Abstract
The reprogramming of fibroblasts to induced pluripotent stem cells raises the possibility that a somatic cell can be reprogrammed to an alternative, differentiated fate without first becoming a stem/progenitor cell. Recent work has shown that fibroblasts can be reprogrammed to other, terminally differentiated cells with a combination of several transcription factors. Here, we report that a combination of four developmental transcription factors; GATA4, SF-1, NGFI-B, and COUP TF2; efficiently reprogrammed human foreskin fibroblasts into functional induced Leydig-like cells (iLCs). The iLCs expressed Leydig-specific markers and secreted testosterone in vitro. We found that GATA4 and SF-1 were particularly critical for Leydig-specific markers expression and that GATA4, SF-1, and NGFI-B were necessary to generate functional iLCs that secreted testosterone. These findings demonstrate that fibroblasts can be directly converted into iLCs with a few, defined factors and may provide insight into potential therapies to treat testosterone deficiency.
Keywords: Human foreskin fibroblasts, induced Leydig-like cells, reprogramming
Introduction
Leydig cells are the primary source of testosterone in males. Testosterone is essential for development of the male reproductive system and maintenance of male reproductive functions [1-3]. Testosterone deficiency in adults is associated with increased body fat, decreased muscle mass, increased fatigue, depressed mood, decreased cognitive function [4], and reduced immune response [5]. Thus, the formation and maintenance of a functional Leydig cell population throughout adult life is of fundamental importance. Since 1940, testosterone therapy has been used clinically in men and women [6]. However, most of these patients require therapy throughout their lives and are at risk of experiencing a number of side effects [7-10].
Leydig-like cells, differentiated from stem cells, including stem Leydig cells (SLCs) [11], mesenchymal stem cells (MSCs) [12-14], embryonic stem cells (ESCs) [15-17], as well as induced pluripotent stem cells (iPSCs) [18], have a steroidogenic capacity. However, the approach is limited by the small number of stem cell sources, the low efficiency of differentiation, the risk of tumor formation, and the possibility of cellular rejection.
The generation of iPSCs suggests that a specific combination of defined factors could epigenetically alter the global gene expression of a cell and allow greater plasticity of cell type. In fact, since the groundbreaking discovery of the iPSCs [19], numerous approaches of direct cell reprogramming have been documented, culminating in the development of induced cellular types for neurons [20], cardiomyocytes [21-23], hepatocytes [24,25], oligodendrocyte precursors [26], and astrocytes [27]. In addition, adult, pancreatic exocrine cells have been reprogrammed to B-cells in vivo [28]. We consequently propose that differentiated somatic cells can be directly reprogrammed into functional iLCs by defined factors.
In this study, we examined whether key developmental Leydig regulators could reprogram human foreskin fibroblasts (HFFs) into iLCs. Four candidate factors; GATA4, SF-1, NGFI-B, and COUP-TF2; were screened and we identified a specific combination of three transcription factors; SF-1, GATA4 and NGFI-B (SGN pool); that was sufficient to generate functional iLCs in vitro directly from HFFs.
Materials and methods
Cell culture and transfection
We obtained the foreskin of healthy boys from the operating room of Shanghai Children’s Medical Center. The foreskin was washed alternately with chloramphenicol lotion and phosphate buffer saline (PBS; HyClone) several times. The corium layer and subcutaneous connective tissue was pruned away. Then, the tissue was minced into 3-4 mm pieces with a sterile scalpel and incubated with collagenase I (Sigma Aldrich) solution [1-2 mg/ml in DMEM/F12 (HyClone)] for 4-18 hours at 37°C. The dispersed cells were passed through a 100-nylon mesh and washed several times by centrifugation in PBS. After the final wash step, the cell pellet was resuspended in DMEM/F12. Cells were seeded into culture vessels with DMEM/F12 containing 10% fetal bovine serum (HyClone) and 1% penicillin-streptomycin (HyClone). The culture medium was changed every 2 days. We chose the first three generations of fibroblasts for transfection, which were infected with GATA4, SF-1, NGFI-B, and COUP TF2 (4F pool) overnight, and, subsequently, green fluorescence protein (GFP) gene expression was monitored by fluorescence microscopy.
Plasmid construction
Human GATA4, SF-1, NGFI-B, and COUP TF2 cDNA were amplified by polymerase chain reaction (PCR) using the primers provided in Table S1. The transcription factors were cloned into the lentiviral pGMLV-CMV-MCS-EF1-Zs Green 1 Vector (Genomeditech), and confirmed by sequencing. Four lentiviral particles were packaged with two other homologous helper plasmids into NIH 293T cells to generate lentivirus following the manufacturer’s protocol.
RNA extraction and quantitative RT-PCR
Total RNA was isolated with TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. One microgram of total RNA was reverse transcribed into cDNA using the PrimescriptTM RT Reagent kit (TaKaRa). Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) was used to perform the quantitative RT-PCR (qRT-PCR) assay. The primers that were used are listed in Table S2. The Stratagene Mx3000P system (Agilent Technologies) with MxPro QPCR Software was used to collect the PCR data.
Western blotting
Western blot analysis was conducted as below in brief, cells were lysed in Pierce IP Lysis Buffer (Thermo) in the presence of a 1% protease inhibitor. Protein samples were normalized for protein concentration and applied to a 10% SDS-PAGE gel. Twenty µg of each protein were analyzed. For immunoblotting analysis, proteins in the SDS gels were transferred to a polyvinylidene difluoride membrane by an electroblot apparatus. The membranes were blocked with a blocking solution (5% nonfat dry milk protein solution in Tris-buffered saline solution containing 0.1% Tween 20, TBS-T). The membranes were incubated with primary antibodies (CYP11A1, ab75497; CYP17A1, ab125022) in the blocking solution at 4°C overnight, washed with TBS-T six times (5 min each), and incubated with secondary antibodies at room temperature for 1 h. The membranes were then washed with TBS-T three times (5 min each) and subjected to enhanced chemiluminescence detection. Protein expression was normalized to b-Actin (ab8227).
Cell proliferation assay by WST-8
Cells were plated into 96 well plates at a density of 1500 per well at day 0. On days 1, 2, and 3, 10 μl of WST-8 reagent (Dojindo, Mashiki-machi, Kumamoto, Japan) were added to each well, the cells were incubated at 37°C for 4 h, and then absorbance at 450 nm was measured by a microplate reader (Synergy 2, BioTek).
Immunofluorescence
For immunofluorescence experiments, cells were fixed in methyl alcohol for 10 min and washed thrice in PBS. Cells were blocked in a solution of PBS containing 1% bovine serum albumin (BSA; Sigma) and 0.25% Triton X-100 (Sigma) for 30 min at room temperature. The primary and secondary antibodies were diluted in solutions of PBS containing 1% BSA and 0.25% Triton X-100. Primary antibodies (CYP11A1, ab75497; CYP17A1, ab125022; HSD3B1, ab 167417) were incubated overnight at 4°C followed by incubation with secondary antibodies for 1 h at room temperature. Nuclei were stained with DAPI (Invitrogen).
Oil Red O staining
Cell culture medium was completely removed, and the cells were rinsed with PBS. After complete aspiration of the PBS, 10% formaldehyde solution was added to the cells, and they were incubated for 10 min at room temperature. After removing the formaldehyde solution, the fixed cells were gently washed with PBS. The Oil Red O solution was added to the wells, and the samples were incubated for 60 min at room temperature, washed with PBS, and hematoxylin stained.
Testosterone concentration assay
Concentrations of testosterone in the culture medium were measured with the Access Testosterone assay (BECKMAN COULTER).
Statistical analyses
All experiments were performed at least three times. Log 10 transformation was applied to the qRT-PCR data and they are expressed as mean ± one standard deviation of the mean. Statistical analyses were performed with a paired t-test. The alpha value level for t-test statistical significance was P < 0.05.
Results
A screen for Leydig-fate-inducing factors
Multiple transcription factors are presumably required to reprogram fibroblasts to Leydig-like cells. We therefore cloned a total of four genes, referred to as the 4F pool, that have important roles in Leydig cell development or that have been implicated in epigenetic reprogramming. A pool of lentiviruses containing all four genes was prepared to infect HFFs obtained from healthy children. To characterize the dynamics involved in Leydig-like cell reprogramming, we analyzed the conversion at different time points by quantitative RT-PCR (qRT-PCR) (Figure 1B-E). The results revealed that the expression of mRNA encoding the steroidogenic enzymes, including steroidogenic acute regulatory protein, cytochrome P450 family 11 subfamily A member 1 (CYP11A1), cytochrome P45017A1 (CYP17A1), 3 beta- and steroid delta-isomerase 1 (HSD3B1), was increased in iLCs as compared with cells that received the mock treatment, with epithelial cell marker E-Cadherin and mesenchymal cell marker Vimentin changing indistinctively (Figure 1B-E). The expression of these enzymes is required for the synthesis of gonadal steroid hormones. Consistent with qRT-PCR, Western blot showed that at 3 and 4 weeks after infection, CYP11A1 and CYP17A1 expression was maintained (Figure 1F, 1G). The full figure of western blot is shown in Figure S5. We consequently believe that GATA4, SF-1, NGFI-B, and COUP TF2 induce fibroblasts toward the Leydig lineage. We also characterized the expression of the four transgenes in iLCs at different time points. qRT-PCR confirmed that there was a pronounced upregulation of the total amount of mRNA for the four genes derived from the endogenous genes and the transgenes in the first week, with SF-1 rising more than fifteen fold, GATA4 thirteen fold, NGFI-B sevenfold, COUP TF2 fivefold in iLCs relative to HFFs transfected with GFP (Figure 2). The total mRNA for the four genes in iLCs then declined gradually toward the levels in HFFs at 4 weeks, indicating that the TFs would be silenced after reprogramming was achieved.
Figure 1.
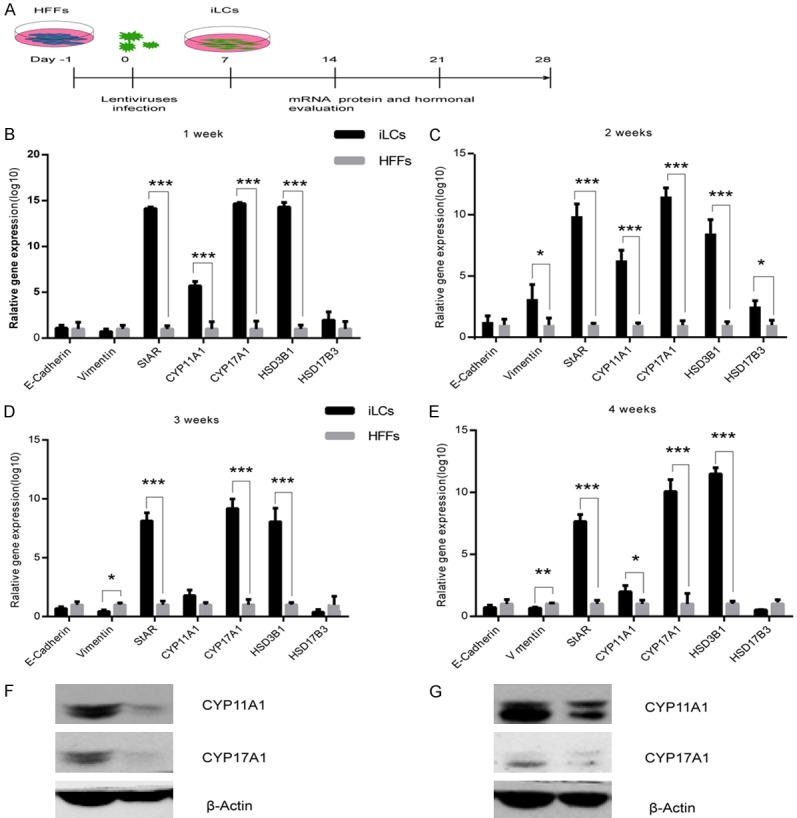
Gene expression analysis of the induced Leydig-like cells (iLCs). A: Scheme of the experimental procedures. B-E: Kinetics analysis of the expression of key genes related using quantitative RT-PCR. The copy number of the mRNA of each gene was normalized to that of the housekeeping gene GAPDH. Data represent mean ± standard deviation (SD) of triplicate experiments. **P < 0.01, ***P < 0.001 as compared with the group of HFFs transfected with GFP. F and G: Western blotting for protein expression of CYP11A1 and CYP17A1 in iLCs at 3 and 4 weeks after infection.
Figure 2.
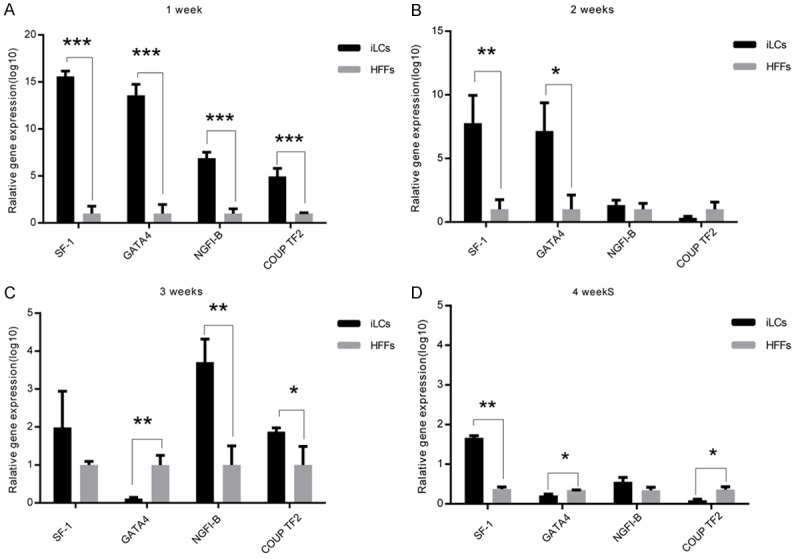
Kinetic analysis of the expression of the four transgenes. The copy number of the mRNA of each gene was normalized to that of the housekeeping gene GAPDH. Data represent mean ± standard deviation (SD) of triplicate experiments. *P < 0.05, **P < 0.01, ***P < 0.001 as compared with the group of HFFs. (A) 1 week after transfection, (B) 2 weeks after transfection, (C) 3 weeks after transfection, and (D) 4 weeks after transfection.
Characterization of 4-factor Leydig-like cells
The iLCs changed their morphology to one that was smaller and rounder relative to primary HFFs (Figure 3A, 3B). As shown in Figure 3C, in the first 3 days after transfection, the iLCs group exhibited a faster growth rate than the control group and then remained constant. Removal of GATA4 resulted in growth retardation, while removal of the other factors yielded comparable growth rates (Figure 3D). These results suggest that GATA4 plays an important role during cell proliferation, while the other 3 factors may have inhibitive effects. GFP expression was used as an indicator of the expression of the four factors in the iLCs. As assessed by flow cytometry, 57.09% of the total induced fibroblasts were GFP+ (Figure S1). The expression of Leydig-specific markers; specifically HSD3B1, CYP11A1, and CYP17A1; was examined by immunostaining. Some of the cells identified as GFP+ expressed Leydig-specific genes (Figure 4). The cells were also positive for Oil Red O staining confirming that the iLCs had the lipid metabolism process (Figure 5). To determine if the Leydig-like cells possessed the functional properties characteristic of adult Leydig cells, we measured the hormones secreted into the medium at time points of 1, 2, 3, and 4 weeks after transfection. At 2 weeks, the testosterone level reached a peak of 1.90 ng/ml and then decline to the low ebb of 0.147 ng/ml at 4 weeks. In comparison, testosterone could not be detected in the control group (Figure 6). Upon closer examination, testosterone was first detected on day 3 and strongly up-regulated on day 6 until the peak level of 3.69 ng/ml on day 12 (Figure 6). These observations suggest that the combination of four factors transduced a number of HFFs into functional Leydig-like cells.
Figure 3.
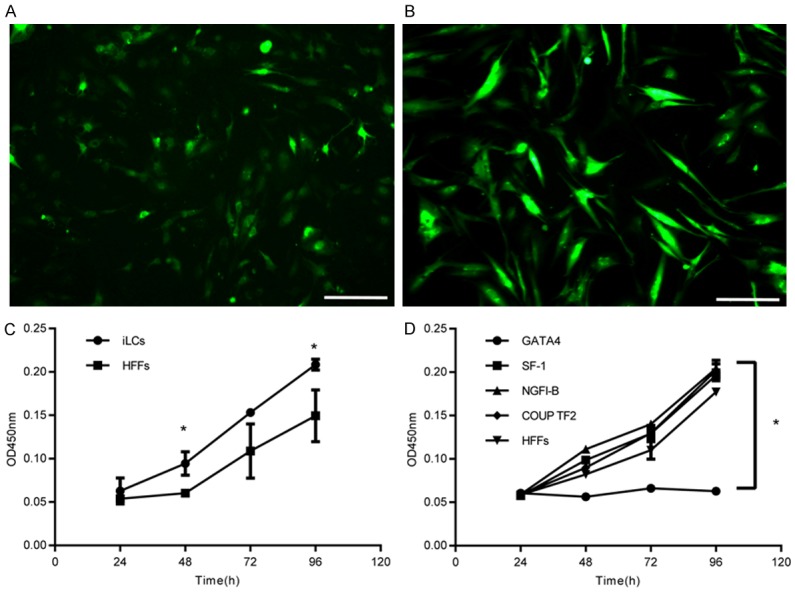
The morphology of iLCs under the microscope and the growth rate. (A) iLCs of 2 weeks under the fluorescence microscope. (B) HFFs transfected with GFP at 2 weeks after transfection. Scale bars in (A and B) represent 200 µm. (C and D) Cell growth assay at day 3 after transfection. Data represent mean ± SD of triplicate experiments. *P < 0.05 as compared with the group of HFFs. iLCs refers to the 4TFs group; HFFs refers to the control group; and GATA4, SF-1, NGFI-B, and COUP TF2, respectively, refer to removing each of the 4 TFs.
Figure 4.
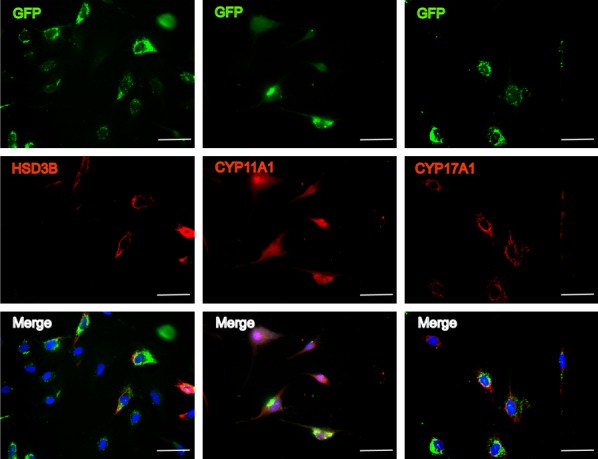
Immunofluorescent staining of HSD3B1, CYP11A1, and CYP17A1. The results confirmed the expression of the Leydig steroidogenic markers at 2 weeks after infection. Nuclei were stained with DAPI (blue). Scale bars, 50 µm.
Figure 5.
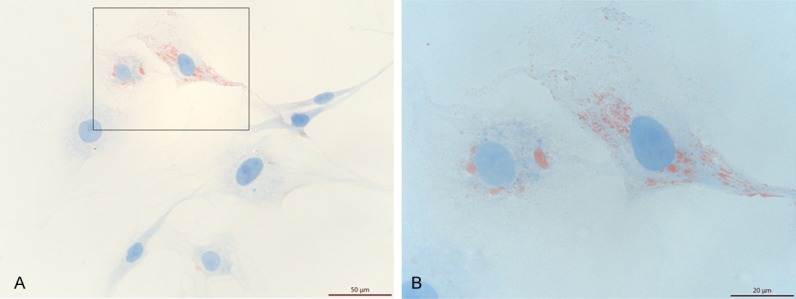
Oil red O staining of iLCs at 2 weeks after infection.
Figure 6.
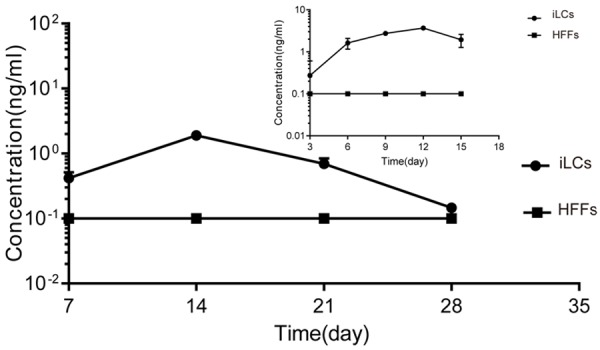
Analysis of testosterone production during culture. The line at 0.1 ng/ml represents the test sensitivity of the Access Testosterone kit. The testosterone concentration of the HFFs was under the line. All quantitative data were obtained from three independent experiments and are presented as mean ± SD.
GATA4, SF-1, and NGFI-B are sufficient for Leydig-like cell induction
The relative contribution of each of the four genes toward the generation of Leydig-like cells was determined by removing each gene from the pool and assessing the efficiency of reprogramming. Only the omission of COUP TF2 had little effect on cell culture supernatant testosterone levels (Figure S2). As shown in Figure S3, although removal of any gene from the 4F pool did not affect Leydig-specific markers expression, the mRNA levels were significantly impacted. Removal of GATA4 or SF-1 resulted in obviously changes in mRNA, with SF-1 mRNA levels reduced to a greater extent than GATA4 mRNA levels.
Discussion
In this study, HFFs were successfully converted to iLCs with 4 TFs (GATA4, SF-1, NGFI-B, and COUP TF2). GATA4 is a member of GATA-binding protein family of transcription factors with zinc fingers, is involved in the embryonic development of various organs and plays a crucial role in hematopoiesis [29], cardiogenesis [30] and in the early development of the testis in male sexual differentiation and in steroidogenesis [31]. SF-1, also known as steroidogenic factor-1, belongs to the nuclear receptor superfamily and is a tissue-specific regulator of the transcription of a smart of genes that are involved in steroidogenesis, reproduction, and male sexual differentiation [32,33]. Previous work suggests that SF-1 can initiate a genetic program that enables ESCs, MSCs, and iPSCs to acquire steroidogenic capacity and then produce a variety of steroidal hormones [13,16-18,34]. NGFI-B regulates testosterone production of Leydig cells in the rest state or when subjected to hormone stimulation [35,36]. Qin J et al. find that COUP TF2 plays roles in progenitor Leydig cell formation and early testis organogenesis. The ablation of COUP TF2 during pre-pubertal stages of male development results in infertility, hypogonadism and spermatogenetic arrest [37]. Herein, we provided evidence that these factors can activate the expression of mature Leydig-specific markers, such as CYP11A1, CYP17A1, HSD3B1, and hydroxysteroid 17-beta dehydrogenase 3 (HSD17B3), necessary for the acquisition of the excretory functional properties of mature Leydig cells.
Unlike iPSCs [19], we found that the conversion to iLCs in our study was efficient and fast, with the excretion function starting at day 3 and reaching peak levels at day 12 after transfection. The latter result is consistent with other work on reprogramming, for example, neuronal induction [20]. Based upon this phenomenon, the reprogramming likely occurs without first passing the pluripotency stage. Consistent with this, no expression of the pluripotency markers OCT4, SOX2, or NANOG was detected at day 3 after transfection (Figure S4).
In addition, the iLCs did not appear to require retroviral gene silencing and became independent of the expression of the exogenous transgenes, as indicated by a reduction in the expression level of the 4 TFs over the course of time. As the 4F pool primarily plays a role during the development of Leydig cells, expression in adult Leydig cell is generally low. Potentially, auto-regulatory feedback and feed forward activation of downstream transcriptional regulators could reinforce the expression of important cell-fate-determining genes and help to stabilize the induced transcriptional program. Once reprogramming was achieved, the TFs were silenced within the iLCs with the need for outside interference.
As stated earlier, our qRT-PCR results indicated that when COUP TF2 or NGFI-B was removed, the relevant mRNA level was comparative to the 4F pool, whereas when SF-1 was removed, the relevant mRNA level declined significantly, approximately tenfold lower than the level achieved by removing GATA4 and approximately one hundredfold lower than the 4F pool. This result suggests that SF-1 plays a critical role in the reprogramming course. GATA4 is also important, potentially because of its effect on cell proliferation. Furthermore, COUP TF2 was the only gene from the 4F pool that had a little effect on testosterone secretion; and there was no difference in testosterone levels when comparing the 4F and SGN pool at the 1 week time point after transfection. Thus, efficient conversion was achieved with the three factors of the SGN pool.
Although we succeeded in converting fibroblasts to iLCs in vitro, the conversion seemed to be transient, with the Leydig-specific markers transiently expressed and the testosterone level reaching a transient peak. We also lack a sorting method to obtain pure fully reprogrammed passage cells. Future studies will focus on using the knock-in technique to sort the reprogrammed cells to obtain a stable cell line for clinical use. Despite these limitations, the generation of iLCs is fast, of high-efficiency, and devoid of tumorigenic pluripotent stem cells, a severe complication of induced pluripotent stem cell approaches in regenerative medicine.
In general, this study demonstrated that somatic cells, such as HFFs, can be converted into functional Leydig-like cells through overexpression of only a minimal set of the three factors, GATA4, SF-1, NGFI-B. Furthermore, the iLCs may prove to be a novel and powerful system for studying cellular plasticity, disease modeling, and regenerative medicine.
Acknowledgements
This research was supported by the grants from the National Natural Science Foundation of China (81270689) and the municipal health and family planning commission of Shanghai (201440034). We are grateful for Professor Zhen Zhang’s assistance with the experimental methods.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Chen H, Ge RS, Zirkin BR. Leydig cells: from stem cells to aging. Mol Cell Endocrinol. 2009;306:9–16. doi: 10.1016/j.mce.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2–13. doi: 10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Midzak AS, Chen H, Papadopoulos V, Zirkin BR. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol Cell Endocrinol. 2009;299:23–31. doi: 10.1016/j.mce.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014;16:192–202. doi: 10.4103/1008-682X.122336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobjer J, Katrinaki M, Tsatsanis C, Lundberg Giwercman Y, Giwercman A. Negative association between testosterone concentration and inflammatory markers in young men: a nested cross-sectional study. PLoS One. 2013;8:e61466. doi: 10.1371/journal.pone.0061466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesser MA. Testosterone propionate therapy in one hundred cases of angina pectoris. J Clin Endocrinol Metab. 1946;6:549–557. doi: 10.1210/jcem-6-8-549. [DOI] [PubMed] [Google Scholar]
- 7.Traish A. Testosterone therapy in men with testosterone deficiency: are we beyond the point of no return? Investig Clin Urol. 2016;57:384–400. doi: 10.4111/icu.2016.57.6.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thirumalai A, Berkseth KE, Amory JK. Treatment of hypogonadism: current and future therapies. F1000Res. 2017;6:68. doi: 10.12688/f1000research.10102.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieschlag E, Behre HM, Bouchard P, Corrales JJ, Jones TH, Stalla GK, Webb SM, Wu FC. Testosterone replacement therapy: current trends and future directions. Hum Reprod Update. 2004;10:409–419. doi: 10.1093/humupd/dmh035. [DOI] [PubMed] [Google Scholar]
- 10.Lanfranco F, Zitzmann M, Simoni M, Nieschlag E. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol (Oxf) 2004;60:500–507. doi: 10.1111/j.1365-2265.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 11.Peak TC, Haney NM, Wang W, DeLay KJ, Hellstrom WJ. Stem cell therapy for the treatment of Leydig cell dysfunction in primary hypogonadism. World J Stem Cells. 2016;8:306–315. doi: 10.4252/wjsc.v8.i10.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing X, Zhang Z, Zhong L, Ju G, Zou X, Zhu Y, Sun J. Differentiation of human umbilical cord mesenchymal stem cells into steroidogenic cells in vitro. Exp Ther Med. 2016;12:3527–3534. doi: 10.3892/etm.2016.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gondo S, Okabe T, Tanaka T, Morinaga H, Nomura M, Takayanagi R, Nawata H, Yanase T. Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of steroidogenic factor 1. Endocrinology. 2008;149:4717–4725. doi: 10.1210/en.2007-1808. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZY, Xing XY, Ju GQ, Zhong L, Sun J. Mesenchymal stem cells from human umbilical cord ameliorate testicular dysfunction in a male rat hypogonadism model. Asian J Androl. 2016;19:543–547. doi: 10.4103/1008-682X.186186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Su Z, Xu W, Luo J, Liang R, Xiang Q, Zhang Q, Ge RS, Huang Y. Directed mouse embryonic stem cells into leydig-like cells rescue testosterone-deficient male rats in vivo. Stem Cells Dev. 2015;24:459–470. doi: 10.1089/scd.2014.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadhav U, Jameson JL. Steroidogenic factor-1 (SF-1)-driven differentiation of murine embryonic stem (ES) cells into a gonadal lineage. Endocrinology. 2011;152:2870–2882. doi: 10.1210/en.2011-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyamoto K, Yazawa T, Mizutani T, Imamichi Y, Kawabe SY, Kanno M, Matsumura T, Ju Y, Umezawa A. Stem cell differentiation into steroidogenic cell lineages by NR5A family. Mol Cell Endocrinol. 2011;336:123–126. doi: 10.1016/j.mce.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Sonoyama T, Sone M, Honda K, Taura D, Kojima K, Inuzuka M, Kanamoto N, Tamura N, Nakao K. Differentiation of human embryonic stem cells and human induced pluripotent stem cells into steroid-producing cells. Endocrinology. 2012;153:4336–4345. doi: 10.1210/en.2012-1060. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Yang Z, Zhao ZA, Shen Z. Direct reprogramming of fibroblasts into cardiomyocytes. Stem Cell Res Ther. 2017;8:118. doi: 10.1186/s13287-017-0569-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 25.Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 26.Najm FJ, Lager AM, Zaremba A, Wyatt K, Caprariello AV, Factor DC, Karl RT, Maeda T, Miller RH, Tesar PJ. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat Biotechnol. 2013;31:426–433. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caiazzo M, Giannelli S, Valente P, Lignani G, Carissimo A, Sessa A, Colasante G, Bartolomeo R, Massimino L, Ferroni S, Settembre C, Benfenati F, Broccoli V. Direct conversion of fibroblasts into functional astrocytes by defined transcription factors. Stem Cell Reports. 2015;4:25–36. doi: 10.1016/j.stemcr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss MJ, Orkin SH. GATA transcription factors: key regulators of hematopoiesis. Exp Hematol. 1995;23:99–107. [PubMed] [Google Scholar]
- 30.Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K, Matsuoka R, Cohen JC, Srivastava D. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424:443–447. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- 31.Viger RS, Taniguchi H, Robert NM, Tremblay JJ. Role of the GATA family of transcription factors in andrology. J Androl. 2004;25:441–452. doi: 10.1002/j.1939-4640.2004.tb02813.x. [DOI] [PubMed] [Google Scholar]
- 32.Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 1999;22:125–126. doi: 10.1038/9629. [DOI] [PubMed] [Google Scholar]
- 33.Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA, Stallings N, Schimmer BP. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res. 2002;57:19–36. doi: 10.1210/rp.57.1.19. [DOI] [PubMed] [Google Scholar]
- 34.Crawford PA, Sadovsky Y, Milbrandt J. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Mol Cell Biol. 1997;17:3997–4006. doi: 10.1128/mcb.17.7.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song KH, Park JI, Lee MO, Soh J, Lee K, Choi HS. LH induces orphan nuclear receptor Nur77 gene expression in testicular Leydig cells. Endocrinology. 2001;142:5116–5123. doi: 10.1210/endo.142.12.8525. [DOI] [PubMed] [Google Scholar]
- 36.Gu Y, Zhang X, Sun D, Zhao H, Cai B, Gao C, Gao L, Cui Y, Tang Z, Jin B. The stimulative effect of Yangjing capsule on testosterone synthesis through Nur77 pathway in Leydig cells. Evid Based Complement Alternat Med. 2015;2015:408686. doi: 10.1155/2015/408686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin J, Tsai MJ, Tsai SY. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS One. 2008;3:e3285. doi: 10.1371/journal.pone.0003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


