Abstract
Background
Autosomal dominant familial cortical myoclonic tremor and epilepsy (FCMTE) is characterized by distal tremulous myoclonus, generalized seizures, and signs of cortical reflex myoclonus. FCMTE has been described in over 100 pedigrees worldwide, under several different names and acronyms. Pathological changes have been located in the cerebellum. This systematic review discusses the clinical spectrum, treatment, pathophysiology, and genetic findings.
Methods
We carried out a PubMed search, using a combination of the following search terms: cortical tremor, myoclonus, epilepsy, benign course, adult onset, familial, and autosomal dominant; this resulted in a total of 77 studies (761 patients; 126 pedigrees) fulfilling the inclusion and exclusion criteria.
Results
Phenotypic differences across pedigrees exist, possibly related to underlying genetic differences. A “benign” phenotype has been described in several Japanese families and pedigrees linked to 8q (FCMTE1). French patients (5p linkage; FCMTE3) exhibit more severe progression, and in Japanese/Chinese pedigrees (with unknown linkage) anticipation has been suggested. Preferred treatment is with valproate (mind teratogenicity), levetiracetam, and/or clonazepam. Several genes have been identified, which differ in potential pathogenicity.
Discussion
Based on the core features (above), the syndrome can be considered a distinct clinical entity. Clinical features may also include proximal myoclonus and mild progression with aging. Valproate or levetiracetam, with or without clonazepam, reduces symptoms. FCMTE is a heterogeneous disorder, and likely to include a variety of different conditions with mutations of different genes. Distinct phenotypic traits might reflect different genetic mutations. Genes involved in Purkinje cell outgrowth or those encoding for ion channels or neurotransmitters seem good candidate genes.
Keywords: Familial, cortical, myoclonus, tremor, epilepsy, genetics
Introduction
Autosomal dominant familial cortical myoclonic tremor and epilepsy (FCMTE) has over the years accumulated several names and acronyms including autosomal dominant cortical myoclonus and epilepsy (ADCME), benign adult familial myoclonic epilepsy (BAFME), cortical tremor (Crt Tr), familial adult myoclonic epilepsy (FAME), familial cortical myoclonic tremor (FCMT), familial cortical tremor with epilepsy (FCTE), familial essential myoclonus and epilepsy (FEME), familial benign myoclonus epilepsy of adult onset (FMEA), and heredofamilial tremor and epilepsy (HTE) (Table 1).1 FCMTE will in the manuscript serve as the acronym for all abbreviations, as suggested in 2005,2 and as currently classified by the HUGO Gene Nomenclature Committee (HGNC).3 The syndrome was first described in the 1990s in Japan, in families with hereditary tremor (distal tremulous myoclonus) and epilepsy.4–7 Subsequently, over a hundred pedigrees have been identified in different countries, which have proven to be genetically heterogenic in linkage studies.
Table 1. Familial Cortical Tremor/Myoclonus Syndromes.
| Acronym | |
|---|---|
| ADCME | Autosomal dominant cortical myoclonus and epilepsy |
| BAFME | Benign adult familial myoclonic epilepsy |
| Crt Tr | Cortical tremor |
| FAME | Familial adult myoclonic epilepsy |
| FCMT | Familial cortical myoclonic tremor |
| FCMTE | Familial cortical myoclonic tremor with epilepsy |
| FCTE | Familial cortical tremor with epilepsy |
| FEME | Familial essential myoclonus and epilepsy |
| FMEA | Familial benign myoclonus epilepsy of adult onset |
| HTE | Heredofamilial tremor and epilepsy |
Symptoms of FCMTE are tremor-like cortical myoclonus, which can mimic essential tremor (ET), and epileptic seizures. Distal tremulous movements are usually the first symptom, usually with an onset in the second or third decade. Seizures, most often unprovoked generalized tonic–clonic seizures without aura, start later in life, frequently in the third or fourth decade. Diagnosis is based on clinical characteristics and family history, and is supported by electrophysiological investigations, after exclusion of other tremulous disorders and epileptic syndromes. Treatment is symptomatic, usually with anti-epileptic drugs such as valproate and clonazepam.
Additional symptoms have also been described, possibly extending the spectrum of the disease. These include mild cognitive decline8–12 but also psychiatric symptoms,13 nystagmus,14 and migraine.15–18 Clinical anticipation has been described in Asian pedigrees.19–21 It is unknown which of these symptoms are part of FCMTE or are co-incidental, and which symptoms might differentiate between underlying genetic mutations.
FCMTE maps to different loci including 8q23.3-q24.13 (Online Mendelian Inheritance in Man [OMIM],22 HGNC,23 FCMTE1) in Japanese families24–26 and a Chinese pedigree;27 2p11.1-q12.2 (OMIM,28 HGNC,23 FCMTE2) in multiple Italian pedigrees,8,9,17,29–33 a Spanish family,29,34 and a family from New Zealand/Australia with ancestors from Austria;16 5p15.31-p15 (OMIM,35 HGNC,23 FCMTE3) in a French family,29,36–38 a Dutch pedigree,39 and two Chinese pedigrees;40,41 and 3q26.32-3q28 (OMIM,42 FCMTE4) in a Thai family.43 Also, an autosomal recessive form of FCMTE (OMIM,44 FCMTE5) has been identified in an Egyptian family where a mutation has been localized in the contactin 2 (CNTN2) gene, which is associated with potassium channel stability.45 In autosomal dominant FCMTE, several possible gene mutations have been proposed as causative on the FCMTE loci.27,46–49 Recently, a mutation was found in a Dutch FCMTE3 pedigree in the catenin delta 2 (CTNND2) gene, supported by functional tests.39
Neurophysiological, imaging, genetic, and pathology findings have shed some light on the pathophysiology underlying FCMTE. A cortical origin of the myoclonus in FCMTE is proven by electrophysiological investigation consistent with reduced cortical inhibition.14 Pathology findings in a Dutch FCMTE pedigree indicate Purkinje cell changes, resembling the abnormal neuronal morphology in Ctnnd2 mutant mice.1,39,50 Congruent imaging findings include decreased fiber density,51 functional connectivity alterations52 and gray matter loss in the cerebellar motor area.53 It has been hypothesized that, via the cerebello-thalamo-cortical loop, reduced input from the cerebellum can lead to cortical hyperexcitability.54 However, the question arises if the cerebellum is the primary generator of cortical hyperexcitability in all FCMTE types. Alternatively, cortical functional changes could underlie the symptoms, with or without coexisting cerebellar pathology.
This review will bring together evidence concerning the clinical spectrum including treatment on the one hand, and, on the other hand, pathophysiological and genetic studies, adding the latest insights.1 The current review aims to not only summarize studies into FCMTE, but also be of guidance to the clinician encountering a patient with suspected FCMTE. A second aim is to link insights from pathophysiological studies to genetic studies to better understand the underlying disease mechanisms and be a guide for future research and better treatment of these patients.
Methods
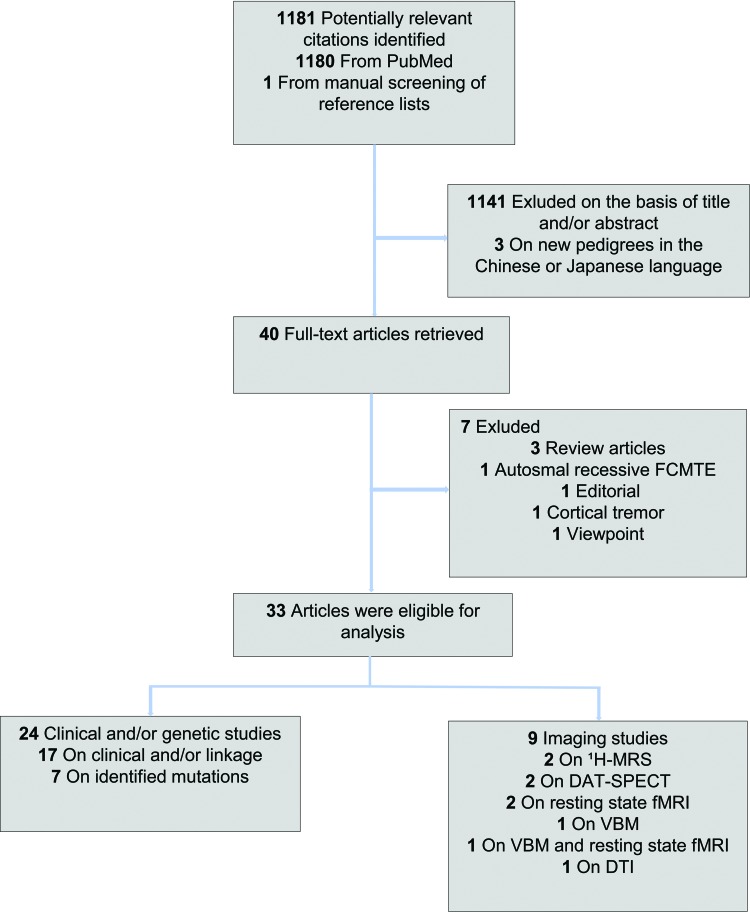
We conducted a PubMed search on November 15, 2017, for the period January 1, 2011, to November 15, 2017, using a combination of the following search terms: cortical tremor, myoclonus, epilepsy, benign course, adult onset, familial, autosomal dominant, and the different acronyms for FCMTE fully written; see Appendix A for complete search terms. The search delivered a total of 1,180 articles, and one additional from reference lists, from which 33 fulfilled the selection criteria (flow chart, Figure 1). The search conducted for our previous review in 2011 had already revealed a total of 44 publications published before 2011,1 which are also included in the current review (Tables 2 and 3).
Figure 1. Article Selection Flowchart. Literature search for the identification of new pedigrees, genetic/linkage or imaging studies since our last review.1 Thirty-three articles were eligible for analysis: 17 reported on clinical and electrophysiology findings with or without linkage analysis, nine reported on neuroimaging, and seven reported on new potential pathogenic mutations. Abbreviations: DAT-SPECT, Dopamine Transporter, Single Photon Emission Computed Tomography; DTI, Diffusion Tensor Imaging; FCMTE, Familial Cortical Myoclonus Tremor And Epilepsy; fMRI, Functional Magnetic Resonance Imaging; 1H-MRS, Proton Magnetic Resonance Spectroscopy; VBM, Voxel Based Morphometry.
Table 2. Described Pedigrees (2011–2017) with Core Disease Characteristics.
| Descent | Genetics | Origin, # Family | Clinical features | Electrophysiology | Imaging (cases) | Additional Symptoms, Other Findings | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Myoclonus | Seizures | Seizure Type | JLA | Giant SEP | LLR | EEG | |||||
| Age at Onset mean and (range) | |||||||||||
| Asian | 8q, SLC30A8 | Chinese, 127 | 26 (13–36) | 34 (29–40) | GTC | n.d. | + | + | n.a. | n.a. (1) | No CA; M lower limbs (n:2) |
| 8q, UBR5 | Japanese, 158 | ? | ? | G | n.d. | n.d. | n.d. | n.d. | n.a. (1) | M worse aging | |
| 22q, PLA2G6 | Chinese, 147 | 29 (21–38) | 39 (36–46) | G, GTC, Ph | n.d, | + | + | PSW, PMR | n.a. (1) | M rest; Head; Lower limbs (n:1) | |
| 5p | Chinese, 240,41 | >18 | > 30 | G, GTC, Ph | n.d. | + | n.d. | PSW, PRA | n.d. | Non-progressive; Headache | |
| 3q | Thai, 143 | 19 (10–33) | 25 (19–33) | G, GTC | + | + | + | PSW, SW, PPR, PMR | n.d. | Early-onset seizures | |
| 8q, n.d. | Chinese, 921 | 31 (15–59) | 36 (19–64) | GTC, Ph | n.d. | + | + | G-E, Sp, Sw | n.d. | S before M (n:5); Headache; M worse aging; | |
| Higher severe M, AED use | Night blindness; Mild cognitive decline; CA | ||||||||||
| n.d. | Chinese, 155 | 30 (20–40) | 39 (28–48) | GTC | n.d. | n.d. | n.d. | E, Sp, Sw | + (7) | Rest fMRI abnormalities | |
| n.d. | Indian, 4856 | 25 maj (14–40) | GTC, CP, Ph | n.d. | + | n.d. | PSW, F, PPR | n.a. (48) | Anxiety (83%); Unique HLA Nadar community | ||
| Seizures possibly first symptom 48% | |||||||||||
| n.d. | Indian, 157 | >15 | ? | GTC | n.d. | n.d. | n.d. | PSW, Sw, PPR | n.a. (3) | Cognitive decline; M axial; M worse aging | |
| European | 2p | Italian, 117 | 28 (19–40) | 34 (5–63) | GTC, M, Ph | + | + | + | G-E, PSW, Sw, PPR | n.a. (?) | Psychiatric comorbidity; M worse aging; |
| Gait disability; Cognitive decline (n:2); Migraine; | |||||||||||
| TMS reduction rest motor threshold | |||||||||||
| 2p | New Zealand/Australia, 116 | Median 15 (4–60) | Median 44 (18–76) | GTC, F | + | + | + | PSW, PMR | n.a. (7), + (1) | Migraine; M head, legs, ‘drop attacks’; M worse aging; | |
| European ancestors | Severe M before S around sleep onset | ||||||||||
| 2p, ACMSD | Spain, 148 | >17 (17–23) | 19 (17–22) | GTC, PSG | n.d. | + | + | PSW, PPR | + (2) | Parkinsonism (n:1); Cognitive decline (n:1); | |
| M, Ph | Mild ataxia unaffected patient (n:1) | ||||||||||
| 2p, ADRA2B | Italian, 146 | (18–50) | ? | GTC, CP, F | + | + | + | G paroxysmal activity | n.a. (3) | Cognitive imparity (n:1); Age-related dementia | |
| n.d. | South Africa, 158 | 16 (12–20) | 39 (30–45) | GTC | n.d. | + | + | G-E, TLE, PSW | n.d. | ADL and MRS affected by M severity; | |
| European descent | Gait dysfunction; Facial M (n:2); M worse aging; | ||||||||||
| No (ataxia/cognitive decline) after 30 years | |||||||||||
| n.d. | Turkish, 118 | > 13 (13–16) | > 17 (14–17) | GTC, F, A, M | n.d. | + | – | n.d. | n.a. (3) | Frequent M seizures (1× month); Migraine; | |
| Higher than ET and JME | Drop attacks; Psychiatric comorbidity | ||||||||||
Abbreviations: A, Absence; ACMSD, Aminocarboxymuconate Semialdehyde Decarboxylase; ADL, Activities of Daily Living; ADRA2B, α2-Adrenergic Receptor Subtype B; AED, Anti-epileptic Drug; CA, Clinical Anticipation; CP, Complex Partial; EEG, Electroencephalography; ET, Essential Tremor; F, Focal; fMRI, Functional Magnetic Resonance Imaging; G, Generalized; G-E, Generalized Epileptiform; giant SEP, Giant Somatosensory Evoked Potential; GTC, Generalized Tonic–Clonic; HLA, Humane Leukocyte Antigen; JLA, Jerk Locked Back Averaging; LLR, Long Latency Reflex; JME, Juvenile Myoclonic Epilepsy; M, Myoclonic/Myoclonus; maj, Majority; MRS, Myoclonus Rating Scale; n, Number; n.a., No Abnormalities; n.d., Not Done; Ph, Photosensitivity; PLA2G6, Phospholipase A2 Group 6; PMR, Photomyoclonic Response; PPR, Photoparoxysmal Response; PRA, Paroxysmal Rate Abnormalities; PSG, Partial Secondarily Generalized; PSW, Polyspike-Wave Complexes; S, Seizures; SLC30A8, Solute Carrier Family 30 (zinc transporter), Member 8; Sp, Spikes; Sw, Slow Waves; SW, Spike Wave Complexes; TLE, Temporal Lobe Epileptiform; TMS, Transcranial Magnetic stimulation; UBR5, Ubiquitin Protein Ligase E3 Component n-Recognin 5; +, Abnormal; –, Normal; # family, Number of Described Families; ?, Not Known.
Table 3. Described Pedigrees (up until 2011) with Core Disease Characteristics1.
| Descent | Genetics | Origin, # Family | Clinical features | Electrophysiology | Structural | Additional Symptoms, Other Findings | Summary | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tremor, Myoclonus | Seizures | Seizure Type | JLA | giant SEP | LLR | EEG | Imaging (cases) | PA (cases) | |||||
| Age at Onset (mean) | |||||||||||||
| Asian | 8q | Japanese, 524,25 | 18–45 | ? | GTC | + | + | + | G-PSW, PPR, | atr (3) n.a. | n.d. | – | Classical phenotype |
| maj >30 | PSW, PMR | (>14) | Age at onset at adulthood | ||||||||||
| Infrequent seizures | |||||||||||||
| n.d. | Japanese, 355–7,68,69,83,99–104 | 16–70 | 17–54 | GTC, Ph | + | + | + | PSW, PPR, SW, Sp | atr (3) n.a. (25) | n.a. (4) | Rare: night blindness, | No other neurological signs | |
| maj >25 | maj >30 | inf (11) | behavioral arrest | Electrophysiological abnormalities | |||||||||
| Excl 2p, 8q | Chinese, 166 | 5–? | ? | GTC, M | n.d. | n.d. | n.d. | M, SW, PSW | n.d. | n.d. | Schizophrenia in family | Classical phenotype with | |
| (34) | Slow waves | earlier age of onset in the | |||||||||||
| 13/? | youngest generations | ||||||||||||
| Presymptomatic changes | |||||||||||||
| detected | |||||||||||||
| European | 2p | Italian, 78,9,30–33 | 11–50 | 12–59 | G, GTC, Ph, | + | + | + | Sp, SW, PPR, | atr (3) | n.d. | Visuospatial impairment; | Symptoms appear earlier |
| maj >20 | maj >25 | CP, M | Also in presymptomatic | PMR, GPA, PSW, SW | n.a. (27) | Eyelid twitching; Voice | Complex partial seizures | ||||||
| 3/79 | tremor; Cognitive | Mild cognitive impairment | |||||||||||
| Absent in 1 pedigree9 | impairment; TMS cortical | ||||||||||||
| hyperexcitability, normal | |||||||||||||
| sensorimotor integration | |||||||||||||
| n.d. | Italian, 112 | 12–57 | 5–18 | GTC, Abs | + | + | + | Sp, SW, PPR | atr (2) | n.d. | – | ||
| Turkish, 115 | 29–? | 30 | GTC | n.d. | n.d. | n.d. | G-Sp, SW, PPR | n.a. (1) | n.d. | Migraine | |||
| 5p | French, 136–38 | 10–47 (30.8) | 24–41 | GTC, Ph, | + | + | + | Sp, PPR, PS | n.d. | n.d. | Progression in gait symptoms; Sensitivity to exercise; GTC preceding M (5/16); | Later onset | |
| (29.1) | CM, PS | Dysarthria; Ophthalmic migraine; | No cognitive impairmentGait disordersIndication of progression | ||||||||||
| 5p, CTNND2 | Dutch, 12,11,14,39,50,77 | 12– 45 (23.5) | 13– 44 (43) | GTC, M, Ph | – | + | + | SW, PPR | atr (2) | +(3) | TMS cortical hyperexcitability; | Cerebellar dysfunction | |
| n.a. (2) | nystagmus; slight cognitive decline | Frequent seizures; Cognitive impairment; Progression in symptoms | |||||||||||
| Excl 2p, 8q | Spanish, 181 | 30–60 (41) | 30–67 (44.6) | GTC | + | + | + | G-PSW | n.a. (5) | n.d. | Childhood onset, Pyramidal signs | ||
| Italian, 162 | 3–12 | 23–34 | GTC, CM | + | + | + | SW, PMR, Sp | n.d | n.d | Prominent photic induced | |||
| Ph | myoclonus and epilepsy; | ||||||||||||
| Changing symptoms with age; | |||||||||||||
| Mild axial ataxia; Behavioral disorder | |||||||||||||
| South African, 210 | 13–31 (20.9) | ? | GTC | + | + | + | Abnormal background, | atr (8) | +(1) | Frequent seizures; | |||
| Intermarriage with | PSW, Sp | n.a. (2) | Cognitive impairment; Signs of | ||||||||||
| European settlers | pyramidal and cerebellar dysfunction; | ||||||||||||
| Progression in symptoms | |||||||||||||
Abbreviations: Abs, Absence; atr, Atrophy; CM, Cortical Myoclonus; CP, Complex Partial; CTNND2, Catenin Delta 2; EEG, Electroencephalography; excl, Excluded; G, Generalized; GPA, Generalized Paroxysmal Activity; giant SEP, Giant Somatosensory Evoked Potential; GTC, Generalized Tonic–Clonic; inf, Infarct; JLA, Jerck Locked Back Averaging; LLR, Long Latency Reflex; maj, Majority; M, Myoclonic; n.a., No Abnormalities; n.d., Not Done; PA, Pathology; Ph, Photosensitivity; PMR, Photomyoclonic Response; PPR, Photoparoxysmal Response; PSW, Polyspike-Wave Complexes; PS, Partial Seizures; Sp, Spikes; SW, Spike-Wave Complexes; TMS, Transcranial Magnetic Stimulation; +, Abnormal; –, Normal; #, Number Of Described Families; ?, Not Known.
The table was originally published in our previous review. 1We have added the table to the manuscript with minimal changes.
Included were reports published in English describing pedigrees with the following diagnostic criteria as previously reported:1 1) distal action and postural tremor/fine myoclonus; 2) generalized tonic–clonic seizures and/or electrophysiological features of cortical reflex myoclonus including giant somatosensory evoked potentials (SEPs) and/or enhanced long loop reflexes (LLRs/C reflex); and 3) a family history of tremor/epilepsy consistent with autosomal dominant inheritance. Excluded were pedigrees and cases with another cause for tremor and epilepsy (including autosomal recessive FCMTE45), structural lesions on magnetic resonance imaging (MRI), and additional symptoms such as clear parkinsonism, dementia, and/or ataxia pointing to a different syndrome.
Results
In total 77 publications (126 pedigrees; 761 patients) were retrieved from the combined current PubMed search and the 2011 search.1 For an overview of additional pedigrees since our last review1 see Table 2. Table 3 shows the pedigrees previously identified.1 Since 2011, 70 additional pedigrees in 33 publications, including 48 from India, have been extensively described (providing clinical and electrophysiological data, Table 2).16–18,21,27,40,41,43,46–48,55–59 Descriptions of the pedigrees comply with autosomal dominant inheritance. The reports used uniform criteria for diagnosing FCMTE, and one paper explicitly stated they used the diagnostic criteria from our last review.55 The reports vary in detail in their description of the neurological examination, the presence or absence of cerebellar signs, of cognitive assessment, comorbid psychiatric disorders, effect of anti-epileptic drugs on tremor/epileptic seizures, drug dosage, clinical anticipation, structural imaging, and electrophysiological investigation including jerk-locked back averaging (JLA). Owing to insufficient clinical/electrophysiological data, several new families were not included in Table 2.13,19,20,29,60 The results section covers both the previously identified and novel pedigrees.
The FCMTE phenotype; core characteristics and less common findings
Clinical spectrum
FCMTE usually starts with tremor-like, cortical myoclonus of the distal limbs, with onset in the second or third decade (range 3–70 years). Epileptic seizures usually start in the third or fourth decade (range 5–76 years), for which patients often seek medical attention. However, epileptic seizures can also be the first symptom.17,21,43,56
The tremulous movements are in fact small, high-frequency myoclonic jerks, induced by posture or action. They can be mild, but can also be more incapacitating.17,27,47,57,58 When present in the lower limbs, these may lead to gait disorders17,36–38,58,61 and even “drop attacks”.16,18 These tremor-like movements during action can easily be mistaken for ET. Electrophysiological measures can distinguish FCMTE from ET, showing short irregular electromyography (EMG) bursts during action and a cortical drive. Stimulus-sensitive myoclonus, frequently present in FCMTE, may be triggered or worsened by alcohol, emotional stress, sleep deprivation, tactile stimuli, and photic/phonic stimulation.16,17,21,37,47,56,57,62 Twitching movements can involve facial/axial muscles.16,21,57,58 Alcohol responsiveness has been reported in two pedigrees.16,58 Myoclonus may remain stable, but long-term follow-up is not always present.18,40,41 Also, myoclonus has been reported to worsen with progressing age.10,16,17,21,36,57,58,63
Epileptic seizures are usually generalized tonic–clonic seizures, but can also be complex partial seizures.8,37,46,56 Recently the latter were renamed as focal seizures with impaired awareness (International League Against Epilepsy [ILAE] 2017 classification).64 Moreover, they can be drug resistant.46 Focal seizures with mesiotemporal symptoms or motor symptoms (déjà vu and fear) have been reported in four patients.16,46 Generalized seizures mostly start without a warning sign but sometimes are preceded by severe myoclonus.16,56 Also, myoclonic seizures have been described.11,17,18 Seizures are reported to be induced by sleep deprivation, photic stimulation, stress, excitement,16,37,47,62 or snow.21 Frequency is usually low. Over five seizures per year is uncommon,13,16,17,21,27,43,47,58 but more severe cases (≥10 seizures per year) have been described.10,18,37
Additional clinical findings, possibly extending the clinical spectrum, are listed below.
- Cerebellar findings include in French pedigrees mild ataxia and gait instability,17,37,38,62 (downbeat) nystagmus increasing with hyperventilation in a Dutch pedigree,14 and dysarthria in South African/French pedigrees.10,38 Ataxia is not a feature of FCMTE16,17,27,47,57,58,60,65 but subtle cerebellar signs have not explicitly been ruled out.
- Mild cognitive decline, not including dementia, has been noted in some papers.8–12,52,57
- Psychiatric comorbidity has been noted in seven Italian families (FCMTE2),13,17 anxiety in Indian patients,56 schizophrenia in a Chinese pedigree,66 and psychiatric comorbidity in a Turkish family.18 State anxiety (measured by a scoring list), depression, and personality disturbances (hypochondriasis and schizophrenia) were more prevalent in Italian FCMTE pedigrees than juvenile myoclonic epilepsy and healthy controls.13
- Clinical anticipation has been recognized in Japanese patients with unknown linkage: tremor started at a younger age and seizures newly appeared in the next generation.19,20 Possibly, maternal transmission is associated with this phenomenon.20 In Chinese patients paternal transmission was noted in the earlier onset of tremor but not of seizures.21
- Other findings include migraine,15–18 ophthalmic migraine,37 headaches,40 logopenic syndrome,67 visual intolerance,61 frontal dysfunction,61 night blindness,68 motionless state,69 absence seizures,18 parkinsonism,48 reduced verbal fluency,70 sensitivity to glucose deprivation or vibration as an aggravating factor,37,38 and visuospatial impairment.9,52
Electrophysiological findings
Additional investigations in patients suspected of FCMTE to aid the diagnosis include electroencephalography (EEG), EMG, SEP, LLR, JLA, and corticomuscular (EEG-EMG) coherence analysis.
EEG findings may include generalized (poly) spikes and waves, photoparoxysmal response, and photomyogenic responses (Tables 2 and 3). EMG shows arrhythmic/semi-rhythmic high-frequency (≥10 per second) burst-like discharges of about 50 ms, which can be synchronous between agonist and antagonist muscles, typical for cortical myoclonus.71
Often features of cortical reflex myoclonus are present, including giant SEP, enhanced LLR, and/or cortical spikes preceding myoclonus with JLA.71 EEG-EMG coherence analysis indicated strong corticomuscular coherences around 20 Hz with a cortical drive.72 With aging giant SEP and JLA cortical spike amplitude can be enhanced in FCMTE patients.16,73 Not all patients have giant SEPs or LLR, possibly due to anti-epileptic drug (AED) use,33 although giant SEP amplitude can also increase with AED use.21
Imaging
Structural imaging with MRI is normal in most cases. Although slight cerebellar atrophy (e.g., vermis)10 has been described.9–11,48 In one patient corticospinal degeneration was present.48 In three patients basal ganglia/periventricular white matter changes were observed.10
Treatment
The treatment of FCMTE consists of the prevention of epileptic seizures and/or reduction of troublesome tremor/myoclonus mainly with AEDs and benzodiazepines. Valproate is the drug most often reported to be effective with or without clonazepam.13,16,17,27,43,47,48,56,58,60,65,74 Because of teratogenicity, this should not be prescribed to women of childbearing age. Newer AEDs with a good effect are levetiracetam13,17,37,38,43,46,48,58,74,75 and lamotrigine.37,58 Other drugs prescribed to prevent seizures in FCMTE are phenobarbital, phenytoin, carbamazepine, clobazam, oxcarbazepine, and primidone.1,16,40,43,47,48,58,60,74 One report observed a reduction in tremulous movements from a β-blocker.16 One to three AEDs usually prevents seizures or reduces them to once a year. Gabapentin has been reported to precipitate myoclonic status.76
Pathophysiology
Cortical hyperexcitability can be considered the hallmark of FCMTE. However, cerebellar changes have been described in neuroimaging and pathology studies.
Cortical hyperexcitability
Electrophysiological findings in FCMTE point to cortical hyperactivity and a cortical origin of the tremulous myoclonic movements. Functional (f)MRI-EMG also indicated cortical activity linked to the tremulous movements.77 Transcranial magnetic stimulation (TMS) in European pedigrees revealed a reduction in short interval cortical inhibition compared with healthy controls, reflecting hyperexcitability of the cortex.9,14 In Japanese FCMTE patients the giant SEP was enhanced, lacking the long-term depression effect seen in healthy controls after quadripulse TMS over the primary motor cortex.78 However, post-mortem pathology studies in three Dutch cases revealed limited involvement of the sensorimotor cortex.1,14,50
Cerebellar involvement
Evidence for cerebellar involvement differs between FCMTE types (Table 4, imaging overview). For FCMTE1 and 4 there are no imaging or pathology studies available. In FCMTE2 a proton magnetic resonance spectroscopy (MRS) study indicated cerebellar dysfunction.79
Table 4. Imaging Findings across Pedigrees.
| Reference | Origin, Linkage | Imaging Modality | Design | Cerebellum | Other Findings in FCMTE |
|---|---|---|---|---|---|
| Buijink et al.53 | Dutch, 5p | MRI (VBM) | 8 FCMTE, 45 ET, 39 HS | Total volume; Crus I; lobules IX, X ⬇ | n.d. |
| Total/local cerebellar volume | |||||
| Buijink et al.51 | Dutch, 5p | MRI (DTI) | 7 FCMTE, 8 ET, 5 HS | Cerebellum ⬇ | n.d. |
| Fractional anisotropy; MDV | |||||
| Magnin et al.67 | French, 5p | DAT-SPECT, MRI | Case report (n:1) | Atrophy: cerebellum ⬇ | Atrophy: cortical structures ⬇ |
| Magnin et al.61 | Case report (n:2) | DAT: striatum; cortical perfusion ⬇ | |||
| Striano et al.79 | Italian, 2p | 1H-MRS | 11 FCMTE, 11 HS, Neurochemical ratios | Cho/Cr ratio ⬆ | n.a. |
| Long et al.52 | Chinese, excl. 2p, 8q | RS fMRI (BOLD) | 11 FCMTE, 15 HS | DN: R crus I with L frontal + R lobule IX ⬇ | n.d. |
| Functional connectivity | AN: L lobule VIII with temporal, | ||||
| R putamen and L crus I ⬆ | |||||
| CN: L lobule VIIb and R frontal ⬆ | |||||
| Long et al.65 | Chinese, excl. 2p, 8q | 1H-MRS | 12 FCMTE, 12 HS, Neurochemical ratios | NAA/Cho ratio ⬇ | n.a. |
| Zeng et al.70 | Chinese, excl. 2p, 8q | RS fMRI + VBM | 11 FCMTE, 15 HS | n.d. | Gray matter: R hippocampus; R temporal ⬇ |
| Gray matter; Functional connectivity | L orbitofrontal; L prefrontal ⬇ | ||||
| FC: R hippocampus with R parietal ⬆ | |||||
| cingulate, L precuneus, L precentral gyrus ⬆ | |||||
| Wang et al.55 | Chinese, n.d. | RS fMRI | 7 FCMTE, 7 ET, 10 HS | n.d. | R fusiform gyrus; cingulate ⬇ |
| BOLD; ALFF | Frontal lobe ⬆ |
Abbreviations: ALFF, Amplitude Of Low Frequency Fluctuation; AN, Attention Network; BOLD, Blood-Oxygen-Level Dependent; Cho, Choline; CN, Control Network; Cr, Creatinine; DAT, Dopamine Transporter; DAT-SPECT: Dopamine Transporter, Single Photon Emission Computed Tomography; DN, Default Network; DTI, Diffusion Tensor Imaging; ET, Essential Tremor; excl, Exclusion; FCMTE, Familial Cortical Myoclonus Tremor And Epilepsy; FC, Functional Connectivity; HS, Healthy Subjects; 1H-MRS, Proton Magnetic Resonance Spectroscopy; L, Left; MDV, Mean Diffusivity Volume; MRI, Magnetic Resonance Imaging; n, Number; n.a., No Abnormalities; NAA, n-Acetylaspartate; n.d., Not Done; R, Right; RS fMRI, Resting State Functional Magnetic Resonance Imaging; VBM, Voxel Based Morphometry; ⬆ Increase; ⬇ Decrease.
In the Dutch FCMTE3 pedigree, with a CTNND2 gene mutation39 several findings point to cerebellar changes. These include, in certain family members, a downbeat nystagmus upon hyperventilation14 and in three deceased cases there was Purkinje cell loss in the cerebellar cortex and abnormal morphology of Purkinje cells during pathological examination.1,14,50
In family members of these deceased Dutch patients a mutation was found in the CTNND2 gene that led to abnormal neuronal sprouting in mice neurons, resembling the aforementioned cerebellar pathology.39 Moreover, imaging findings in the same pedigree showed decreased cerebellar fiber density51 and gray matter loss in the cerebellar motor area.53 In a Chinese pedigree in which 8q and 2p linkage was excluded, MRS indicated cerebellar dysfunction65 and a fMRI study indicated alterations between the cerebellum and supratentorial structures after network analysis.52 A South African pathology case, from a pedigree in which 8q and 2p linkage was excluded, revealed focal Purkinje cell loss, neuronal atrophy in the dentate nucleus, and neuronal loss with gliosis in the olives and pallidum.10
Other findings
A functional MRI/voxel-based morphometry (VBM) study indicated gray matter loss in cortical and subcortical structures with connectivity alterations.70 In a resting state fMRI study, compared to ET and healthy controls, the right fusiform gyrus and the posterior cingulate cortex showed decreased amplitude of low-frequency fluctuation (ALFF), and the frontal lobe showed increased ALFF that correlated with disease duration.55 In two FCMTE patients with gait difficulties but no benefit from L-dopa in one, MRI showed frontal atrophy and single positron emission computed tomography (SPECT) showed dopamine depletion.61 In a FCMTE patient with a language disorder and short-term memory problems, MRI showed frontal atrophy and SPECT indicated loss of dopamine transporters in the left striatum.67
Genetic linkage, proposed mutations
In several FCMTE pedigrees, causative mutations have been proposed (Table 5). In a Dutch pedigree a mutation in CTNND2, supported by functional tests, has recently been discovered.39 However, functional tests have not been performed for every mutation.80 Linkage studies indicated several loci: 8q23.3-8q24.13 (FCMTE1)24–27; 2p11.1-q12.2 (FCMTE2)8,9,16,17,29–34; 5p15.31-p15 (FCMTE3)29,36–41; and 3q26.32-3q28 (FCMTE4).43 Furthermore, there are several families (Chinese, Spanish, Italian, South African) in which 2p and/or 8q linkage was excluded.10,62,66,81 Below we summarize the findings in FCMTE1–4 and FCMTE-like disorders.
Table 5. Mutations Found in Different Pedigrees.
| Reference | FCMTE linkage | Origin | Gene, Chromosome | Patients/pedigrees | Mutation | Causative? |
|---|---|---|---|---|---|---|
| Cen et al.27 | FCMTE1 | Chinese | SLC30A8, 8q24.11 | N:2, N1 | Missense, p.Y69F, c.206A>T | No expression in the brain |
| Felix Marti-Masso et al.48 | FCMTE2 | Spanish | ACMSD, 2q21.3 | N:3, N:1 | SNV, p.Trp26Stop, c.77G>A | Linked to PD, PME, ULD |
| De Fusco et al.46 | FCMTE2 | Italian | ADRA2B, 2q11.2 | N:2, N:2 | Indel, c.675_686delTGGTGGGG | H: gain of function, altered cortical |
| CTTTinsGTTTGGCAG | rhythmic activity | |||||
| Rootselaar et al.39 | FCMTE3 | Dutch | CTNND2, 5p15 | N:3, N:1 | Missense, p.Glu1044Lys | Abnormal neuronal sprouting in mice |
| Gao et al.47 | FCMTE# | Chinese | PLA2G6, 22q13.1 | N:3, N1 | Missense, p.Ala159Thr, c.475C > T | Linked to INAD, KS, EODP |
| Kato et al.59 | FCMTE# | Japanese | UBR5, 8q22.3 | N5, N1 | Missense, p.Arg1907His, c.5720G>A | Linked to AS, ARJP |
| Russel et al.49 | FCM# | Canada | NOL3, 16q22.1 | N:1, N:1 | Missense, c.61G>C | No giant SEP or symptoms in Nol3- mice |
Abbreviations: ACMSD, Aminocarboxymuconate Semialdehyde Decarboxylase; ADRA2B, α2-Adrenergic Receptor Subtype B; ARJP, Autosomal Recessive Inherited Juvenile Parkinsonism; AS, Angelman Syndrome; CTNND2, Catenin Delta 2; EODP, Early Onset Dystonia Parkinsonism; FCMTE, Familial Cortical Myoclonic Tremor And Epilepsy; FCM, Familial Cortical Myoclonus; giant SEP, Giant Somatosensory Evoked Potential; H, Hypothesis; INAD, Infantile Neuroaxonal Dystrophy; KS, Karak Syndrome; N, Number; NOL3, Nucleolar Protein 3; PD, Parkinson Disease; PLA2G6, Phospholipase A2 Group 6; PME, Progressive Myoclonus Epilepsy; SLC30A8, Solute Carrier Family 30 (zinc transporter), Member 8; SNV, Single Nucleotide Variant; UBR5, Ubiquitin Protein Ligase E3 Component n-recognin 5; ULD, Unverricht–Lundborg Disease; -, Negative; #, Unknown linkage.
Linkage to the FCMTE1 locus on chromosome 8q23.3-8q24.13 was found in several Japanese families24–26 and one Chinese family.27 In the Chinese pedigree a single nucleotide variant (SNV) was identified in the SLC30A8 (solute carrier family 30 [zinc transporter]) gene after whole-exome sequencing (WES).27 However, there is no expression of this gene in the brain, making it irrelevant.27 Besides, sequence and copy number variant analysis of the genes located on the 8q23.3-8q24.13 locus did not reveal any mutation.26
Linkage to the FCMTE2 locus on chromosome 2p11.1-q12.2 was found in Italian,8,9,17,29–33 Spanish,29,34 and Australian/New Zealand pedigrees (with European ancestors).16 A founder haplotype was identified in Italian FCMTE2 pedigrees from the same geographical area.17,29 A mutation in two Italian family members within the FCMTE2 locus was found on the α2-adrenergic receptor subtype B (ADRA2B) gene with next-generation sequencing (NGS).46 Identity by descent mapping (IBD), which is able to detect genetic loci with an ancestral segment among pedigree unrelated individuals, refined the FCMTE2 locus to 2p11.1-q11.1 without excluding the ADRA2B mutation.29 In a Spanish pedigree, WES indicated an SNV in the aminocarboxymuconate semialdehyde decarboxylase (ACMSD) gene encoding for an enzyme that is part of the kynurenine pathway of tryptophan and linked to neurodegenerative diseases and epilepsy.48
FCMTE3, with linkage to 5p15.31-p15, has been identified in two Chinese families,40,41 a Dutch pedigree,39 and a French family.29,36–38 In the Dutch family after WES, a mutation was found in the CTNND2 gene.39 Knockdown of Ctnnd2 in cortical mouse neurons led to abnormal neuronal sprouting which resembled abnormal Purkinje cell morphology in deceased FCMTE patients.39 However, in the French family a mutation in SEMA5A (semaphorin-5A) and CTNND2 genes was excluded using direct sequencing.36 Carr et al.10 have named their South African pedigree FAME3; however, only analysis to exclude the linkage areas 2p and 8q was performed.
FCMTE4, with linkage to 3q26.32-3q28, has recently been discovered in a Thai family.43 No causative gene has yet been identified. Two candidate genes have been proposed for FCMTE4: HTR3D (5-hydroxytryptamine receptor 3D) and KCNMB3 (calcium-activated potassium channel subunit beta-3), which encode for ion channel receptors.29
Other proposed mutations include a missense mutation on 8q22.3 in ubiquitin protein ligase E3 component n-recognin 5 (UBR5) within a Japanese pedigree, linked to Angelman syndrome and autosomal recessive juvenile parkinsonism.59 In a Chinese family, a missense mutation at 22q13.1 in PLA2G6 (phospholipase A2 Group VI) was found using WES.47 Pathogenicity is hypothetical. PLA2G6 mutations have also been linked to infantile neuroaxonal dystrophy and the enzyme encoded by PLA2G6 is involved in the synthesis of arachidonic acid; abnormal metabolism of arachidonic acid could lead to neuronal hyperexcitability.47
Genetic findings in FCMTE-like disorders
In a family from Canada with familial cortical myoclonus without seizures (a potential new disorder), linkage analysis and WES identified a missense mutation on 16q22.1 in the nucleolar protein 3 (NOL3) gene.49 Pathogenicity is doubtful because Nol3 knockout mice did not exhibit myoclonic symptoms and had normal SEPs.49 Moreover there is an autosomal recessive form of FCMTE, caused by a single base pair deletion in CNTN2, crucial for the stability of potassium channels.45
Discussion
In this systematic review we included a total of 126 FCMTE pedigrees (761 patients), including 70 additional FCMTE pedigrees (275 patients) since our 2012 review.1 Here we discuss the diagnostic criteria, clinical spectrum, and treatment. Also, we suggest features that might relate a phenotype to a specific genotype, and focus on the pathophysiology combining neurophysiological, imaging, and pathology studies, as well as on potential candidate genes.
Diagnostic criteria and clinical spectrum
The pedigrees presented in this review share the core features of FCMTE, including autosomal dominant inheritance, distal myoclonic tremor with signs of cortical reflex myoclonus, and/or generalized tonic–clonic seizures. Myoclonus is typically the first symptom, while seizures, usually occurring later in the disease course, are a common reason for patients to seek medical care. However, seizures might in a number of cases be the first symptom.56
EEG findings in FCMTE include generalized spikes/waves and photoparoyxsmal or photomyogenic responses. Other possible clinical features include mild progression of symptoms with aging,16,17,21,57–59 and proximal myoclonus, potentially leading to gait disorders, across linkage types or European/Asian descent.16,17,37,46,56–58,61,65,67 Both are not major inclusion criteria but phenomena that are frequently observed and seem to be part of the disease spectrum.
Focal EEG abnormalities and a slower posterior dominant rhythm might be features of FCMTE.56,74 Also, EEG may show generalized spikes and waves preceding later onset of epilepsy.58
FCMTE is differentiated from other tremor-like disorders (including ET) with electrophysiological recordings.32 FCMTE can be differentiated from the progressive myoclonus epilepsy syndromes and juvenile myoclonic epilepsy (JME) in the absence of severe cognitive decline and absence of severe ataxia.82 Unlike FCMTE, JME presents with myoclonus in the morning and shows no features of cortical reflex myoclonus.82
Criteria for the diagnosis of FCMTE are the following:
Distal action and postural tremor/fine myoclonus, accompanied by generalized tonic-clonic seizures in at least one family member. Also, mild progression of symptoms with aging and proximal muscle myoclonus can be present.
Electrophysiological measures support the diagnosis: giant SEP and LLR point to cortical hyperexcitability; polymyography showing discharges of <50 ms suggest cortical myoclonus; and EEG-EMG coherence analysis or JLA can support a cortical origin of the tremulous movements.
Autosomal dominant inheritance of epilepsy and “tremor”/myoclonus within the family.
No other cause for tremor, epilepsy. No other symptoms must be present like ataxia, parkinsonism, dementia, dystonia, spasticity.
Are phenotypical differences related to specific mutations?
Differences in symptomatology have been reported, possibly linked to different mutations.1 Japanese and Chinese pedigrees, linked to 8q (FCMTE1; also known as BAFME) suffer from a more “benign” form of FCMTE with age of onset in the third decade, infrequent seizures, and no cognitive decline.24,25,27 Usually, FCMTE1 patients present with rhythmic distal myoclonus of the upper extremities, but more extensive involvement of the lower extremities and facial muscles has also been reported.24,68,83
Pedigrees with linkage to 2p (FCMTE2) exhibit a more severe form of myoclonus (proximal involvement) that progresses with aging and leads to gait disability.16,17,46 Specific for Italian 2p pedigrees, psychiatric comorbidity is frequently present.13 Atypical symptoms in pedigrees with a possibly pathogenic mutation were present in Spanish 2p ACMSD patients (n:1 parkinsonism and mild ataxia)48 and in 2p ADRA2B patients (focal seizures and age-related dementia).46
Linkage to 5p (FCMTE3) in two Chinese pedigrees is related to a benign course with age of onset in the fourth decade, and, during short-term follow-up, no disease progression.40,41 However, in French FCMTE3 pedigrees, progression of symptoms, gait disability, frontal syndrome, cognitive changes, and logopenic syndrome were described.61,67 Also, in a Dutch FCMTE3 pedigree subjective cognitive decline was present.39
In a Thai pedigree, with linkage to 3q (FCMTE4), myoclonus presented earlier, in the second decade, and seizures started in the third decade.43 No cognitive decline was present.43
In patients with unknown linkage the following findings were reported: clinical anticipation was present in Asian pedigrees (measured with rating scales).19–21 Mild cognitive decline was present in Asian patients.21,57 European patients (or with European ancestors) suffered from mild cognitive decline,8–12,17,39,47,48 focal seizures,16,46 mild ataxia and/or gait instability,16,17,37,38,48 nystagmus,14 dysarthria,10,38 and migraine.15–18 Atypical features were also described in a Turkish family, including absence seizures, early onset of seizures (14–17 years), negative LLR (while using AEDs), and frequent myoclonic seizures (once a month).18
There are a number of possible explanations for the reported phenotypical differences between pedigrees/FCMTE types. Firstly, different mutations, extensively described in a limited number of pedigrees, could lead to clinical diversity.39,46–48,59 For instance, more severe disease course,17,36 cognitive decline,67 and/or the pathological/neuroimaging changes in the cerebellum1,51,53 might be related to specific FCMTE mutations. Secondly, the clinical differences could be co-incidental or based on comorbidity, either related or unrelated to FCMTE. Other possible explanations might be differences in genetic trait other than FCMTE; heterogenic prescription regimes, including AED use per country, or environmental factors. A third explanation is reporting bias. Documentation, investigations, and follow-up differ substantially between pedigrees.
Pathophysiology
The primary symptoms of FCMTE, cortical myoclonus, and epilepsy seem to have their origin in the sensorimotor cortex. Electrophysiology findings indicate cortical hyperexcitability with generalized spikes and waves on EEG, giant SEP, LLR, and reduced cortical inhibition in TMS studies9,14,78 and cortical activity preceding the onset of movement (EEG-EMG back averaging and coherence analysis), confirmed with combined EMG/fMRI.72,77,84
Cerebellar changes might be a key feature of FCMTE. Pathology findings in three Dutch patients1,14,50 and one South African patient10 report severe loss of Purkinje cells with dendritic sprouts, neuronal loss in the dentate nucleus and microglia activation with limited changes in the sensorimotor cortex. Use of AEDs might result in loss of Purkinje cells. Notwithstanding, cerebellar pathology findings in FCMTE patients differ from changes seen in patients with chronic idiopathic epilepsy who used phenytoin85 and rats chronically exposed to valproic acid.86 Also, Dutch patients who did not use AEDs had a downbeat nystagmus upon hyperventilation, indicating cerebellar dysfunction.14 Moreover, a mutation in CTNND2 was found in the same Dutch pedigree, probably related to FCMTE3, as the gene CTNND2 is located on the same linkage area.39 A mutant version of this gene was responsible for abnormal neuronal sprouting in mouse neurons, resembling cerebellar pathology findings in the same Dutch pedigree.39 In a French FCMTE3 pedigree a mutation in CTNND2 was excluded using direct sequencing.37 It could be there are several mutations located on the same linkage area or the mutation was undetectable due to the technique used.
Pathological cerebellar involvement is in line with cerebellar signs in European FCMTE pedigrees; these include downbeat nystagmus,14 mild ataxia (although possibly difficult to judge in the presence of tremulous myoclonus),37,38 dysarthria,10,38 and imaging findings revealing cerebellar involvement.51,53,79
Further evidence for cerebellar involvement in FCMTE arises from imaging studies in FCMTE, indicating functional connectivity changes between the cerebellum and cortical/subcortical structures.52 Supratentorial gray matter loss outside the motor circuit might be secondary.70 Also, cerebellar atrophy and ataxia have frequently been noted in patients with epilepsy.87 Repetitive TMS over the cerebellar cortex was able to reduce seizure frequency in drug-resistant epilepsy.87,88 The cerebellum might be involved in psychiatric disorders.89 In several FCMTE pedigrees anxiety and depressive comorbidity was reported.13,56 With aging, Purkinje cells become atrophic in healthy adults and the amount of white matter is reduced in the cerebellum.90 The degeneration of Purkinje cells might be enhanced in FCMTE patients leading to defective compensatory input to cortical structures. Clinically, this could explain the later onset of seizures and the worsening of symptoms with aging.16 Moreover, cortical hyperexcitability seems to increase with aging in Japanese patients, reflected by higher giant SEP amplitude.73
Pathology, imaging, genetic, and clinical findings indicate cerebellar changes. This offers a possible explanation for the decreased cortical inhibition. Deficient stimulation of the dentate nucleus by Purkinje cells in the cerebellum may lead to increased cortical facilitation via the cerebello-thalamo-cortical loop, a hypothesis already raised for cortical myoclonus and epilepsy.54,87,91 Purkinje cell changes might however not be the (sole) explanation for the symptoms observed in FCMTE.
The cerebellar changes might be specific for certain FCMTE types, with the strongest evidence for FCMTE3. Future research is needed to indicate whether cerebellar involvement, including pathological cerebellar changes, is a general finding in FCMTE. In some FCMTE types it might be co-existent, secondary due to primary cortical pathology, or even absent.
Possible candidate genes
Possible candidate causative genes include a trinucleotide repeat expansion or channelopathy.80 Purkinje cell changes in deceased Dutch FCMTE patients show striking similarities with those found in spinocerebellar ataxia (SCA) type 6, characterized by ataxia and downbeat nystagmus.1,50 Patients from the same Dutch FCMTE pedigree also have a downbeat nystagmus.14 SCA type 6 is caused by a CAG repeat in a calcium channel CACNA1A heavily expressed in the cerebellum.92 Other observations that might also point to a trinucleotide repeat expansion or channelopathy underlying FCMTE include in Asian FCMTE pedigrees, progressively earlier onset of the disease with increasing severity in successive generations19–21; presence of migraine15–18 analogues to hemiplegic migraine with mutations in calcium channels93; and the recognition that certain epilepsy syndromes are channelopathies.45,94 Recently, an autosomal recessive form of FCMTE has been recognized with a single base pair deletion in CNTN2, crucial for the stability of potassium channels.45 A channelopathy could involve cerebellar cells and/or induce functional cortical changes.50,92
Several genes have been identified as potentially causative in FCMTE. A gain of function mutation in the ADRA2B gene has been found in Italian FCMTE2 pedigrees that could potentially alter the neuronal firing pattern and even reduce gamma-aminobutyric acid (GABA) neurotransmission.46 A reduction in cortical GABA neurotransmission has been associated with cortical myoclonus in rats, progressive myoclonus epilepsy and Unverricht–Lundborg disease characterized by cortical myoclonus and ataxia along with seizures.91 Treatment with AEDs increasing GABA neurotransmitter levels can relieve the symptoms of FCMTE.
Another neurotransmitter implied in the pathophysiology of FCMTE2 is serotonin.48 A mutation in the ACMSD gene, encoding for an enzyme that is part of the kynurenine pathway, might lead to the accumulation of waste products.48 In mice, seizures could be induced when injecting these kynurenines in the brain ventricles.95
In Dutch FCMTE3 patients, a mutation in CTNND2, supported by functional tests in mice, seems to be responsible for abnormal neuronal sprouting.39 Hypothetically, the pathology findings in the cerebellum and the disease itself are caused by the CTNND2 mutation.39
Other proposed mutations/linkage studies have not yet elucidated the pathophysiology of FCMTE and have provided conflicting results, including proposed mutations outside the known linkage areas (UBR5, PLA2G6)47,59 and a pedigree from Canada (NOL3 mutation)49 without seizures, possibly reflecting another illness. The lack of progress might be due to the inability of next-generation sequencing techniques to detect exon rearrangements, trinucleotide repeat expansion, and copy number variants.80 Even a deletion in an intron might lead to defective splicing of a gene as in Unverricht–Lundborg disease.96 New algorithms and WGS might lead to a breakthrough.97,98
Future genetic studies should focus on mutations in proteins expressed in the cerebellum, involved in neuronal outgrowth, calcium, sodium, or potassium signaling, GABA neurotransmission, and genes which interact with the SCAs or other diseases with cortical myoclonic symptoms.80
Limitations
Reporting bias may have influenced our results. The number of patients and follow-up differ between pedigrees. Across studies, rating scales for motor symptoms and cognitive functioning differ or have not been reported. Therefore, symptoms might have been underreported, for instance cerebellar dysfunction and cognitive deterioration in certain pedigrees.14 Additional investigations (MRI, electrophysiology, pathology) have not always been performed, in some cases leading to diagnostic uncertainty, but more often raising questions with respect to generalizability of findings across pedigrees. For instance, both the imaging and the pathology findings involving cerebellar changes have largely been confined to a South African patient (linkage exclusion 2p, 8q), a Dutch pedigree (FCMTE3), and Chinese patients (linkage exclusion 2p, 8q) making the generalizability of the findings problematic.10,39,51–53,65,70 Several mutations (ACMSD, ADRA2B, UBR5, PLA2G6) have been proposed but pathogenicity has still to be proven.46–48,59
Conclusion
FCMTE, also known under different names and acronyms, is a clinical entity not (yet) listed by the ILAE. It is characterized by cortical tremor/fine myoclonus and generalized tonic–clonic seizures with autosomal dominant inheritance. Proximal myoclonus and mild progression with aging are part of the spectrum. Electrophysiology recordings show features of cortical reflex myoclonus. Valproate (not recommended in women of childbearing age) or levetiracetam, with or without clonazepam reduce symptoms. Gabapentin should be prescribed cautiously.
FCMTE is a heterogeneous disorder, and is likely to include a variety of different conditions with mutations of different genes. Additional symptoms have frequently been reported and can be co-incidental or based on genetic differences. Pathophysiological mechanisms remain to be elucidated, but pathology, genetic, and imaging studies have given clues that indicate cerebellar involvement. The cerebellar changes might lead to reduced cortical inhibition. Alternatively, underlying genetic changes induce both cerebellar and cortical changes. Genetic heterogeneity is present in linkage studies. Several causative genes have been suggested. However, functional tests have not always been performed.
Pathophysiology and genetic studies indicate that future genetic studies should focus on mutations involving Purkinje cell outgrowth, channelopathies, or genes responsible for neurotransmitter synthesis.
Appendix A
PubMed search
Conducted on 15-11-2017, articles were sought from 01-01-2011 to 15-11-2017.
((((cortical tremor myoclonus) OR cortical tremor epilepsy) OR (((((“Tremor”[Mesh]) AND “Myoclonus”[Mesh]) AND “Epilepsy”[Mesh])) OR ((cortical tremor [tiab] AND epilepsy [tiab] AND myoclonus [tiab])))) OR (((((((((((“Epilepsy, Myoclonic, Benign Adult Familial, Type 1”[Supplementary Concept]) OR (“Tremor”[Mesh] AND “Epilepsy”[Mesh])) OR (“Tremor”[Mesh]) AND “Myoclonus”[Mesh])) OR (“Epilepsy”[Mesh] AND “Myoclonus”[Mesh])) OR (autosomal dominant [tiab] AND “Epilepsy”[Mesh]) AND “Myoclonus”[Mesh])) OR (autosomal dominant [tiab] AND “Tremor”[Mesh] AND “Myoclonus”[Mesh])) OR (familial [tiab] AND “Epilepsy”[Mesh] AND “Myoclonus”[Mesh])) OR (familial [tiab] AND “Tremor”[Mesh] AND “Myoclonus”[Mesh])) OR (benign course [tiab] AND “Epilepsy”[Mesh] OR benign course [tiab] AND epilepsy [tiab])) OR (”Tremor”[Mesh] AND “Myoclonus”[Mesh] OR tremor [tiab] AND myoclonus [tiab] OR epilepsy [tiab] AND myoclonus [tiab])))) OR (Autosomal dominant cortical myoclonus and epilepsy OR Benign adult familial myoclonic epilepsy OR Cortical tremor OR Familial adult myoclonic epilepsy OR Familial cortical myoclonic tremor OR Familial cortical myoclonic tremor with epilepsy OR Familial cortical tremor with epilepsy OR Familial essential myoclonus and epilepsy OR Familial benign myoclonus epilepsy of adult onset OR Heredofamilial tremor and epilepsy).
Footnotes
Funding: None.
Financial Disclosures: None.
Conflicts of Interest: The authors report no conflicts of interest.
Ethics Statement: Not applicable for this category of article.
References
- 1.Sharifi S, Aronica E, Koelman JH, Tijssen MA, Van Rootselaar AF. Familial cortical myoclonic tremor with epilepsy and cerebellar changes: description of a new pathology case and review of the literature. Tremor Other Hyperkinet Mov. 2012:2. doi: 10.7916/D8ST7NKK. doi: 10.7916/D8ST7NKK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Rootselaar AF, van Schaik IN, van den Maagdenberg AM, Koelman JH, Callenbach PM, Tijssen MA. Familial cortical myoclonic tremor with epilepsy: a single syndromic classification for a group of pedigrees bearing common features. Mov Disord. 2005;20:665–673. doi: 10.1002/mds.20413. doi: 10.1002/mds.20413. [DOI] [PubMed] [Google Scholar]
- 3.HGNC Database, HUGO Gene Nomenclature Committee (HGNC), EMBL Outstation - Hinxton, European Bioinformatics Institute, Wellcome Trust Genome Campus. www.genenames.org Accessed December 12, 2017.
- 4.Yasuda T. Benign adult familial myoclonic epilepsy (BAFME) Kawasaki Med J. 1991;17:1–13. doi: 10.11482/KMJ-E17. [Google Scholar]
- 5.Inazuki G, Naito H, Ohama E, Kawase Y, Honma Y, Tokiguchi S, et al. [A clinical study and neuropathological findings of a familial disease with myoclonus and epilepsy--the nosological place of familial essential myoclonus and epilepsy (FEME)] Seishin Shinkeigaku Zasshi. 1990;92:1–21. [PubMed] [Google Scholar]
- 6.Ikeda A, Kakigi R, Funai N, Neshige R, Kuroda Y, Shibasaki H. Cortical tremor: a variant of cortical reflex myoclonus. Neurology. 1990;40:1561–1565. doi: 10.1212/wnl.40.10.1561. doi: 10.1212/WNL.40.10.1561. [DOI] [PubMed] [Google Scholar]
- 7.Kudo J, Kudo T, Yamauchi T. [Seven families with heredofamilial tremor and epilepsy] Rinsho Shinkeigaku. 1984;24:1–8. [PubMed] [Google Scholar]
- 8.Guerrini R, Bonanni P, Patrignani A, Brown P, Parmeggiani L, Grosse P, et al. Autosomal dominant cortical myoclonus and epilepsy (ADCME) with complex partial and generalized seizures: A newly recognized epilepsy syndrome with linkage to chromosome 2p11.1-q12.2. Brain. 2001;124((Pt 12)):2459–2475. doi: 10.1093/brain/124.12.2459. doi: 10.1093/brain/124.12.2459. [DOI] [PubMed] [Google Scholar]
- 9.Suppa A, Berardelli A, Brancati F, Marianetti M, Barrano G, Mina C, et al. Clinical, neuropsychological, neurophysiologic, and genetic features of a new Italian pedigree with familial cortical myoclonic tremor with epilepsy. Epilepsia. 2009;50:1284–1288. doi: 10.1111/j.1528-1167.2008.01976.x. doi: 10.1111/j.1528-1167.2008.01976.x. [DOI] [PubMed] [Google Scholar]
- 10.Carr JA, van der Walt PE, Nakayama J, Fu YH, Corfield V, Brink P, et al. FAME 3: a novel form of progressive myoclonus and epilepsy. Neurology. 2007;68:1382–1389. doi: 10.1212/01.wnl.0000260063.46425.7e. doi: 10.1212/01.wnl.0000260063.46425.7e. [DOI] [PubMed] [Google Scholar]
- 11.van Rootselaar F, Callenbach PM, Hottenga JJ, VermeulenHans FLM, SpeelmanOebele HD, Brouwer OF, et al. A Dutch family with ‘familial cortical tremor with epilepsy’. Clinical characteristics and exclusion of linkage to chromosome 8q23.3-q24.1. J Neurol. 2002;249:829–834. doi: 10.1007/s00415-002-0729-x. doi: 10.1007/s00415-002-0729-x. [DOI] [PubMed] [Google Scholar]
- 12.Elia M, Musumeci SA, Ferri R, Scuderi C, Gracco SD, Bottitta M, et al. Familial cortical tremor, epilepsy, and mental retardation: a distinct clinical entity? Arch Neurol. 1998;55:1569–1573. doi: 10.1001/archneur.55.12.1569. doi: 10.1001/archneur.55.12.1569. [DOI] [PubMed] [Google Scholar]
- 13.Coppola A, Caccavale C, Santulli L, Balestrini S, Cagnetti C, Licchetta L, et al. Psychiatric comorbidities in patients from seven families with autosomal dominant cortical tremor, myoclonus, and epilepsy. Epilepsy Behav. 2016;56:38–43. doi: 10.1016/j.yebeh.2015.12.038. doi: 10.1016/j.yebeh.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 14.van Rootselaar AF, van der Salm SM, Bour LJ, Edwards MJ, Brown P, Aronica E, et al. Decreased cortical inhibition and yet cerebellar pathology in 'familial cortical myoclonic tremor with epilepsy. Mov Disord. 2007;22:2378–2385. doi: 10.1002/mds.21738. doi: 10.1002/mds.21738. [DOI] [PubMed] [Google Scholar]
- 15.Saka E, Saygi S. Familial adult onset myoclonic epilepsy associated with migraine. Seizure. 2000;9:344–346. doi: 10.1053/seiz.2000.0402. doi: 10.1053/seiz.2000.0402. [DOI] [PubMed] [Google Scholar]
- 16.Crompton DE, Sadleir LG, Bromhead CJ, Bahlo M, Bellows ST, Arsov T, et al. Familial adult myoclonic epilepsy: recognition of mild phenotypes and refinement of the 2q locus. Arch Neurol. 2012;69:474–481. doi: 10.1001/archneurol.2011.584. doi: 10.1001/archneurol.2011.584. [DOI] [PubMed] [Google Scholar]
- 17.Licchetta L, Pippucci T, Bisulli F, Cantalupo G, Magini P, Alvisi L, et al. A novel pedigree with familial cortical myoclonic tremor and epilepsy (FCMTE): clinical characterization, refinement of the FCMTE2 locus, and confirmation of a founder haplotype. Epilepsia. 2013;54:1298–1306. doi: 10.1111/epi.12216. doi: 10.1111/epi.12216. [DOI] [PubMed] [Google Scholar]
- 18.Aydin Ozemir Z, Oguz Akarsu E, Matur Z, Oge AE, Baykan B. Autosomal dominant cortical tremor, myoclonus, and epilepsy syndrome mimicking juvenile myoclonic epilepsy. Noro Psikiyatr Ars. 2016;53:272–275. doi: 10.5152/npa.2016.14841. doi: 10.5152/npa.2016.14841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hitomi T, Kondo T, Kobayashi K, Matsumoto R, Takahashi R, Ikeda A. Clinical anticipation in Japanese families of benign adult familial myoclonus epilepsy. Epilepsia. 2012;53:e33–36. doi: 10.1111/j.1528-1167.2011.03349.x. doi: 10.1111/j.1528-1167.2011.03349.x. [DOI] [PubMed] [Google Scholar]
- 20.Hitomi T, Kobayashi K, Jingami N, Nakagawa T, Imamura H, Matsumoto R, et al. Increased clinical anticipation with maternal transmission in benign adult familial myoclonus epilepsy in Japan. Epileptic Disord. 2013;15:428–432. doi: 10.1684/epd.2013.0608. doi: 10.1684/epd.2013.0608. [DOI] [PubMed] [Google Scholar]
- 21.Cen Z, Huang C, Yin H, Ding X, Xie F, Lu X, et al. Clinical and neurophysiological features of familial cortical myoclonic tremor with epilepsy. Mov Disord. 2016;31:1704–1710. doi: 10.1002/mds.26756. doi: 10.1002/mds.26756. [DOI] [PubMed] [Google Scholar]
- 22.Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University; Baltimore, MD: MIM number {601068}: {08/03/2017} https://omim.org/ [Google Scholar]
- 23.HGNC Database, HUGO Gene Nomenclature Committee (HGNC), EMBL Outstation - Hinxton, European Bioinformatics Institute, Wellcome Trust Genome Campus. www.genenames.org Accessed May 13, 2017.
- 24.Mikami M, Yasuda T, Terao A, Nakamura M, Ueno S, Tanabe H, et al. Localization of a gene for benign adult familial myoclonic epilepsy to chromosome 8q23.3-q24.1. Am J Hum Genet. 1999;65:745–751. doi: 10.1086/302535. doi: 10.1086/302535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plaster NM, Uyama E, Uchino M, Ikeda T, Flanigan KM, Kondo I, et al. Genetic localization of the familial adult myoclonic epilepsy (FAME) gene to chromosome 8q24. Neurology. 1999;53:1180–1183. doi: 10.1212/wnl.53.6.1180. doi: 10.1212/WNL.53.6.1180. [DOI] [PubMed] [Google Scholar]
- 26.Mori S, Nakamura M, Yasuda T, Ueno S, Kaneko S, Sano A. Remapping and mutation analysis of benign adult familial myoclonic epilepsy in a Japanese pedigree. J Hum Genet. 2011;56:742–747. doi: 10.1038/jhg.2011.93. doi: 10.1038/jhg.2011.93. [DOI] [PubMed] [Google Scholar]
- 27.Cen ZD, Xie F, Lou DN, Lu XJ, Ouyang ZY, Liu L, et al. Fine mapping and whole-exome sequencing of a familial cortical myoclonic tremor with epilepsy family. Am J Med Genet B Neuropsychiatr Genet. 2015;168:595–599. doi: 10.1002/ajmg.b.32337. doi: 10.1002/ajmg.b.32337. [DOI] [PubMed] [Google Scholar]
- 28.Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University; Baltimore, MD: https://omim.org/ MIM number: {607876}: {07/03/2017} [Google Scholar]
- 29.Henden L, Freytag S, Afawi Z, Baldassari S, Berkovic SF, Bisulli F, et al. Identity by descent fine mapping of familial adult myoclonus epilepsy (FAME) to 2p11.2-2q11.2. Hum Genet. 2016;135:1117–1125. doi: 10.1007/s00439-016-1700-8. doi: 10.1007/s00439-016-1700-8. [DOI] [PubMed] [Google Scholar]
- 30.de Falco FA, Striano P, de Falco A, Striano S, Santangelo R, Perretti A, et al. Benign adult familial myoclonic epilepsy: genetic heterogeneity and allelism with ADCME. Neurology. 2003;60:1381–1385. doi: 10.1212/01.wnl.0000055874.24000.4a. doi: 10.1212/01.WNL.0000055874.24000.4A. [DOI] [PubMed] [Google Scholar]
- 31.Madia F, Striano P, Di Bonaventura C, de Falco A, de Falco FA, Manfredi M, et al. Benign adult familial myoclonic epilepsy (BAFME): evidence of an extended founder haplotype on chromosome 2p11.1-q12.2 in five Italian families. Neurogenetics. 2008;9:139–142. doi: 10.1007/s10048-008-0118-4. doi: 10.1007/s10048-008-0118-4. [DOI] [PubMed] [Google Scholar]
- 32.Striano P, Chifari R, Striano S, De Fusco M, Elia M, Guerrini R, et al. A new benign adult familial myoclonic epilepsy (BAFME) pedigree suggesting linkage to chromosome 2p11.1-q12.2. Epilepsia. 2004;45:190–192. doi: 10.1111/j.0013-9580.2004.39903.x. doi: 10.1111/j.0013-9580.2004.39903.x. [DOI] [PubMed] [Google Scholar]
- 33.Striano P, Madia F, Minetti C, Striano S, Zara F. Electroclinical and genetic findings in a family with cortical tremor, myoclonus, and epilepsy. Epilepsia. 2005;46:1993–1995. doi: 10.1111/j.1528-1167.2005.00346.x. doi: 10.1111/j.1528-1167.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- 34.Saint-Martin C, Bouteiller D, Stevanin G, Popescu C, Charon C, Ruberg M, et al. Refinement of the 2p11.1-q12.2 locus responsible for cortical tremor associated with epilepsy and exclusion of candidate genes. Neurogenetics. 2008;9:69–71. doi: 10.1007/s10048-007-0107-z. doi: 10.1007/s10048-007-0107-z. [DOI] [PubMed] [Google Scholar]
- 35.Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University; Baltimore, MD: https://omim.org/ MIM number: {613608}: {10/26/2010} [Google Scholar]
- 36.Depienne C, Magnin E, Bouteiller D, Stevanin G, Saint-Martin C, Vidailhet M, et al. Familial cortical myoclonic tremor with epilepsy: the third locus (FCMTE3) maps to 5p. Neurology. 2010;74:2000–2003. doi: 10.1212/WNL.0b013e3181e396a8. doi: 10.1212/WNL.0b013e3181e396a8. [DOI] [PubMed] [Google Scholar]
- 37.Magnin E, Vidailhet M, Depienne C, Saint-Martin C, Bouteiller D, LeGuern E, et al. Familial cortical myoclonic tremor with epilepsy (FCMTE): Clinical characteristics and exclusion of linkages to 8q and 2p in a large French family. Rev Neurol (Paris) 2009;165:812–820. doi: 10.1016/j.neurol.2009.05.014. doi: 10.1016/j.neurol.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Bourdain F, Apartis E, Trocello JM, Vidal JS, Masnou P, Vercueil L, et al. Clinical analysis in familial cortical myoclonic tremor allows differential diagnosis with essential tremor. Mov Disord. 2006;21:599–608. doi: 10.1002/mds.20725. doi: 10.1002/mds.20725. [DOI] [PubMed] [Google Scholar]
- 39.van Rootselaar AF, Groffen AJ, de Vries B, Callenbach PMC, Santen GWE, Koelewijn S, et al. delta-Catenin (CTNND2) missense mutation in familial cortical myoclonic tremor and epilepsy. Neurology. 2017;89:2341–2350. doi: 10.1212/WNL.0000000000004709. doi: 10.1212/WNL.0000000000004709. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Sun W, Chen Q, Li J, Hu G. Genetic analysis of a Chinese family provides further evidence for linkage of familial cortical myoclonic tremor with epilepsy to 5p15.31-p15. Neurol India. 2015;63:215–219. doi: 10.4103/0028-3886.156283. doi: 10.4103/0028-3886.156283. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Hu X, Chen Q, Zhang Y, Zhang Y, Hu G. A Chinese benign adult familial myoclonic epilepsy pedigree suggesting linkage to chromosome 5p15.31-p15.1. Cell Biochem Biophys. 2014;69:627–631. doi: 10.1007/s12013-014-9843-5. doi: 10.1007/s12013-014-9843-5. [DOI] [PubMed] [Google Scholar]
- 42.Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University; Baltimore, MD: https://omim.org/ MIM number: {615127}: {08/02/2017} [Google Scholar]
- 43.Yeetong P, Ausavarat S, Bhidayasiri R, Piravej K, Pasutharnchat N, Desudchit T, et al. A newly identified locus for benign adult familial myoclonic epilepsy on chromosome 3q26.32-3q28. Eur J Hum Genet. 2013;21:225–228. doi: 10.1038/ejhg.2012.133. doi: 10.1038/ejhg.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University; Baltimore, MD: https://omim.org/ MIM number: {615400}: {08/14/2017} [Google Scholar]
- 45.Stogmann E, Reinthaler E, Eltawil S, Etribi MAE, Hemeda M, Nahhas NE, et al. Autosomal recessive cortical myoclonic tremor and epilepsy: association with a mutation in the potassium channel associated gene CNTN2. Brain. 2013;136:1155–1160. doi: 10.1093/brain/awt068. doi: 10.1093/brain/awt068. [DOI] [PubMed] [Google Scholar]
- 46.De Fusco M, Vago R, Striano P, Di Bonaventura C, Zara F, Mei D, et al. The alpha2B-adrenergic receptor is mutant in cortical myoclonus and epilepsy. Ann Neurol. 2014;75:77–87. doi: 10.1002/ana.24028. doi: 10.1002/ana.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao L, Li L, Ye J, Zhu X, Shen N, Zhang X, et al. Identification of a novel mutation in PLA2G6 gene in a Chinese pedigree with familial cortical myoclonic tremor with epilepsy. Seizure. 2016;41:81–85. doi: 10.1016/j.seizure.2016.07.013. doi: 10.1016/j.seizure.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Marti-Masso JF, Bergareche A, Makarov V, Ruiz-Martinez J, Gorostidi A, de Munain AL, et al. The ACMSD gene, involved in tryptophan metabolism, is mutated in a family with cortical myoclonus, epilepsy, and parkinsonism. J Mol Med (Berl) 2013;91:1399–1406. doi: 10.1007/s00109-013-1075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell JF, Steckley JL, Coppola G, Hahn AFG, Howard MA, Kornberg Z, et al. Familial cortical myoclonus with a mutation in NOL3. Ann Neurol. 2012;72:175–183. doi: 10.1002/ana.23666. doi: 10.1002/ana.23666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Rootselaar AF, Aronica E, Jansen Steur EN, Rozemuller-Kwakkel JM, de Vos RA, Tijssen MA. Familial cortical tremor with epilepsy and cerebellar pathological findings. Mov Disord. 2004;19:213–217. doi: 10.1002/mds.10662. doi: 10.1002/mds.10662. [DOI] [PubMed] [Google Scholar]
- 51.Buijink AW, Caan MW, Tijssen MA, Hoogduin JM, Maurits NM, van Rootselaar AF. Decreased cerebellar fiber density in cortical myoclonic tremor but not in essential tremor. Cerebellum. 2013;12:199–204. doi: 10.1007/s12311-012-0414-2. [DOI] [PubMed] [Google Scholar]
- 52.Long L, Zeng LL, Song Y, Shen H, Fang P, Zhang L, et al. Altered cerebellar-cerebral functional connectivity in benign adult familial myoclonic epilepsy. Epilepsia. 2016;57:941–948. doi: 10.1111/epi.13372. doi: 10.1111/epi.13372. [DOI] [PubMed] [Google Scholar]
- 53.Buijink AW, Broersma M, van der Stouwe AM, Sharifi S, Tijssen MAJ, Speelman JD, et al. Cerebellar atrophy in cortical myoclonic tremor and not in hereditary essential tremor-a voxel-based morphometry study. Cerebellum. 2016;15:696–704. doi: 10.1007/s12311-015-0734-0. doi: 10.1007/s12311-015-0734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Striano P, Louis ED, Manto M. Autosomal dominant cortical tremor, myoclonus, and epilepsy: is the origin in the cerebellum? Editorial. Cerebellum. 2013;12:145–146. doi: 10.1007/s12311-012-0419-x. doi: 10.1007/s12311-012-0419-x. [DOI] [PubMed] [Google Scholar]
- 55.Wang P, Luo C, Dong L, Bin Y, Ma S, Yao D, et al. Altered intrinsic brain activity in patients with familial cortical myoclonic tremor and epilepsy: an amplitude of low-frequency fluctuation study. J Neurol Sci. 2015;351:133–139. doi: 10.1016/j.jns.2015.03.005. doi: 10.1016/j.jns.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Mahadevan R, Viswanathan N, Shanmugam G, Sankaralingam S, Essaki B, Chelladurai RP. Autosomal dominant cortical tremor, myoclonus, and epilepsy (ADCME) in a unique south Indian community. Epilepsia. 2016;57:e56–59. doi: 10.1111/epi.13303. doi: 10.1111/epi.13303. [DOI] [PubMed] [Google Scholar]
- 57.Sharma CM, Nath K, Kumawat BL, Khandelwal D. Autosomal dominant cortical tremor, myoclonus, and epilepsy (ADCME): Probable first family from India. Ann Indian Acad Neurol. 2014;17:433–436. doi: 10.4103/0972-2327.144025. doi: 10.4103/0972-2327.144025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Coller R, van Rootselaar AF, Schutte C, van der Meyden CH. Familial cortical myoclonic tremor and epilepsy: description of a new South African pedigree with 30 year follow up. Parkinsonism Relat Disord. 2017;38:35–40. doi: 10.1016/j.parkreldis.2017.02.016. doi: 10.1016/j.parkreldis.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 59.Kato T, Tamiya G, Koyama S, Nakamura T, Makino S, Arawaka S, et al. UBR5 Gene mutation is associated with familial adult myoclonic epilepsy in a Japanese family. ISRN Neurol. 2012;2012:508308. doi: 10.5402/2012/508308. doi: 10.5402/2012/508308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toyota T, Akamatsu N, Tanaka A, Tsuji S, Uozumi T. Electroencephalographic features of benign adult familial myoclonic epilepsy. Clin Neurophysiol. 2014;125:250–254. doi: 10.1016/j.clinph.2013.08.002. doi: 10.1016/j.clinph.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Magnin E, Vidailhet M, Ryff I, Ferreira S, Labauge P, Rumbach L. Fronto-striatal dysfunction in type 3 familial cortical myoclonic tremor epilepsy occurring during aging. J Neurol. 2012;259:2714–2719. doi: 10.1007/s00415-012-6575-6. doi: 10.1007/s00415-012-6575-6. [DOI] [PubMed] [Google Scholar]
- 62.Gardella E, Tinuper P, Marini C, Guerrini R, Parrini E, Bisulli F, et al. Autosomal dominant early-onset cortical myoclonus, photic-induced myoclonus, and epilepsy in a large pedigree. Epilepsia. 2006;47:1643–1649. doi: 10.1111/j.1528-1167.2006.00636.x. doi: 10.1111/j.1528-1167.2006.00636.x. [DOI] [PubMed] [Google Scholar]
- 63.Coppola A, Santulli L, Del Gaudio L, Minetti C, Striano S, Zara F, et al. Natural history and long-term evolution in families with autosomal dominant cortical tremor, myoclonus, and epilepsy. Epilepsia. 2011;52:1245–1250. doi: 10.1111/j.1528-1167.2011.03017.x. doi: 10.1111/j.1528-1167.2011.03017.x. [DOI] [PubMed] [Google Scholar]
- 64.Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–530. doi: 10.1111/epi.13670. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 65.Long L, Song Y, Zhang L, Hu C, Gong J, Xu L, et al. A case-control proton magnetic resonance spectroscopy study confirms cerebellar dysfunction in benign adult familial myoclonic epilepsy. Neuropsychiatr Dis Treat. 2015;11:485–491. doi: 10.2147/NDT.S77910. doi: 10.2147/NDT.S77910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deng FY, Gong J, Zhang YC, Wang K, Xiao SM, Lia YN, et al. Absence of linkage to 8q23.3-q24.1 and 2p11.1-q12.2 in a new BAFME pedigree in China: indication of a third locus for BAFME. Epilepsy Res. 2005;65:147–152. doi: 10.1016/j.eplepsyres.2005.05.006. doi: 10.1016/j.eplepsyres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 67.Magnin E. Logopenic syndrome and corticobasal dysfunction in a “benign” type 3 familial cortical myoclonic tremor with epilepsy. Seizure. 2015;25:84–86. doi: 10.1016/j.seizure.2015.01.004. doi: 10.1016/j.seizure.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 68.Manabe Y, Narai H, Warita H, Hayashi T, Shiro Y, Sakai K, et al. Benign adult familial myoclonic epilepsy (BAFME) with night blindness. Seizure. 2002;11:266–268. doi: 10.1053/seiz.2001.0606. doi: 10.1053/seiz.2001.0606. [DOI] [PubMed] [Google Scholar]
- 69.Morita S, Miwa H, Kondo T. [A case of the familial essential myoclonus and epilepsy presenting behavioral arrest] No To Shinkei. 2003;55:345–348. [PubMed] [Google Scholar]
- 70.Zeng LL, Long L, Shen H, Fang P, Song Y, Zhang L, et al. Gray matter loss and related functional connectivity alterations in a Chinese family with benign adult familial myoclonic epilepsy. Medicine (Baltimore) 2015;94:e1767. doi: 10.1097/MD.0000000000001767. doi: 10.1097/MD.0000000000001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kojovic M, Cordivari C, Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4:47–62. doi: 10.1177/1756285610395653. doi: 10.1177/1756285610395653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Rootselaar AF, Maurits NM, Koelman JH, van der Hoeven JH, Bour LJ, Leenders KL, et al. Coherence analysis differentiates between cortical myoclonic tremor and essential tremor. Mov Disord. 2006;21:215–222. doi: 10.1002/mds.20703. doi: 10.1002/mds.20703. [DOI] [PubMed] [Google Scholar]
- 73.Hitomi T, Ikeda A, Kondo T, Imamura H, Inouchi M, Matsumoto R, et al. Increased cortical hyperexcitability and exaggerated myoclonus with aging in benign adult familial myoclonus epilepsy. Mov Disord. 2011;26:1509–1514. doi: 10.1002/mds.23653. doi: 10.1002/mds.23653. [DOI] [PubMed] [Google Scholar]
- 74.Hitomi T, Kobayashi K, Sakurai T, Ueda S, Jingami N, Kanazawa K, et al. Benign adult familial myoclonus epilepsy is a progressive disorder: no longer idiopathic generalized epilepsy. Epileptic Disord. 2016;18:67–72. doi: 10.1684/epd.2016.0807. doi: 10.1684/epd.2016.0807. [DOI] [PubMed] [Google Scholar]
- 75.Striano P, Manganelli F, Boccella P, Perretti A, Striano S. Levetiracetam in patients with cortical myoclonus: a clinical and electrophysiological study. Mov Disord. 2005;20:1610–1614. doi: 10.1002/mds.20530. doi: 10.1002/mds.20530. [DOI] [PubMed] [Google Scholar]
- 76.Striano P, Coppola A, Madia F, Pezzella M, Zara F, Striano S. Life-threatening status epilepticus following gabapentin administration in a patient with benign adult familial myoclonic epilepsy. Epilepsia. 2007;48:1995–1998. doi: 10.1111/j.1528-1167.2007.01198.x. doi: 10.1111/j.1528-1167.2007.01198.x. [DOI] [PubMed] [Google Scholar]
- 77.van Rootselaar AF, Maurits NM, Renken R, Koelman JHTM, Hoogduin JM, Leenders KL, et al. Simultaneous EMG-functional MRI recordings can directly relate hyperkinetic movements to brain activity. Hum Brain Mapp. 2008;29:1430–1441. doi: 10.1002/hbm.20477. doi: 10.1002/hbm.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakatani-Enomoto S, Hanajima R, Hamada M, Terao Y, Matsumoto H, Shiroat Y, et al. Somatosensory-evoked potential modulation by quadripulse transcranial magnetic stimulation in patients with benign myoclonus epilepsy. Clin Neurophysiol. 2016;127:1560–1567. doi: 10.1016/j.clinph.2015.07.029. doi: 10.1016/j.clinph.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 79.Striano P, Caranci F, Di Benedetto R, Tortora F, Zara F, Striano S. (1)H-MR spectroscopy indicates prominent cerebellar dysfunction in benign adult familial myoclonic epilepsy. Epilepsia. 2009;50:1491–1497. doi: 10.1111/j.1528-1167.2008.01900.x. doi: 10.1111/j.1528-1167.2008.01900.x. [DOI] [PubMed] [Google Scholar]
- 80.Cen ZD, Xie F, Xiao JF, Luo W. Rational search for genes in familial cortical myoclonic tremor with epilepsy, clues from recent advances. Seizure. 2016;34:83–89. doi: 10.1016/j.seizure.2015.12.004. doi: 10.1016/j.seizure.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 81.Labauge P, Amer LO, Simonetta-Moreau M, Attané F, Tannier C, Clanet M, et al. Absence of linkage to 8q24 in a European family with familial adult myoclonic epilepsy (FAME) Neurology. 2002;58:941–944. doi: 10.1212/wnl.58.6.941. doi: 10.1212/WNL.58.6.941. [DOI] [PubMed] [Google Scholar]
- 82.Striano P, Zara F. Autosomal dominant cortical tremor, myoclonus and epilepsy. Epileptic Disord. 2016;18((S2)):139–144. doi: 10.1684/epd.2016.0860. doi: 10.1684/epd.2016.0860. [DOI] [PubMed] [Google Scholar]
- 83.Okino S. Familial benign myoclonus epilepsy of adult onset: a previously unrecognized myoclonic disorder. J Neurol Sci. 1997;145:113–118. doi: 10.1016/s0022-510x(96)00245-6. doi: 10.1016/S0022-510X(96)00245-6. [DOI] [PubMed] [Google Scholar]
- 84.van Rootselaar AF, Renken R, de Jong BM, Hoogduin JM, Tijssen MA, Maurits NM. fMRI analysis for motor paradigms using EMG-based designs: a validation study. Hum Brain Mapp. 2007;28:1117–1127. doi: 10.1002/hbm.20336. doi: 10.1002/hbm.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crooks R, Mitchell T, Thom M. Patterns of cerebellar atrophy in patients with chronic epilepsy: a quantitative neuropathological study. Epilepsy Res. 2000;41:63–73. doi: 10.1016/s0920-1211(00)00133-9. doi: 10.1016/S0920-1211(00)00133-9. [DOI] [PubMed] [Google Scholar]
- 86.Sobaniec-Lotowska ME. Ultrastructure of Purkinje cell perikarya and their dendritic processes in the rat cerebellar cortex in experimental encephalopathy induced by chronic application of valproate. Int J Exp Pathol. 2001;82:337–348. doi: 10.1046/j.1365-2613.2001.00206.x. doi: 10.1046/j.1365-2613.2001.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marcian V, Filip P, Bares M, Brazdil M. cerebellar dysfunction and ataxia in patients with epilepsy: coincidence, consequence, or cause? Tremor Other Hyperkinet Mov. 2016;6 doi: 10.7916/D8KH0NBT. doi: 10.7916/D8KH0NBT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brighina F, Daniele O, Piazza A, Giglia G, Fierro B. Hemispheric cerebellar rTMS to treat drug-resistant epilepsy: case reports. Neurosci Lett. 2006;397:229–233. doi: 10.1016/j.neulet.2005.12.050. doi: 10.1016/j.neulet.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 89.Phillips JR, Hewedi DH, Eissa AM, Moustafa AA. The cerebellum and psychiatric disorders. Front Public Health. 2015;3:66. doi: 10.3389/fpubh.2015.00066. doi: 10.3389/fpubh.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andersen BB, Gundersen HJ, Pakkenberg B. Aging of the human cerebellum: a stereological study. J Comp Neurol. 2003;466:356–365. doi: 10.1002/cne.10884. doi: 10.1002/cne.10884. [DOI] [PubMed] [Google Scholar]
- 91.Ganos C, Kassavetis P, Erro R, Edwards MJ, Rothwell J, Bhatia KP. The role of the cerebellum in the pathogenesis of cortical myoclonus. Mov Disord. 2014;29:437–443. doi: 10.1002/mds.25867. doi: 10.1002/mds.25867. [DOI] [PubMed] [Google Scholar]
- 92.Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62–69. doi: 10.1038/ng0197-62. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- 93.Huang Y, Xiao H, Qin X, Nong Y, Zou D, Wu Y. The genetic relationship between epilepsy and hemiplegic migraine. Neuropsychiatr Dis Treat. 2017;13:1175–1179. doi: 10.2147/NDT.S132451. doi: 10.2147/NDT.S132451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lascano AM, Korff CM, Picard F. Seizures and epilepsies due to channelopathies and neurotransmitter receptor dysfunction: a parallel between genetic and immune aspects. Mol Syndromol. 2016;7:197–209. doi: 10.1159/000447707. doi: 10.1159/000447707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lapin IP. Stimulant and convulsive effects of kynurenines injected into brain ventricles in mice. J Neural Transm. 1978;42:37–43. doi: 10.1007/BF01262727. doi: 10.1007/BF01262727. [DOI] [PubMed] [Google Scholar]
- 96.Joensuu T, Kuronen M, Alakurtti K, Tegelberg S, Hakala P, Aalto A, et al. Cystatin B: mutation detection, alternative splicing and expression in progressive myclonus epilepsy of Unverricht-Lundborg type (EPM1) patients. Eur J Hum Genet. 2007;15:185–193. doi: 10.1038/sj.ejhg.5201723. doi: 10.1038/sj.ejhg.5201723. [DOI] [PubMed] [Google Scholar]
- 97.Abel HJ, Duncavage EJ. Detection of structural DNA variation from next generation sequencing data: a review of informatic approaches. Cancer Genet. 2013;206:432–440. doi: 10.1016/j.cancergen.2013.11.002. doi: 10.1016/j.cancergen.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao W, Chen JJ, Perkins R, Wang Y, Liu Z, Hong H, et al. A novel procedure on next generation sequencing data analysis using text mining algorithm. BMC Bioinformatics. 2016;17:213. doi: 10.1186/s12859-016-1075-9. doi: 10.1186/s12859-016-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Okuma Y, Shimo Y, Shimura H, Hatorib K, Hattorib T, Tanakab S, et al. Familial cortical tremor with epilepsy: an under-recognized familial tremor. Clin Neurol Neurosurg. 1998;100:75–78. doi: 10.1016/s0303-8467(98)00003-1. doi: 10.1016/S0303-8467(98)00003-1. [DOI] [PubMed] [Google Scholar]
- 100.Terada K, Ikeda A, Mima T, Kimura K, Nagahama Y, Kamioka Y, et al. Familial cortical myoclonic tremor as a unique form of cortical reflex myoclonus. Mov Disord. 1997;12:370–377. doi: 10.1002/mds.870120316. doi: 10.1002/mds.870120316. [DOI] [PubMed] [Google Scholar]
- 101.Yokochi M, Takaoka S, Kawano H, Mori H, Shirai T, Imai H, et al. [A 77-year-old woman with myoclonus and epilepsy] No To Shinkei. 1993;45:1173–1185. [PubMed] [Google Scholar]
- 102.Okuma Y, Shimo Y, Hatori K, Hattori T, Tanaka S, Mizuno Y. Familial cortical tremor with epilepsy. Parkinsonism Relat Disord. 1997;3:83–87. doi: 10.1016/s1353-8020(97)00001-1. doi: 10.1016/S1353-8020(97)00001-1. [DOI] [PubMed] [Google Scholar]
- 103.Oguni E, Hayashi A, Ishii A, Mizusawa H, Shoji S. A case of cortical tremor as a variant of cortical reflex myoclonus. Eur Neurol. 1995;35:63–64. doi: 10.1159/000117093. doi: 10.1159/000117093. [DOI] [PubMed] [Google Scholar]
- 104.Nagayama S, Kishikawa H, Yukitake M, Matsui M, Kuroda Y. [A case of familial myoclonus showing extremely benign clinical course] Rinsho Shinkeigaku. 1998;38:430–434. [PubMed] [Google Scholar]