Abstract
Objective: Herpes zoster (HZ) can develop into postherpetic neuralgia (PHN), which is a chronic neuropathic pain (NP). Whether the chronification from HZ to PHN induced brain functional or structural change is unknown and no study compared the changes of the same brains of patients who transited from HZ to PHN. We minimized individual differences and observed whether the chronification of HZ to PHN induces functional and pain duration dependent grey matter volume (GMV) change in HZ-PHN patients. Methods: To minimize individual differences induced error, we enrolled 12 patients with a transition from HZ to PHN. The functional and structural changes of their brains between the two states were identified with resting-state functional MRI (rs-fMRI) technique (i.e., the regional homogeneity (ReHo) and fractional aptitude of low-frequency fluctuation (fALFF) method) and the voxel based morphometry (VBM) technology respectively. The correlations between MRI parameters (i.e., ΔReHo, ΔfALFF and ΔVBM) and Δpain duration were analyzed too. Results: Compared with HZ brains, PHN brains exhibited abnormal ReHo, fALFF and VBM values in pain matrix (the frontal lobe, parietal lobe, thalamus, limbic lobe and cerebellum) as well as the occipital lobe and temporal lobe. Nevertheless, the activity of vast area of cerebellum and frontal lobe significantly increased while that of occipital lobe and limbic lobe showed apparent decrease when HZ developed to PHN. In addition, PHN brain showed decreased GMV in the frontal lobe, the parietal lobe and the occipital lobe but increased in the cerebellum and the temporal lobe. Correlation analyses showed that some of the ReHo, fALFF and VBM differential areas (such as the cerebellum posterior lobe, the thalamus extra-nuclear and the middle temporal gyrus) correlated well with Δpain duration. Conclusions: HZ chronification induced functional and structural change in cerebellum, occipital lobe, temporal lobe, parietal lobe and limbic lobe. These changes may be correlated with HZ-PHN chronification. In addition, these changes could be reasons of refractory chronic pain of PHN.
Keywords: Postherpetic neuralgia (PHN), herpes zoster (HZ), resting-state functional magnet resonance imaging (rsfMRI), regional homogeneity (ReHo), fractional amplitude of low frequency fluctuation (fALFF), voxel-based morphometry (VBM), pain chronnification, brain
Introduction
Herpes Zoster (HZ) causes some complications, among which the postherpetic neuralgia (PHN) is the most common and refractory one [1]. PHN is a neuropathic pain (NP) which lasts more than one month [2] or three months [3,4] following an outbreak of shingles. PHN is an economic burden of the gradually aging society [5], which affects the quality of life [6] and increases the risk of anxiety, depression and suicide [7,8]. Although the HZ vaccines are promising [4], efficient analgesia and treatment are limited [9]. In addition, not like some types of NP, PHN animal model is not well enough [10,11] to mimic the Varicella-Zoster virus (VZV) caused NP in animals. Therefore, it is not easy to study the molecular mechanisms of HZ and PHN in animals, which hampered the understanding of HZ-PHN chronification and the clinical neuroimaging studies are crucial to reveal the brain change.
However, the functional and structural change between HZ and PHN brain is not clear, although we reported that HZ and PHN brain displayed functional differences in pain matrix (frontal lobe, insular and cerebellum etc.), occipital lobe, temporal lobe and brainstem [12]. Some studies have explored functional changes in PHN brain relative to normal control by using functional magnetic resonance imaging (fMRI) [13-15]. Besides sensory-discriminative areas [16], brain areas associated with emotion, hedonics and reward (striatum, amygdale etc.) were also activated in PHN brain [13]. PHN brain showed increased cerebral blood flow (CBF) in S1 area, insula, thalamus, inferior parietal lobule, amygdala and striatum and increased CBF in the frontal cortex [17]. Functional connectivity (FC) analysis detected altered connections between putamen and some regions in PHN patients [14]. Small-world network analysis found PHN brain showed decreased local efficiency in brain areas related to sense, memory and emotion [15]. We found PHN patients exhibited abnormal spontaneous brain activity in the pain matrix as well as the temporal lobe and brainstem as compared with healthy controls [18].
The regional homogeneity (ReHo) [19,20] and the fractional amplitude of the low frequency fluctuations (fALFF) [21,22] are reliable indices to evaluate resting-state brain activity [23-25]. ReHo represents the local coherence of local spontaneous neuronal activity, while fALFF reflects the resting-state brain activity, which had been proved to be more gray matter specific and sensitive to BOLD signal [21].
Voxel-based morphometry (VBM) is a popular approach for identifying grey matter volume (GMV) changes in diseases with chronic pain such as the trigimental neuralgia [26,27], fibromyalgia [28,29] and chronic back pain [30].
The brain activity and GMV may be varied among HZ patients and PHN patients, especially considering the fact that most of the HZ and PHN patients are old people, and decades of different habits may have influenced their brain function and GMV, therefore, we used the self-controlled study to eliminate the individual difference induced experimental interferences.
In the present study, we enrolled 12 HZ patients who became PHN at last, the MRI scans were taken at the HZ and PHN states. The ReHo, fALFF and VBM methods were employed to detect brain activity and GMV changes after HZ-PHN chronification.
Methods
Participants
This MRI study was approved by the Ethics Committee of the local hospital. Informed consents were obtained from all participants. All of the 12 right handed patients were recruited from the Pain Medicine Department of the local hospital from December 2014 to March 2017. The diagnosis of HZ and PHN was based on the International Association for the Study of Pain (IASP) criteria [31]. Spontaneous pain intensity was evaluated by using the Visual Analogue Scale (VAS). All of the patients recruited in both states claimed intense pain (VAS scores ≥5), no antidepressants or antipsychotic drugs were taken before MRI scans. All HZ patients underwent pain for less than three months after the HZ rash (shingles) and all PHN patients reported persistent pain for more than three months after the HZ rash (shingles). All of these patients showed no visible brain structural abnormalities in the MRI structural images.
Image acquisition
The MRI experiments were implemented on a GE Signa HDxT 3.0 T MRI scanner (GE Company, USA) with a standard eight channel head coil as we reported [12,18]. fMRI data were acquired using an echo-planar image (EPI) sequence with parameters as follows: thickness/gap = 4.0/0 mm, matrix = 64 × 64, TR = 2000 ms, TE = 40 ms, flip angle = 90°, field of view (FOV) = 240 × 240 mm. A total of 210 time points and 33 axial slices were obtained in 7 min. High-resolution anatomic 3D T1 (TR = 5.8 ms, TE = 1.8 ms, flip angle = 12°, thickness/gap = 1.0/0 mm, 146 sagittal slices, FOV = 256 × 256 mm, matrix = 256 × 256) images were also acquired.
Image processing
Preprocessing was performed by using the Data Processing Assistant for Resting-State fMRI (DPARSF, http://rest.restfmri.net/forum/DPARSF) [32] and SPM 8 (Wellcome Department, University College of London, UK) software based on MATLAB R2012a (MathWorks, USA).
DPARSF was used for data processing following the steps below: The first 10 volumes of the fMRI images were discarded to allow for the scanner calibration and participants adaptation in the scan, and the remaining 200 volumes were further analyzed. Processing steps included slice timing, head-motion correction, spatial normalization to the Montreal Neurological Institute (MNI) space and resampling with a 3 × 3 × 3 mm3 resolution. Participants with head motion >2.0 mm of translation or >2.0° of rotation in any direction were excluded from further processing. The linear trend of the fMRI data was removed. For ReHo analysis, the band-pass filtering (0.01-0.08 Hz) was conducted to discard high-frequency physiological noise and the frequency drift lower than 0.01 Hz [33]. Resting State fMRI Data Analysis Toolkit (REST, http://rest.restfmri.net) [22] was used for the conduct of subsequent steps: Individual ReHo map was generated by calculating the KCC of the time series of a given voxel with those of its neighbors (26 voxels) in a voxel-wise way [19,34]. Afterwards, a whole-brain mask was adopted to remove the non-brain tissues. For standardization purposes, the individual ReHo maps were divided by their own global mean KCC within the whole-brain mask. Then spatial smoothing was performed on the standardized individual ReHo map with a Gaussian kernel of 4 mm full-width at half maximum (FWHM) [35].
fALFF analysis was conducted as previously described [21,24]. First, the resampled images were smoothed with a 4 mm Gaussian kernel. Then the frequency band filtering was set as 0.01-0.08 Hz, and the time courses were converted to the frequency band using a Fast Fourier Transform. The mean and standard deviation of each individual’s ReHo and fALFF value was calculated by DPARSF within the whole brain mask.
The VBM analysis was performed by using SPM 8 as we reported [36]. The procedure included the following steps: (1) checking for scanner artifacts and gross anatomical abnormalities for each subject; (2) setting the image origin to the anterior commissure; (3) segmenting the images into grey matter, white matter and cerebrospinal fluid (CSF) images; (4) using the DARTEL toolbox on SPM8 to produce a high-dimensional normalization protocol; (5) checking for homogeneity across the sample and applying a 8 mm full width at half-maximum (FWHM) Gaussian kernel standard smoothing. After this pre-processing, modulated, smoothed, normalized images were obtained for statistical analysis.
Statistical analysis
Demographic and clinical data were analyzed using Prism 7.0 (GraphPad Software Inc, USA). Two-sample t-tests were used for detecting the age differences. χ2 test was applied for comparison of gender ratio. The criteria for all statistical significance were set as P<0.05.
For ReHo and fALFF comparison, paired t-tests were conducted in a whole-brain voxel-wise way with REST. To determine the significance of ReHo and fALFF between two groups, multiple comparison correction was performed by Monte Carlo simulations [37] by using the REST AlphaSim utility [22]. Voxels with P<0.05 (two-tailed, corrected with AlphaSim method: rmm = 4 mm, cluster size was estimated according to the whole brain mask; http://afni.nih.gov/afni/docpdf/AlphaSim.pdf) were regarded as brain areas with significant difference.
For VBM comparison, paired t-tests between two states were performed. Statistical maps were corrected for multiple comparisons by AlphaSim (P<0.05, rmm = 4 mm, cluster size was estimated according to the grey matter mask) on the voxel level.
REST Slice Viewer, which is a routine for the display of statistic results [22], was used to generate result graphs. Brain areas were overlaid on structural brain images. A color-bar was set to illustrate the statistic values [38].
Results
Demographic and clinical features
Clinical characteristics of patients in the HZ and PHN state are listed in Table 1. The comparison of VAS score and pain duration are listed in Table 2. Both the VAS score and pain duration showed significant differences between the HZ and PHN state.
Table 1.
Demographic and clinical variables of 12 patients with HZ transited PHN
| No. | Age (year) | Gender | Location of lesion | Pain duration 1 (month) | Pain duration 2 (month) | Time gap (month) | VAS 1 | VAS 2 |
|---|---|---|---|---|---|---|---|---|
| 1 | 66 | F | Left V2-3 | 0.25 | 12 | 11.75 | 9 | 5 |
| 2 | 65 | F | Right V3 | 0.66 | 9 | 8.34 | 9 | 6 |
| 3 | 66 | M | Right V1 | 0.66 | 6 | 5.34 | 5 | 4 |
| 4 | 60 | F | Right T6-9 | 1.00 | 6 | 5.00 | 7 | 4 |
| 5 | 76 | M | Left C2-6 | 0.66 | 12 | 11.34 | 7 | 5 |
| 6 | 62 | M | Right L1-2 | 2.00 | 9 | 7.00 | 6 | 6 |
| 7 | 73 | M | Right V2 | 0.66 | 9 | 8.34 | 5 | 4 |
| 8 | 59 | M | Right T5-7 | 0.66 | 6 | 5.34 | 7 | 4 |
| 9 | 71 | F | Right V1 | 1.00 | 9 | 8.00 | 5 | 4 |
| 10 | 71 | M | Left C2-4 | 2.00 | 9 | 7.00 | 5 | 4 |
| 11 | 72 | F | Left T2-4 | 2.00 | 13 | 11.00 | 5 | 5 |
| 12 | 54 | M | Right V1 | 0.50 | 15 | 14.50 | 8 | 5 |
M = male; F = female; C = cervical; T = thoracic; L = lumbar; S = sacral; V1: ophthalmic branch; V2: maxillary branch; V3: mandibular branch; VAS: visual analogue scale; Pain duration 1: pain duration at the time of the first MRI scan (HZ state); Pain duration 2: pain duration at the time of the second MRI scan (PHN state); Time gap: pain duration between the two MRI scans.
Table 2.
Comparison of clinical variables in HZ and PHN state (mean ± SD)
| HZ | PHN | P | |
|---|---|---|---|
| VAS | 6.50 ± 1.57 | 4.67 ± 0.78 | 0.0015 |
| Pain duration (month) | 1.00 ± 0.63 | 9.58 ± 2.91 | <0.0001 |
PHN: postherpetic neuralgia; HZ: herpes zoster; VAS: visual analogue scale.
Comparison of ReHo between PHN and HZ state
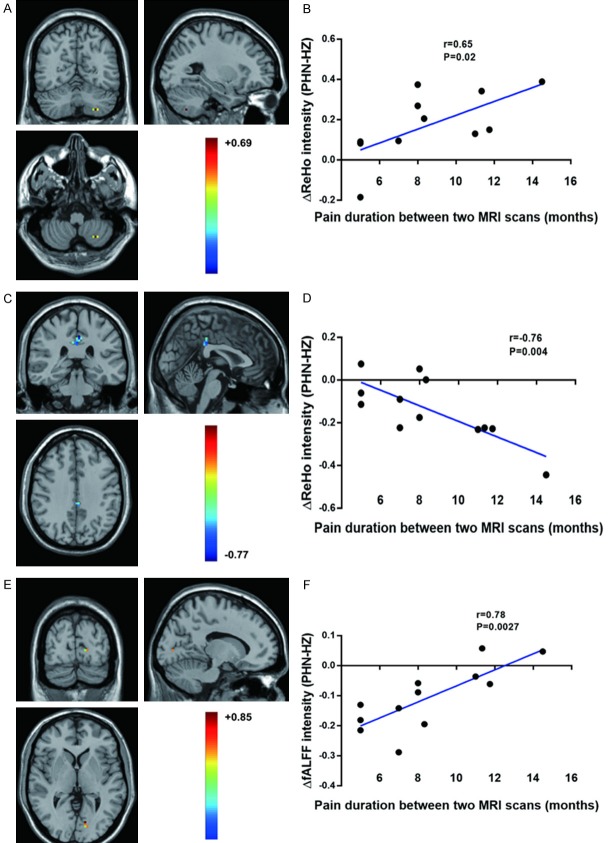
As shown in Figure 1 and Table 3, compared with HZ brain, PHN brain showed significantly increased ReHo mainly in the bilateral cerebellum (posterior lobe, inferior semi-lunar lobule) and the middle frontal gyrus. Lower ReHo values were observed in the right sub-lobar area (such as the putamen, the extra-nuclear and the lentiform nucleus) and the bilateral limbic lobe (mainly in the anterior-, posterior- and middle-cingulate).
Figure 1.
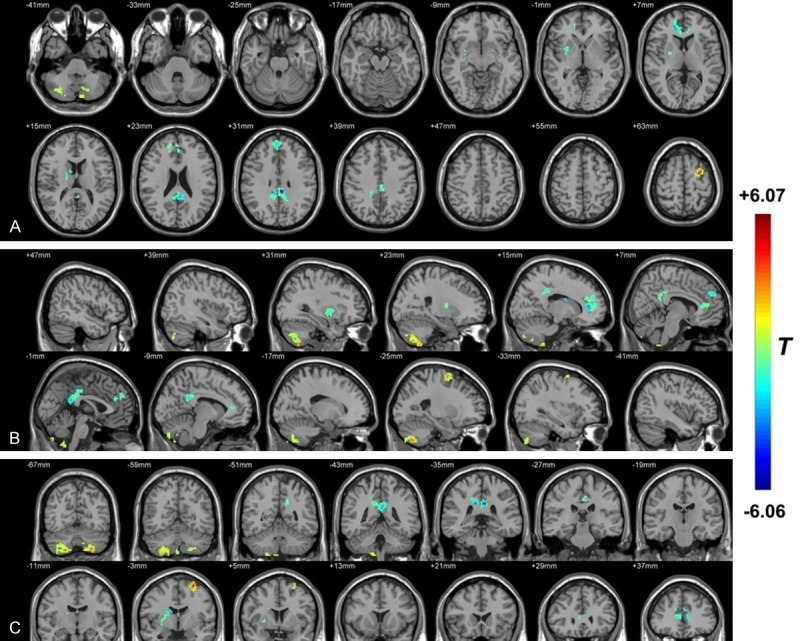
The distribution of ReHo differential brain areas in the transversal sections (A), sagittal sections (B) and coronal sections (C) after trasition from HZ to PHN. The warm colors indicate higher ReHo, and cool colors indicate lower ReHo in PHN brain than that of HZ brain (P<0.05, AlphaSim corrected, paired t test, n = 12). Compared with HZ brain, PHN brain showed significantly increased ReHo mainly in the bilateral cerebellum (posterior lobe and inferior semi-lunar lobule) and the middle frontal gyrus. Lower ReHo values were observed in the right sub-lobar area (such as the putamen, the extra-nuclear and the lentiform nucleus) and the bilateral limbic lobe (mainly in the anterior-, posterior- and middle-cingulate). The detailed information for each cluster and their peak T values and coordinates are listed in Table 3.
Table 3.
Clusters with different ReHo or fALFF values between PHN and HZ patients
| Region (R: right; L: left) | Peak MNI coordinate | Peak T value | Voxel number | Brain volume (mm3) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| PHN>HZ (ReHo) | ||||||
| Cerebelum_8_R (aal) | 21 | -66 | -54 | 4.66 | 452 | 12204 |
| Frontal_Sup_L (aal) | -30 | 0 | 66 | 6.07 | 68 | 1836 |
| PHN<HZ (ReHo) | ||||||
| Putamen_R (aal) | 18 | -6 | 12 | -3.92 | 61 | 1647 |
| Cingulum_Ant_R (aal) | 18 | 45 | 6 | -3.90 | 198 | 5346 |
| Cingulum_Post_L (aal) | -3 | -33 | 30 | -5.66 | 209 | 5643 |
| PHN>HZ (fALFF) | ||||||
| Frontal_Inf_Orb_R (aal) | 21 | 18 | -12 | 4.44 | 80 | 2160 |
| Frontal_Inf_Tri_L (aal) | -54 | 33 | 9 | 6.34 | 65 | 1755 |
| Frontal_Sup_Medial_R (aal) | 9 | 51 | 39 | 3.76 | 73 | 1971 |
| Frontal_Mid_L (aal) | -27 | 0 | 63 | 4.70 | 123 | 3321 |
| PHN<HZ (fALFF) | ||||||
| Lingual_R (aal) | 12 | -72 | -6 | -4.87 | 87 | -4.878 |
| Calcarine_L (aal) | -3 | -78 | 15 | -6.35 | 236 | -6.35 |
| Occipital_Mid_R (aal) | 45 | -78 | 0 | -4.19 | 110 | -4.19 |
ReHo: regional homogeneity; fALFF: fractional aplitude of low-frequency fluctuation; PHN: postherpetic neuralgia; HZ: herpes zoster; MNI: Montreal Neurological Institute; aal: anatomical automatic labeling.
Comparison of fALFF between PHN and HZ state
As shown in Figure 2 and Table 3, compared with HZ brain, PHN brain showed significantly increased fALFF mainly in the bilateral frontal lobe (bilateral inferior frontal gyrus, bilateral middle frontal gyrus, right superior frontal gyrus and Precentral_L (aal or anatomical automatic labeling)). Lower fALFF values were observed in the occipital lobe (such as the lingual gyrus, Calcarine_L (aal), middle occipital gyrus, Occipital_inf_bilateral (aal) and Occipital_sup_L (aal)) and the temporal lobe (mainly in the middle temporal gyrus and the inferior temporal gyrus).
Figure 2.
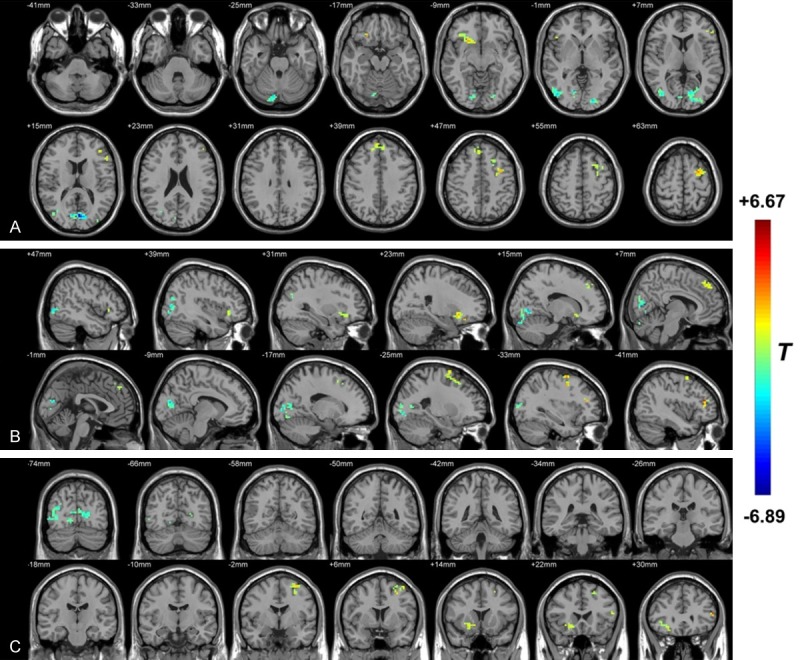
The distribution of fALFF differential brain areas in the transversal sections (A), sagittal sections (B) and coronal sections (C) after the transition from HZ to PHN. The warm colors indicate higher fALFF, and cool colors indicate lower fALFF in PHN brain than that of HZ brain (P<0.05, AlphaSim corrected, paired t test, n = 12). Compared with HZ brain, PHN brain showed significantly increased fALFF mainly in the bilateral frontal lobe (bilateral inferior frontal gyrus, bilateral middle frontal gyrus, right superior frontal gyrus and Precentral_L (aal)). Lower fALFF values were observed in the occipital lobe (such as the lingual gyrus, Calcarine_L (aal), middle occipital gyrus, Occipital_inf_bilateral (aal) and Occipital_sup_L (aal)) and the temporal lobe (mainly in the middle temporal gyrus and the inferior temporal gyrus). The detailed information for each cluster and their peak T values and coordinates are listed in Table 3. aal: anatomical automatic labeling.
Correlation analyses of ΔReHo/ΔfALFF and Δpain duration (PHN-HZ)
The correlation analysis of the ΔReHo (ReHo (PHN) - ReHo (HZ)) and pain duration (= pain duration PHN (at the second scan) - pain duration HZ (at the first scan)) indicated that ΔReHo values of the left cerebellum (i.e., the inferior semi-lunar lobule and the cerebellum posterior lobe) were positively correlated with pain duration between the two MRI scans, while the left posterior- and middle-cingulated gyrus were negatively correlated with pain duration (Table 4; Figure 3A-D). In addition, the ΔfALFF (fALFF (PHN) - fALFF (HZ)) of the left occipital lobe (Calcarine_L (aal) and Occipital_Sup_L (aal)) was positively correlated with pain duration (Table 4; Figure 3E and 3F).
Table 4.
Correlation between ΔReHo/ΔfALFF and Δpain duration
| Region (R: right; L: left) | Peak MNI coordinate | Peak R value | Voxel number | Brain volume (mm3) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| + correlation (ReHo) | ||||||
| Cerebelum_8_L (aal) | -27 | -63 | -51 | 0.69 | 3 | 81 |
| - correlation (ReHo) | ||||||
| Cingulum_Mid_L (aal) | -3 | -33 | 42 | -0.77 | 17 | 459 |
| + correlation (fALFF) | ||||||
| Calcarine_L (aal) | -12 | -78 | 3 | 0.84 | 4 | 108 |
| - correlation (fALFF) | ||||||
| None | ||||||
ReHo: regional homogeneity; VAS: visual analogue scale; MNI: Montreal Neurological Institute; ΔReHo = ReHo (PHN) - ReHo (HZ); ΔfALFF = fALFF (PHN) - fALFF (HZ); MNI: Montreal Neurological Institute; aal: anatomical automatic labeling.
Figure 3.
Correlation analysis of the ΔReHo/ΔfALFF and Δpain duration (PHN-HZ). ΔReHo values of the left cerebellum (i.e., the inferior semi-lunar lobule and the cerebellum posterior lobe) were positively correlated with pain duration between the two MRI scans (A, B), while those of the left posterior- and middle-cingulated gyrus were negatively correlated with pain duration (C, D). In addition, the ΔfALFF values of the left occipital lobe (Calcarine_L (aal) and Occipital_Sup_L (aal)) were positively correlated with pain duration (E, F). ΔReHo = ReHo (PHN) - ReHo (HZ); ΔfALFF = fALFF (PHN) - fALFF (HZ); Pain duration = pain duration at the second MRI scan (the PHN period) - pain duration at the first scan (the HZ period). Pearson correlation analysis, n = 12.
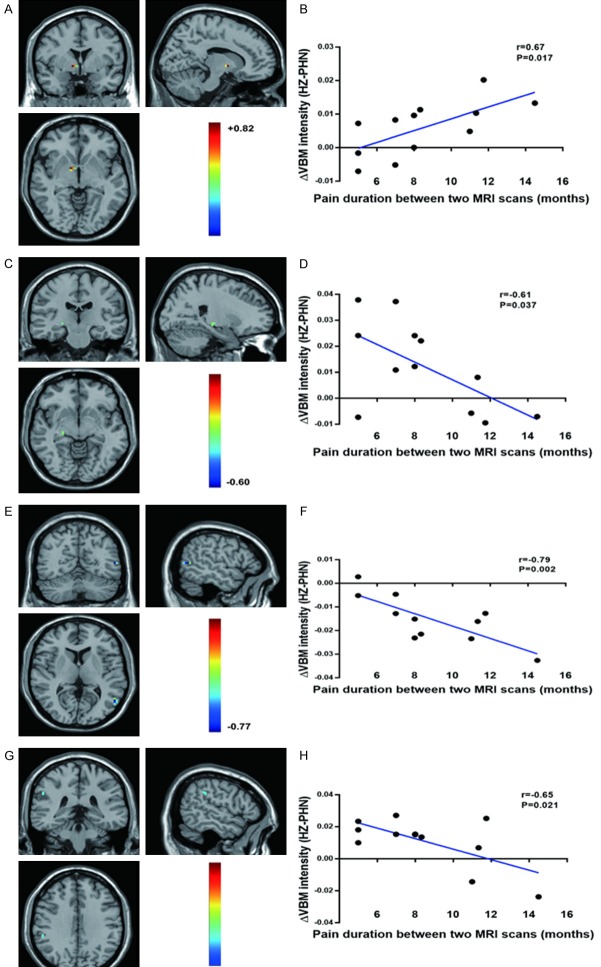
Comparison of VBM between PHN and HZ brain
As shown in Figure 4 and Table 5, compared with HZ, PHN brain showed significantly decreased VBM values mainly in the right limbic lobe (the hippocampus and parahippocampa gyrus), the right extra-nuclear, the right frontal lobe (superior frontal gyrus and medial frontal gyrus) the thalamus, the occipital lobe (Calcarine_R (aal) and Cuneus_R (aal)), the parietal lobe (postcentral gyrus, inferior parietal lobule and SupraMarginal_R (aal)) and the frontal lobe (bilateral precentral gyrus, middle frontal gyrus and Frontal_Sup_R (aal)). Higher VBM values were observed in the bilateral cerebellum (the inferior semi-lunar lobule, the posterior lobe and the cerebellar tonsil) and the temporal lobe (inferior temporal gyrus and middle temporal gyrus).
Figure 4.
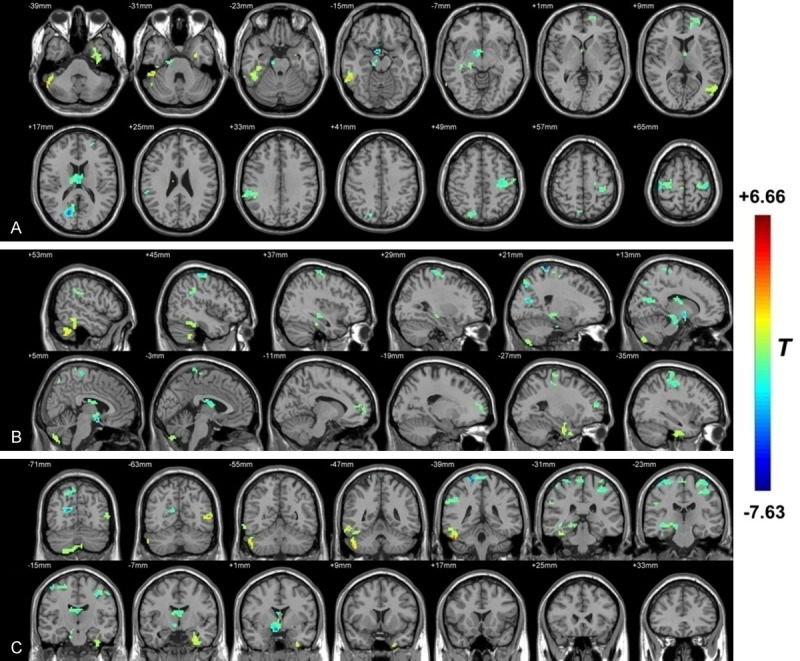
The distribution of VBM differential brain areas in the transversal section (A), sagittal section (B) and coronal section (C). The warm colors indicate higher VBM, and cool colors indicate lower VBM in PHN brain than that of HZ brain (P<0.05, AlphaSim corrected, paired t test, n = 12). Compared with HZ, PHN brain showed significantly decreased VBM values mainly in the right limbic lobe (the hippocampus and parahippocampa gyrus), the right extra-nuclear, the right frontal lobe (superior frontal gyrus and medial frontal gyrus), the thalamus, the occipital lobe (Calcarine_R (aal) and Cuneus_R (aal)), the parietal lobe (postcentral gyrus, inferior parietal lobule and SupraMarginal_R (aal)) and the frontal lobe (bilateral precentral gyrus, middle frontal gyrus and Frontal_Sup_R (aal)). Higher VBM values were observed in the bilateral cerebellum (the inferior semi-lunar lobule, the posterior lobe and the cerebellar tonsil) and the temporal lobe (inferior temporal gyrus and middle temporal gyrus). The detailed information for each cluster and their peak T values and coordinates are listed in Table 5. aal: anatomical automatic labeling.
Table 5.
Clusters with different VBM values between PHN and HZ brain
| Region (R: right; L: left) | Peak MNI coordinate | Peak T value | Voxel number | Brain volume (mm3) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| PHN<HZ | ||||||
| Hippocampus_R (aal) | 12 | -21 | -15 | -3.65 | 96 | 2592 |
| Extra-Nuclear_R (aal) | 6 | 0 | -15 | -5.47 | 61 | 1647 |
| Frontal_Mid_L (aal) | -27 | 54 | 15 | -3.80 | 61 | 1647 |
| Thalamus_R (aal) | 0 | -12 | 18 | -3.75 | 95 | 2565 |
| Calcarine_R (aal) | 18 | -72 | 18 | -5.54 | 70 | 1890 |
| SupraMarginal_R (aal) | 60 | -36 | 24 | -3.33 | 72 | 1944 |
| Precentral_L (aal) | -33 | -18 | 48 | -3.54 | 156 | 4212 |
| Precentral_R (aal) | 27 | -27 | 69 | -3.72 | 71 | 1917 |
| Postcentral_R (aal) | 21 | -42 | 78 | -7.33 | 90 | 2430 |
| PHN>HZ | ||||||
| Cerebellum Posterior Lobe | 15 | -81 | -57 | 2.81 | 2619 | 2619 |
| Cerebelum_Crus2 (aal) | 51 | -48 | -48 | 3.88 | 1836 | 1836 |
| Temporal_Inf_L (aal) | -33 | 0 | -42 | 3.46 | 2160 | 2160 |
| Cerebelum_Crus1_R (aal) | 51 | -39 | -33 | 5.06 | 2943 | 2943 |
| Temporal_Mid_L (aal) | -57 | -66 | 6 | 4.75 | 1836 | 1836 |
PHN: postherpetic neuralgia; HZ: herpes zoster; MNI: Montreal Neurological Institute; aal: anatomical automatic labeling.
Correlation analyses of ΔVBM and Δpain duration between PHN and HZ patients
The correlation analyses of the ΔVBM (VBM (PHN) - VBM (HZ)) and Δpain duration (PHN - HZ) indicated that ΔVBM values of the right extra-nuclear was positively correlated with Δpain duration (Table 6; Figure 5A, 5B), while the right parahippocampal gyrus, the temporal lobe (the middle temporal gyrus) and the parietal lobe (i.e., the SupraMarginal_R (aal)) were negatively correlated with pain duration (Table 6; Figure 5C-H).
Table 6.
Correlation between ΔVBM and pain duration
| Region (R: right; L: left) | Peak MNI coordinate | Peak R value | Voxel number | Brain volume (mm3) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| + correlation | ||||||
| Extra-Nuclear_R (aal) | 12 | 0 | -3 | 0.82 | 4 | 108 |
| - correlation | ||||||
| Hippocampus_R (aal) | 27 | -24 | -9 | -0.61 | 4 | 108 |
| Temporal_Mid_L (aal) | -54 | -63 | 6 | -0.77 | 6 | 162 |
| SupraMarginal_R (aal) | 54 | -36 | 33 | -0.64 | 3 | 81 |
ReHo: regional homogeneity; VAS: visual analogue scale; MNI: Montreal Neurological Institute; aal: anatomical automatic labeling.
Figure 5.
Correlation analysis of the ΔVBM and Δpain duration. ΔVBM values of the right extra-nuclear were positively correlated with Δpain duration (A, B), while the right parahippocampal gyrus (C, D), the temporal lobe (the middle temporal gyrus, E, F) and the parietal lobe (i.e., the SupraMarginal_R (aal), G, H) were negatively correlated with pain durations. ΔVBM = VBM (PHN) - VBM (HZ); Δpain duration = pain duration at the second MRI scan (the PHN period) - the pain duration at the first scan (the HZ period). Pearson correlation analysis, n = 12.
Discussion
As evidenced by ReHo, fALFF and VBM results, compared with HZ brain, PHN brains showed different brain activity and GMV in several brain regions. Most of these differential brain areas, such as the frontal lobe, cerebellum, thalamus, cingulated gyrus and parietal lobe, belong to the “pain matrix”, which was defined as regions that exhibited a reliable activation in response to increasing levels of pain [16,39-41].
The pain matrix includes somatosensory area, supplementary motor area, cerebellum, forebrain, thalamus, insula, anterior cingulate gyrus (ACC), posterior parietal cortex, periaqueductal grey and striatum [42,43]. Our results indicated that besides regions of pain matrix, the occipital lobe, the temporal lobe and some other regions of the limbic lobe (i.e., the cingulate cortex and the hippocampus) were also involved. The functional differences of PHN brain are most similar as we found in the HZ and PHN patients (not self-controlled study, with independent t tests) [12]. In addition, the brain areas with structural change are concordant with the brain activity changed ones. These suggest that the functional change could be resulted from structural change.
In this study, the cerebellum changed a lot both in the functional and structural aspect. It was activated in the PHN state and the GMV increased profoundly. Cerebellum is part of pain matrix and always activated in painful events in healthy humans [44] and in patients with chronic pain [45]. For example, neuralgia (mononeuropathy) patients showed an increased rCBF in the cerebellum [46]. Kim et al. found that cerebellar activity correlated well with rat NP development in an eight-week longitudinal FDG microPET study [47]. More interestingly, the cerebellum activity seemed to be correlated with depression [48]. Abnormal cerebellar response to the anticipation of pain has been suggested to be a potential marker for depression [49]. Patients with depression showed increased activity in the cerebellum [50]. These literatures suggest that pain and depression may share a common mechanism within the cerebellum [48].
It is reported that in chronic pain states, the GMV of cerebellum could be increased or decreased. For example, it was increased in patients with fibromyalgia [51] and cluster headache [52], but decreased in trigeminal neuralgia [26,27] and burn moth syndrome [53]. Bocci et al. found that cerebellar direct current stimulation (tcDCS) could modulate pain perception and its cortical correlates [54], and thought cerebellum holds the potential to be one of the targets to defeat chronic pain.
In this study, the bilateral limbic lobe (mainly the anterior-, posterior- and middle-cingulate gyrus) showed lower ReHo values in PHN brain. In addition, the ReHo values decreased in the left posterior- and middle-cingulate gyrus and these decreases were negatively correlated with pain duration. Furthermore, PHN brain showed lower VBM values in the right limbic lobe (the hippocampus and parahippocampa gyrus) as compared with HZ brain. These results indicate that PHN deactivated limbic lobe such as cingulate cortex and decreased the GMV of some areas such as hippocampus. It is reported that limbic regions of the pain matrix encode emotional aspects of pain perception, and the primary sensory region encodes the intensity and location of pain sensation [55,56]. Chronic pain studies in rodents showed functional changes in limbic regions (the hippocampus [57-60], amygdale [61], striatum [62], and frontal cortex [63-65]). Whole-brain network analysis of NP rats showed FC changes within areas of the limbic system and between the limbic and nociceptive systems [66]. Recent human imaging studies [13,15,67,68] displayed the same trend. Although the effects of PHN on the GMV in limbic lobe are not clear, chronic pain induced alterations in limbic lobe in fibromyalgia patients [69] and hippocampus in migraine patients [70] have been widely reported.
In this study, the temporal lobe of PHN brain displayed lower fALFF values and higher VBM values relative to HZ brain. In addition, the GMV increase in temporal lobe (the middle temporal gyrus) was positively correlated with PHN duration. Although the temporal lobe is considered to be one of the brain regions of pain integration [71,72], some studies reported its functional changes in NP patients and animal models. The medial temporal lobes are involved in pain perception and modulation [73]. The middle temporal lobe was activated in patients with chronic cluster pain [52]. In addition, fibromyalgia patients performed a reduced deactivation in the temporal lobe when they received stimulation [74], which may be one of the causes of allodynia in this disease.
Most of the pain-related VBM studies indicated that the temporal lobe displayed a decrease GMV in chronic pain [75-77] such as trigeminal neuralgia [26] and fibromyalgia [78]. Sinding et al. [53] detected a significant increase of GMV in the temporal gyrus in burning mouth syndrome patients. We found a significant increase in GMV in the middle temporal and inferior temporal gyrus. In addition, the middle temporal GMV increase positively correlated with pain duration. These results suggest that the temporal lobe may be involved in the HZ-PHN transition.
Many occipital lobe areas showed lower brain activity relative to HZ brain in this study. In addition, the brain activity decrease degree in the occipital lobe was positively correlated with pain duration. The Calcarine_R (aal) and Cuneus_R (aal) of the occipital lobe in PHN brain also showed significantly decreased VBM, which indicated that PHN induces profound changes in occipital lobe, and these changes could be one reason of the intractable pain of PHN. In painful events, occipital activity showed some abnormalities. For example, a rsfMRI study found that PSPD patients displayed reduced occipital ReHo signal [79]. Karibe et al. found the rCBF in the occipital lobe of PSPD patients (with chronic pain) was significantly lower than that of healthy controls [80]. Electrical stimulation of the rat occipital lobe reduced pain intensity, which may be related to the anatomical connection between occipital lobe and the pain descending inhibition system [81]. The occipital lobe is inhibited in NP rat and NP patients. Cauda et al. used independent component analysis (ICA) and detected a significant activity reduction in occipital lobe of patients with diabetic NP relative to healthy controls [82]. Kim et al. [83] observed the metabolism decrease in the occipital lobe and cerebellum in rats with spinal nerve ligation (SNL, a NP model) by using PET.
To date, only a few studies had reported structural changes of the occipital lobe in chronic pain condition. The GMV of the occipital lobe in migraine patients was found reduced [84]. We previously found that PHN patients showed abnormal microstructure in the occipital lobe as evidenced by decreased diffusional kurtosis imaging (DKI) intensity [38]. Thus, the effect of chronic pain on occipital lobe structure requires further investigation.
Besides pain processing, the ReHo and fALFF differential brain areas hold additional functions. For example, the frontal lobe was associated with depression and anxiety [85]. The limbic system were involved in sleep control [86]. Pain is an integrated feeling of sensory, affective and cognitive dimensions [87]. Geha et al. [13] analyzed the BOLD signal of PHN patients and detected that brain areas with BOLD change was not restricted to the sensory-discriminative areas, but also the emotion, reward and punishment related brain regions. It was reported that chronic pain and neuropsychiatric disease such as depression [88] and axiety [89], congnitive disfunctions [90] and sleep disorder [91] were highly comorbid. Indeed, up to 50% of patients with chronic pain exhibited symptoms of anxiety or depression [92], whereas in some studies the number reached to 75% [93]. Importantly, the prevalence of depression increased with greater pain severity [88]. This reminds us that when we handle PHN, we cannot be confined to analgesia, the mental health evaluation is necessary.
Rodriguez-Raecke et al. [76] reported that a long-term suffering of pain may cause changes in brain plasticity. They found the GMV reduction in brain areas such as anterior cingulate gyrus (ACC) in patients with hip arthritis recovered in hip replacement subgroups (no pain for 6 weeks after surgery group and 4 months group). Meanwhile, we think these changes in PHN brain may be some reasons for the refractory NP in PHN patients.
More detecting time points in PHN state may be useful to observe the functional and GMV change with the prolongation of PHN. In addition, fMRI study using conventional software may hold a high false-positive rate [94], alternative neuroscience technologies are warranted to identify the functional and structural changes in HZ and PHN brains.
Conclusions
The chronification from HZ to PHN induces brain activity changes and alters the GMV of several brain areas. These differential brain areas are not only regions of pain matrix (cerebellum etc.) but also the temporal lobe and occipital lobe. Functional and structural changes in the cerebellum, occipital lobe, parietal lobe, temporal lobe, frontal lobe and limbic lobe may be not only the results of the chronification from HZ to PHN, but also the reasons for the refractory pain of PHN.
Disclosure of conflict of interest
None.
References
- 1.Cohen JI. Clinical practice: herpes zoster. N Engl J Med. 2013;369:255–263. doi: 10.1056/NEJMcp1302674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmader K. Postherpetic neuralgia in immunocompetent elderly people. Vaccine. 1998;16:1768–1770. doi: 10.1016/s0264-410x(98)00137-6. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JI. Herpes zoster. N Engl J Med. 2013;369:255–263. doi: 10.1056/NEJMcp1302674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keating GM. Shingles (herpes zoster) vaccine (zostavax®): a review in the prevention of herpes zoster and postherpetic neuralgia. Biodrugs. 2016;30:243–254. doi: 10.1007/s40259-016-0180-7. [DOI] [PubMed] [Google Scholar]
- 5.Friesen KJ, Falk J, Alessi-Severini S, Chateau D, Bugden S. Price of pain: population-based cohort burden of disease analysis of medication cost of herpes zoster and postherpetic neuralgia. J Pain Res. 2016;9:543. doi: 10.2147/JPR.S107944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickering G, Gavazzi G, Gaillat J, Paccalin M, Bloch K, Bouhassira D. Is herpes zoster an additional complication in old age alongside comorbidity and multiple medications? Results of the post hoc analysis of the 12-month longitudinal prospective observational ARIZONA cohort study. BMJ Open. 2016;6:e009689. doi: 10.1136/bmjopen-2015-009689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sah DW, Ossipo MH, Frank P. Neurotrophic factors as novel therapeutics for neuropathic pain. Nat Rev Drug Discov. 2003;2:460–472. doi: 10.1038/nrd1107. [DOI] [PubMed] [Google Scholar]
- 8.Denkinger MD, Lukas A, Nikolaus T, Peter R, Franke S ActiFE study group. Multisite pain, pain frequency and pain severity are associated with depression in older adults: results from the ActiFE Ulm study. Age Ageing. 2014;43:510–514. doi: 10.1093/ageing/afu013. [DOI] [PubMed] [Google Scholar]
- 9.Snedecor SJ, Sudharshan L, Cappelleri JC, Sadosky A, Desai P, Jalundhwala Y, Botteman M. Systematic review and meta-analysis of pharmacological therapies for pain associated with postherpetic neuralgia and less common neuropathic conditions. Int J Clin Pract. 2014;68:900–918. doi: 10.1111/ijcp.12411. [DOI] [PubMed] [Google Scholar]
- 10.Lei Y, Sun Y, Lu C, Ma Z, Gu X. Activated glia increased the level of proinflammatory cytokines in a resiniferatoxin-induced neuropathic pain rat model. Reg Anesth Pain Med. 2016;41:744–749. doi: 10.1097/AAP.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 11.Wu CH, Yuan XC, Gao F, Li HP, Cao J, Liu YS, Yu W, Tian B, Meng XF, Shi J, Pan HL, Li M. Netrin-1 contributes to myelinated afferent fiber sprouting and neuropathic pain. Mol Neurobiol. 2016;53:5640–5651. doi: 10.1007/s12035-015-9482-x. [DOI] [PubMed] [Google Scholar]
- 12.Cao S, Li Y, Deng W, Qin B, Zhang Y, Xie P, Yuan J, Yu B, Yu T. Local brain activity differences between herpes zoster and postherpetic neuralgia patients: a resting-state functional MRI study. Pain Physician. 2017;20:E687–E699. [PubMed] [Google Scholar]
- 13.Geha PY, Baliki MN, Chialvo DR, Harden RN, Paice JA, Apkarian AV. Brain activity for spontaneous pain of postherpetic neuralgia and its modulation by lidocaine patch therapy. Pain. 2007;128:88–100. doi: 10.1016/j.pain.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geha PY, Baliki MN, Wang X, Harden RN, Paice JA, Apkarian AV. Brain dynamics for perception of tactile allodynia (touch-induced pain) in postherpetic neuralgia. Pain. 2008;138:641–656. doi: 10.1016/j.pain.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Liu J, Li L, Du M, Fang W, Wang D, Jiang X, Hu X, Zhang J, Wang X. A study on small-world brain functional networks altered by postherpetic neuralgia. Magn Reson Imaging. 2014;32:359–365. doi: 10.1016/j.mri.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Hao Y, Du M, Wang X, Zhang J, Manor B, Jiang X, Fang W, Wang D. Quantitative cerebral blood flow mapping and functional connectivity of postherpetic neuralgia pain: a perfusion fMRI study. Pain. 2013;154:110–118. doi: 10.1016/j.pain.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Cao S, Song G, Zhang Y, Xie P, Tu Y, Li Y, Yu T, Yu B. Abnormal local brain activity beyond the pain matrix in postherpetic neuralgia patients: a resting-state functional MRI study. Pain Physician. 2017;20:E303–E314. [PubMed] [Google Scholar]
- 19.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 20.Zuo XN, Xu T, Jiang L, Yang Z, Cao XY, He Y, Zang YF, Castellanos FX, Milham MP. Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. Neuroimage. 2013;65:374–386. doi: 10.1016/j.neuroimage.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fryer SL, Roach BJ, Ford JM, Turner JA, van Erp TG, Voyvodic J, Preda A, Belger A, Bustillo J, O’Leary D. Relating intrinsic low-frequency BOLD cortical oscillations to cognition in schizophrenia. Neuropsychopharmacology. 2015;40:2705–14. doi: 10.1038/npp.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haag LM, Heba S, Lenz M, Glaubitz B, Höffken O, Kalisch T, Puts NA, Edden RA, Tegenthoff M, Dinse H, Schmidt-Wilcke T. Resting BOLD fluctuations in the primary somatosensory cortex correlate with tactile acuity. Cortex. 2015;64:20–28. doi: 10.1016/j.cortex.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui Y, Jiao Y, Chen YC, Wang K, Gao B, Wen S, Ju S, Teng GJ. Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes. 2014;63:749–760. doi: 10.2337/db13-0519. [DOI] [PubMed] [Google Scholar]
- 26.Obermann M, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, Yoon MS, Theysohn N, Blex S, Diener HC, Katsarava Z. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage. 2013;74:352–358. doi: 10.1016/j.neuroimage.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 27.Li M, Yan J, Li S, Wang T, Zhan W, Wen H, Ma X, Zhang Y, Tian J, Jiang G. Reduced volume of gray matter in patients with trigeminal neuralgia. Brain Imaging Behav. 2017;11:486–492. doi: 10.1007/s11682-016-9529-2. [DOI] [PubMed] [Google Scholar]
- 28.Lin C, Lee SH. Gray matter atrophy within the default mode network of fibromyalgia: a meta-analysis of voxel-based morphometry studies. Biomed Res Int. 2016;2016:7296125. doi: 10.1155/2016/7296125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T. Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain-cognition interaction. Brain. 2008;131:3222–3231. doi: 10.1093/brain/awn229. [DOI] [PubMed] [Google Scholar]
- 30.Fritz HC, McAuley JH, Wittfeld K, Hegenscheid K, Schmidt CO, Langner S, Lotze M. Chronic back pain is associated with decreased prefrontal and anterior insular gray matter: results from a population-based cohort study. J Pain. 2016;17:111–118. doi: 10.1016/j.jpain.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Merskey H, Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. 2 edition. Seattle: International Association for the Study of Pain; 1994. [Google Scholar]
- 32.Yan C, Zang Y. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greicius MD, Ben K, Reiss AL, Vinod M. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Kadivar A, Pluta J, Dunlop J, Wang Z. Test-retest stability analysis of resting brain activity revealed by blood oxygen level-dependent functional MRI. J Magn Reson Imaging. 2012;36:344–354. doi: 10.1002/jmri.23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Li K, Zhang Q, Zeng Y, Dai W, Su Y, Wang G, Tan Y, Jin Z, Yu X. Short-term effects of escitalopram on regional brain function in first-episode drug-naive patients with major depressive disorder assessed by resting-state functional magnetic resonance imaging. Psychol Med. 2014;44:1417–1426. doi: 10.1017/S0033291713002031. [DOI] [PubMed] [Google Scholar]
- 36.Tu Y, Yu T, Wei Y, Sun K, Zhao W, Yu B. Structural brain alterations in hemifacial spasm: a voxel-based morphometry and diffusion tensor imaging study. Clin Neurophysiol. 2016;127:1470–1474. doi: 10.1016/j.clinph.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 37.Ledberg A, Åkerman S, Roland PE. Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage. 1998;8:113–128. doi: 10.1006/nimg.1998.0336. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Yu T, Qin B, Li Y, Song G, Yu B. Microstructural abnormalities in gray matter of patients with postherpetic neuralgia: a diffusional kurtosis imaging study. Pain Physician. 2016;19:E601–611. [PubMed] [Google Scholar]
- 39.Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, Kadetoff D, Ingvar M. Cognitive behavioral therapy increases painevoked activation of the prefrontal cortex in patients with fibromyalgeia. Pain. 2012;153:1495–1503. doi: 10.1016/j.pain.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- 41.Seifert F, Maihöfner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci. 2009;66:375–390. doi: 10.1007/s00018-008-8428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Larrea L, Peyron R. Pain matrices and neuropathic pain matrices: a review. Pain. 2013;154:S29–S43. doi: 10.1016/j.pain.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Helmchen C, Mohr C, Erdmann C, Petersen D, Nitschke M. Differential cerebellar activation related to perceived pain intensity during noxious thermal stimulation in humans: a functional magnetic resonance imaging study. Neurosci Lett. 2003;335:202–206. doi: 10.1016/s0304-3940(02)01164-3. [DOI] [PubMed] [Google Scholar]
- 45.Jensen KB, Regenbogen C, Ohse MC, Frasnelli J, Freiherr J, Lundström JN. Brain activations during pain: a neuroimaging meta-analysis of patients with pain and healthy controls. Pain. 2016;157:1279–1286. doi: 10.1097/j.pain.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain. 1995;63:225–236. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- 47.Kim J, Shin J, Oh JH, Jung HH, Kim YB, Cho ZH, Chang JW. Longitudinal FDG microPET imaging of neuropathic pain: does cerebellar activity correlate with neuropathic pain development in a rat model? Acta Neurochir (Wien) 2015;157:1051–1057. doi: 10.1007/s00701-015-2415-7. [DOI] [PubMed] [Google Scholar]
- 48.Moulton EA, Schmahmann JD, Becerra L, Borsook D. The cerebellum and pain: passive integrator or active participator? Brain Res Rev. 2010;65:14–27. doi: 10.1016/j.brainresrev.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith KA, Ploghaus A, Cowen PJ, McCleery JM, Goodwin GM, Smith S, Tracey I, Matthews PM. Cerebellar responses during anticipation of noxious stimuli in subjects recovered from depression. Br J Psychiatry. 2002;181:411–415. doi: 10.1192/bjp.181.5.411. [DOI] [PubMed] [Google Scholar]
- 50.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi H, Yuan C, Dai Z, Ma H, Sheng L. Gray matter abnormalities associated with fibromyalgia: a meta-analysis of voxel-based morphometric studies. Semin Arthritis Rheum. 2016;46:330–337. doi: 10.1016/j.semarthrit.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Naegel S, Holle D, Desmarattes N, Theysohn N, Diener HC, Katsarava Z, Obermann M. Cortical plasticity in episodic and chronic cluster headache. Neuroimage Clin. 2014;6:415–423. doi: 10.1016/j.nicl.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinding C, Gransjoen AM, Schlumberger G, Grushka M, Frasnelli J, Singh PB. Grey matter changes of the pain matrix in patients with burning mouth syndrome. Eur J Neurosci. 2016;43:997–1005. doi: 10.1111/ejn.13156. [DOI] [PubMed] [Google Scholar]
- 54.Bocci T, Santarcangelo E, Vannini B, Torzini A, Carli G, Ferrucci R, Priori A, Valeriani M, Sartucci F. Cerebellar direct current stimulation modulates pain perception in humans. Restor Neurol Neurosci. 2015;33:597–609. doi: 10.3233/RNN-140453. [DOI] [PubMed] [Google Scholar]
- 55.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 56.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 57.del Rey A, Yau HJ, Randolf A, Centeno MV, Wildmann J, Martina M, Besedovsky HO, Apkarian AV. Chronic neuropathic pain-like behavior correlates with IL-1β expression and disrupts cytokine interactions in the hippocampus. Pain. 2011;152:2827–2835. doi: 10.1016/j.pain.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32:5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carmen RG, Esther B, Cristina AD, Madrigal JLM, Perez-Nievas BG, Juan Carlos L, Juan Antonio M. Stress increases the negative effects of chronic pain on hippocampal neurogenesis. Anesth Analg. 2015;121:1078–88. doi: 10.1213/ANE.0000000000000838. [DOI] [PubMed] [Google Scholar]
- 60.Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology. 2011;36:979–992. doi: 10.1038/npp.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ren W, Palazzo E, Maione S, Neugebauer V. Differential effects of mGluR7 and mGluR8 activation on pain-related synaptic activity in the amygdala. J Ind Microbiol Biotechnol. 2011;61:1334–1344. doi: 10.1016/j.neuropharm.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goffer Y, Xu D, Eberle SE, D’amour J, Lee M, Tukey D, Froemke RC, Ziff EB, Wang J. Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J Neurosci. 2013;33:19034–19044. doi: 10.1523/JNEUROSCI.2454-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pais-Vieira M, Aguiar P, Lima D, Galhardo V. Inflammatory pain disrupts the orbitofrontal neuronal activity and risk-assessment performance in a rodent decision-making task. Pain. 2012;153:1625–1635. doi: 10.1016/j.pain.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009;106:2423–2428. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seminowicz DA, Laferriere AM. MRI structural brain changes associated with sensory and emotional function in a rat model of longterm neuropathic pain. Neuroimage. 2009;47:1007–1014. doi: 10.1016/j.neuroimage.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baliki MN, Chang PC, Baria AT, Centeno MV, Apkarian AV. Resting-sate functional reorganization of the rat limbic system following neuropathic injury. Sci Rep. 2014;4:6186. doi: 10.1038/srep06186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mansour AR, Baliki MN, Huang L, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV. Brain white matter structural properties predict transition to chronic pain. Pain. 2013;154:2160–2168. doi: 10.1016/j.pain.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lutz J, Jager L, de Quervain D, Krauseneck T, Padberg F, Wichnalek M, Beyer A, Stahl R, Zirngibl B, Morhard D, Reiser M, Schelling G. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusiontensor and volumetric imaging study. Arthritis Rheum. 2008;58:3960–3969. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- 70.Liu J, Lan L, Mu J, Zhao L, Yuan K, Zhang Y, Huang L, Liang F, Tian J. Genetic contribution of catechol-O-methyltransferase in hippocampal structural and functional changes of female migraine sufferers. Hum Brain Mapp. 2015;36:1782–1795. doi: 10.1002/hbm.22737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt-Wilcke T, Hierlmeier S, Leinisch E. Altered regional brain morphology in patients with chronic facial pain. Headache. 2010;50:1278–1285. doi: 10.1111/j.1526-4610.2010.01637.x. [DOI] [PubMed] [Google Scholar]
- 72.Weissman-Fogel I, Moayedi M, Tenenbaum HC, Goldberg MB, Freeman BV, Davis KD. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain. 2011;152:384–396. doi: 10.1016/j.pain.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 73.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb Cortex. 2011;21:719–726. doi: 10.1093/cercor/bhq146. [DOI] [PubMed] [Google Scholar]
- 74.Schreiber KL, Loggia ML, Kim J, Cahalan CM, Napadow V, Edwards RR. Painful after-sensations in fibromyalgia are linked to catastrophizing and differences in brain response in the medial temporal lobe. J Pain. 2017;18:855–867. doi: 10.1016/j.jpain.2017.02.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rocca MA, Ceccarelli A, Falini A, Colombo B, Tortorella P, Bernasconi L, Comi G, Scotti G, Filippi M. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37:1765–1770. doi: 10.1161/01.STR.0000226589.00599.4d. [DOI] [PubMed] [Google Scholar]
- 76.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57. e42. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, Leinisch E, Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain. 2007;132(Suppl 1):S109–116. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 79.Huang T, Zhao Z, Yan C, Lu J, Li X, Tang C, Fan M, Luo Y. Altered spontaneous activity in patients with persistent somatoform pain disorder revealed by regional homogeneity. PLoS One. 2016;11:e0151360. doi: 10.1371/journal.pone.0151360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karibe H, Arakawa R, Tateno A, Mizumura S, Okada T, Ishii T, Oshima K, Ohtsu M, Hasegawa I, Okubo Y. Regional cerebral blood flow in patients with orally localized somatoform pain disorder: a single photon emission computed tomography study. Psychiatry Clin Neurosci. 2010;64:476–482. doi: 10.1111/j.1440-1819.2010.02119.x. [DOI] [PubMed] [Google Scholar]
- 81.Reis GM, Dias QM, Silveira JW, Del Vecchio F, Garcia-Cairasco N, Prado WA. Antinociceptive effect of stimulating the occipital or retrosplenial cortex in rats. J Pain. 2010;11:1015–1026. doi: 10.1016/j.jpain.2010.01.269. [DOI] [PubMed] [Google Scholar]
- 82.Cauda F, Sacco K, Duca S, Cocito D, D’Agata F, Geminiani GC, Canavero S. Altered resting state in diabetic neuropathic pain. PLoS One. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim CE, Kim YK, Chung G, Im HJ, Lee DS, Kim J, Kim SJ. Identifying neuropathic pain using (18)F-FDG micro-PET: a multivariate pattern analysis. Neuroimage. 2014;86:311–316. doi: 10.1016/j.neuroimage.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 84.Jin C, Yuan K, Zhao L, Zhao L, Yu D, von Deneen KM, Zhang M, Qin W, Sun W, Tian J. Structural and functional abnormalities in migraine patients without aura. NMR Biomed. 2013;26:58–64. doi: 10.1002/nbm.2819. [DOI] [PubMed] [Google Scholar]
- 85.Vincent V, Bagot RC, Cahill ME, Deveroux F, Robison AJ, Dietz DM, Barbara F, Michelle MR, Ku SM, Eileen H. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of ΔFosB. J Neurosci. 2014;34:3878–3887. doi: 10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Z, Ferretti V, Güntan İ, Moro A, Steinberg EA, Ye Z, Zecharia AY, Yu X, Vyssotski AL, Brickley SG. Neuronal ensembles sufficient for recovery sleep and the sedative actions of [alpha] 2 adrenergic agonists. Nat Neurosci. 2015;18:553–561. doi: 10.1038/nn.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Melzack R. From the gate to the neuromatrix. Pain. 1999;(suppl 6):S121–126. doi: 10.1016/S0304-3959(99)00145-1. [DOI] [PubMed] [Google Scholar]
- 88.Currie SR, Jianli W. More data on major depression as an antecedent risk factor for first onset of chronic back pain. Psychol Med. 2005;35:1275–1282. doi: 10.1017/S0033291705004952. [DOI] [PubMed] [Google Scholar]
- 89.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- 90.Coppieters I, Ickmans K, Cagnie B, Nijs J, De PR, Noten S, Meeus M. Cognitive performance is related to central sensitization and health-related quality of life in patients with chronic whiplash-associated disorders and fibromyalgia. Pain Physician. 2015;18:E389–E401. [PubMed] [Google Scholar]
- 91.Morin CM, Gibson D, Wade J. Self-reported sleep and mood disturbance in chronic pain patients. Clin J Pain. 1998;14:311–314. doi: 10.1097/00002508-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 92.Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesisstress framework. Psychol Bull. 1996;119:95–110. [Google Scholar]
- 93.Sigtermans MJ, van Hilten JJ, Bauer MC, Arbous MS, Marinus J, Sarton EY, Dahan A. Ketamine produces effective and long-term pain relief in patients with complex regional pain syndrome type 1. Pain. 2009;145:304–311. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 94.Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–5. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]