervision. Acta Facultatis Medicae Naissensis. 2010;27(1):33-7. Available from: https://pdfs.semanticscholar.org/3202/8fd4cd45fd0418bcfe476eb90acb86f8bdc0.pdf [Google Scholar]
- 104. Nikolić A, Klun I, Bobić B, Ivović V, Vujanić M, Zivković T, et al. Human giardiasis in Serbia: asymptomatic vs symptomatic infection. Parasite. 2011;18(2):197-201. 10.1051/parasite/2011182197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nikolić A, Bobić B, Katić-Radivojević S, Klun I, Djurković-Djaković O. Giardia infection in humans and pets in Serbia. In: Mas-Coma S, editor. Multidisciplinarity for Parasites Vectors and Parasitic Diseases: Proceedings of the IX European Multicolloquium of Parasitology, Vol 2; 2004 Jul 18-23; Valencia, Spain. Bologna: Medimond; 2004. p. 179-183. [Google Scholar]
- 106.Spahić Š. Učestalost crevnih parazita u školske dece u opštini Novi Pazar u odnosu na životnu sredinu i konfesionalnu pripadnost. [Intestinal parasitic infections in schoolchildren in Novi Pazar in relation to the environment and confessional affiliation]. [MS thesis]. Belgrade: Military Medical Academy, Belgrade; 2006. Serbian. [Google Scholar]
- 107. Mišić Z, Katić-Radivojević S, Kulišić Z. Cryptosporidium infection in weaners, bull calves and postparturient cows in the Belgrade area. Acta Vet (Beogr). 2002;52(1):37-42. 10.2298/AVB0201037M [DOI] [Google Scholar]
- 108. Mišić Z, Abe N. Subtype analysis of Cryptosporidium parvum isolates from calves on farms around Belgrade, Serbia and Montenegro, using the 60 kDa glycoprotein gene sequences. Parasitology. 2007;134(3):351-8. 10.1017/S0031182006001508 [DOI] [PubMed] [Google Scholar]
- 109. Mišić Z, Katić-Radivojević S, Kulišić Z. Cryptosporidium infection in nursing, weaning and post-weaned piglets and sows in the Belgrade district Serbia. Acta Vet (Beogr). 2003;53(5-6):361-6. 10.2298/AVB0306361M [DOI] [Google Scholar]
- 110. Nikolić A, Dimitrijević S, Katić-Radivojević S, Klun I, Bobić B, Djurković-Djaković O. High prevalence of intestinal zoonotic parasites in dogs from Belgrade, Serbia--short communication. Acta Vet Hung. 2008;56(3):335-40. 10.1556/AVet.56.2008.3.7 [DOI] [PubMed] [Google Scholar]
- 111. Sommer MF, Beck R, Ionita M, Stefanovska J, Vasić A, Zdravković N, et al. Multilocus sequence typing of canine Giardia duodenalis from South Eastern European countries. Parasitol Res. 2015;114(6):2165-74. 10.1007/s00436-015-4405-3 [DOI] [PubMed] [Google Scholar]
- 112. Nikolić A, Dimitrijević S, Djurković-Djaković O, Bobić B, Maksimović-Mihajlović O. Giardiasis in dogs and cats in the Belgrade area. Acta Vet (Beogr). 2002;52(1):43-8. 10.2298/AVB0201043N [DOI] [Google Scholar]
- 113.Lažetić V, Ilić T, Ilić V, Dimitrijević S. Parazitske bolesti mačaka na beogradskom Području sa posebnim osvrtom na zoonoze. [Parasitic diseases in cats in Belgrade area with special emphasis on zoonoses]. Arhiv veterinarske medicine. 2012; 5(2):53-66. Serbian. Available from: http://niv.ns.ac.rs/StariSajt/tr31084/fajlovi/12/78.pdf
- 114.National Institute of Public Health of the Republic of Slovenia. Epidemiološko spremljanje nalezljivih bolezni v Sloveniji. Leta 2003-2015. [Annual Epidemiological Report on Communicable Diseases in Slovenia. Years 2003-2015]. Ljubljana: National Institute of Public Health. [Accessed 18 Dec 2017]. Slovenian. Available from: http://www.nijz.si/sl/epidemiolosko-spremljanje-nalezljivih-bolezni-letna-in-cetrtletna-porocila
- 115.Šoba B, Islamović S, Skvarč M, Caccio SM. Multilocus genotyping of Giardia duodenalis (Lambl, 1859) from symptomatic human infections in Slovenia. Folia Parasitol (Praha). 2015;62:062. 10.14411/fp.2015.062 PMID:26580803 [DOI] [PubMed]
- 116. Stantic-Pavlinic M, Xiao L, Glaberman S, Lal AA, Orazen T, Rataj-Verglez A, et al. Cryptosporidiosis associated with animal contacts. Wien Klin Wochenschr. 2003;115(3-4):125-7. 10.1007/BF03040292 [DOI] [PubMed] [Google Scholar]
- 117. Soba B, Logar J. Genetic classification of Cryptosporidium isolates from humans and calves in Slovenia. Parasitology. 2008;135(11):1263-70. 10.1017/S0031182008004800 [DOI] [PubMed] [Google Scholar]
- 118. Van Lith L, Šoba B, Vizcaino VV, Svard S, Sprong H, Tosini F, et al. A real-time assemblage-specific PCR assay for the detection of Giardia duodenalis assemblages A, B and E in fecal samples. Vet Parasitol. 2015;211(1-2):28-34. 10.1016/j.vetpar.2015.04.017 [DOI] [PubMed] [Google Scholar]
- 119. Kváč M, Hromadová N, Květoňová D, Rost M, Sak B. Molecular characterization of Cryptosporidium spp. in pre-weaned dairy calves in the Czech Republic: absence of C. ryanae and management-associated distribution of C. andersoni, C. bovis and C. parvum subtypes. Vet Parasitol. 2011;177(3-4):378-82. 10.1016/j.vetpar.2010.11.048 [DOI] [PubMed] [Google Scholar]
- 120. Guzman-Herrador B, Carlander A, Ethelberg S, Freiesleben de Blasio B, Kuusi M, Lund V, et al. Waterborne outbreaks in the Nordic countries, 1998 to 2012. Euro Surveill. 2015;20(24):21160. 10.2807/1560-7917.ES2015.20.24.21160 [DOI] [PubMed] [Google Scholar]
- 121. Cacciò SM, Chalmers RM. Human cryptosporidiosis in Europe. Clin Microbiol Infect. 2016;22(6):471-80. 10.1016/j.cmi.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 122. Ryan U, Xiao L, Read C, Zhou L, Lal AA, Pavlásek I. Identification of novel Cryptosporidium genotypes from the Czech Republic. Appl Environ Microbiol. 2003;69(7):4302-7. 10.1128/AEM.69.7.4302-4307.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ondráčková Z, Kváč M, Sak B, Květoňová D, Rost M. Prevalence and molecular characterization of Cryptosporidium spp. in dairy cattle in South Bohemia, the Czech Republic. Vet Parasitol. 2009;165(1-2):141-4. 10.1016/j.vetpar.2009.06.035 [DOI] [PubMed] [Google Scholar]
- 124. Kváč M, Sak B, Hanzlíková D, Kotilová J, Květoňová D. Molecular characterization of Cryptosporidium isolates from pigs at slaughterhouses in South Bohemia, Czech Republic. Parasitol Res. 2009. c;104(2):425-8. 10.1007/s00436-008-1215-x [DOI] [PubMed] [Google Scholar]
- 125. Kváč M, Hanzlíková D, Sak B, Květoňová D. Prevalence and age-related infection of Cryptosporidium suis, C. muris and Cryptosporidium pig genotype II in pigs on a farm complex in the Czech Republic. Vet Parasitol. 2009. a;160(3-4):319-22. 10.1016/j.vetpar.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 126. Jeníková M, Němejc K, Sak B, Květoňová D, Kváč M. New view on the age-specificity of pig Cryptosporidium by species-specific primers for distinguishing Cryptosporidium suis and Cryptosporidium pig genotype II. Vet Parasitol. 2011;176(2-3):120-5. 10.1016/j.vetpar.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 127. Němejc K, Sak B, Květoňová D, Hanzal V, Janiszewski P, Forejtek P, et al. Cryptosporidium suis and Cryptosporidium scrofarum in Eurasian wild boars (Sus scrofa) in Central Europe. Vet Parasitol. 2013. a;197(3-4):504-8. 10.1016/j.vetpar.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wagnerová P, Sak B, McEvoy J, Rost M, Matysiak AP, Ježková J, et al. Genetic diversity of Cryptosporidium spp. including novel identification of the Cryptosporidium muris and Cryptosporidium tyzzeri in horses in the Czech Republic and Poland. Parasitol Res. 2015;114(4):1619-24. 10.1007/s00436-015-4353-y [DOI] [PubMed] [Google Scholar]
- 129. Holubová N, Sak B, Horčičková M, Hlásková L, Květoňová D, Menchaca S, et al. Cryptosporidium avium n. sp. (Apicomplexa: Cryptosporidiidae) in birds. Parasitol Res. 2016;115(6):2243-51. 10.1007/s00436-016-4967-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Němejc K, Sak B, Květoňová D, Hanzal V, Jeníková M, Kváč M. The first report on Cryptosporidium suis and Cryptosporidium pig genotype II in Eurasian wild boars (Sus scrofa) (Czech Republic). Vet Parasitol. 2012;184(2-4):122-5. 10.1016/j.vetpar.2011.08.029 [DOI] [PubMed] [Google Scholar]
- 131. Němejc K, Sak B, Květoňová D, Kernerová N, Rost M, Cama VA, et al. Occurrence of Cryptosporidium suis and Cryptosporidium scrofarum on commercial swine farms in the Czech Republic and its associations with age and husbandry practices. Parasitol Res. 2013. b;112(3):1143-54. 10.1007/s00436-012-3244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kodádková A, Kvác M, Ditrich O, Sak B, Xiao L. Cryptosporidium muris in a reticulated giraffe (Giraffa camelopardalis reticulata). J Parasitol. 2010;96(1):211-2. 10.1645/GE-2212.1 [DOI] [PubMed] [Google Scholar]
- 133. Kotková M, Němejc K, Sak B, Hanzal V, Květonová D, Hlásková L, et al. Cryptosporidium ubiquitum, C. muris and Cryptosporidium deer genotype in wild cervids and caprines in the Czech Republic. Folia Parasitol (Praha). 2016;63:63. 10.14411/fp.2016.003 [DOI] [PubMed] [Google Scholar]
- 134. Máca O, Pavlásek I. First finding of spontaneous infections with Cryptosporidium baileyi and C. meleagridis in the red-legged partridge Alectoris rufa from an aviary in the Czech Republic. Vet Parasitol. 2015;209(3-4):164-8. 10.1016/j.vetpar.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 135. Máca O, Pavlásek I. Cryptosporidium infections of ring-necked pheasants (Phasianus colchicus) from an intensive artificial breeding programme in the Czech Republic. Parasitol Res. 2016;115(5):1915-22. 10.1007/s00436-016-4933-5 [DOI] [PubMed] [Google Scholar]
- 136. Kváč M, McEvoy J, Loudová M, Stenger B, Sak B, Květoňová D, et al. Coevolution of Cryptosporidium tyzzeri and the house mouse (Mus musculus). Int J Parasitol. 2013. b;43(10):805-17. 10.1016/j.ijpara.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hůrková L, Hajdusek O, Modrý D. Natural infection of Cryptosporidium muris (Apicomplexa: Cryptosporiidae) in Siberian chipmunks. J Wildl Dis. 2003;39(2):441-4. 10.7589/0090-3558-39.2.441 [DOI] [PubMed] [Google Scholar]
- 138. Hofmannová L, Hauptman K, Huclová K, Květoňová D, Sak B, Kváč M. Cryptosporidium erinacei and C. parvum in a group of overwintering hedgehogs. Eur J Protistol. 2016;56(56):15-20. 10.1016/j.ejop.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 139. Kváč M, Hořická A, Sak B, Prediger J, Salát J, Širmarová J, et al. Novel Cryptosporidium bat genotypes III and IV in bats from the USA and Czech Republic. Parasitol Res. 2015;114(10):3917-21. 10.1007/s00436-015-4654-1 [DOI] [PubMed] [Google Scholar]
- 140. Zemanová I, Husník R, Svobodová V. Giardia intestinalis u psů – výskyt, zoonotický potenciál a využití endoskopické diagnostiky. [Giardia intestinalis in dogs – occurrence, zoonotic potential and use of endoscopic diagnostic]. Veterinarstvi. 2005;55:319-25. Czech. Available from: http://vetweb.cz/giardia-intestinalis-u-psu-vyskyt-zoonoticky-potencial-a-vyuziti-endoskopicke-diagnostiky/ [Google Scholar]
- 141. Dubná S, Langrová I, Nápravník J, Jankovská I, Vadlejch J, Pekár S, et al. The prevalence of intestinal parasites in dogs from Prague, rural areas, and shelters of the Czech Republic. Vet Parasitol. 2007;145(1-2):120-8. 10.1016/j.vetpar.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 142. Strnadová P, Svobodová V, Vernerová E. Protozoální infekce jehňat a kůzlat na farmách v České republice. [Protozoal infections of lambs and goats on farms in the Czech Republic]. Veterinarstvi. 2008;58:451-8. Czech. Available from: http://vetweb.cz/protozoalni-infekce-jehnat-a-kuzlat-na-farmach-v-ceske-republice/ [Google Scholar]
- 143.Pavlásek I, Hess L, Stehlík I. Stika V. První nálezy Giardia spp. u koní v České republice. [First finding of Giardia spp. in horses in the Czech Republic]. Veterinární medicína. 1995;40(3):81-6. Czech. Available from: http://www.medvik.cz/link/bmc96002788 [PubMed]
- 144. Pavlásek I, Nágl I, Vácha J, Lávicka M. První nálezy Giardia spp. u srnce obecného (Capreolus capreolus L.). [The first finding of Giardia spp. in roebucks (Capreolus capreolus L.)]. Vet Med (Praha). 1993;38(6):381-4. Czech. [PubMed] [Google Scholar]
- 145.Stojecki K, Sroka J, Cacciò SM, Cencek T, Dutkiewicz J, Kusyk P. Prevalence and molecular typing of Giardia duodenalis in wildlife from eastern Poland. Folia Parasitol. 2015;62:042. 10.14411/fp.2015.042 [DOI] [PubMed]
- 146. Stojecki K, Sroka J, Cencek T, Dutkiewicz J. Epidemiological survey in Łęczyńsko-Włodawskie Lake District of eastern Poland reveals new evidence of zoonotic potential of Giardia intestinalis. Ann Agric Environ Med. 2015. b;22(4):594-8. 10.5604/12321966.1185759 [DOI] [PubMed] [Google Scholar]
- 147. Rzeżutka A, Kaupke A. Occurrence and molecular identification of Cryptosporidium species isolated from cattle in Poland. Vet Parasitol. 2013;196(3-4):301-6. 10.1016/j.vetpar.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 148. Rzeżutka A, Kaupke A, Kozyra I, Pejsak Z. Molecular studies on pig cryptosporidiosis in Poland. Pol J Vet Sci. 2014;17(4):577-82. 10.2478/pjvs-2014-0086 [DOI] [PubMed] [Google Scholar]
- 149. Majewska AC, Solarczyk P, Tamang L, Graczyk TK. Equine Cryptosporidium parvum infections in western Poland. Parasitol Res. 2004;93(4):274-8. 10.1007/s00436-004-1111-y [DOI] [PubMed] [Google Scholar]
- 150. Majewska AC, Werner A, Sulima P, Luty T. Prevalence of Cryptosporidium in sheep and goats bred on five farms in west-central region of Poland. Vet Parasitol. 2000;89(4):269-75. 10.1016/S0304-4017(00)00212-0 [DOI] [PubMed] [Google Scholar]
- 151. Jaros D, Zygner W, Jaros S, Wędrychowicz H. Detection of Giardia intestinalis assemblages A, B and D in domestic cats from Warsaw, Poland. Pol J Microbiol. 2011;60(3):259-63. [PubMed] [Google Scholar]
- 152. Zygner W, Jaros D, Skowrońska M, Bogdanowicz-Kamirska M, Wedrychowicz H. [Prevalence of Giardia intestinalis in domestic dogs in Warsaw]. Wiad Parazytol. 2006;52(4):311-5. Polish. [PubMed] [Google Scholar]
- 153. Solarczyk P, Majewska AC. A survey of the prevalence and genotypes of Giardia duodenalis infecting household and sheltered dogs. Parasitol Res. 2010;106(5):1015-9. 10.1007/s00436-010-1766-5 [DOI] [PubMed] [Google Scholar]
- 154.Bajer A, Bednarska M. Cryptosporidium spp. and Giardia spp. infections in sled dogs. Med Weter. 2007;63:681-7. Polish. Available from: http://medycynawet.edu.pl/index.php/archives/121/1278-summary-medycyna-wet-63-6-681-687-2007
- 155. Majewska AC, Graczyk TK, Słodkowicz-Kowalska A, Tamang L, Jędrzejewski S, Zduniak P, et al. The role of free-ranging, captive, and domestic birds of Western Poland in environmental contamination with Cryptosporidium parvum oocysts and Giardia lamblia cysts. Parasitol Res. 2009;104(5):1093-9. 10.1007/s00436-008-1293-9 [DOI] [PubMed] [Google Scholar]
- 156. Paziewska A, Bednarska M, Niewegłowski H, Karbowiak G, Bajer A. Distribution of Cryptosporidium and Giardia spp. in selected species of protected and game mammals from North-Eastern Poland. Ann Agric Environ Med. 2007;14(2):265-70. [PubMed] [Google Scholar]
- 157. Majewska AC, Solarczyk P, Moskwa B, Cabaj W, Jankowska W, Nowosad P. Giardia prevalence in wild cervids in Poland. Ann Parasitol. 2012;58(4):207-9. [PubMed] [Google Scholar]
- 158. Perec-Matysiak A, Buńkowska-Gawlik K, Zaleśny G, Hildebrand J. Small rodents as reservoirs of Cryptosporidium spp. and Giardia spp. in south-western Poland. Ann Agric Environ Med. 2015;22(1):1-5. 10.5604/12321966.1141359 [DOI] [PubMed] [Google Scholar]
- 159. Kloch A, Bajer A. Natural infections with Cryptosporidium in the endangered spotted souslik (Spermophilus suslicus). Acta Parasitol. 2012;57(1):13-9. 10.2478/s11686-012-0006-9 [DOI] [PubMed] [Google Scholar]
- 160. Bajer A. Between-year variation and spatial dynamics of Cryptosporidium spp. and Giardia spp. infections in naturally infected rodent populations. Parasitology. 2008;135(14):1629-49. 10.1017/S0031182008004952 [DOI] [PubMed] [Google Scholar]
- 161. Pilarczyk B, Smugała M, Binerowska B, Tomza-Marciniak A, Bąkowska M, Tylkowska A. Prevalence of intestinal parasites of Polish Konik horses – comparison between domestic horses and imported from the Netherlands. Bulletin of the Veterinary Institute in Pulawy. 2010;54:171-4. [Google Scholar]
- 162. Mircean V, Györke A, Cozma V. Prevalence and risk factors of Giardia duodenalis in dogs from Romania. Vet Parasitol. 2012;184(2-4):325-9. 10.1016/j.vetpar.2011.08.022 [DOI] [PubMed] [Google Scholar]
- 163. Adriana G, Zsuzsa K, Mirabela Oana D, Mircea GC, Viorica M. Giardia duodenalis genotypes in domestic and wild animals from Romania identified by PCR-RFLP targeting the gdh gene. Vet Parasitol. 2016;217:71-5. 10.1016/j.vetpar.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 164. Mircean V, Titilincu A, Vasile C. Prevalence of endoparasites in household cat (Felis catus) populations from Transylvania (Romania) and association with risk factors. Vet Parasitol. 2010;171(1-2):163-6. 10.1016/j.vetpar.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 165. Mircean V, Györke A, Jarca A, Cozma V. Prevalence of Giardia species in stool samples by ELISA in household cats from Romania and risk factors. J Feline Med Surg. 2011;13(6):479-82. 10.1016/j.jfms.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lalošević V, Lalošević D, Terzić M. Prevalenca kriptosporidioze kod domaćih životinja. Letopis naučnih radova. [Prevalence of cryptosporidiosis in domestic animals]. 2007;31(1):153-7. Serbian. Available from: http://scindeks-clanci.ceon.rs/data/pdf/0546-8264/2007/0546-82640701153L.pdf
- 167. Mišić Z, Katić-Radivojević S, Kulišić Z. Cryptosporidium infection in lambs and goat kids in Serbia. Acta Vet (Beogr). 2006;56(1):49-54. 10.2298/AVB0601049M [DOI] [Google Scholar]
- 168. Šoba B, Petrovec M, Mioč V, Logar J. Molecular characterisation of Cryptosporidium isolates from humans in Slovenia. Clin Microbiol Infect. 2006;12(9):918-21. 10.1111/j.1469-0691.2006.01465.x [DOI] [PubMed] [Google Scholar]


