Abstract
Objective: Uighur medicine Abnormal Savda Munziq (ASMq) shows cardioprotective effect in myocardial ischemia/reperfusion injury (IRI) in animal models, but the molecular mechanism of this effect is not clear. The present study investigates the regulation of nuclear factor kappa B (NF-kappa B) pathway by ASMq in IRI rat models. Methods: Male Sprague-Dawley rats were divided into three groups: the NF-κB gene knockout group (n = 5); the NF-κB transgenic group (n = 8); and the control group (n = 5). All rats were treated with ASMq for 21 days before underwent IRI surgical procedure. Blood and tissue samples were collected for the RT-PCR, ELISA, western blot and other examination. Results: Expression of NF-κB p65mRNA and protein were down-regulated in the NF-κB knockout rats but up-regulated in the transgenic rats comparing with the controls (P<0.05). The upstream NF-κB kinase expressions, the downstream inflammatory cytokines, and the myocardial injury markers were all changed in accordance with the NF-κB gene modification (all P values <0.05). AMSq treatment relieved IRI in the NF-κB knockout rats. Conclusion: Inhibition on NF-κB signaling pathway may alleviate IRI in rats under ASMq treatment.
Keywords: Ischemic reperfusion injury, NF-κB pathway, abnormal savda munziq, treatment, pro-inflammatory cytokines, rat
Introduction
Myocardial ischemia/reperfusion injury (IRI) is the tissue damage caused by no blood flow to the heart (ischemia) and the blood reperfusion itself [1]. In patients with myocardial infarction, acute myocardial ischemic injury is treated by timely and effective myocardial reperfusion using either thrombolytic therapy or primary percutaneous coronary intervention [2]. However, the process of myocardial reperfusion itself can independently induce cardiomyocyte death [3-5]. Animal model of acute myocardial infarction suggests that myocardial IRI accounts for up to 50% of the myocardial infarct final size [1]. IRI is a main contributor to adverse outcomes after cardiac surgery or circulatory arrest. A couple of factors act in concert to mediate the detrimental effect of IRI, such as oxidative stress, intracellular Ca2+ overload, the rapid restoration of physiological pH at the time of reperfusion, and, inflammation [2]. More recently, inflammation has become a therapeutic target of lethal reperfusion injury and the results are controversial.
Abnormal Savda Munziq (ASMq) is a herbal formula in Uighur medicine, and mainly consists of stokesia laevis, hyssopus, lavender, adiantaceae, fumaria officinalis, jujube, and alhagi pseudoalhagi desv. The ASMq exhibits anti-oxidant, anti-inflammatory, and anti-thrombosis effect and has been widely used in treating gastrointestinal cancer, diabetes, cardiovascular diseases or chronic asthma [6-11]. Our previous studies demonstrate that ASMq has cardioprotective effect in abnormal savda animal model, and that ASMq dose-dependently improves the pathological changes in myocardial fibers and the general condition in rats [12,13]. Although the anti-inflammation effect and IRI protection of ASMq have been observed in animals, the molecular mechanism of these effects is not clear yet.
Nuclear factor kappa B (NF-κB) is a transcription factor that is composed of five subunits (p50/p105, p52/p100, p65 (RelA), RelB and c-Rel) and can regulate downstream gene expression [14]. The effect of quercetin on IRI of peripheral nerve is mediated by inhibition of NF-κB pathway [15]. Chinese medicine gualou xiebai banxia decoction is reported to be able reduce rat myocardial IRI through inhibition of NF-кB activation [16].
Herein, to examine the effect of ASMq on IRI injury and the potential involvement of NF-кB pathway, we treated NF-κB knockout rats, transgenic rats, and control rats with ASMq for 21 days before they underwent IRI surgery. The subsequent tissue damages and protein expression changes in myocardial tissues in each group were assessed after ASMq treatment.
Materials and methods
Animals
Male Sprague-Dawley rats weighing between 250 and 295 g and aging between 8 and 12 weeks were purchased from ViewSolid Biotech Lt. Co. (Beijing, China). Rats were pair-housed, maintained at a controlled temperature (23 ± 3°C) and humidity (50 ± 20%), and subjected to a 12 h light-dark cycle. These rats included the NF-κB gene knockout rats (the NF-κB knockout group; n = 5), the NF-κB transgenic rats (the NF-κB transgenic group; n = 8), and the normal rats (the control group; n = 5). The experimental protocol was approved by the ethical review board of Xinjiang Medical University. All experiments were performed in accordance with Guide for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology.
ASMq treatment
ASMq was intragastrically administered in all rats for 21 days before the IRI surgery. The optimal dose for IRI protection in rats was 5.07 g/kg (5.0 g ASMq solved in 1 ml distilled water), equivalent to 2 folds the dosage of clinical use. The animal general condition including body temperature, body weight, food intake, water intake, urine and stool properties were observed and recorded during the drug administration.
ECG monitor and IRI surgical procedure
All animals underwent surgical procedure to establish IRI model. Briefly, the rats were anesthetized by an intraperitoneal injection of sodium pentobarbital at the dose of 30 mg/kg. Five-lead ECGs (Techman Soft, Chengdu, China) were recorded using four needle electrodes subcutaneously implanted in each hind/forelimb and one electrode placed on the right side of the chest. The ECG (paper speed: 100 mm/s, voltage: 20 mm/mV) was recorded for 5 min as the baseline. The arrhythmia was monitored for 1 min by ECG at -30, -10, -5, 3, 5, 10, 15, 30, 45, 60, 75, and 90 min in the surgery. Tracheostomy tube ventilator assisted breathing was used, with the parameters as follows: a respiratory rate of 50 breaths/min, a tidal volume of 20 ml/kg, and an I:E ratio of 1:1 (the ratio of inspiration duration to expiration duration) [17,18].
The left coronary artery was ligated using a 6-0 silk suture (Johnson & Johnson NJ, USA) through a 15 mm opening at the 4th intercostal space. A plain knot was tied after calmly breathing for 10 min, and the knot was left in-situ for 30 min. Ischemia was confirmed in all rats by discoloration of the heart surface and ST elevation (≥0.1 mV) on the ECG recording. After 30 min, the knot was released and the reperfusion was verified by reddening of the previously discolored area of the heart surface and the ECG recordings. The reperfusion continued for 120 min before the animal was sacrificed to obtain the myocardial infarction tissue.
Quantitative RT-PCR
Total RNA was extracted from 100 mg of rat myocardial infarction tissue with Trizol reagent (Invitrogen). RNA purity was assessed by the absorbance ratio at 260 nm and 280 nm (1.8≤A260/A280≤2.1). cDNA was prepared from samples of 1 μg of RNA with Fast Quant RT Kit (Tiangen, Beijing, China) according to the instructions of the manufacturer. PCR was performed in 20 μL of reaction solution containing 1 μg of cDNA and the appropriate primers from Oebiotech (Oebiotech, Urumqi, China) (Table 1) using a SYBR green PCR kit (Qiagen, MD, USA). PCR conditions were as follows: denaturation at 95°C for 2 min, 40 cycles of 15 s at 95°C, 60 s at 60°C, 60 s at 72°C, and a final extension at 72°C for 2 min. The mRNA relative expression was calculated using the 2-ΔΔCt method [19]. All reactions were performed in triplicate.
Table 1.
Primers used in the RT-PCR
| Primer (5’ to 3’) | |
|---|---|
| P65-Forward | CTGGCCATGGACGATCTGTT |
| P65-Reverse | GCACTTGTAACGGAAACGCA |
| HSP70-Forward | AATGCGCTCGAGTCCTATGC |
| HSP70-Reverse | CTCAGCCAGCGTGTTAGAGT |
| CRP78-Forward | CCTATTCCTGCGTCGGTGTA |
| CRP78-Reverse | GACGTGAGTTGGTTCTTGGC |
| NIK-Forward | ACGTGATACCCTGAGTTCCG |
| NIK-Reverse | TGGACCTTGACACCGTTGAA |
| IKKα-Forward | CGCTCTCTTGTAGGATCCAGTC |
| IKKα-Reverse | CTAACGTCTCGCCATCTTGAGG |
| PKC zeta-Forward | ACTCCTGCTTCCAGACAACG |
| PKC zeta-Reverse | TCAGCAGCATAGAACCTGGC |
| beta actin-Forward | CCCATCTATGAGGGTTACGC |
| beta actin-Reverse | TTTAATGTCACGCACGATTTC |
ELISA
Blood samples were collected from rat right ventricular. The serum levels of IL-lβ, IL-6, IL-10, intercellular cell adhesion molecule (ICAM), E-cadherin (E-cad), TNF-α, troponin (cTn-T), and integrin beta-4 (ITGB4) were measured by the ELISA kit (Cusabio Co. Ltd., Wuhan, China) according to the manufacturer’s instructions. The creatine kinase (CK-MB), lactate dehydrogenase (LDH), and nitric oxide (NO) was detected using the corresponding kits (Jiancheng bioengineering, Nanjing, China), and the malondialdehyde (MDA) was detected by MDA kit (Beyotime, Shanghai, China).
Western blot
The protein was extracted from tissue samples by RIPA lysis (Boster, Wuhan, China) according to the manufacturer’s instruction. After determination of the protein concentration using BCA Protein Assay Kit (Tiangen, Beijing, China), proteins (40 μg lane-1, with 6 uL marker) were then electrophoresed through a 10% SDS-PAGE and transferred to a PVDF membrane (Millipore, UK) for 70 min under 100 V voltage. The membrane was blocked with 5% skim milk in TBST at room temperature for 1 h. The blots were stained with rabbit polyclonal primary (CRP78, HSP70, IKKa, NIk, PKC-zeta, and p (PKC-zeta), 1:500; p (IKKa), 1:1000; p65, 1:200; Abcam, Cambridge, MA, USA) at 4°C overnight and secondary (1:10000; Pierce Goat Anti-Rabbit IgG, Thermo Scientific, USA) antibodies for 1 h at room temperature. The intensity of the bands was scanned and analyzed using Clinx Chemiscope 3000 (Clinx, Shanghai, China).
HE staining
Rat myocardium tissue was prepared by fixation in 4% formaldehyde solution. The tissues were then dehydrated in a graded series of ethanol solutions, embedded in paraffin and cut into sections (6 μm). The hematoxylin and eosin (HE) staining was performed according to routine procedure. The degree of pathological damage was evaluated under a light microscope.
Electron microscopy observation
The ultrastructural observation of myocardium was performed using an electron microscopy accordingly to routine procedure.
Statistical analysis
Data were presented as mean values ± SEM. Data was processed by SPSS17.0 software (SPSS, Chicago, IL, USA). Differences between groups were tested using either a one-way analysis of variance or an unpaired Student’-test. P<0.05 was considered to be statistical significant.
Results
Clinical characteristics of rats
All rats received ASMq treatment before the IRI surgery. The ECG results confirmed the IRI procedures were successful. The average infarction size of the transgenetic group was greater than that of the controls and the NF-κB knockout rats, suggesting greater IRI injury in the transgenetic group.
Ultrastructural analysis of the myocardium
Rat IRI tissues were examined by electron microscopy (Figure 1). The peri-infarct myocardial area of the IRI in the NF-κB transgenic rats showed disrupted endothelial linings of microvessels, red blood cell leakage, and vacuoles, indicating endothelial damage (Figure 1C). In contrast, in the NF-κB knockout rats, the pathological changes were significantly improved (Figure 1B). The HE staining results (Figure 2) showed some myocardial vacuolar degeneration in the NF-κB knockout rats, vacuolar degeneration and some lymphocytes infiltration in the controls, and vacuolar degeneration and cell proliferation, vascular dilation in the transgenetic rats.
Figure 1.
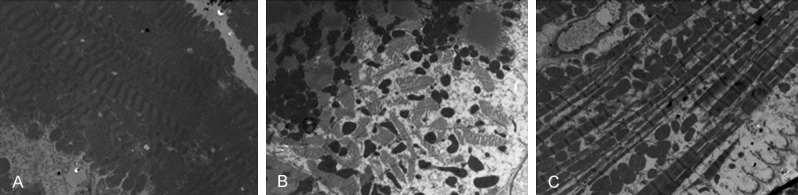
Ultrastructure changes of rat myocardia. The ultrastructure of rat myocardia was observed under an electron microscopy. A: Control rat; B: NF-κB knockout rat; C: NF-κB transgenic rats.
Figure 2.
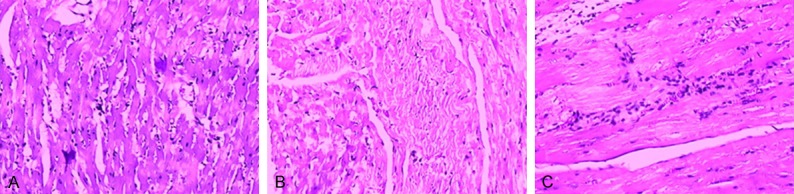
Pathological changes of rat myocardia. The hematoxylin and eosin staining was performed and the representative results were shown. A: Control rat; B: NF-κB knockout rat; C: NF-κB transgenic rats.
The HSP70, GRP78, P65, NIK, IKK-alpha mRNA expressions
The relative expression of HSP70, GRP78, P65, NIK (NF-κB inducing kinase), and IKKα (IκB kinase alpha) mRNA was down-regulated in the NF-κB knockout rats while were up-regulated in the transgenic rats comparing to the controls (Figure 3). The only exception was PKC-zeta. The results suggest that NF-κB transduction pathway plays a vital role in regulation of these mRNA expressions.
Figure 3.
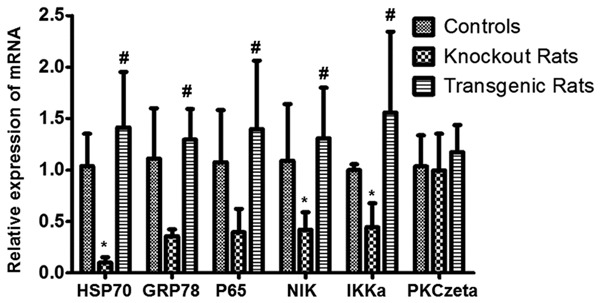
Analysis of HSP70, GRP78, p65, NIK, IKKa, and PKCzeta mRNA. The relative expression of mRNA among the controls, the NF-κB knockout rat group and the NF-κB transgenic rat group were detected with RT-qPCR. *indicating comparison with the control group, P<0.05; #indicating comparison with the NF-κB knockout rat group, P<0.05.
The pro-inflammatory cytokines expressions among groups
The pro-inflammatory cytokines of IL-1β, IL-6, IL-10, and TNF-α were significantly decreased in the NF-κB knockout rats while were up-regulated in the transgenic rats when comparing with the controls (Figure 4). The results suggest that the inflammation is inhibited in the NF-κB knockout rats but is more severe in the transgenic rats.
Figure 4.
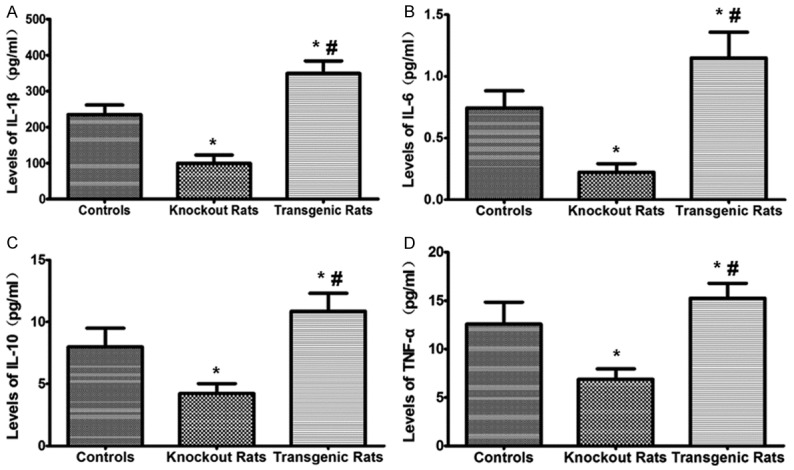
Analysis of IL-1β, IL-6, IL-10 and TNF-α levels in the serum of rats. ELISA was used to detect these cytokines in serum. *indicating comparison with the control group, P<0.05; #indicating comparison with the NF-κB knockout rat group, P<0.05.
The adhesion molecular expressions among groups
The adhesion molecules ICAM, E-Cad, ITGB4 were significantly decreased in the NF-κB knockout rats while were up-regulated in the transgenic rats comparing to the controls (Figure 5). The results suggest that NF-κB pathway regulated the adhesion molecular expression during the IRI modeling and plays a role in the inflammatory responses.
Figure 5.
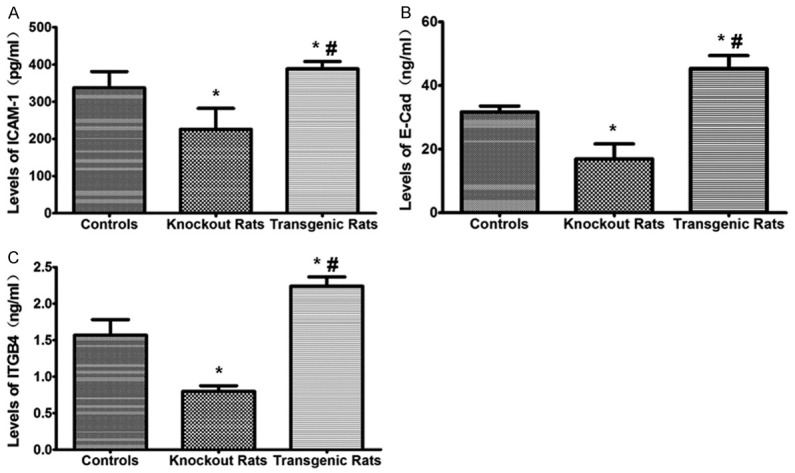
Analysis of intercellular cell adhesion molecule (ICAM), E-Cadherin (E-Cad), and integrin beta-4 (ITGB4) levels. The levels of adhesion molecules among groups were measured using ELISA test. *indicating comparison with the control group, P<0.05; #indicating comparison with the NF-κB knockout rat group, P<0.05.
The cardiac marker expressions among groups
The levels of cTn-T and CK-MB were significantly decreased in the NF-κB knockout rats while were up-regulated in the transgenic rats comparing to the controls (Figure 6). The results suggest that the myocardial infarction is more severe in the transgenic rats, and the NF-κB knockout rats suffered less injury compared with the controls. The level of LDH did not show the above changes. The reason is possibly because the peak time of LDH is about 72 h after myocardial infarction, so the changes in our observation window are not evident.
Figure 6.
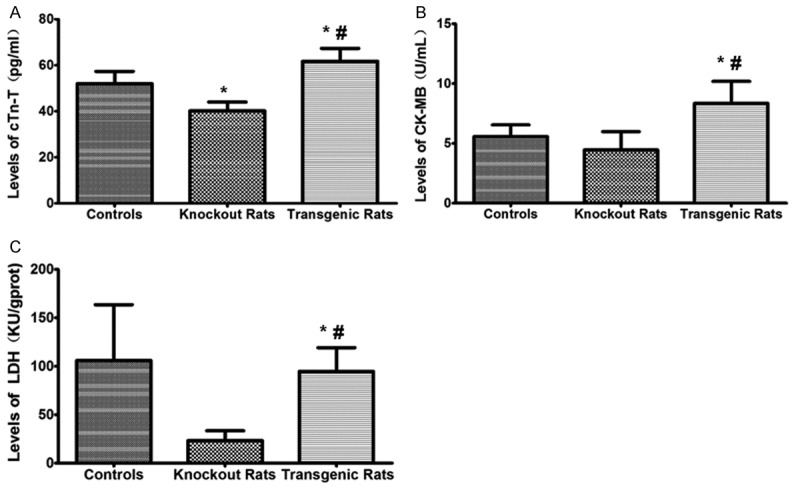
Analysis of creatine kinase (CK-MB), lactate dehydrogenase (LDH), and troponin (cTn-T) levels. The levels of CK-MB, LDH, and cTn-T were detected with corresponding kits. *indicating comparison with the control group, P<0.05; #indicating comparison with the NF-κB knockout rat group, P<0.05.
The oxidative stress among groups
The expression of MDA and NO were significantly decreased in the NF-κB knockout rats while were up-regulated in the transgenic rats comparing to the controls (Figure 7). These results suggest the myocardium oxidative stress in the transgenic rats was elevated. And knockout of the NF-κB gene can reduce the oxidative stress in the experimental setting.
Figure 7.
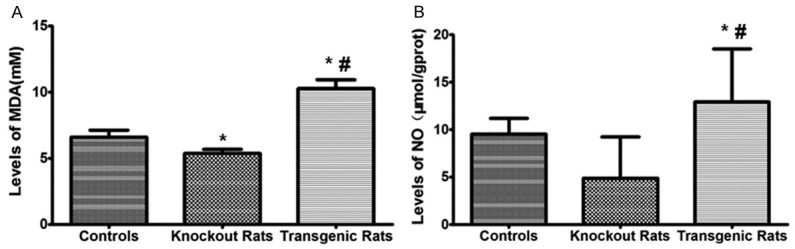
Analysis of malondialdehyde (MDA) and nitric oxide (NO) levels in myocardial infarction tissues. MDA level was detected by MDA and NO level was measured by NO ELISA kit. *indicating comparison with the control group, P<0.05; #indicating comparison with the NF-κB knockout rat group, P<0.05.
The HSP70, GRP78, P65, NIK, IKKα protein expression by western blot
The genetic modification altered the expression of HSP70, GRP78, P65, NIK, IKK-alpha protein among different groups, as shown in Figure 8 and Supplementary Figure 1. HSP70, GRP78 expression in the transgenic rats was up-regulated and it suggested greater endoplasmic reticulum (ER) stress. P65 expression was higher in the transgenic rats as expected. IKKα and NIK are inducers of NF-κB pathway and they lead to NF-κB activation. The phosphorylated IKKα was down-regulated in the NF-κB knockout rats, suggesting an inhibition of NF-κB activation in these rats. The protein expressions were not always coincided with the mRNA expression among the three groups, particularly, the expression of phosphorylated PKC-zeta showed a reverse trend comparing with the mRNA expression.
Figure 8.
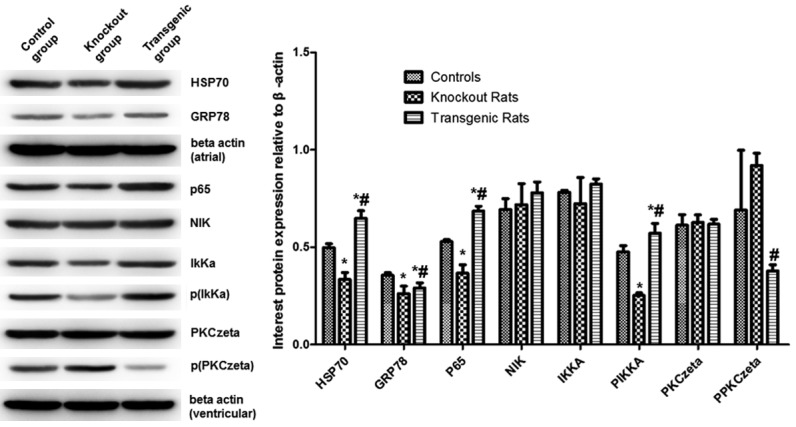
Analysis of related protein levels. Protein expression was detected by western blot. Representative and quantitative western blot results were shown on the right and left, respectively. *indicating comparison with the control group, P<0.05; #indicating comparison with the NF-κB knockout rat group, P<0.05.
Discussion
IRI has been a thorny problem for cardiac surgeries and understanding the molecular mechanism of myocardial IRI is of great clinical significance. At present, a number of cell signaling pathways are reported to be involved in the pathogenesis of IRI, including NF-κB, Janus and JAK/PKC, and mitogen-activated protein kinase [20]. Our previous studies have demonstrated that the Uighur medicine ASMq can protect myocardial IRI in rat models [12,13]. The current study showed that NF-κB signaling pathway was vital to IRI in rats under ASMq treatment.
Inflammatory immune response is initiated from ischemia and continues over several hours into reperfusion, thus contributing to a potential second therapeutic window for reducing myocardial injury size [21-23]. Anti-inflammation agents have shown clinically promising benefit in protecting renal, nerve, and liver IRI [15,24,25]. The ASMq has anti-inflammation and anti-oxidative effects and exhibits protection against myocardial IRI in our previous investigation [12,13].
NF-κB pathway can be activated by alternative processes that induced by IKKα or NIK respectively [26,27]. PKC zeta is also reported to participate in NF-κB activation [28]. NF-κB activation can promote inflammatory gene expression, including IL-1β, IL-6, IL-10 and TNF-α, and adhesion factors such as intercellular adhesion molecule ICAM-1 [29]. Increased expression of adhesion molecules attracts more neutrophils and lymphocytes, leading injury to vascular endothelial cells and myocardial cells [30]. In our study, we measured the oxidative stress, ER stress during IRI. Meanwhile, NF-κB upstream kinase expressions, NF-κB p65 expression, the regulated downstream inflammatory cytokines, as well as the cardiac injury markers were also monitored. As the results shown, the injury stresses were alleviated by the knockout of NF-κB gene, the pro-inflammatory cytokines and cardiac markers were down-regulated, and less severe myocardial injuries in these rats were observed by pathological findings. The average infarction size was smaller, and the lymphocytes infiltration in the myocardium was less evident in the NF-κB knockout rats when comparing with the NF-κB transgenic rats. These results suggest that the myocardial injury protection effect of ASMq treatment may be mediated by the NF-κB pathway.
Limited number of experimental animal constrained us from a thorough comparison of the treatment effect of ASMq, which is the main weakness of this study. But in our preliminary/previous experiment, we have found that ASMq could inhibit the activation of NF-κB. We postulate that the protection effect of ASMq on myocardial IRI might be acted through NF-κB signaling pathway. And the results confirmed that NF-κB is a key regulator of the IRI injury in rats.
Conclusion
The NF-κB upstream and downstream gene expression was altered after IRI. Inflammatory responses were reduced in the NF-κB knockout rats during IRI model. We suggest that the inhibition on NF-κB signaling pathway activation may alleviate IRI in rats under ASMq treatment.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant number: 81460755].
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth. 2012;16:123–132. doi: 10.1177/1089253211436350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piper HM, Garcia-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38:291–300. doi: 10.1016/s0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- 5.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 6.Yusup A, Upur H, Umar A, Berke B, Moore N. Ethanol extract of abnormal savda munziq, a herbal preparation of traditional uighur medicine, inhibits Caco-2 cells proliferation via cell cycle arrest and apoptosis. Evid Based Complement Alternat Med. 2012;2012:926329. doi: 10.1155/2012/926329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Upur H, Chen Y, Kamilijiang M, Deng W, Sulaiman X, Aizezi R, Wu X, Tulake W, Abudula A. Identification of plasma protein markers common to patients with malignant tumour and Abnormal Savda in Uighur medicine: a prospective clinical study. BMC Complement Altern Med. 2015;15:9. doi: 10.1186/s12906-015-0526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halmurat U, Askar Y, Ilhamjan S, Obul K, Roxangul S. [Gene polymorphisms in Uighur patients with Abnormal Savda] . Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2003;20:77–78. [PubMed] [Google Scholar]
- 9.Tursun K, Asmtula D, Smayil M, Upur D, Upur H. [The relationship between asthma patients with abnormal Savda in Uyghur medicine and the gene polymorphism of IL-4] . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33:1076–1080. [PubMed] [Google Scholar]
- 10.Yin P, Mohemaiti P, Chen J, Zhao X, Lu X, Yimiti A, Upur H, Xu G. Serum metabolic profiling of abnormal savda by liquid chromatography/mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871:322–327. doi: 10.1016/j.jchromb.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 11.Mamtimin B, Upur H, Hao FH, Matsidik A, Rahim R. Plasma metabonomic analysis with (1)H nuclear magnetic resonance revealing the relationship of different tumors and the disease homology theory of traditional Uyghur medicine. Chin J Integr Med. 2011;17:111–115. doi: 10.1007/s11655-011-0638-x. [DOI] [PubMed] [Google Scholar]
- 12.Abudunaibi M, Mulati A, Aisikaer S, Zulifeiya M, Qiao J, Gulibositan M, Aili A, Halmurat U. Myocardial protective effects of Munziq in myocardial ischemia-reperfusion injury rats with abnormal savda syndrome. Genet Mol Res. 2015;14:3426–3435. doi: 10.4238/2015.April.15.6. [DOI] [PubMed] [Google Scholar]
- 13.Maimaitiaili A, Shabiti A, Abudureheman M, Musha Z, Jun Q, Maimaitiaili G, Aibibula A, Upur H. Effects of different doses of Savda Munziq on myocardial ischemia-reperfusion injury in rats with abnormal Savda syndrome. Genet Mol Res. 2014;13:4729–4735. doi: 10.4238/2014.July.2.2. [DOI] [PubMed] [Google Scholar]
- 14.Nabel GJ, Verma IM. Proposed NF-kappa B/I kappa B family nomenclature. Genes Dev. 1993;7:2063. doi: 10.1101/gad.7.11.2063. [DOI] [PubMed] [Google Scholar]
- 15.Gholami M, Khayat ZK, Anbari K, Obidavi Z, Varzi A, Boroujeni MB, Alipour M, Niapoor A, Gharravi AM. Quercetin ameliorates peripheral nerve ischemia-reperfusion injury through the NF-kappa B pathway. Anat Sci Int. 2017;92:330–337. doi: 10.1007/s12565-016-0336-z. [DOI] [PubMed] [Google Scholar]
- 16.Zhang HM, Tang DL, Tong L, Sun MJ, Sui Y, Zhu HY, Cao HX. Gualou xiebai banxia decoction inhibits NF-kappa B-dependent inflammation in myocardial ischemia-reperfusion injury in rats. J Tradit Chin Med. 2011;31:338–343. doi: 10.1016/s0254-6272(12)60015-6. [DOI] [PubMed] [Google Scholar]
- 17.Hou YM, Wen H, Zhang C. Study on animal experiment. Xinjiang People’s Health Publishing House; 2011. [Google Scholar]
- 18.Wen H, Hou YM, Zhang C. Human disease animal model research and experimental animal management. Science Press; 2012. [Google Scholar]
- 19.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Yu Z, Huang X, Gao Y, Wang X, Gu J, Xue S. Peroxisome proliferator-activated receptor gamma (PPARgamma) mediates the protective effect of quercetin against myocardial ischemia-reperfusion injury via suppressing the NF-kappaB pathway. Am J Transl Res. 2016;8:5169–5186. [PMC free article] [PubMed] [Google Scholar]
- 21.Rochitte CE, Lima JA, Bluemke DA, Reeder SB, McVeigh ER, Furuta T, Becker LC, Melin JA. Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation. 1998;98:1006–1014. doi: 10.1161/01.cir.98.10.1006. [DOI] [PubMed] [Google Scholar]
- 22.Zhao ZQ, Nakamura M, Wang NP, Velez DA, Hewan-Lowe KO, Guyton RA, Vinten-Johansen J. Dynamic progression of contractile and endothelial dysfunction and infarct extension in the late phase of reperfusion. J Surg Res. 2000;94:133–144. doi: 10.1006/jsre.2000.6029. [DOI] [PubMed] [Google Scholar]
- 23.King TD, Song L, Jope RS. AMP-activated protein kinase (AMPK) activating agents cause dephosphorylation of Akt and glycogen synthase kinase-3. Biochem Pharmacol. 2006;71:1637–1647. doi: 10.1016/j.bcp.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HM, Jang HJ, Kim SS, Kim HJ, Lee SY, Oh MY, Kwan HC, Jang DS, Eom DW. Protective effect of eupatilin pretreatment against hepatic ischemia-reperfusion injury in mice. Transplant Proc. 2016;48:1226–1233. doi: 10.1016/j.transproceed.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Zou YR, Zhong X, Deng HD, Pu L, Peng K, Wang L. Erythropoietin pretreatment ameliorates renal ischaemia-reperfusion injury by activating PI3K/Akt signalling. Nephrology (Carlton) 2015;20:266–272. doi: 10.1111/nep.12384. [DOI] [PubMed] [Google Scholar]
- 26.Bonizzi G, Bebien M, Otero DC, Johnson-Vroom KE, Cao Y, Vu D, Jegga AG, Aronow BJ, Ghosh G, Rickert RC, Karin M. Activation of IKKalpha target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. EMBO J. 2004;23:4202–4210. doi: 10.1038/sj.emboj.7600391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG, Edwards SW, McDowell HP, Unitt JF, Sullivan E, Grimley R, Benson N, Broomhead D, Kell DB, White MR. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- 28.Savkovic SD, Koutsouris A, Hecht G. PKC zeta participates in activation of inflammatory response induced by enteropathogenic E. coli. Am J Physiol Cell Physiol. 2003;285:C512–521. doi: 10.1152/ajpcell.00444.2002. [DOI] [PubMed] [Google Scholar]
- 29.Rao J, Yue S, Fu Y, Zhu J, Wang X, Busuttil RW, Kupiec-Weglinski JW, Lu L, Zhai Y. ATF6 mediates a pro-inflammatory synergy between ER stress and TLR activation in the pathogenesis of liver ischemia-reperfusion injury. Am J Transplant. 2014;14:1552–1561. doi: 10.1111/ajt.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Browder W, Kao RL. Early activation of transcription factor NF-kappaB during ischemia in perfused rat heart. Am J Physiol. 1999;276:H543–552. doi: 10.1152/ajpheart.1999.276.2.H543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


