Abstract
Potassium bromate (KBrO3) is used in many countries in cosmetic and food industries. In this work, we investigated in male Sprague-Dawley rats, the effect of four graded oral doses of KBrO3 (5, 15, 45 and 135 mg/kg/day for 28 days) on renal function tests, inflammation, oxidative damage, and apoptosis, as well as on histopathology, using several traditional and novel renal injury biomarkers in plasma, urine and renal tissues. We also tested the possible ameliorative action of the renoprotective prebiotic agent gum acacia (GA) on the actions of KBrO3 when given concomitantly with it in the drinking water at a concentration of 15%w/v. Taken together, the results indicated that treatment with KBrO3 at the 45 and 135 mg/kg doses caused a significant dose-dependent nephrotoxicity, as evident by the measured renal structural and functional indices and biomarkers of toxicity. GA co-treatment significantly abated most of the indices and biomarkers of the renal toxicity caused by KBrO3, suggesting a beneficial effect and its possible inclusion in edible products where KBrO3 is still used.
Keywords: Potassium bromate, kidneys, gum acacia, Sprague-Dawley rats
Introduction
Potassium bromate (KBrO3) is an oxidizing agent that is commonly used in cosmetic products (such as permanent hair weaving solutions and dying of textiles), and as a food additive, and is a major tap water pollutant. In several countries, including the United States, it is still used (legally and illegally) as a bread and cake improver even though it has been associated with the development of several organ damage [1-3]. Acute intoxication to KBrO3 in humans can results in renal failure, neuropathological disorders and thrombocytopenia, while chronic intoxications have been linked to the development of several renal and nonrenal tumors [4-7].
The nephrotoxicity caused by KBrO3 has been attributed to its ability to trigger the production of reactive oxygen species (ROS), lipid peroxidation and 8-hydroxyguanosine modification in renal DNA [8-11]. The oxidative stress induced by KBrO3 far exceeds the cellular antioxidative defense capacity leading to marked nephrotoxicity in humans and animals and carcinogenicity in experimental animals. Therefore, the search for safe and effective synthetic and/or naturally occurring ROS scavengers and antioxidants is of major clinical importance. Some of these such as rutin, taurine and Nymphaeaalba L. have demonstrated potential protective effects against KBrO3 induced nephrotoxicity [12-14].
Gum Acacia (GA) is a well-known dietary fiber with several uses in the pharmaceutical, cosmetic and food industries. It is an easily obtained, very safe and relatively cheap prebiotic agent, that has been shown to possess nephroprotective actions in humans, rats and mice [15,16]. In several studies GA demonstrated an anti-inflammatory and antioxidative properties making it a strong apoptosis scavenger [17-20].
Reports in the literature showed that KBrO3 was used to induce nephrotoxicity via either subcutaneous or intraperitoneal route of administration. However, human exposure to KBrO3 occurs orally. We only came across two reports that used KBrO3 via oral route to induce nephrotoxicity in rats as a single dose of 100 mg/Kg in male Wistar rats and as twice weekly doses of 20 mg/Kg for four weeks in male Sprague-Dawley rats for four weeks [13,21]. Also, as far as we are aware, GA has not been used as a potential agent in treatment of KBrO3 induced nephrotoxicity. Therefore, the present study aimed at investigating, using several traditional and novel biochemical and histological parameters, the nephrotoxic effect of four graded oral doses of KBrO3 in rats and possible ameliorative effect of co-treatment of GA thereon.
Materials and methods
Animals
Male Sprague-Dawley rats (9-10 weeks old, weighing 200±10 g) were housed in a room kept at a temperature of 22±2°C, relative humidity of about 60%, with a 12 h light-dark cycle (lights on at 6:00). Free access to water and standard pellet chow diet containing 0.85% phosphorus, 1.12% calcium, 0.35% magnesium, 25.3% crude protein and 2.5 IU/g vitamin D3 (Oman Flour Mills, Muscat, Oman) were provided. Ethical approval for conducting the work was sought and obtained from Sultan Qaboos University Animal Ethics Committee, and all procedures involving animals and their care were carried out in accordance with international laws and policies (EEC Council directives 2010/63/EU, 22 September 2010 and NIH Guide for the Care and Use of Laboratory Animals, NIH Publications, 8th edition, 2011).
Experimental design
After an acclimatization period of one week, rats (n = 60) were randomly distributed into ten equal groups and treated for four consecutive weeks.
The 1st group (Control) continued to receive tap water and the same diet without treatment until the end of the study.
The 2nd group was given normal food and GA (SUPERGUMTM EM 10) in the drinking water at a concentration of 15%w/v.
The 3rd, 5th, 7th, and 9th groups were given normal food, tap water and KBrO3 at oral doses of 5, 15, 45 and 135 mg/kg/day, respectively.
The 4th, 6th, 8th, and 10th groups were treated as the 3rd, 5th, 7th, and 9th groups, respectively, except that they were also concomitantly treated with GA as in the 2nd group.
The dose of GA was chosen based on our previous experiments with this prebiotic, while the selected doses of KBrO3 and treatment duration were selected to bracket, as much as possible, the doses previously used by others [22-25].
During the treatment period, the rats were weighed weekly, and a day before the last day of treatment, they were placed individually in metabolic cages to collect the urine voided in the last 24 h. At the end of the treatment, the rats were anesthetized with ketamine (75 mg/kg) and xylazine (5 mg/kg) intraperitoneally, and blood (about 6 mL) was collected from the anterior vena cava and placed into heparinized tubes and centrifuged at 900 g at 4°C for 15 min to separate plasma. The plasma and urine were stored at -80°C pending analysis. The two kidneys were excised, blotted on filter paper and weighed. The right kidney and most of the left one were rapidly dipped in liquid nitrogen and kept frozen at -80°C for conducting biochemical tests. A small piece of the left kidney was placed in formol-saline for subsequent histopathological examination.
Physiological and biochemical measurements
The body weights of all rats were recorded on a weekly basis during the experimental period as described before [26]. Plasma and urine osmolality were measured by the freezing point depression method (-70°C) using a Digimatic osmometer (Osmomat 3000, Gonotec GmbH, Berlin, Germany). Plasma neutrophil gelatinase-associated lipocalin (NGAL) activity was measured by an ELISA method using kits obtained from BioPorto Diagnostics (Gentofte, Denmark). Urinary N-acetyl-β-glucosaminidase (NAG) activity was measured by kits from Diazyme (Poway, CA, USA). Other plasma and urinary biochemical biomarkers of renal function (creatinine, urea, creatinine clearance, and albumin) were analyzed by using an automated machine (Mindray BS-120 Chemistry Analyzer, Shenzhen, China). Plasma enzymes lactate dehydrogenase (LDH), aspartate amino transferase (AST) and alanine amino transferase (ALT), used as indices of tissue damage, were also measured using an automated machine (Mindray BS-120 Chemistry Analyzer, Shenzhen, China).
The supernatants of renal homogenates were separated into two portions. The first was used for the measurement of superoxide dismutase (SOD) and total antioxidant capacity (TAC) as described earlier [26]. The second portion was used to measure TNF-α, clusterin, adiponectin, by ELIZA technique as detailed elsewhere [26].
Histopathology
To confirm the effect of KBrO3, with and without GA co-treatment, on the kidneys, light microscopic investigation of renal histology, were conducted as described before [17]. Briefly, the kidney (4 µm sections) were cut and stained with three stains: Hematoxylin and Eosin (H&E), Masson Trichrome and Sirius Red stains. They were examined for necrosis and fibrosis by a specialist unaware of the treatments.
Immunohistochemistry
Apoptotic cells were stained by anti-caspase 3 antibody using (1:100; Abcam, Ab4051), as previously reported [27]. The number of caspase 3-positive cells in each specimen was also scored. Cells with brown nuclear staining were considered positive, and the number of caspase 3 positive cells were counted in random high-power sections using a light microscope (Olympus BX51, Japan) and incorporating a software analysis system (ArgenitKameram, ver. 2.11.5.1, Istanbul, Turkey). All the counts were converted to number of positive cells per unit area (mm2).
Drugs and chemicals
GA used was SUPERGUMTM EM 10, Lot 101008, 1.1.11 (Sanwa-cho, Toyonaka-shi, Osaka, Japan). KBrO3 was obtained from Sigma (St. Louis, MO, USA). Aqueous solutions of both compounds were prepared freshly every day. The chemical properties of GA have been fully reviewed before [15]. All used chemicals were of analytical reagent grade. SUPERGUMTM EM 10 used was characterized, per the manufacturer, by size fractionation followed by multiple angle laser light scattering (GPC-MALLS) to give its molecular profile. The average molecular weight was 3.43×106, and the content of the arabinogalactan protein (AGP) therein was 26.4%.
Statistical analysis
The data in this work was expressed as means ± SEM, and was analyzed with GraphPad Prism Version 5.03 for Windows software (Graphpad Software Inc., San Diego, USA). Comparisons between the separate groups were conducted by analysis of variance (ANOVA), followed by Tukey’s multiple comparison tests. P values < 0.05 were considered significant.
Results
Table 1 shows body weight changes, relative kidney to body weight, water intake and urine output. Compared to control rats, KBrO3 (5, 15 and 45 mg/kg) caused no significant changes in any of the parameters measured. At a dose of 135 mg/kg, KBrO3 caused significant change in body weight and significant rise in the volume of water drunk and urine voided. GA treatment significantly reduced body weight, as consistently reported before [20-26].
Table 1.
Effects of treatment of rats with KBrO3 separately, or in combination with gum acacia (GA), on some physiological parameters
| Parameters/Treatment | Initial body weight (g) | Final body weight (g) | Change in body wright (%) | Relative kidney weight (%) | Water intake (mL) | Urine output (mL) |
|---|---|---|---|---|---|---|
| Control | 198.17±10.7 | 244.0±8.9 | 23.93±4.09 | 0.74±0.06 | 14.08±1.64 | 7.75±0.54 |
| GA | 199.0±5.09 | 220.0±6.98 | 10.48±1.01 | 0.77±0.03 | 20.0±3.92 | 7.83±1.33 |
| KBrO3 (5 mg/Kg) | 199.83±12.06 | 249.67±5.84 | 26.5±5.45 | 0.76±0.03 | 15.42±1.5 | 7.5±0.96 |
| KBrO3 (5 mg/Kg)+GA | 199.33±8.89 | 203.3±8.47 | 2.08±0.82*,° | 0.79±0.05 | 16.58±2.48 | 7.67±0.76 |
| KBrO3 (15 mg/Kg) | 198.67±11.13 | 248.83±10.03 | 26.35±5.97 | 0.79±0.06 | 13.33±2.0 | 7.5±1.06 |
| KBrO3 (15 mg/Kg)+GA | 200.0±6.61 | 221.67±10.17 | 10.63±2.02 | 0.72±0.03 | 14.17±2.48 | 7.17±0.91 |
| KBrO3 (45 mg/Kg) | 199.0±7.87 | 241.83±3.79 | 22.32±4.3 | 0.81±0.02 | 16.92±2.29 | 7.83±1.22 |
| KBrO3 (45 mg/Kg)+GA | 199.67±11.51 | 219.17±11.44 | 10.03±2.16 | 0.83±0.05 | 11.67±1.96 | 7.5±1.11 |
| KBrO3 (135 mg/Kg) | 205.0±9.57 | 214.17±15.1 | 3.94±3.01* | 0.83±0.01 | 27.0±4.28* | 18.33±2.22* |
| KBrO3 (135 mg/Kg)+GA | 203.5±12.04 | 193.67±15.35* | 5.18±3.06* | 0.69±0.02 | 11.0±1.06° | 5.17±1.01° |
Values in the table are means ± SEM (n = 6). Different doses of KBrO3 were orally administered (in distilled water) for 28 days, and GA (15%W/V) was given concomitantly in the drinking water. On the 28th day of treatment, the rats were placed in metabolic cage to collect urine.
denote significance of different groups vs. control;
denote significance of groups treated with KBrO3 alone vs. it correspondence groups treated with GA.
The plasma activities of ALT, AST and LDH were significantly raised by the highest dose of KBrO3 used (135 mg/kg) but was not significantly affected by other doses or by GA. Co-treatment of rats with GA significantly mitigated the rise in AST activity induced by KBrO3 (135 mg/kg), but not ALT or LDH activities (data not shown).
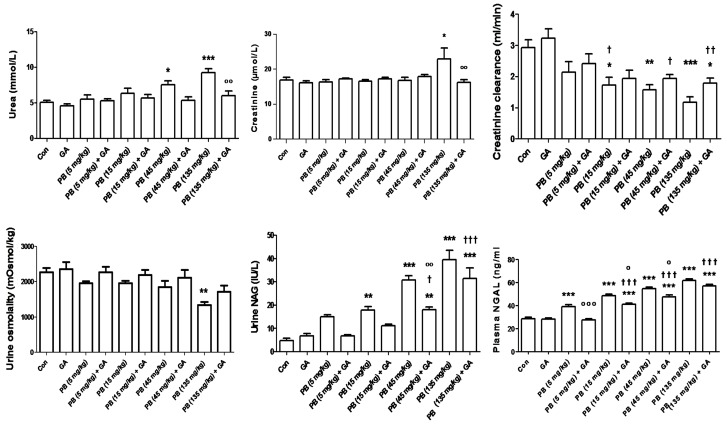
Treatment with KBrO3, at doses of 45 and 135 mg/kg, caused significant increases in the plasma concentrations of urea, which were antagonized by GA treatment (Figure 1). Treatment of rats with KBrO3, at a dose of 135 mg/kg (but not lower doses), significantly raised creatinine concentration, an action that was reversed by GA. Creatinine clearance was dose-dependently diminished by the four graded doses of KBrO3, and these values were antagonized by GA co-treatment.
Figure 1.
The plasma concentration of urea and creatinine, creatinine clearance, urine osmolality, urinary N-Acetyl-β-D-. glucosaminidase (NAG) and plasma neutrophil gelatinase-associated lipocalin (NGAL) in control (Con) rats and rats treated with different doses of KBrO3 (PB) with or without gum acacia (GA). Each column and vertical bar represents mean ± SEM (n = 6). Different superscripts indicate significance as follows: *denotes significance of different groups vs. Control group: where *P < 0.05, **P < 0.001, ***P < 0.0001. †denotes significance of GA alone vs. its corresponding groups treated with PB: where †P < 0.05, ††P < 0.001, †††P < 0.0001. °denotes significance of groups treated with PB alone vs. its corresponding groups treated with GA: where °P < 0.05, °°P < 0.001, °°°P < 0.0001.
Urine osmolality was not significantly affected by any treatment except in the group that was given KBrO3 at a dose of 135 mg/kg. Urinary NAG and plasma NGAL activities were increased in a dose-dependent fashion by KBrO3, and this action was significantly antagonized by GA at all doses of KBrO3 for plasma NGAL and at 45 and 135 mg/Kg for urinary NAG.
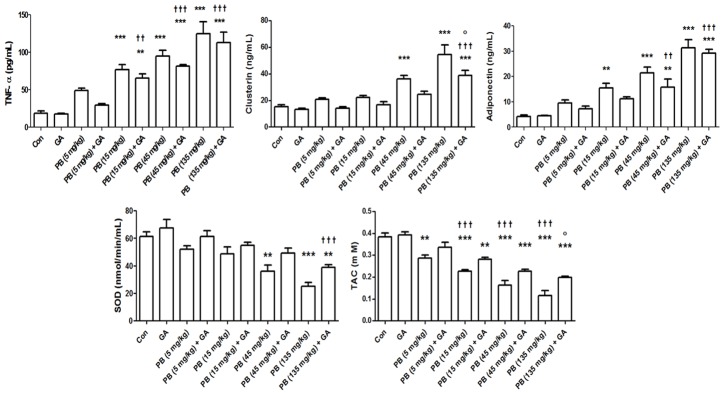
Treatment of rats with KBrO3 induced dose-dependent increases in TNF-α, clusterin and adiponectin concentrations, and dose-dependent reductions in SOD activity and TAC (Figure 2). GA co-treatment in both cases antagonized these actions either marginally at some doses or significantly at other doses.
Figure 2.
The plasma concentration of TNF-α, clusterin, and adiponectin, and the renal concentration or activity of total antioxidant capacity (TAC) and superoxide dismutase (SOD) in control (Con) rats, and rats treated with different doses of KBrO3 (PB) with or without gum acacia (GA). Each column and vertical bar represents mean ± SEM (n = 6). Different superscripts indicate significance as follows: *denotes significance of different groups vs. Control group: where **P < 0.001, ***P < 0.0001. †denotes significance of GA alone vs. its corresponding groups treated with PB: where ††P < 0.001, †††P < 0.0001. °denotes significance of groups treated with PB alone vs. its corresponding groups treated with GA: where °P < 0.05.
The histopathological changes in kidneys induced by KBrO3 are shown in Figure 3 (H&E), Figure 4 (Masson Trichrome) and Figure 5 (Sirius Red stain). It is evident that the administration of GA significantly reduced inflammatory cell infiltration, apoptotic cell numbers, and tubular cast and injuries (Figure 3D, 3F, 3H and 3J). The deposition of extracellular matrix in kidneys from rats treated with KBrO3 at doses of 45 and 135 mg/kg groups are shown in sections stained with Masson’s trichrome and Sirius Red stains (Figures 4G, 4I, 5G and 5I, respectively). The kidneys of rats treated with KBrO3 at doses of 15 and 45 mg/kg (Figures 4E and 5E) showed less fibrosis compared to the 135 mg/kg group, suggesting that the effect of KBrO3 is dose-dependent. Co-treatment with GA significantly mitigated the renal interstitial fibrosis induced by KBrO3 (Figures 4F, 4H, 4J, 5F, 5H and 5J).
Figure 3.
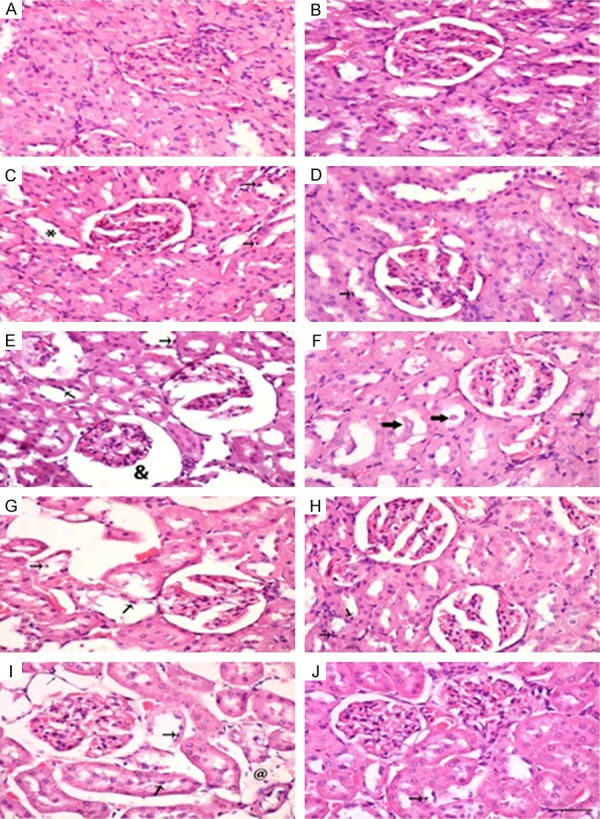
Hematoxylin and Eosin staining of renal tissues of control (Con) rats and rats treated with different doses of KBrO3 (PB) with or without gum acacia (GA). Different sections in the figure represent the following groups: (A) Con; (B) GA; (C) PB 5 mg/kg; (D) PB 5 mg/kg plus GA; (E) PB 15 mg/kg; (F) PB 15 mg/kg plus GA; (G) PB 45 mg/kg; (H) PB 45 mg/kg plus GA; (I) PB 135 mg/kg; and (J) PB 135 mg/kg plus GA. Sections (A, B and D) showed normal kidney histological appearance. Section (C) showed minimal histopathological degeneration. ‘*’ indicate dilated tubule. Sections (E, G and I) showed apoptotic cells (thick arrow), and necrotic tubule ‘@’. Partial ameliorations in necrotic and degenerative field were seen in sections (F, H and J) (thick arrows: tubular cast; thin arrows: apoptotic cells). (×400; Bars: 65 µm).
Figure 4.
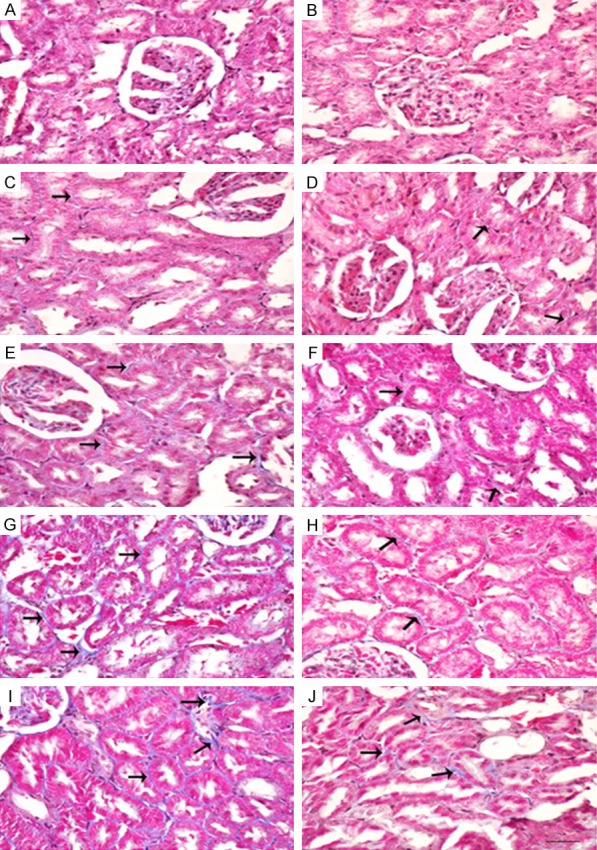
Mallory’s trichrome staining of renal tissues of control (Con) rats and rats treated with different doses of KBrO3 (PB) with or without gum acacia (GA). Different sections in the figure represent the following groups: A: Con; B: GA; C: PB 5 mg/kg; D: PB 5 mg/kg plus GA; E: PB 15 mg/kg; F: PB 15 mg/kg plus GA; G: PB 45 mg/kg; H: PB 45 mg/kg plus GA; I: PB 135 mg/kg; and J: PB 135 mg/kg plus GA. Sections A, B and D: Showed normal kidney histological appearance. Sections C and E: Showed minimal interstitial fibrosis. Sections G and I: Showed large areas of interstitial fibrosis (arrows). Sections F, H and J: Showed decrease in fibrosis (arrows). (×400; Bars: 65 µm).
Figure 5.
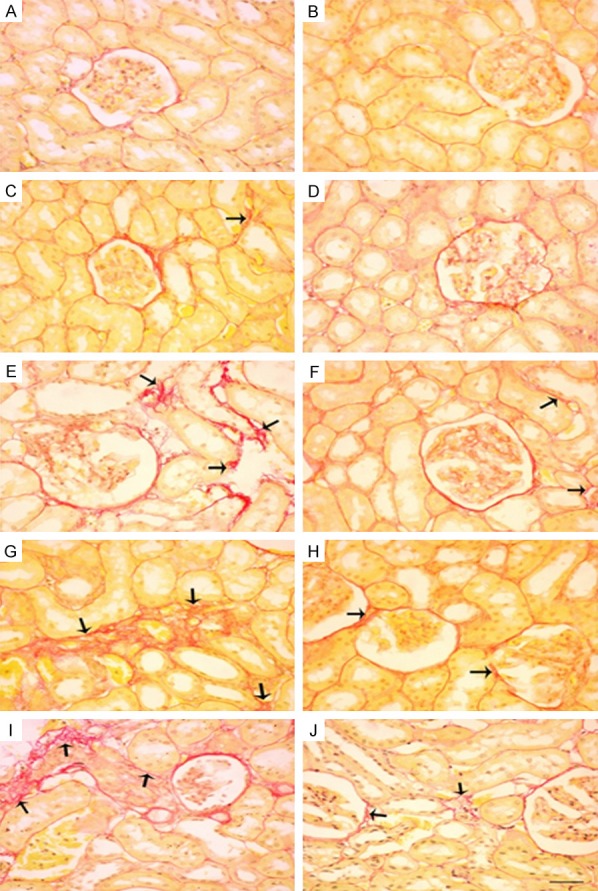
Sirius red staining of renal tissues of control (Con) rats and rats treated with different doses of KBrO3 (PB) with or without gum acacia (GA). Different sections in the figure represent the following groups: A: Con; B: GA; C: PB 5 mg/kg; D: PB 5 mg/kg plus GA; E: PB 15 mg/kg; F: PB 15 mg/kg plus GA; G: PB 45 mg/kg; H: PB 45 mg/kg plus GA; I: PB 135 mg/kg; and J: PB 135 mg/kg plus GA. Sections A, B and D: Showed normal kidney histological appearance. Sections C and E: Showed minimal interstitial fibrosis. Sections G and I: Showed large areas of interstitial fibrosis (arrows). Sections F, H and J: Showed decrease in fibrosis (arrows). (×400; Bars: 65 µm).
Figure 6 shows the effect of different doses of KBrO3, with and without GA on apoptosis in renal tissue. Control and GA-treated groups (Figure 6A and 6B, respectively) showed normal kidney architecture while KBrO3-treated groups (Figure 6C, 6E, 6G and 6I), showed dose-dependent increases in apoptotic cells (arrows). Rats given KBrO3 plus GA (Figure 6D, 6F, 6H and 6J) showed fewer apoptotic cells when each group is compared with its corresponding group treated with KBrO3 alone.
Figure 6.
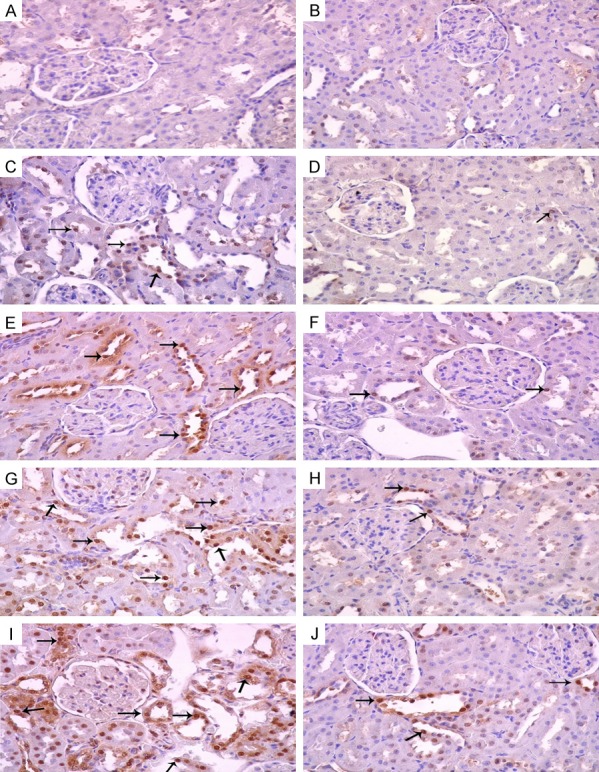
Representative photograph of sections of renal tissue of control rats (A) and rats that have been treated with gum acacia, (GA) (B), potassium bromate at doses of 5, 15, 45 and 135 mg/kg (C, E, G and I), respectively, and GA plus potassium bromate at doses of 5, 15, 45 and 135 mg/kg (D, F, H and J), respectively. Arrows indicate apoptotic cells.
Figure 7 shows the immunohistochemical analysis of some renal sections (anticaspase-3, streptavidin-biotin immunohistochemical method). Control and GA-treated groups had few caspase 3-positive cells. KBrO3-treated groups showed dose-dependent increases in caspase 3-positive cell. GA significantly reduced the caspase 3-positive cells when it was co-administered with KBrO3.
Figure 7.
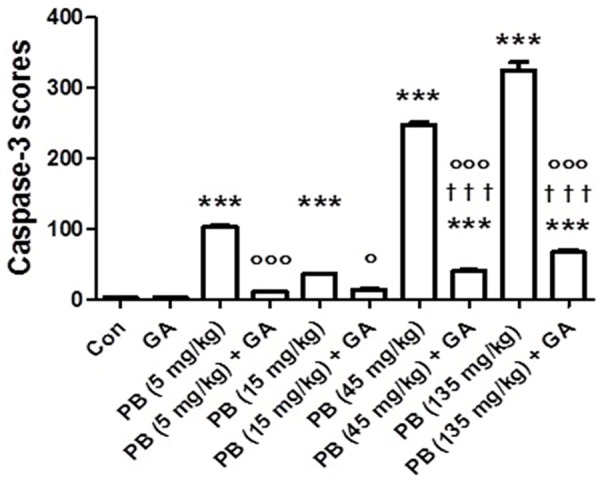
Immunohistochemistry scores of caspase-3 (the number of caspase-3 positive cells per mm2 of kidney tissue) in control (Con) rats and rats treated with different doses of KBrO3 (PB) with or without gum acacia (GA). Each column and vertical bar represents mean ± SEM (n = 6). Different superscripts indicate significance as follows: *denotes significance of different groups vs. Control group: where ***P < 0.0001. †denotes significance of GA alone vs. its corresponding groups treated with PB: where †††P < 0.0001. °denotes significance of groups treated with PB alone vs. its corresponding groups treated with GA: where °P < 0.05, °°°P < 0.0001.
Discussion
Several investigators reported that KBrO3 induced renal impairments in different animal species and strains, and at various doses [27,28]. Others, however, have found little or no evidence of renal impairments in Fischer 334 rats [29]. Our aim here was to study the nephrotoxicity of this agent in male rats of a strain (Sprague-Dawley) that has been used before only once at a single oral dose, using a wider range of oral doses (5 to 135 mg/kg) than has been reported by most researchers before [24]. We also wanted to test if co-treatment with a natural dietary prebiotic GA, previously reported in rodents and humans to abate the severity of gentamicin acute kidney injury (AKI), adenine-induced CKD and CKD patients undergoing hemodialysis, can also mitigate KBrO3 nephrotoxicity [17,24-30].
Taken together, our results indicated that oral administration of KBrO3 at doses of 45 and 135 mg/kg/day for 28 days induced significant renal impairment in male Sprague-Dawley rats. Lower doses (5 and 35 mg/kg/day), however, did not produce significant indication of nephrotoxicity. The literature on KBrO3 nephrotoxicity revealed several discrepancies in the doses of KBrO3 required to induce renal impairment. For example, Ahmad et al. used a single aqueous dose of 100 mg/kg of KBrO3 in adult male Wistar rats and found that it produced several signs of nephrotoxicity, apparently more evident than what we have found with 135 mg/kg/day dose used in this work [21]. On the other hand, Khan et al. reported that KBrO3 in male Sprague-Dawley rats (same strain used in this work) when given at an oral of 20 mg/kg twice a week for 28 days produced severer renal impairments than that found in this work [13]. The reasons for this (and other) discrepancies are not known, but may be due to strain differences, experimental conditions, or to other unknown causes.
The decrease in body weight following KBrO3 treatment may possibly be ascribed to the injured renal tubules, and the ensuing loss of the tubular cells to reabsorb water, leading to dehydration and decrease in body weight. The above action, and the observed polyuria might be the reasons for the loss of body weight.
Several attempts to find possible protective agents against KBrO3-induced organ toxicity, particularly nephrotoxicity, have been reported. The agents tested included rutin, taurine and Nymphaeaalba L [12,13,28]. The common denominator among these agents is that they have strong anti-oxidant capabilities, and it is known that a major mechanism of KBrO3-induced nephrotoxicity is by the production of ROS, which initiates lipid peroxidation and decreases the enzymatic and non-enzymatic antioxidants, an action that will finally culminate in oxidative stress [8,31-32].
In the past few years, several new renal, plasma and urinary nephrotoxicity biomarkers have been tested and approved for use in preclinical studies by the Food and Drug Administration and the European Medicines Agency. In this work, we used both conventional and novel biomarkers, to detect early AKI. There is great interest among nephrologists and scientists to identify novel and sensitive biomarkers for the early detection of signs and symptoms of AKI, especially in the urine, as this biological fluid can easily be obtained non-invasively and in ample quantities [18,33,34]. Among these are pro-inflammatory TNF-α, capspase-3, NAGAL, NAG, clusterin and adiponectin [33,34]. These were dose dependently raised by KBrO3 indicating earlier postulated mechanism of renal damage via the generation of free radicals [10,11]. GA, which is well known for its anti-inflammatory and antioxidative properties, significantly and insignificantly reduced the observed elevation in these biomarkers when it was concomitantly administrated with KBrO3 [15,18,20,24].
In this work, GA was effective in ameliorating several of the parameters measured that have been adversely affected by KBrO3 especially at the higher doses. This may be ascribed to the strong anti-oxidant properties of this agent, as GA is a known dietary prebiotic with an established safety profiles in humans, its inclusion in the edible items that may contain KBrO3, such as flour, bread or cakes may be beneficial, especially in countries that allow the use of KBrO3 in food products, or do not enforce laws regarding its banning [16,23,35]. Further studies exploring the ameliorative and possibly the therapeutic effect of GA on KBrO3 toxicity in other animal strains and body organs are needed.
Acknowledgements
This work was supported by an internal grant from Sultan Qaboos University (IG/MED/PHAR/15/01). Thanks to Professor Gerald Blunden for reading the manuscript, and to the Sultan Qaboos University Animal House staff for looking after the animals used in this work.
Disclosure of conflict of interest
None.
References
- 1.Environmental Protection Agency. Toxicological review of bromate. Washington, DC, USA: Environmental Protection Agency; 2001. (CAS No. 15541-45-4) [Google Scholar]
- 2.Oloyede OB, Sunmonu TO. Potassium bromate content of selected bread samples in Ilorin, central nigeria and its effect on some enzymes of rat liver and kidney. Food Chem Toxicol. 2009;47:2067–2070. doi: 10.1016/j.fct.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Kakehashi A, Wei M, Fukushima S, Wanibuchi H. Oxidative stress in the carcinogenicity of chemical carcinogens. Cancers (Basel) 2013;5:1332–1354. doi: 10.3390/cancers5041332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher N, Hutchinson JB, Berry R, Hardy J, Ginocchio AV, Waite V. Long-term toxicity and carcinogenicity studies of the bread improver potassium bromate 1. Studies in rats. Food Cosmet Toxicol. 1979;17:33–39. doi: 10.1016/0015-6264(79)90156-1. [DOI] [PubMed] [Google Scholar]
- 5.Wolf DC, Crosby LM, George MH, Kilburn SR, Moore TM, Miller RT, DeAngelo AB. Time- and dose-dependent development of potassium bromate-induced tumors in male Fischer 344 rats. Toxicol Pathol. 1998;26:724–729. doi: 10.1177/019262339802600602. [DOI] [PubMed] [Google Scholar]
- 6.Bayomy NA, Soliman GM, Abdelaziz EZ. Effect of potassium bromate on the liver of adult male albino rat and a possible protective role of vitamin C: histological, immunohistochemical, and biochemical study. Anat Rec (Hoboken) 2016;299:1256–1269. doi: 10.1002/ar.23386. [DOI] [PubMed] [Google Scholar]
- 7.Ajarem J, Altoom NG, Allam AA, Maodaa SN, Abdel-Maksoud MA, Chow BK. Oral administration of potassium bromate induces neurobehavioral changes, alters cerebral neurotransmitters level and impairs brain tissue of Swiss mice. Behav Brain Funct. 2016;12:14. doi: 10.1186/s12993-016-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spassova MA, Miller DJ, Nikolov AS. Kinetic modeling reveals the roles of reactive oxygen species scavenging and DNA repair processes in shaping the dose-response curve of KBrO3-induced DNA damage. Oxid Med Cell Longev. 2015;2015:764375. doi: 10.1155/2015/764375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurokawa Y, Takamura N, Matsuoka C, Imazawa T, Matsushima Y, Onodera H, Hayashiet Y. Comparative studies on lipid peroxidation in the kidney of rats, mice and hamsters and on the effect of cysteine, glutathione and diethylmaleate treatment on mortality and nephrotoxicity after administration of potassium bromate. Int J Toxicol. 1987;6:489–501. [Google Scholar]
- 10.Sai K, Uchiyama S, Ohno Y, Hasegawa R, Kurokawa Y. Generation of active oxygen species in vitro by the interaction of potassium bromate with rat kidney cell. Carcinogenesis. 1992;13:333–339. doi: 10.1093/carcin/13.3.333. [DOI] [PubMed] [Google Scholar]
- 11.Chipman JK, Davies JE, Parsons JL, Nair J, O’Neill G, Fawell JK. DNA oxidation by potassium bromate; a direct mechanism or linked to lipid peroxidation? Toxicology. 1998;126:93–102. doi: 10.1016/s0300-483x(97)00174-1. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad MK, Khan AA, Mahmood R. Taurine ameliorates potassium bromate-induced kidney damage in rats. Amino Acids. 2013;45:1109–1121. doi: 10.1007/s00726-013-1563-4. [DOI] [PubMed] [Google Scholar]
- 13.Khan RA, Khan MR, Sahreen S. Protective effects of rutin against potassium bromate induced nephrotoxicity in rats. BMC Complement Altern Med. 2012;12:204. doi: 10.1186/1472-6882-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan M, Sultana S. Inhibition of KBRO3 induced renal oxidative stress and hyperproliferative response by Nymphaea alba in Wistar rats. J Enzyme Inhib Med Chem. 2005;20:275–83. doi: 10.1080/14756360400028119. [DOI] [PubMed] [Google Scholar]
- 15.Ali BH, Ziada A, Blunden G. Biological effects of gum arabic: a review of some recent research. Food Chem Toxicol. 2009;47:1–8. doi: 10.1016/j.fct.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Calame W, Weseler AR, Viebke C, Flynn C, Siemensma AD. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br J Nutr. 2008;100:1269–1275. doi: 10.1017/S0007114508981447. [DOI] [PubMed] [Google Scholar]
- 17.Ali BH, Al-Salam S, Al Za’abi M, NWaly MI, Ramkumar A, Beegam S, Al-Lawati I, Adham SA, Nemmar A. New model for adenine-induced chronic renal failure in mice, and the effect of gum acacia treatment thereon: comparison with rats. J Pharmacol Toxicol Methods. 2013;68:384–393. doi: 10.1016/j.vascn.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Ali BH, Al-Husseni I, Beegam S, Al-Shukaili A, Nemmar A, Schierling S, Queisser N, Schupp N. Effect of gum arabic on oxidative stress and inflammation in adenine-induced chronic renal failure in rats. PLoS One. 2013;8:e55242. doi: 10.1371/journal.pone.0055242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali AA, Ali KE, Fadlalla AE, Khalid KE. The effects of gum arabic oral treatment on the metabolic profile of chronic renal failure patients under regular haemodialysis in Central Sudan. Nat Prod Res. 2008;22:12–21. doi: 10.1080/14786410500463544. [DOI] [PubMed] [Google Scholar]
- 20.Al Za’abi M, Al Busaidi M, Yasin J, Schupp N, Nemmar A, Ali BH. Development of a new model for the induction of chronic kidney disease via intraperitoneal adenine administration, and the effect of treatment with gum acacia thereon. Am J Transl Res. 2015;7:28–38. [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad MK, Naqshbandi A, Fareed M, Mahmood R. Oral administration of a nephrotoxic dose of KBRO3, a food additive alters renal redox and metabolic status and inhibits brush border membrane enzymes in rats. Food Chem. 2012;134:980–5. doi: 10.1016/j.foodchem.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Ballmaier B, Epe B. Oxidative DNA damage induced by potassium bromate under cell-free conditions and in mammalian cells. Carcinogenesis. 1995;16:335–342. doi: 10.1093/carcin/16.2.335. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad MK, Khan AA, Ali SN, Mahmood R. Chemoprotective effect of taurine on potassium bromate-induced DNA damage, DNA-protein cross-linking and oxidative stress in rat intestine. PLoS One. 2015;10:e0119137. doi: 10.1371/journal.pone.0119137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali BH, Al Balushi K, Al-Husseini I, Mandel P, Nemmar A, Schupp N, Ribeiro DA. Gum acacia mitigates genetic damage in adenine-induced chronic renal failure in rats. Eur J Clin Invest. 2015;45:1221–1227. doi: 10.1111/eci.12501. [DOI] [PubMed] [Google Scholar]
- 25.Ali BH, Al-Salam S, Al Za’abi M, Al Balushi KA, Ramkumar A, Waly MI, Yasin J, Adham SA, Nemmar A. Does swimming exercise affect experimental chronic kidney disease in rats treated with gum acacia? PLoS One. 2014;9:e102528. doi: 10.1371/journal.pone.0102528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Suleimani YM, Al Mahruqi AS, Al Za’abi M, Shalaby A, Ashique M, Nemmar A, Ali BH. Effect of diesel exhaust particles on renal vascular responses in rats with chronic kidney disease. Environ Toxicol. 2015;19:498–506. doi: 10.1002/tox.22258. [DOI] [PubMed] [Google Scholar]
- 27.Topcu-Tarladacalisir Y, Sapmaz-Metin M, Karaca T. Curcumin counteracts cisplatin-induced nephrotoxicity by preventing renal tubular cell apoptosis. Ren Fail. 2016;38:1741–1748. doi: 10.1080/0886022X.2016.1229996. [DOI] [PubMed] [Google Scholar]
- 28.Ben Saad H, Driss D, EllouzChaabouni S, Boudawara T, Zeghal KM, Hakim A, Ben Amara I. Vanillin mitigates potassium bromate-induced molecular, biochemical and histopathological changes in the kidney of adult mice. Chem Biol Interact. 2016;25:102–113. doi: 10.1016/j.cbi.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Dodd DE, Layko DK, Cantwell KE, Willson GA, Thomas RS. Subchronic toxicity evaluation of potassium bromate in Fischer 344 rats. Environ Toxicol Pharmacol. 2013;36:1227–1234. doi: 10.1016/j.etap.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Al-Majed AA, Mostafa AM, Al-Rikabi AC, Al-Shabanah OA. Protective effects of oral arabic gum administration on gentamicin-induced nephrotoxicity in rats. Pharmacol Res. 2002;46:445–451. doi: 10.1016/s1043661802001251. [DOI] [PubMed] [Google Scholar]
- 31.Nishioka H, Fujii H, Sun B, Aruoma OI. Comparative efficacy of oligonol, catechin and (-)-epigallocatechin 3-O-gallate in modulating the potassium bromate-induced renal toxicity in rats. Toxicology. 2006;226:181–187. doi: 10.1016/j.tox.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Abd-Allah AR, Al-Majed AA, Mostafa AM, Al-Shabanah OA, Din AG, Nagi MN. Protective effect of arabic gum against cardiotoxicity induced by doxorubicin in mice: a possible mechanism of protection. J Biochem Mol Toxicol. 2002;16:254–259. doi: 10.1002/jbt.10046. [DOI] [PubMed] [Google Scholar]
- 33.Ennulat D, Adler S. Recent successes in the identification, development, and qualification of translational biomarkers: the next generation of kidney injury biomarkers. Toxicol Pathol. 2015;43:62–69. doi: 10.1177/0192623314554840. [DOI] [PubMed] [Google Scholar]
- 34.Al Za’abi M, Ali BH, AL Othman ZA, Ali I. Analyses of acute kidney injury biomarkers by ultra-high performance liquid chromatography with mass spectrometry. J Sep Sci. 2016;39:69–82. doi: 10.1002/jssc.201500982. [DOI] [PubMed] [Google Scholar]
- 35.Ali BH, Inuwa I, Al Za’abi M, Al Bahlani S, Al Issaei H, Ramkumar A, Madanagopal T, Nemmar A, Malheiros DM, Zatz R. Renal and myocardial histopathology and morphometry in rats with adenine-induced chronic renal failure: influence of gum acacia. Cell Physiol Biochem. 2014;34:818–828. doi: 10.1159/000363045. [DOI] [PubMed] [Google Scholar]