Abstract
The Polycomb Repressive Complex 2 (PRC2) component PHD Finger Protein 19 (phf19) gene has been identified to be associated with rheumatoid arthritis (RA) risk. Here we show that Phf19 is highly expressed in murine germinal centers (GCs) and RA patients. To investigate the function of Phf19 in lymphocytes, we generated RAG1-deficient mice reconstituted with Phf19 or control-vector transduced bone marrow (BM) cells. Lymphogenesis in primary lymphoid tissues of Phf19-RM is normal, however, Phf19-RM form enlarged GCs and generate more antibody-secreting cells (ASCs). Overexpression of Phf19 promotes proliferation and survival of GC B cells and Tfh cells in vivo. The uncovered Phf19-dependent targets include the genes encoding cyclin D2, the prosurvival factor Bcl-xL and CD40-CD40 ligand axis, their regulation by Phf19 could partially elucidate the advantages observed in Phf19-overexpressing GCs. Our results underscore an unrecognized but critical function for Phf19 in GCs formation and antibody generation, and implicate the potential role of Phf19 in RA pathogenesis.
Keywords: Phf19, rheumatoid arthritis, murine germinal centers, follicular helper T cells
Introduction
RA is a systemic autoimmune disease with intra-articular inflammation as a dominant feature affecting up to 1% of the population [1]. Previous research has revealed that autoantibodies including anti-cyclic citrullinated protein antibody (anti-CCP) are present in almost 70% RA patients at the early stages of disease, indicating the central role of autoreactive humoral immune responses in the initiation of RA [2]. Somatically mutated high-affinity autoantibodies are a hallmark of RA pathogenesis, and GCs are critical in this production of autoantibodies, as they are critical sites to sustain a high rate of somatic hypermutation of activated B cells and antibody affinity maturation dependent on follicular helper T (Tfh) cells [3].
The introduction of B-cell-depleting therapy was a great success for the treatment of RA [4]. By binding to CD20, rituximab depletes subpopulations of peripheral B cells through several postulated mechanisms, including cell-mediated and complement-dependent cytotoxicity and promotion of apoptosis. The clinical trial of rituximab has demonstrated significant efficacy and adequate safety in modifying the symptoms of RA and has provided further evidence of the role of B cells in the disease pathogenesis. Thus identifying regulators of B-cell activation and antibody production is pivotal for a better understanding of the pathogenesis of RA.
Transcriptome analysis of mouse B cells has identified that PRC2 component Phf19 is significantly upregulated in activated B cells [5]. In addition, a large-scale RA genetic study has also identified several SNPs at the gene locus of phf19, suggesting its association with RA risk [6].
In this paper, we aim to explore the function of Phf19 in the process of GCs formation and antibody production. Measurement of transcriptional and translational level of Phf19 showed its induction in GCs and RA patients. By gain of function experiments, Phf19 was proved to positively regulate the GCs reaction without influencing the development of mature lymphocytes. Mechanically, Phf19 promoted the proliferation and survival of GC B cells and Tfh cells by targeting ccnd2, Bcl-xL, and CD40-CD40L. Our findings indicate a pivotal role for Phf19 in GCs formation and ASCs generation, and suggest that Phf19 could be recognized as a new regulator in the autoreactive antibody-initiated RA pathogenesis.
Materials and methods
Mice
C57BL/6 (Jax 664), Rag-1 deficient (Rag1-/-) mice (Jax2216) mice were obtained from the Jackson Laboratory and bred under specific pathogen-free conditions. All mice used were >8 weeks old.
Human samples
SF from RA patients was collected by routine knee joint paracentesis. Peripheral blood samples were collected from RA patients and healthy donors. 4 ml of SF and 10 ml of peripheral blood were collected. All patients in the study fulfilled the American College of Rheumatology criteria for the classification of RA. All study protocols and consent forms were approved by the Institutional Medical Ethics Committee of Henan Luoyang Orthopedic Hospital (Henan Provincial Rehabilitation Hospital).
Retroviral transduction of hematopoietic stem cells and bone marrow transplantation
The coding sequence of Phf19 was amplified by PCR from C57BL/6 genomic DNA and inserted into pMSCV-IRES-GFP retroviral vector. Retrovirus-containing supernatant was generated with the Plat-E packaging cell line. Bone marrow cells were extracted from the femur of C57BL/6 mice and cultured for 24 hours in RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin in the presence of 50 ng/ml mSCF, 20 ng/ml IL-3, and 50 ng/ml IL-6 (all from R&D Systems) before spin-infected with retroviral supernatants and 4 μg/ml polybrene (Sigma). After infection, cells were cultured for additional 24 hours and then used for reconstituting sublethally irradiated Rag1-/- recipient mice. The reconstitutions were completed 8 weeks after transplantation. Chimeric mice had frequencies of 30%-50% of lymphocyte cells being transduced, as assessed by GFP expression.
Immunization and flow cytometry
1×108 sheep red blood cells (SRBCs) were intraperitoneally injected into C57BL/6 mice or BM chimera mice. Splenocytes and BM cells were prepared, stained for surface molecules, and sorted on FACS Aria III or analyzed on FACS LSR (BD Biosciences). The following antibodies and reagents were used for cell surface staining: anti-B220-AF700 (BD Biosciences), anti-CD4-APC-Cy7 (BD Biosciences), anti-CD95-PE (eBiosciences), anti-GL7-Pacific blue (BD Biosciences), anti-CD138-BV510 (BD Biosciences), anti-CXCR5-PE-Cy7 (BD Biosciences), anti-PD-1-APC (eBiosciences), anti-PD-1-PE (eBiosciences), anti-CD40-PE-Cy7 (BD Biosciences), anti-CD40L-PE (eBiosciences).
Cells of BM, thymus, and spleen from normal lymphocyte development analysis were prepared, stained for surface molecules, and analyzed on FACS LSR (BD Biosciences). The following antibodies and reagents were used for cell staining: anti-B220-AF700 (BD Biosciences), anti-IgM-Pacific blue (BD Biosciences), anti-CD4-PE (eBiosciences), anti-CD8-APC (BD Biosciences), anti-CD3-PE-Cy7 (eBiosciences).
Evaluation of cell proliferation and apoptosis
BM chimera mice used for proliferative and survival analysis of GC B cells and Tfh cells were first administrated with BrdU (BD biosciences) 6 hours before sacrifice. Splenocytes were prepared and surface stained with GC or Tfh markers, further subjected to intracellular staining with anti-BrdU-APC (BD Biosciences), anti-Ki67-APC (BD Biosciences), anti-active caspase-3-AF647 (BD Biosciences), or Annexin V/PI double staining (BD Biosciences) according to the manufacturer’s instructions.
Quantitative RT-PCR
Murine B cells and T cells populations were sorted into RNA extraction reagent (Qiagen) and human lymphocytes from RA patients and healthy controls were lysed directly with RNA extraction reagent (Qiagen). Total RNA was extracted according to the manufacturer’s instructions. First-strand cDNA was synthesized with Oligo (dT) primers and MultiScribe™ MuLV (Thermo Fisher). Quantification of mouse and human Phf19, Bcl-xl, and ccnd2 transcripts in indicated cell populations was performed with the SYBR Green PCR Master Mix (Invitrogen) and ABI 7500 system (Applied biosystems). Each sample was assessed in triplecates and the analysis was repeated with a second set of samples. Expression of gapdh was used for copy number normalization. The relative expression of phf19, bcl-xl, and ccnd2 was determined with the comparative threshold cycle method. The primer pairs used throughput this study were listed and presented as Supplementary Table 1.
Western blotting
Sorted B cell populations and T cell populations were lysed with RIPA buffer containing 1% protease inhibitor and the cell lysates were centrifuged at 15000×g for 15 min at 4°C. Supernatants were collected and the protein concentrations were determined by BCA assay kit (Beyotime). The whole cell proteins were applied to 10% SDS-PAGE gels and then transferred to nitrocellulose membranes. After incubation with the appropriate specific primary and secondary antibodies, Western blot bands were quantified by using Odyssey infrared imaging system (Li-Cor Inc., Lincoln, NE, USA).
Immunofluorescence microscopy
Immunized mouse spleen cryo-sections (8 μm) were fixed with acetone and blocked with 5% fetal bovine serum. B cell follicles were revealed with anti-IgD-Pacific Blue (BioLegend), GC B cells were revealed with anti-Bcl6-APC (BD Biosciences), and Phf19+ cells were revealed with rabbit anti-Phf19 purified primary antibody (Santa Cruz) and appropriate secondary antibody. All images were obtained at ×100 magnification.
Statistical analysis
Levels of statistical significance between means were calculated by unpaired two-tailed Student’s t test, unless indicated otherwise. A value of P less than 0.05 indicated a statistically significant difference; NS indicated no significant difference. Data in each group were expressed as mean ± s.e.m.
Results
Phf19 is induced in GC reactions and RA patients
Known as a member of polycomb group proteins, the previous studies of Phf19 are largely emphasized on its functions and mechanisms responsible for regulating metazoan development, cellular differentiation and maintenance. For instance, a recently published study has profoundly demonstrated that Phf19 functions as a transcriptional repressor conferring its silencing activities on mouse embryonic stem cell (mESC) genes by binding the H3K36me3 via its Tudor domain to access active chromatin, whereby potentiating normal mESCs differentiation [7]. However, so far the role of Phf19 is rarely discussed in the immune system, until an RNA-seq data was recently reported which revealed the transcriptional levels of Phf19 in different B cell subsets are identical to those of some essential GC genes Bcl6, Bach2 and Pou2af1, suggesting a possible role of Phf19 necessary for the GC program, an important part of the B cell humoral immune response [5]. Another clue arouses our interests of studying Phf19 in GC reactions stemmed from a large-scale RA genetic study in white North American RA patients, wherein, Phf19 has been identified as a risk factor for RA, which are usually accompanied by excess of GC-derived autoantibody [6]. To investigate whether Phf19 underlies GC reactions, we first examined the expression pattern of Phf19 in normal C57BL/6 mice immunized with classic T-dependent antigen sheep red blood cells (SRBCs) to induce strong humoral immune responses (Figure 1A, 1B). The GC reaction in spleen reaches its maximum by day 7 and maintains high levels until day 10 post immunization [8]. Analysis of Phf19 mRNA and protein expression level in B cells and T cells during different activation stages indicated that Phf19 was specifically upregulated in GC B cells, while Tfh cells also maintained certain mRNA level of Phf19, these results suggest a dominant expression pattern of Phf19 in GC program (Figure 1A, 1B). Enhanced Phf19 expression in GCs was further confirmed by immunohistochemical (IHC) analysis on spleen sections from SRBC-immunized mice (Figure 1C). In IHC image, Phf19 was strongly found in the nuclei of most of GC B cells and T cells, as shown by specific markers of high expression of Bcl-6 and null expression of IgD [9], whereas, Phf19 was detected weak in other lymphocytes in white pulps. Therefore, Phf19 expression is induced in GC B and Tfh cells in murine GC reactions.
Figure 1.
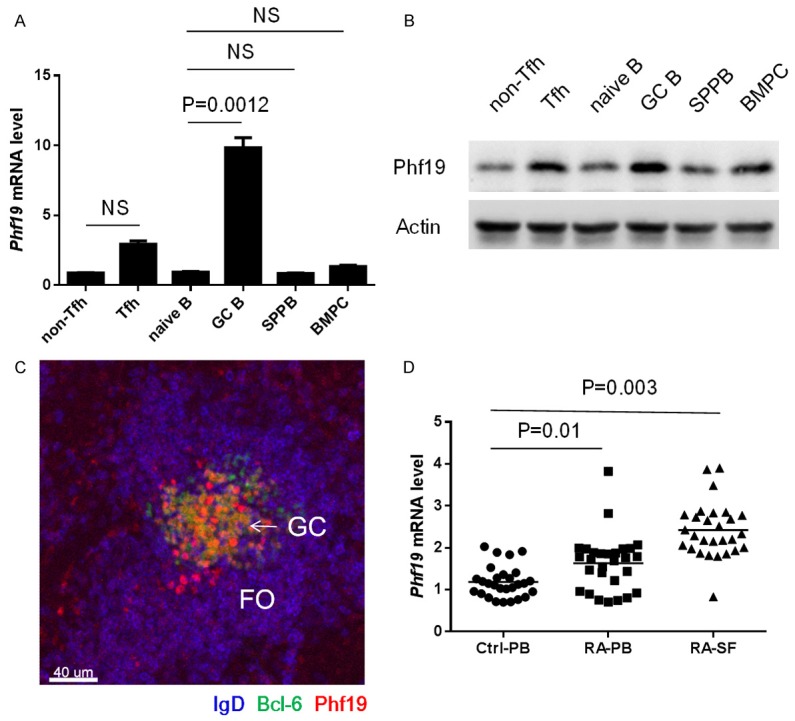
Phf19 is induced in GC reactions and RA patients. Real-time PCR (A) and Immunoblot (B) analysis of Phf19 expression in mouse splenic naïve B cells, GC B cells, plasma cells, bone marrow plasma cells, follicular helper T cells and non-follicular helper T cells obtained from 5 SRBC immunized C57BL/6 mice. Mouse GAPDH was used as reference control in Real-time PCR analysis and β-Actin was used as loading control for Immunoblot analysis, respectively. (C) Frozen splenic sections were simultaneously stained with anti-Phf19, anti-Bcl-6 and anti-IgD. One typical image was shown here. FO, B cell follicle; GC, germinal center. Original magnification, ×100. Scale bar, 40 um. (D) Real-time PCR analysis of Phf19 expression in peripheral blood (PB; n = 30) and synovial fluid (SF; n = 30) of RA patients and PB (n = 28) of healthy controls. Human GAPDH was used as reference control. Data between two groups were compared and the P value were shown as indicated. NS, not significant. Data are representative of three independent experiments.
Next, to test whether Phf19 is connected with lymphocyte activation in RA patients, we compared the expression level of Phf19 in lymphocytes from synovial fluid (SF) and peripheral blood (PB) of 30 RA patients with that in lymphocytes from PB of 28 healthy controls (Figure 1D). Quantitative RT-PCR analysis revealed that Phf19 was markedly increased in lymphocytes from both PB and SF samples of RA patients, suggesting that Phf19 upregulation is important during B cells and T cells activation in RA patients.
Phf19 is irrelevant with the development of B and T cells
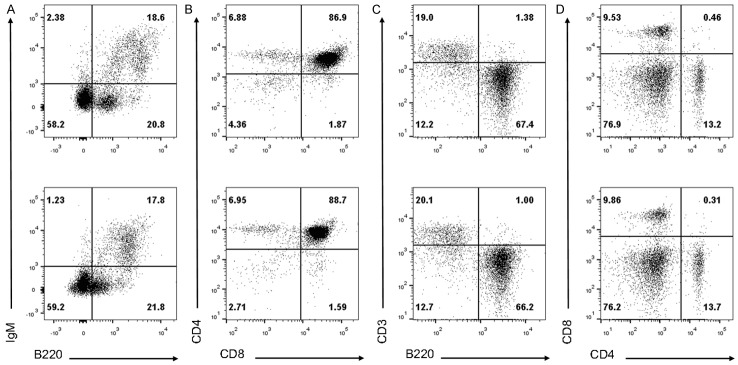
Considering that Phf19 is an important transcriptional regulator controlling cell development and differentiation, we initially wondered whether it could also exert similar functions in lymphocytes, especially B cells and T cells. To investigate the function of Phf19 in the development of B cells and T cells, lymphocytedeficient RAG1-/- mice were reconstituted with BM cells transduced with control or Phf19-expressing retrovirus (ctrl-RM or Phf19-RM). As RAG1-deficient lymphocytes cannot develop into a mature form, all mature lymphocytes developed in ctrl-RM or Phf19-RM are derived from donor BM cells [10]. Flow cytometric analysis of BM cells and thymocytes from ctrl-RM and Phf19-RM mice 8 weeks post transplantation showed no abnormalities in their early lymphocyte development (Figure 2A, 2B). In addition, the percentages of B220+ B cells, CD3+ T cells, CD4+ and CD8+ T cells in spleens from Phf19-RM mice were also unaffected compared to those from ctrl-RM mice (Figure 2C, 2D). Although strong Phf19 expression can be detected in activated lymphocytes in RA patients and murine GC structures, these data demonstrate that Phf19 is not essential for lymphocyte development in primary and secondary lymphoid tissues.
Figure 2.
Phf19 is irrelevant with the development of B and T cells. Representative flow cytometry analysis of bone marrow (BM) cells (A), thymocytes (B), and splenocytes (C and D) harvested from 5 ctrl-RM (upper row) and 5 phf19-RM (bottom row) mice by staining with indicated biomarker antibodies for identifying B or T cells. Numbers in each panel indicate relative percentages of positive cells within a quadrant. Data are representative of three independent experiments.
Phf19 is required for GC formation
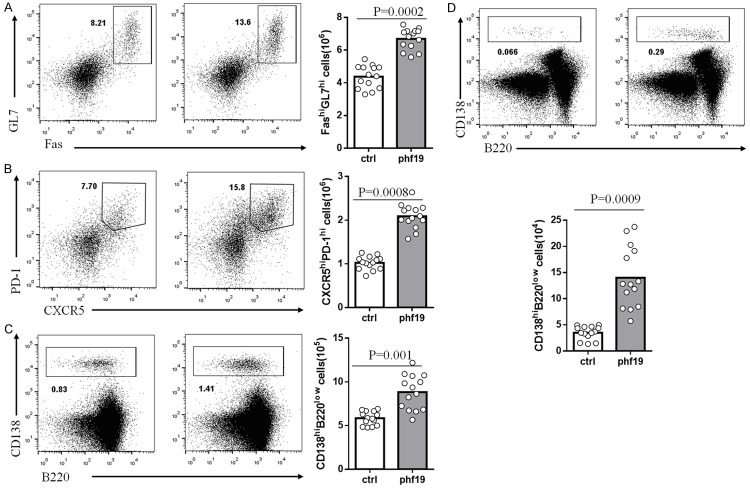
We then analyzed the function of Phf19 in GCs formation in Phf19-RM and ctrl-RM mice by immunizing them with SRBCs. Formation of GC B cells and Tfh cells were analyzed by flow cytometry on day 7 post immunization. The percentage of GC B cells in splenic B220+ B cell pool of Phf19-RM mice was significantly increased compared to that of ctrl-RM mice (Figure 3A). Meanwhile, the percentages of Tfh cells in total CD4+ T cells also showed obvious augment in Phf19-RM mice (Figure 3B). Moreover, a significant increase was also found in the number of CD138+B220low antibody-secreting cells in spleen of Phf19-RM mice on day 7 (Figure 3C). To further investigate the role of Phf19 in long-term high-affinity antibody production, the number of BM plasma cells was compared between Phf19-RM and ctrl-RM mice on day 15 post immunization. As results, a larger population of CD138+B220low plasma cells was identified in Phf19-RM mice than that in ctrl-RM mice (Figure 3D). These evidences collectively indicate that Phf19 is essential for the differentiation of mature B cells into GC B cells and further into antibody-producing long-lived plasma cells, and is also pivotal for the maintenance of Tfh cells during GC responses.
Figure 3.
Phf19 is required in lymphocytes for GC formation. Representative flow cytometry and total number statistical analysis of GC (A), Tfh (B), spleen plasma cells (C), and bone marrow plasma cells (D) from 14 SRBC-immunized ctrl-RM and 14 Phf19-RM mice by staining with indicated biomarker antibodies. The numbers in the figures indicate percentages of FashiGL7hi GC B cells relative to total B220+ cells (A), CXCR5+PD-1+ Tfh cells relative to total CD4+ cells (B), CD138hiB220low plasma cells relative to total splenocytes (C), and CD138hiB220low plasma cells relative to total BM cells (D). The total number of GC, Tfh, spleen plasma cells and bone marrow plasma cells in 14 ctrl-RM and 14 Phf19-RM mice were compared and the P value were shown.
Phf19 is essential for both proliferation and survival of GC B and Tfh cells
GC B cells undergo rapid round of proliferation and apoptosis [11], this prompts us to determine whether Phf19 is able to intrinsically regulate the proliferation or survival of GC B cells. The capacity of proliferation of GC B cells from SRBC-immunized Phf19-RM and ctrl-RM mice was examined using pulse labeling with 5-bromo-2’-deoxyuridine (BrdU), a thymidine analog which can be incorporated into cells undergoing DNA synthesis [12], and immunofluorescence staining with Ki67 [13], a protein expressed in dividing cells. The proportion of BrdU+ cells in GC B cells from Phf19-RM mice increased markedly than that from ctrl-RM mice after a 6-hour labeling with BrdU (Figure 4A), and the percentage of Ki67+ cells in GC B cells maintained at a similar level between these two groups of mice (Figure 4B), these indicate that Phf19 is able to accelerate the normal cell cycle of GC B cells. Then the resistant capacity for apoptosis of GC B cells from SRBC-immunized Phf19-RM and ctrl-RM mice was assessed by measurement of active caspase-3 and DNA fragmentation. The frequency of GC B cells with either activated caspase-3 (Active Csp3+) or Annexin V+PI- was notably different for Phf19-RM and ctrl-RM mice (Figure 4C, 4D), and GC B cells from Phf19-RM were more resistant to apoptosis than those from ctrl-RM mice. Similarly, enforced Phf19 expression in Tfh cells also had promoting effects on the proliferation and anti-apoptosis capacity in Tfh cells (Figure 4A-D). Thus, these data indicate a requirement for Phf19 in B cell and T cell survival and proliferation during GC formation.
Figure 4.
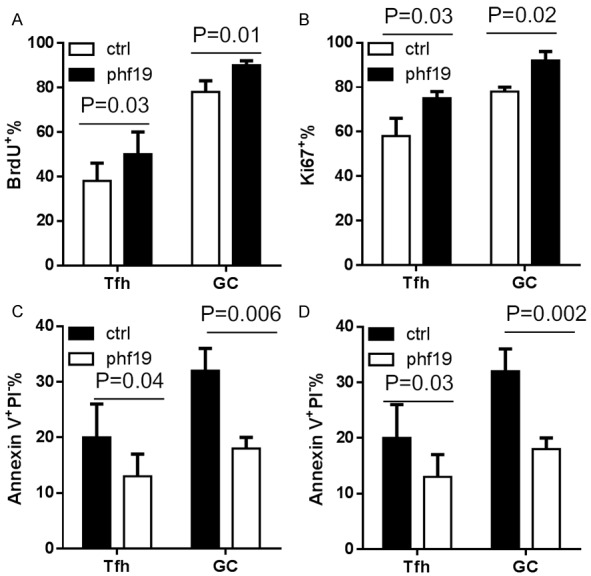
Phf19 is required for proliferation and survival of GC B cells and Tfh cells. Frequencies of Brdu+ cells (A), Ki67+ cells (B), active caspase3+ cells (C), and Annexin V+PI- cells (D) in GC B cells and Tfh cells obtained from 14 SRBC-immunized ctrl-RM and 14 Phf19-RM mice. All data were presented as a percentage of the indicated positive cells relative to either GC B cells or Tfh cells. Data in two groups were compared and P value were shown as indicated.
Phf19 promotes cell cycle and prosurvival signaling in GC cells and CD40-CD40L-dependent engagement of GC B cells with Tfh cells
GC B cell precursors entry into and progression through the cell cycle is tightly regulated by the coordinated activities of cyclin-dependent kinases and their regulatory cyclins [11]. We next characterized differences in cyclins gene expression in Phf19-RM versus ctrl-RM GC B cells by real-time PCR analysis. We identified that the expression of cyclin D2 was increased progressively in Phf19-RM GC B cells than that in ctrl-RM GC B cells (Figure 5A). The suppression and promotion of GC B cell apoptosis is regulated by Bcl-2 family members, and Bcl-xL is a member of the Bcl-2 family of prosurvival molecules [14]. Bcl-xL was upregulated approximately three fold in Phf19-RM GC B cells than that in ctrl-RM GC B cells (Figure 5B). Similarly, enforced Phf19 expression also upregulated expressions of cyclin D2 and Bcl-xL in Tfh cells (Figure 5A, 5B).
Figure 5.
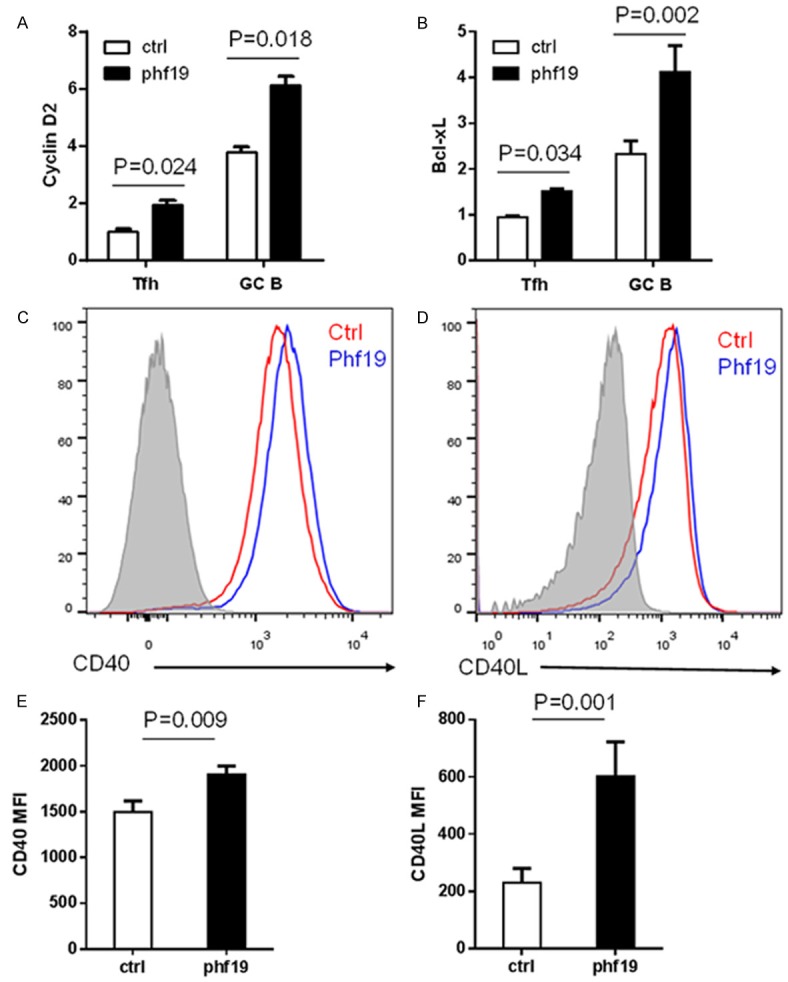
Phf19 promotes the expression of cyclin D2 and Bcl-xL in GC cells and CD40-CD40L-dependent engagement of GC B cells with Tfh cells. Real-time PCR analysis of cyclin D2 (A) and Bcl-xL (B) expression in Tfh cells and GC B cells from 5 SRBC-immunized ctrl-RM and 5 Phf19-RM mice. Mouse GAPDH was used as reference control. Representative flow cytometry of CD40 expression on GC B cells (C) and CD40L expression on Tfh cells (D) from 14 SRBC-immunized ctrl-RM and 14 Phf19-RM mice. Statistics of mean fluorescent intensity (MFI) of CD40 on GC B cells (E) and CD40L on Tfh cells (F) in these mice were shown here. Data in two groups were compared and the P value were shown.
Acquisition of T cell help has been identified as another dominant force in GC reaction. GC B cells receive cognate T cell help mostly in the form of CD40-CD40 ligand engagement [15]. To evaluate the influence of Phf19 in the cognate T cell help signaling, expression of CD40 and CD40L by GC B cells and Tfh cells were characterized by fluorescence-activated cell sorting (FACS). Phf19-RM derived GC B cells showed an increase of CD40 surface expression (Figure 5C, 5E), and activated CXCR5+PD-1+ Tfh cells consistently increased their CD40L surface expression (Figure 5D, 5F).
Together, these data suggest that cyclins, Bcl-xL, and CD40-CD40L could be potentially transcriptional targets, direct or indirect, of Phf19 activity to promote GC B cell proliferation, survival, and differentiation into high-affinity antibody-secreting cells during GC phase.
Discussion
Genetic polymorphisms of regulators of the immune response can affect the risk or severity of RA. Several SNPs in strong linkage disequilibrium, one in the phf19 3’ UTR region (rs1837) and one in the intron region (rs1056567), have been associated with increased risk of RA [6]; however, until now a mechanistic understanding of how these SNPs contribute to RA pathogenesis has been lacking. Our data demonstrate that lymphocytes from both SF and periphery blood of RA patients express higher levels of Phf19 than those from periphery blood of healthy controls. However, the way by which these SNPs in phf19 gene locus alter the expression or function of Phf19 needs further detailed investigation.
RA is a chronic inflammatory autoimmune disease hallmarked by synovial inflammation [16]. Within the inflamed synovium, T cells and B cells frequently accumulate underneath the synovial surface and form ectopic lymphoid follicles containing GC, which is a typical histological feature of RA synovium [17]. The development of these structures appears to contribute to the pathogenesis of RA by local production of GC-derived autoantibody, rheumatoid factor. From this point of view, delineating the underlying mechanism tightly regulating the magnitude and duration of the GC response could contribute to our understanding of the potentially destructive consequences associated with excessive GC reaction and autoantibody production caused by RA susceptible genes.
In our animal model study, strong Phf19 expression could be detected in GCs containing mostly GC B cells and Tfh cells, which play fundamental roles in GC responses. In addition, the expression of Phf19 in other B cell subsets including naïve B cells, plama cells in spleen and bone marrow, and non-Tfh cells, suggests a tight association of Phf19 with GC program. Furthermore, significant upregulation of Phf19 in RA patients and GCs in animal model points to a possibility that high expression of Phf19 could be involved in the excess ectopic GC formation in humans and ultimately contributes to the pathogenesis of RA.
Due to the excessive expression of Phf19 in RA patients, we set to investigate the role of Phf19 in animal models using gain-of-function experiments. For high expression of Phf19 is owned by not only B cells, but also T cells, we took use of the sublethal Rag1-deficient mice as BM transplantation recipient mice, whose lymphocytes including B cells and T cells are totally derived from transplanted Phf19 or control-transduced BM cells, while other radio-resistant and non-resistant cells remained in the sublethal host could develop normally and provide right indications for the development and differentiation of B cells and T cells. Thus the phenotypes we observed in the Phf19-RM mice could be B cell and T cell intrinsic. Using Phf19-RM and control-RM mice, we showed that Phf19 is essential for GC responses. Overexpression of Phf19 specifically in lymphocytes including B cells and T cells induced strong expansion of GC B cells and correlated enhancement of spleen and BM long-term antibody-producing cells, and increasing number of Tfh cells. During the GC responses, B cells undergo rapid clonal expansion, somatic hypermutation, and class-switch recombination to promote the generation and export of high-affinity plasma cells and memory cells [18]. Given that only GC B cells and Tfh cells but not antibody-producing cells either in spleen or BM upregulate Phf19 expression, Phf19 may be an essential regulator for the induction, magnitude or duration of GC responses without influencing the intrinsic plasma cell differentiation program.
Our in vivo data suggest that the growth advantage governed by Phf19-overexpressing GC B cells is attributed to the rapid progression through cell cycle, characterized by high BrdU incorporation and high ratio of Ki67+ GC B cells, and the prosurvival effect provided by their strong anti-apoptotic capacity. Similarly, enforced Phf19 expression in Tfh cells also had promoting effects on the proliferation and anti-apoptosis capacity in Tfh cells. Due to the observation that Phf19-RM mice did not show any abnormal development of B cells and T cells during their development and maturation, we speculate a model whereby the profound prosurvival and proliferative functions of Phf19 is limited in GCs, where these advantages are acquired by GC B cells and Tfh cells through their sophisticated transcription and translation programs orchestrated by Phf19.
GC dark and light zones segregate cells undergoing somatic hypermutation and antigen-driven selection, respectively [18]. Thus Phf19 could not only drive more proliferative centroblasts in the dark zone, but also induce re-proliferation of nondividing centrocytes in the light zone. At the same time, the high expression of Phf19 prevents these cells from apoptosis. By breaking the balance between proliferation and apoptosis of GCs, Phf19 is very likely to induce excessive high-affinity antibody production, which may further contribute to the abundant autoreactive antibody production during the pathogenesis of RA.
Progression through gap-phase 1 is the most important thing occurs during cell cycle and is regulated by the induced expression of D-type cyclins and their subsequent association with and activation of cyclin-dependent kinases 4 and 6, which inactivate the retinoblastoma tumor suppressor and commit cells to progression through the cell cycle [19]. Mechanistically, we have identified cyclin D2 as a Phf19-dependent target during GC B cell proliferation. Phf19 could significantly induce ccnd2 expression to drive the rapid progression of GC B cells through cell cycle, especially G1 phase. The suppression of B cell apoptosis is regulated by Bcl-2 and Bcl-xL, they prevent apoptosis by protecting mitochondrial membrane integrity and preventing the release of cytochrome c into the cytoplasm [20]. We also have identified Bcl-xL as a Phf19-dependent target during the clonal selection and apoptosis of GC B cells after immunization and the maintenance of the GC reactions.
Except for the direct prosurvival and proliferation accelerating functions of Phf19 in GCs, signaling through cell-cell contact and information transduction achieved mostly with the help from Tfh cells is also important for GC B cells survival and differentiation. CD40-CD40L ligation is the simplest but most profound help provided by Tfh cells that contributes to pro-mitotic signalling in GC B cells [15]. Our data proved that Phf19 could induce CD40 and CD40L expressed by GC B cells and Tfh cells respectively, suggesting the enhanced interact of GC B cells and Tfh cells through CD40-CD40L ligation and promoted GC responses.
Together with our findings that Phf19-RM mice had higher antibody responses and generated more GC B cells after immunization, these data suggest that Phf19-dependent targets, directly or indirectly, including cyclin D2, Bcl-xL, CD40-CD40L, are fundamental in regulating the GC responses. Further evidence is needed to explore whether Phf19 could influence activities of master regulator of GC B cells and Tfh cells, Bcl6, directly or indirectly to alter the GC responses [21]. It is also interesting to compare the contributions of Phf19 for GC B cells and Tfh cells to GC response respectively. For the further investigation of the role for Phf19 on B cells and T cells, sublethal BCR-deficient and TCR-deficient mice could probably be used as recipients as Rag1-deficient mice did.
In summary, our data provide evidence demonstrating that Phf19 could exert a critical regulatory function to raise GC responses by regulating key molecules associated with proliferation, survival and differentiation of GC B cells and Tfh cells. The identification of Phf19 in promoting GC-derived antibodies could lead to a validated conclusion that excessive Phf19 expression contributes to superfluous autoantibody productions in RA patients, this may explain why SNPs found in Phf19 locus are associated with RA and how these SNPs could contribute to the increased incidencse and severity of RA and other inflammatory diseases.
Acknowledgements
This study was supported by Henan Province Traditional Chinese Medicine Research Project (2016ZY2080).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Jacoby RK, Jayson MI, Cosh JA. Onset, early stages, and prognosis of rheumatoid arthritis: a clinical study of 100 patients with 11-year follow-up. Br Med J. 1973;2:96–100. doi: 10.1136/bmj.2.5858.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 3.Corsiero E, Nerviani A, Bombardieri M, Pitzalis C. Ectopic lymphoid structures: powerhouse of autoimmunity. Front Immunol. 2016;7:430. doi: 10.3389/fimmu.2016.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 5.Ikura K, Hanai K, Shinjyo T, Uchigata Y. HDL cholesterol as a predictor for the incidence of lower extremity amputation and wound-related death in patients with diabetic foot ulcers. Atherosclerosis. 2015;239:465–469. doi: 10.1016/j.atherosclerosis.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Chang M, Rowland CM, Garcia VE, Schrodi SJ, Catanese JJ, van der Helm-van Mil AH, Ardlie KG, Amos CI, Criswell LA, Kastner DL, Gregersen PK, Kurreeman FA, Toes RE, Huizinga TW, Seldin MF, Begovich AB. A large-scale rheumatoid arthritis genetic study identifies association at chromosome 9q33.2. PLoS Genet. 2008;4:e1000107. doi: 10.1371/journal.pgen.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brien GL, Gambero G, O’Connell DJ, Jerman E, Turner SA, Egan CM, Dunne EJ, Jurgens MC, Wynne K, Piao L, Lohan AJ, Ferguson N, Shi X, Sinha KM, Loftus BJ, Cagney G, Bracken AP. Polycomb PHF19 binds H3K36me3 and recruits PRC2 and demethylase NO66 to embryonic stem cell genes during differentiation. Nat Struct Mol Biol. 2012;19:1273–1281. doi: 10.1038/nsmb.2449. [DOI] [PubMed] [Google Scholar]
- 8.Mittler RS, Bailey TS, Klussman K, Trailsmith MD, Hoffmann MK. Anti-4-1BB monoclonal antibodies abrogate T cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy. J Exp Med. 1999;190:1535–1540. doi: 10.1084/jem.190.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nature Immunology. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 10.Shaw AC, Swat W, Ferrini R, Davidson L, Alt FW. Activated Ras signals developmental progression of recombinase-activating gene (RAG)-deficient pro-B lymphocytes. J Exp Med. 1999;189:123–129. doi: 10.1084/jem.189.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 13.Hall PA, Richards MA, Gregory WM, d’Ardenne AJ, Lister TA, Stansfeld AG. The prognostic value of Ki67 immunostaining in non-Hodgkin’s lymphoma. J Pathol. 1988;154:223–235. doi: 10.1002/path.1711540305. [DOI] [PubMed] [Google Scholar]
- 14.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. Bcl-XL and Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 16.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 17.Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann N Y Acad Sci. 2003;987:140–149. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- 18.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 20.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 21.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.