Abstract
The use of low-intensity pulsed ultrasound (LIPUS) is a promising approach to promote osteogenesis. However, few studies have reported the influence of this technique on the osseointegration of endosseous implants, especially regarding different implant topographies. We focused on how the initial interaction between cells and the titanium surface is enhanced by LIPUS and the potential regulatory mechanisms. The bone marrow mesenchymal stem cells (BMSCs) of rats were cultured on two types of titanium surfaces (polished surface, Flat and large grain blast acid etched, SLA) under LIPUS stimulation or control conditions. The cell proliferation on the implant surfaces was significantly promoted by LIPUS, which stimulated the increase in the number of microfilaments, pseudopodia formed and extracellular matrix mineralization nodules compared with those in the control group. The expression of osteogenesis-related genes, including OPN, OCN, BMP-2, ALP, Runx2 and Col-1, were up-regulated on all the surfaces by LIPUS stimulation. Our findings suggest that LIPUS enhances osteoblast differentiation from BMSCs on titanium surfaces. The use of LIPUS might be a potential adjuvant treatment to improve the osseointegration process.
Keywords: Low-intensity pulsed ultrasound, bone marrow mesenchymal stem cells, osseointegration, implant topography
Introduction
Implant dentures can effectively restore the oral function in patients with the absence of dentition. With the development of technology, the achievement ratio of implant dentures was greatly improved; however, due to deficiency or low-quality of bone mass, the failure of implantation still occurs [1]. Recently, many studies have shown that rapid and successful osseointegration is a critical factor necessary for implant fixation [2,3]. Implant surface modifications, drug delivery systems and biophysical stimulation have been considered to achieve better osseointegration [4,5]. Because of a non-invasive and locally applied strategy, biophysical stimulation is the research topic of interest to enhance bone regeneration around implants among these methods.
Low-intensity pulsed ultrasound (LIPUS) is a type of high-frequency sound wave that could be transmitted in vivo through mechanical vibration [6-8]. It has been demonstrated to have beneficial therapeutic effects on a variety of bone- and cartilage-related disorders [8-11]. Recently, many animal and clinical studies have shown that the frequency of 0.5-1.5 MHz, intensity of 30-200 mW/m2 ultrasound intervention will promote bone healing, deposition and growth [12]. Although considerable research on the osteogenic effects of LIPUS has been reported, few studies have evaluated the osteogenic effects of LIPUS on titanium implant surfaces or the underlying mechanism.
It is known to all that bone marrow mesenchymal stem cells (BMSCs) had the potentiality of multi-directional differentiation. To explore whether the LIPUS can promote BMSC differentiation toward osteogenesis, primary BMSC culture under no stimulation (Control) and LIPUS stimulation were evaluated on implant surfaces in this work. Two representative implant surface topographies, SLA and Flat, were used as substrates. The proliferation and differentiation of BMSCs, observation of the microfilaments, pseudopodia formed, the number of mineralization nodules with or without LIPUS stimulation were investigated. Furthermore, to define the initial molecular mechanism associated with BMSC differentiation activated by LIPUS, the expression profile of alkaline phosphatase (ALP), osteocalcin (OCN), type 1 collagen (COL-1), bone morphogenetic protein-2 (BMP-2) and runt-related transcription factor 2 (Runx2) were also investigated.
Materials and methods
Cell culture
The animal procedures were approved by the Ethics Committee of the Jinan Military General Hospital. Briefly, BMSCs were obtained by digesting the marrow cavity of Wistar rats that weighed between 70 and 80 g [13,14]. The complete BMSCs were cultured in low-glucose DMEM supplemented with 10% bovine calf serum (BCS, Gibco) and antibiotics (penicillin, 100 U/ml; streptomycin, 100 µg/ml; Sigma). Cells were grown in a humidified atmosphere with 5% CO2 and 95% air at 37°C. Cells from passage 3 were used.
Specimen preparation
Pure titanium was cut into 10 mm × 10 mm × 1.0 mm and 20 mm × 20 mm × 1.0 mm samples through machining. Next, the specimens were polished using 200 #, 400 #, 600 #, 800 #, 1000 #, 1200 # and 1500 # grit of silicon carbide sandpaper. After polishing and cleaning ultrasonically, the substrates were dried in hot air. To prepare sand-blasted SLA surfaces, the substrates were alumina-blasted with large-grit particles (an average grit size of 250 μm) and then acid-etched using a hot solution of HCl/H2SO4 following the proprietary process of Institute Straumann AG [15]. The morphologies of the two surfaces were examined by field-emission scanning electron microscopy (FE-SEM; JEOL JSM-4800, Hitachi Corporation, Japan). All specimens were sterilized by ultraviolet irradiation for 30 min before use.
Cell proliferation
A 1-ml aliquot of the cell suspension was seeded onto each specimen at a density of 2 × 104 cells/ml and then was cultured in DMEM with 10% BCS. After culturing for 1, 3 and 5 days, the level of cell proliferation was assessed using the CCK-8 Detection Kit (Beyotime Institute of Biotechnology) according to the manufacturer’s instructions. The specimens were then incubated with CCK-8 solution at 37°C for 3 h. The optical density (OD) was measured at 450 nm using a spectrophotometer (Bio-Tek). The LIPUS parameters were as follows: intensity: 100 mW/cm2, frequency: 1.0 MHz, duty ratio: 10%, time: 10 min. In the control group, the 24-well cell culture plate was also placed in another solenoid without stimulation.
Cell morphology
FE-SEM
The titanium specimens were divided into four groups-, Flat group, Flat/LIPUS group, SLA group, SLA/LIPUS group. The suspension of BMSCs was seeded onto each specimen at a density of 1 × 104 cells/ml. After culturing in DMEM with 10% BCS for 24 h, the samples were fixed with glutaraldehyde solution for 12 h at 4°C, rinsed with PBS three times and dehydrated in a series of acetonitrile washes (50%, 70%, 80%, 90% and 100%). All samples were dried to the critical point, coated with gold and examined using SEM (Hitachi JSM-4800).
CLSM
Confocal laser scanning microscopy (CLSM; Olympus FV 1000) was used to examine the cell morphology and cytoskeletal arrangement of the BMSCs seeded onto the titanium surfaces. After culturing for 24 h, the specimens were stimulated by LIPUS (intensity: 100 mW/cm2, frequency: 1.0 MHz, duty ratio: 10%, time: 10 min). Cells were fixed in glutaraldehyde solution for 20 min and then were permeabilized with 0.1% Triton X-100. The cells were blocked with VCL (1:100, red fluorescence; Abcam) overnight at 4°C in the dark. Next, the cells were blocked with FITC (1:100, green fluorescence; Sigma) for 1 h. DAPI (1:100, blue fluorescence; Sigma) was used to stain the nuclei for 5 min. The cells were analyzed using CLSM.
Alkaline phosphatase activity assay
Cells were seeded at the density of 2 × 104/ml on titanium specimens and were cultured for 3 d and 7 d, followed by centrifugation at 1000 r/min for 10 min. The reagent was added according to the specification of the alkaline phosphatase assay kit, and a microplate reader measured the OD value at 520 nm. ALP activity was calculated according to the following formula: Activity of alkaline phosphatase (Kim/100 ml) = (OD - blank OD/standard OD - blank OD) × standard phenol concentration (0.02 mg/ml) × 100 ml × samples before dilution.
Alkaline phosphatase and Alizarin red staining
Cells were seeded at the density 2 × 104/ml on titanium specimens and were cultured for 14 d. Ultrasound treatment was performed as previously described, and then the cells were fixed with 4% paraformaldehyde for 20 min. ALP staining was performed using BCIP/NBT solution or 0.1% Alizarin red solution according to the manufacturer’s instructions, followed by observation under a stereoscopic microscope.
Western blot analysis
After culturing for 14 days and 21 days, the protein samples were solubilized in RIPA (Beyotime) containing protease inhibitors (Roche, Switzerland). The concentration of the protein was determined using the BCA kit, and western blot analysis was performed as previously described [16]. Primary antibodies against OPN (1:500, Abcam), Runx2 (1:1000, Abcam), BMP-2 (1:1000, CST) were purchased from Abcam or CST. An antibody against β-actin (Sigma) was used as an internal control.
Osteogenesis-related gene expression
The expression levels of osteogenesis-related genes were evaluated using real-time PCR. The cells were seeded at 2 × 104 cells per well. After 14 days’ and 21 days’ incubation, total RNA was isolated using Trizol reagent (TaKaRa), and RNA was reverse transcribed to cDNA using the PrimeScript RT reagent kit (TaKaRa RR037A). Osteogenesis-related genes, including OCN, OPN, BMP-2, ALP, COL-1 and Runx2, were analyzed by Applied Biosystems 7500 using SYBR Premix Ex Taq IITAKARA (RR820A). The data were analyzed using the iQ 5 optical system software, version 2.0 (Bio-Rad). The relative expression level of each gene of interest was normalized to that of the housekeeping gene GAPDH.
Statistical analysis
The data were analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). One-way ANOVA followed by an SNK post hoc test and paired t test was used to determine the level of significance. p<0.05 was considered significant, and p<0.01 was considered highly significant.
Results
SEM characterization
The surface morphologies of samples were examined by SEM (Figure 1). Residual parallel grooves were observed on the flat samples (Figure 1A), and SLA samples exhibited micropitted morphology. SLA treatment caused the surface to become irregular and induced the formation of micropits (Figure 1B).
Figure 1.
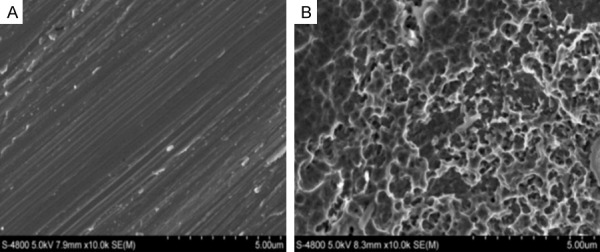
SEM images at 10,000 × showing the microscale topography. A. Flat. B. SLA.
Cell proliferation
According to the results of screening, LIPUS significantly increased cell proliferation on both Flat flat and SLA surfaces (Figure 2). On day 1, no significant difference was found between the LIPUS and Control groups regarding the SLA specimens. However, the cell viabilities on all surfaces in the LIPUS-stimulated group were obviously higher than those for the non-stimulated group on days 3 and 5.
Figure 2.
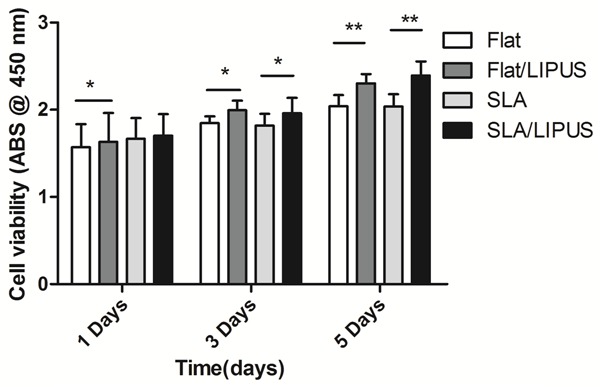
BMSC proliferation after incubation for 1, 3 and 5 days (*P<0.05, **P<0.01).
Cell morphology
SEM
The SEM images revealed that the BMSCs generated more pseudopodia on the titanium surfaces under LIUPS stimulation (Figure 3). Most of the BMSCs on the Flat and SLA specimens exhibited a spindle fibroblast-like shape and spread completely after LIPUS stimulation.
Figure 3.
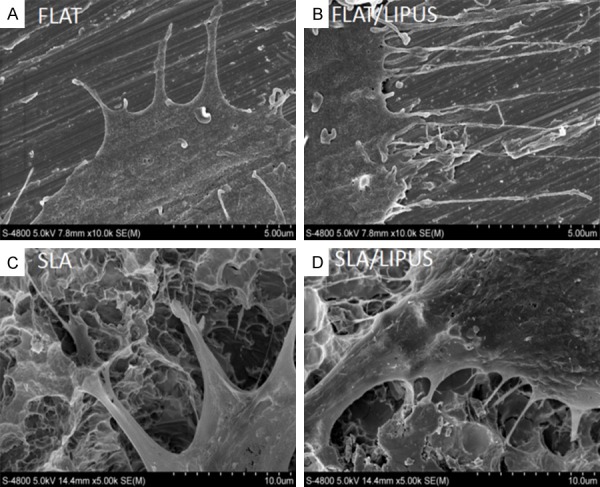
SEM images of BMSC adhesion on two surfaces after 48 h. A. Flat/Control. B. Flat/LIPUS. C. SLA/Control. D. SLA/LIPUS. The images were taken at high magnification (5000 ×) to display the detail of cells adhesion on Flat and Micro surfaces.
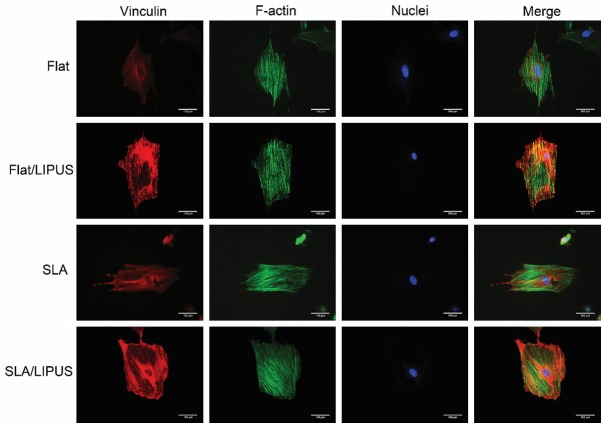
CLSM
The confocal microscopy images show that BMSCs that were stained with vinculin and F-actin to label the cytoskeleton (Figure 4). LIPUS stimulation increased the expression of vinculin on all two surfaces, and the expression in the Flat/LIPUS group was increased significantly. More microfilaments were observed in the SLA/LIPUS group. By contrast, most of the cells in the control group did not exhibit a well-developed cytoskeleton. Additionally, there was no obviously difference between the two surface topographies in affecting cytoskeletal morphology.
Figure 4.
Representative CLSM images of cells stained with red fluorescence to show vinculin, with DAPI to show the nuclei (blue), with F-action to show the actin filaments (green) and with VCL to show the vinculin (red).
ALP activity
As shown in the quantitative experiment in Figure 5A, LIPUS stimulation significantly increased the level of ALP activity only on the SLA surface after 3 days of culture (P<0.01); however, after 7 days of culture, the level of ALP activity was increased on both the flat and SLA surfaces (P<0.05). The results of ALP staining showed that, compared with the control group, LIPUS stimulation led to slightly higher ALP activity on all two surfaces after 2 weeks of osteogenic induction (Figure 5B).
Figure 5.
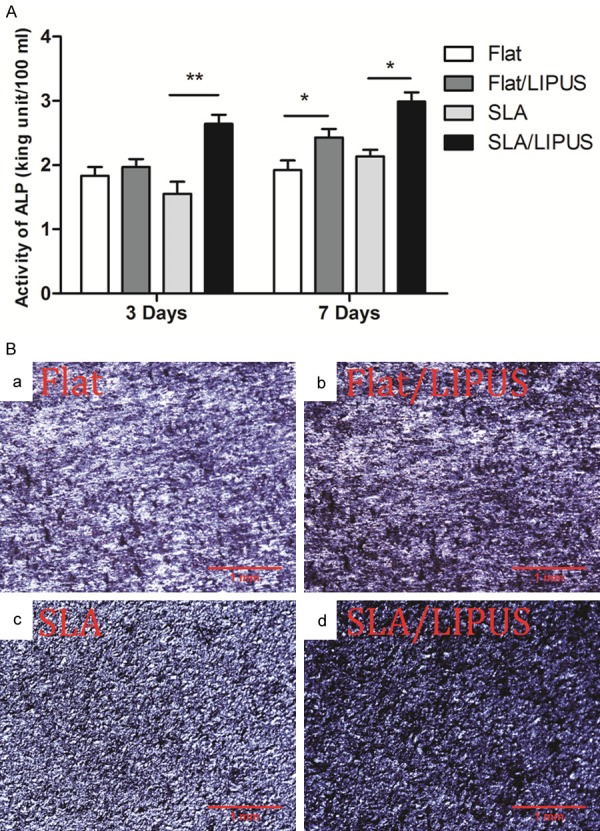
A. ALP activity in different groups cultured for 3 or 7 days. *P<0.05, **P<0.01. B. Optical images of ALP-stained osteoblasts after 2 weeks of incubation.
ECM mineralization
ECM mineralization was assayed by Alizarin red staining (Figure 6). The applied LIPUS led to more mineralized nodules on both Flat and SLA surfaces. In the control group, no obvious difference in matrix mineralization was observed between the Flat and SLA surfaces.
Figure 6.
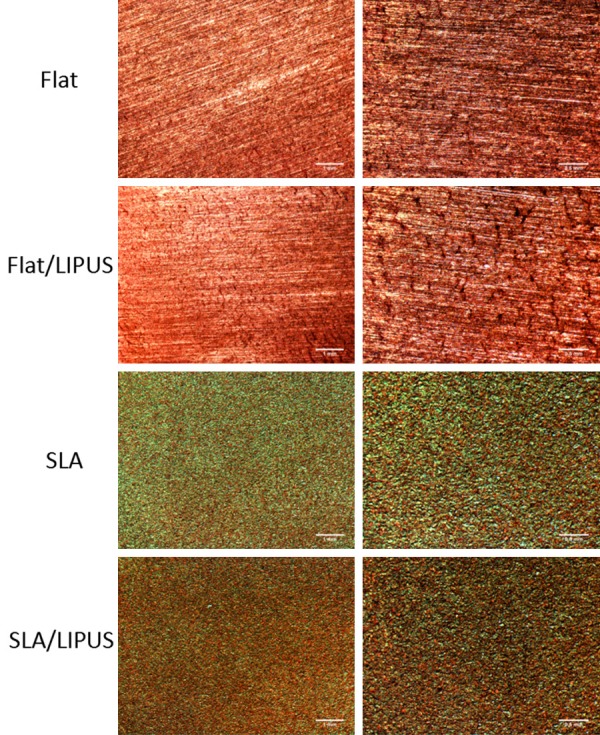
Analysis of ECM mineralization after 3 weeks of incubation. Images taken at low magnification (Bar = 1 mm) and high magnification (Bar = 0.5 mm).
Osteogenesis-related gene expression
The expression levels of osteogenesis-related genes, including OPN, OCN, BMP-2, ALP, Col-1, and Runx2, are presented in Figure 7. Generally, LIPUS stimulation up-regulated the mRNA levels of all osteogenesis-related genes. The expression of OPN was up-regulated slightly after LIPUS stimulation at day 14 (P<0.05) but was significantly up-regulated at day 21 (P<0.01, Figure 7A). Col1 was most responsive to the applied LIPUS, with maximum increases in expression of 5-fold (Flat/LIPUS) at day 14, and 7-fold (Flat/LIPUS) at day 21, over the corresponding controls in the control group (Figure 7E). The expression of OCN was up-regulated by 3-fold (SLA/LIPUS) after LIPUS stimulation at day 14 (Figure 7B). BMP-2 was also up-regulated by LIPUS, with increases of 1.8-fold (SLA/LIPUS) at day 14 (Figure 7C); however, there was no difference between day 14 and day 21. The expression of ALP was up-regulated in both Flat and SLA group after LIPUS stimulation at day 14 but was decreased after day 21 (Figure 7D). Runx2 was also up-regulated by LIPUS in both Flat and SLA groups (Figure 7F).
Figure 7.
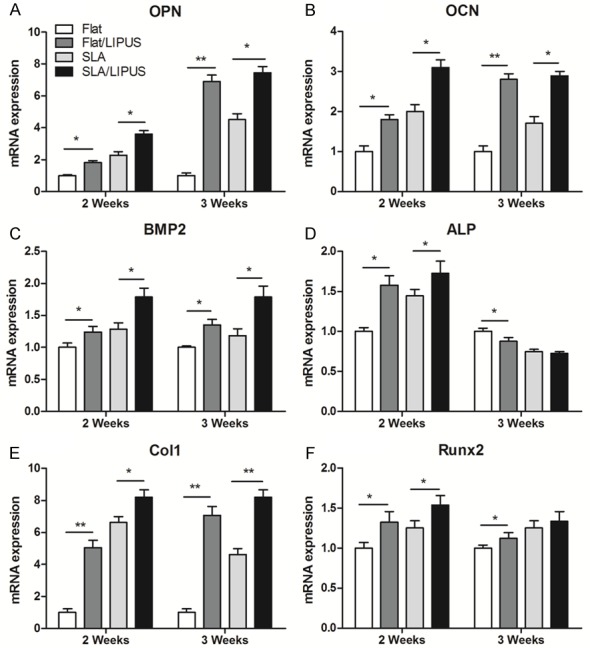
Relative expression levels of (A) OPN, (B) OCN, (C) BMP2, (D) ALP, (E) Col1 and (F) Runx2. *P<0.05, **P<0.01.
Osteogenesis-related protein expression
The expression levels of osteogenesis-related proteins, including OPN, BMP-2 and Runx2, are presented in Figure 8. Similar to the gene expression levels, LIPUS stimulation up-regulated the protein levels of all samples. The expression of OPN was up-regulated slightly after LIPUS stimulation at day 14 but was significantly increased at day 21. BMP2 was most responsive to the applied LIPUS, with maximum increases in expression at day 21 over the corresponding controls in the control group. Runx2 was also slightly up-regulated by LIPUS at day 14; however, its expression showed no difference between day 14 and day 21.
Figure 8.
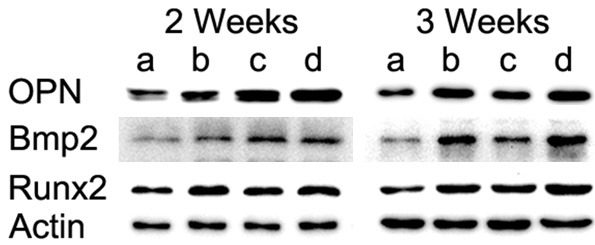
Expression of OPN, BMP2 and Runx2 after osteogenic induction for 2 and 3 weeks.
Discussion
As a safe and non-invasive physical technique, LIPUS has been used in the delayed healing of bone fracture by the FDA [17]. LIPUS has been shown to exert strong effects on bone repair processes through many mechanisms, including stimulating calcification, increasing the blood supply, inhibiting the resorptive phase and increasing the activity of osteoblasts [18-20]. Recently, several studies have demonstrated the positive effects of LIPUS on implants; however, most of them were observation studies in animals [21,22]. Few studies have reported the effects of LIPUS on the functions of osteoblasts when cultured on titanium implants, especially on Flat or SLA surfaces.
BMSCs play an important role in the progress of bone remodeling. Because of the potential of multi-directional differentiation, BMSCs are regarded as the very important cell in bone healing and regeneration [23]. Several studies have shown that LIPUS could up-regulate the expression of bio-markers of ossification and matrix protein in BMSCs, further increasing the number of osteoblasts [24,25]. However, BMSCs are selected as the target cells, and the aim of this study was to evaluate the effects of LIPUS on the functions of BMSCs growing on implant surfaces with different topographies and to understand whether this physical stimulation could make the titanium implant more active for osseointegration in the dental and orthopedic fields.
First, BMSCs are separated from rats, and their character was identified, indicating they were obtained successfully. Second, according to our previous experience [15], two topographies of implants were prepared to use for subsequent detection. Additionally, the optimal parameters of LIPUS stimulation were screened through cell proliferation experiment: intensity: 100 mW/cm2, frequency: 1.0 MHz, duty ratio: 10%, and time: 10 min. Our results demonstrate that, compared with the control group, LIPUS stimulation promoted BMSC activity in all of the tested surfaces (Flat and SLA). The microscopic results indicated that the cells exhibited dramatic microstructures and orientations after LIPUS stimulation. The SEM results showed that increased filipodia were attached to the surfaces in the LIPUS stimulation group. These results were consistent with those of previous studies [26]. However, this stimulation did not affect the pseudopodia form of BMSCs. In the CLSM experiment, more intracellular microfilaments were observed. By contrast, most cells in the control group did not exhibit well-developed cytoskeletons. Moreover, increased expression of VCL by CSLM also proved that LIPUS promoted cell adhesion and extension in both implants on implant topographies in this study.
ALP is presented in the prophase of ossification. Increased expression of ALP in BMSCs is represented as well differentiated osteoblasts. Thus, the activity of ALP in vitro is considered the bio-marker of osteoblast differentiation [27,28]. Our study showed that LIPUS stimulation significantly promotes ALP activity, especially on day 14. However, the expression of ALP is reduced on day 21. Due to the expression of hydroxyapatite, osteocalcin and other genes in the bone mineralization, the activity of ALP was reduced. Additionally, the results of Alizarin red staining showed that more mineralization nodules were observed in the LIPUS stimulation group on day 21, corresponding to the activity of ALP. These results demonstrate that LIPUS can promote the differentiation of BMSCs toward osteoblasts, a finding that was with that in other studies [29-31]. The ALP activity did not differ significantly on the Flat and SLA surfaces between the LIPUS and control groups, but LIPUS stimulation significantly promoted the formation of ECM nodules on the SLA surface. This result is consistent with the findings of previous studies using cell culture plates. Thus, the consensus is that LIPUS exerts a reproducible osteogenic effect.
Regarding the expression of genes associated with osteogenesis, the present study showed that OCN, OPN, ALP, BMP-2, Col-1, and Runx2 were up-regulated in cells on the implant surfaces after LIPUS stimulation. As non-collagen proteins, OCN and OPN were the markers of mature osteoblasts. OPN plays an important role in cell adhesion and mineralization [32,33]. We found that the expression levels of OCN and OPN were significantly increased by LIPUS stimulation, especially at day 21. The same results were also found by western blotting. It has been established that BMPs affect many stages of endochondral bone formation and mediate the recruitment of cells to repair sites through chemotaxis [34,35]. Col-1 is another important marker of osteoblast differentiation. The up-regulation of Col-1 transcription might account for the increased ECM formation by LIPUS. BMP2 is a very important member of the TGF-β superfamily, which regulates Runx2 by activating the BMP/Smad pathway [36,37]. Runx2 is essential for the differentiation of osteoblasts from mesenchymal precursors and the formation of mineralized bone [38,39]. Taking the above into consideration, we speculated that the BMP2 signaling pathway may play an important role in promoting osteoblast functions under LIPUS stimulation. However, to verify the speculation, more work should be performed to elucidate the process between LIPUS stimulation and conformational changes in biomolecules.
It is well known that implant surface morphology affects the attachment, proliferation and differentiation of osteoblasts and osteogenesis-related gene expression [40]. Our results showed that there was no significant difference between Flat and SLA surfaces, but the SLA surface exhibited greater cell proliferation, ECM mineralization and COL-1 gene expression than the Flat surface. The cause may be that the micro-structure increased the surface area of SLA. This result is also consistent with the findings of previous studies [41].
Although we attempted to control other factors that would affect the experiment, some limitations must be considered when interpreting our results. For example, we analyzed ECM mineralization only at day 21. That observation time point is not sufficient to draw the time variation curve of ECM mineralization. Importantly, LIPUS only induces physiological effects within certain parameter windows, but the mechanism remains unclear. Further studies will be required to elucidate the interaction between surface topography and LIPUS stimulation and how the mechanical irritation translates into biochemical signals.
Conclusions
LIPUS can have beneficial effects on the functions of BMSCs on implant surfaces with different topographies (Flat and SLA). This stimulation significantly increased cell proliferation and filipodia on the implant surfaces, resulting in the formation of more intracellular microfilaments. LIPUS can accelerate BMSC proliferation and differentiation on implant surfaces by up-regulating the expression of relevant genes. The results of the study suggest that LIPUS could be used as a potential adjuvant treatment to improve the osseointegration of titanium implants.
Acknowledgements
This study was supported by the Natural Science Foundation of Shandong Province of China (ZR2014HM041) and Natural Science Foundation of China (61471384, 81400573, 6170012037, 81602651). The authors are deeply thankful to Dr. Fabin Han of Liaocheng People’s Hospital of China (Liaocheng, China) for his earnest guidance.
Disclosure of conflict of interest
None.
References
- 1.Korabi R, Shemtov-Yona K, Dorogoy A, Rittel D. The failure envelope concept applied to the bone-dental implant system. Sci Rep. 2017;7:2051. doi: 10.1038/s41598-017-02282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chrcanovic BR, Albrektsson T, Wennerberg A. Bone quality and quantity and dental implant failure: a systematic review and meta-analysis. Int J Prosthodont. 2017;30:219–237. doi: 10.11607/ijp.5142. [DOI] [PubMed] [Google Scholar]
- 3.Yuan Q, Xiong QC, Gupta M, Lopez-Pintor RM, Chen XL, Seriwatanachai D, Densmore M, Man Y, Gong P. Dental implant treatment for renal failure patients on dialysis: a clinical guideline. Int J Oral Sci. 2017;9:125–132. doi: 10.1038/ijos.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng HW, Chien EY, Chien HH. Dental implant bioactive surface modifications and their effects on osseointegration: a review. Biomark Res. 2016;4:24. doi: 10.1186/s40364-016-0078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ustun Y, Erdogan O, Kurkcu M, Akova T, Damlar I. Effects of low-intensity pulsed ultrasound on dental implant osseointegration: a preliminary report. Eur J Dent. 2008;2:254–262. [PMC free article] [PubMed] [Google Scholar]
- 6.Liao JC, Chen WJ, Chen LH, Lai PL, Keorochana G. Low-intensity pulsed ultrasound enhances healing of laminectomy chip bone grafts on spinal fusion: a model of posterolateral intertransverse fusion in rabbits. J Trauma. 2011;70:863–869. doi: 10.1097/TA.0b013e3181e7c13d. [DOI] [PubMed] [Google Scholar]
- 7.Malizos KN, Hantes ME, Protopappas V, Papachristos A. Low-intensity pulsed ultrasound for bone healing: an overview. Injury. 2006;37(Suppl 1):S56–62. doi: 10.1016/j.injury.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 8.Romano CL, Romano D, Logoluso N. Low-intensity pulsed ultrasound for the treatment of bone delayed union or nonunion: a review. Ultrasound Med Biol. 2009;35:529–536. doi: 10.1016/j.ultrasmedbio.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Massari L, Caruso G, Sollazzo V, Setti S. Pulsed electromagnetic fields and low intensity pulsed ultrasound in bone tissue. Clin Cases Miner Bone Metab. 2009;6:149–154. [PMC free article] [PubMed] [Google Scholar]
- 10.Rothenberg J, Jayaram P, Naqvi U, Gober J, Malanga GA. The role of low-intensity pulsed ultrasound on cartilage healing in knee osteoarthritis: a review. PM R. 2017;9:1268–1277. doi: 10.1016/j.pmrj.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Jia XL, Chen WZ, Zhou K, Wang ZB. Effects of low-intensity pulsed ultrasound in repairing injured articular cartilage. Chin J Traumatol. 2005;8:175–178. [PubMed] [Google Scholar]
- 12.Pounder NM, Harrison AJ. Low intensity pulsed ultrasound for fracture healing: a review of the clinical evidence and the associated biological mechanism of action. Ultrasonics. 2008;48:330–338. doi: 10.1016/j.ultras.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Ross CL, Siriwardane M, Almeida-Porada G, Porada CD, Brink P, Christ GJ, Harrison BS. The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Res. 2015;15:96–108. doi: 10.1016/j.scr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derubeis AR, Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32:160–165. doi: 10.1023/b:abme.0000007800.89194.95. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, An Y, Li F, Li D, Jing D, Guo T, Luo E, Ma C. The effects of pulsed electromagnetic field on the functions of osteoblasts on implant surfaces with different topographies. Acta Biomater. 2014;10:975–985. doi: 10.1016/j.actbio.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Wu Q, Yang Z, An Y, Hu H, Yin J, Zhang P, Nie Y, Wu K, Shi Y, Fan D. MiR-19a/b modulate the metastasis of gastric cancer cells by targeting the tumour suppressor MXD1. Cell Death Dis. 2014;5:e1144. doi: 10.1038/cddis.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanna A, Nelmes RT, Gougoulias N, Maffulli N, Gray J. The effects of LIPUS on soft-tissue healing: a review of literature. Br Med Bull. 2009;89:169–182. doi: 10.1093/bmb/ldn040. [DOI] [PubMed] [Google Scholar]
- 18.Poolman RW, Agoritsas T, Siemieniuk RA, Harris IA, Schipper IB, Mollon B, Smith M, Albin A, Nador S, Sasges W, Schandelmaier S, Lytvyn L, Kuijpers T, van Beers LW, Verhofstad MH, Vandvik PO. Low intensity pulsed ultrasound (LIPUS) for bone healing: a clinical practice guideline. BMJ. 2017;356:j576. doi: 10.1136/bmj.j576. [DOI] [PubMed] [Google Scholar]
- 19.Tan L, Ren Y, van Kooten TG, Grijpma DW, Kuijer R. Low-intensity pulsed ultrasound (LIPUS) and pulsed electromagnetic field (PEMF) treatments affect degeneration of cultured articular cartilage explants. Int Orthop. 2015;39:549–557. doi: 10.1007/s00264-014-2542-4. [DOI] [PubMed] [Google Scholar]
- 20.Harrison A, Lin S, Pounder N, Mikuni-Takagaki Y. Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair. Ultrasonics. 2016;70:45–52. doi: 10.1016/j.ultras.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Wang J, Yoshinori A, Paul F, Shen H, Chen J, Sotome S, Liu Z, Shinomiya K. Low-intensity pulsed ultrasound prompts tissue-engineered bone formation after implantation surgery. Chin Med J (Engl) 2014;127:669–674. [PubMed] [Google Scholar]
- 22.Zhou HB, Hou YF, Chen WC, Shen JF, Wang J, Zhu ZM. [The acceleration of titanium implant osseointegration by low intensity pulsed ultrasound: an experimental study in rats] . Zhonghua Kou Qiang Yi Xue Za Zhi. 2011;46:425–430. [PubMed] [Google Scholar]
- 23.Xu L, Lv K, Zhang W, Zhang X, Jiang X, Zhang F. The healing of critical-size calvarial bone defects in rat with rhPDGF-BB, BMSCs, and beta-TCP scaffolds. J Mater Sci Mater Med. 2012;23:1073–1084. doi: 10.1007/s10856-012-4558-x. [DOI] [PubMed] [Google Scholar]
- 24.Zhi Z, Na T, Jue W, Zhihe Z, Lijun T. [Effects of pulsed ultrasound and pulsed electromagnetic field on the extracellular matrix secretion of rat bone marrow mesenchymal stem cell pellets in chondrogenesis] . Hua Xi Kou Qiang Yi Xue Za Zhi. 2016;34:291–294. doi: 10.7518/hxkq.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapi E, Bozkurt M, Selcuk CT, Celik MS, Akpolat V, Isik FB, Bozarslan BH, Celik Y. Comparison of effects of pulsed electromagnetic field stimulation on platelet-rich plasma and bone marrow stromal stem cell using rat zygomatic bone defect model. Ann Plast Surg. 2015;75:565–571. doi: 10.1097/SAP.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 26.Guo G, Ma Y, Guo Y, Zhang C, Guo X, Tu J, Yu ACH, Wu J, Zhang D. Enhanced porosity and permeability of three-dimensional alginate scaffolds via acoustic microstreaming induced by low-intensity pulsed ultrasound. Ultrason Sonochem. 2017;37:279–285. doi: 10.1016/j.ultsonch.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Sodek J, McKee MD. Molecular and cellular biology of alveolar bone. Periodontol 2000. 2000;24:99–126. doi: 10.1034/j.1600-0757.2000.2240106.x. [DOI] [PubMed] [Google Scholar]
- 28.Kassem MS, Mosekilde L, Eriksen EF. [Physiopathology behind age-related bone loss. New insight in the mechanisms of cellular and molecular biology] . Ugeskr Laeger. 1999;161:5663–5666. [PubMed] [Google Scholar]
- 29.Ganzorig K, Kuroda S, Maeda Y, Mansjur K, Sato M, Nagata K, Tanaka E. Low-intensity pulsed ultrasound enhances bone formation around miniscrew implants. Arch Oral Biol. 2015;60:902–910. doi: 10.1016/j.archoralbio.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Gleizal A, Ferreira S, Lavandier B, Simon B, Beziat JL, Bera JC. [The impact of low intensity pulsed ultrasound on mouse skull bone osteoblast cultures] . Rev Stomatol Chir Maxillofac. 2010;111:280–285. doi: 10.1016/j.stomax.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Warden SJ, Favaloro JM, Bennell KL, McMeeken JM, Ng KW, Zajac JD, Wark JD. Low-intensity pulsed ultrasound stimulates a bone-forming response in UMR-106 cells. Biochem Biophys Res Commun. 2001;286:443–450. doi: 10.1006/bbrc.2001.5412. [DOI] [PubMed] [Google Scholar]
- 32.Musial K, Fornalczyk K, Zwolinska D. [Osteopontin (OPN), PDGF-BB (platelet-derived growth factor) and BMP-7 (bone morphogenetic protein) as markers of atherogenesis in children with chronic kidney disease (CKD) treated conservatively--preliminary results] . Pol Merkur Lekarski. 2008;24(Suppl 4):25–27. [PubMed] [Google Scholar]
- 33.Devoll RE, Pinero GJ, Appelbaum ER, Dul E, Troncoso P, Butler WT, Farach-Carson MC. Improved immunohistochemical staining of osteopontin (OPN) in paraffin-embedded archival bone specimens following antigen retrieval: anti-human OPN antibody recognizes multiple molecular forms. Calcif Tissue Int. 1997;60:380–386. doi: 10.1007/s002239900247. [DOI] [PubMed] [Google Scholar]
- 34.Kamiya N, Mishina Y. New insights on the roles of BMP signaling in bone-A review of recent mouse genetic studies. Biofactors. 2011;37:75–82. doi: 10.1002/biof.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zellin G, Hedner E, Linde A. Bone regeneration by a combination of osteopromotive membranes with different BMP preparations: a review. Connect Tissue Res. 1996;35:279–284. doi: 10.3109/03008209609029202. [DOI] [PubMed] [Google Scholar]
- 36.Delecrin J, Deschamp C. [Contribution of animal experiments in preclinical evaluation of BMP. Review of the literature] . Rev Chir Orthop Reparatrice Appar Mot. 2000;86(Suppl 1):153–154. [PubMed] [Google Scholar]
- 37.Hopkins CR. Inhibitors of the bone morphogenetic protein (BMP) signaling pathway: a patent review (2008-2015) Expert Opin Ther Pat. 2016:1–14. doi: 10.1080/13543776.2016.1217330. [DOI] [PubMed] [Google Scholar]
- 38.Salingcarnboriboon R, Tsuji K, Komori T, Nakashima K, Ezura Y, Noda M. Runx2 is a target of mechanical unloading to alter osteoblastic activity and bone formation in vivo. Endocrinology. 2006;147:2296–2305. doi: 10.1210/en.2005-1020. [DOI] [PubMed] [Google Scholar]
- 39.Lian JB, Stein GS. Runx2/Cbfa1: a multifunctional regulator of bone formation. Curr Pharm Des. 2003;9:2677–2685. doi: 10.2174/1381612033453659. [DOI] [PubMed] [Google Scholar]
- 40.Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17:137–146. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 41.Raines AL, Olivares-Navarrete R, Wieland M, Cochran DL, Schwartz Z, Boyan BD. Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy. Biomaterials. 2010;31:4909–4917. doi: 10.1016/j.biomaterials.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]