Abstract
To investigate the clinical efficacy and safety of umbilical cord mesenchymal stem cell (UCMSC) transplantation for treating multiple sclerosis (MS), the patients with MS were recruited and treated with UCMSC. The procedure of preparing UCMSC was in accordance with the standards formulated by the International Society for Cell Biology. Cell surface markers, multiple differentiation potential and safety of UCMSC for transplantation were detected. The number of cells in each infusion was 1 to 2×106 cells/kg. Patients were recruited in accordance with the standards of the International Mesenchymal Stem Cells Transplantation Study Group. After treatment, the clinical therapeutic effects including symptoms, vital signs, clinical attacks, magnetic resonance imaging (MRI), neurological function scores and adverse reactions such as fever, dizziness, and vascular irritation were monitored and evaluated. In addition, the regulatory effects of UCMSC on immune system of patients were also assessed. The results showed that the patients’ symptoms were improved after UCMSC transplantation. No clinical attacks occurred during transplantation. MRI revealed a reduced number of foci and Expanded Disability Status Scale scores were decreased. Some of patients had adverse reactions after transplantation. These adverse effects were not serious and lasted short duration, thus no intervention was conducted and let it be eliminated by itself. The mRNA expression of CD86, IL-2, CTLA-4, and HLADRB1 in peripheral blood was significantly decreased after UCMSC transplantation (P < 0.05). Based on our present studies, UCMSCs would be considered as a safe and alternative option for treatment of MS.
Keywords: Umbilical cord mesenchymal stem cell, multiple sclerosis, transplantation, clinical efficacy
Introduction
Multiple sclerosis (MS) is a demyelinating disease and the most common autoimmune disorder affecting the central nervous system. MS is characterized by demyelination, progressive neurological dysfunction, and remission and relapse. Hormones and immunosuppressants are commonly used to treat MS. However, the long-term use of hormones and immunosuppressants has side effects and cost a fortune. Furthermore, the symptoms were recurrent or become aggravated after drug withdrawal. There is no effective therapy for MS recently. With the disease progression, approximately 50% of affected patients develop chronic progressive disease with a poor prognosis [1-3]. Recent studies have shown that umbilical cord mesenchymal stem cells (UCMSCs) present low immunogenicity, hence can be used in allogeneic transplantation without rejection. UCMSC play an important role in immunomodulation and tissue repair, thereby provide a new strategy for the treatment of autoimmune diseases [4,5]. In this study, an established UCMSC bank for transplantation was employed to patients with MS. The stability, consistence and safety of UCMSC were strictly controlled. The clinical efficacy and adverse effects were evaluated after treatment. In addition, the molecular mechanism involved in UCMSC’s effects was also investigated. Our present studies provide scientific evidence to support the feasibility of this cell therapy.
Materials and methods
Isolation, culture, and identification of UCMSCs
Umbilical cords from healthy pregnant women with no history of infectious, familial, or hereditary diseases were collected in Yan’an Affiliated Hospital of Kunming Medical University with the approval of the Hospital Ethics Committee. Donors and their families provided written informed consent. Umbilical cord was cut up into small pieces with 1 mm3 diameter after umbilical arterial and venous were removed. Subsquently, UCMSC were isolated and cultured though tissue adherent method. Alpha-Modified Eagle Medium (α-MEM) containing 10% fetal bovine serum, 100 U/ml penicillin and 10 U/ml streptomycin was used for cell culture. Once the cultured cells reached 70-90% confluence, all UCMSC were harvested by using TrypLE™ Express (Invitrogen).
Flow cytometry analyze cell surface marker
Cells were incubated with mouse anti-human monoclonal antibodies CD29PE, CD44FITC, CD73PE, CD90FITC, CD105FITC, CD166PE, CD34FITC, CD45PerCP, CD123FITC and HLA DRFITC, as well as the corresponding isotype control for 1 h at room temperature. The antibodies were 1:100 dilution with blocking buffer. Cells were then incubated with goat anti-mouse IgG secondary antibody (1:100 dilution; Life Technologies) in blocking buffer for 1 h at room temperature. Subsquently, the cells were washed for three times with PBS and the level of each marker was determined by FACSCaliburTM flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
The multi-directional differentiation capacity analysis
Cells were seeded in 6-well tissue culture treated plates (3×104 cells/cm2) with α-MEM. The culture medium was changed to differentiation medium after 24 h. Meanwhile, in order to ensure the optimum condition, the growth of cells reached about 80% confluence. Appropriate differentiation medium was indicated as below and thereafter cells were replenished with the corresponding differentiation medium every 3 days. For adipogenesis, cells were cultured in adipogenic medium (MSC go Adipogenic XF™, Biological Industries (BI)) for 3 weeks followed by Oil Red O staining. For osteogenesis, cells were cultivated in osteogenic differentiation medium (MSC go Osteogenic XF™, Biological Industries (BI)) for 10-21 days and 2% Alizarin Red S Staining was performed. For neurogenesis, neurogenic differentiation medium, which was DMEM with 1000 g/l Glucose and Glutamax, 2% FCS, 10-7 M dexamethasone, 0.5 μM linoleic acid, 10 ng/ml platelet-derived growth factor (cat. no. 120-HD, R&D Systems), 10 ng/ml epidermal growth factor (cat. no. 236-EG, R&D Systems), and 50 μg/ml gentamicin, was used. Distribute cell suspension at 10000 cells/cm2 in chamber slides. The following day, the culture medium was replaced with equal volume of neurogenic differentiation medium for inducing neurogenesis) or kept culturing with complete a-MEM for control. Cells were cultured for 2 weeks with replacing fresh medium of the respective type twice weekly, and then were proceed to the evaluation assays. Immunocytochemistry using the primary antibodies against galactoserebriside and neurofilament M (1:50 dilution) was evaluated to neurogenesis.
Karyotype analysis
To eliminate the possibility of chromosomal abnormalities, karyotype analysis were carried out by making metaphase spreads of cells cultured to P7 and P23 generation. The cells were treated with colchicine, and were harvested for fixation with a hypotonic solution. Then the fixed cells were dropped on to a slide, dried and stained to observe a G banding pattern.
Tumor formation experiment
Male BALB/c nude mice (6-8 weeks of age) were randomized into 3 groups: UC-MSCs inoculated group, small cell lung cancer cells SPC-A-1 inoculated group as the positive control group and phosphate buffered saline injected group as control group. Ten mice were in each group. The tumor formation experiment was carried out by axillary subcutaneous inoculation of 1×106 cells. After 8 weeks, tumor formation was observed.
Cell viability and microbial detection
Cell viability was monitored by trypan blue staining. Moreover, the cultured medium from UCMSC and the cell suspension were detected bacteria, mycoplasma and chlamydia.
Patients enrollment on the trial
Patients with MS were recruited based on the standards of the International Mesenchymal Stem Cells Transplantation Study Group. This study was approved by the Ethics Committee of Kunming Yan’an Hospital in China. The inclusion criteria were MS diagnosed in accordance with the 2005 McDonald diagnostic criteria, age of 18 to 65 years, either sex, ineffective or poorly effective treatment with corticosteroids and immunosuppressants. The exclusion criteria were treatment with immunosuppressants 3 months before the trial; treatment with interferon-β, glatiramer acetate, or steroid hormones in the last month; uncontrollable infection; heart, kidney, liver, or other diseases; and pregnancy or lactation. The patients in control group had no significant differences in age, sex, or course of disease compared with the experimental group. The difference in age between the control and experimental groups was ±3 years. The initial Expanded Disability Status Scale (EDSS) score was identical between patients in the control and experimental groups. The patients in control group experienced poor effects of conventional treatment including anti-inflammatories, immunosuppressants, and/or immunomodulators and continued maintenance with the current treatment.
Clinical efficacy and safety evaluation
UCMSC passages 4 to 6 were most suitable for cell transplantation. Intravenous transfusions were performed with 1 to 2×106 cells/kg at 3-month intervals for 7 times. The safety evaluation involved routine blood examination, biochemical test of blood, liver and kidney function tests, urine and stool tests, electrocardiography, chest X-ray examination before and after transplantation. When the trail was finished, the assessment of adverse reactions was carried out. Meanwhile, the clinical efficacy including assessment of changes in symptoms and vital signs, clinical attacks, magnetic resonance imaging (MRI), and neurological function scores was also evaluated.
Real-time polymerase chain reaction (PCR)
Finally, in order to investigate the immunomodulatory effects of UCMSC in patients with MS, peripheral blood was collected before and after cell transplantation. Real-time PCR was used to determine the mRNA expression of CD86, interleukin (IL)-2, IL-17c, Foxp3, CTLA-4, HLA-DRB1, transforming growth factor (TGF)-β1, and TGF-β2.
Statistical analysis
Data are expressed as mean ± standard deviation. Data before and after transplantation were compared using a paired t-test. The data in the experimental and control groups were compared with a two-sample t-test. A P value of < 0.05 was considered to be statistically significant. All data were analyzed with SPSS 17.0 software (SPSS Inc., Chicago, IL).
Results
UCMSC with high purity and high viability
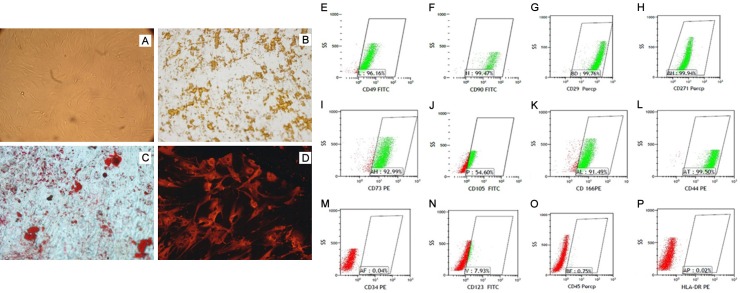
Using the tiled tissue method, cells from passage 5 to 7 were observed and became confluent at 10 to 12 days. UCMSC appeared as fibroblast-like cells under light microscopic examination (Figure 1A). During in vitro induction, UCMSC were able to differentiate into osteoblasts, adipocytes, and nerve cells (Figure 1B-D). UCMSC were positive for CD29, CD44, CD90, CD73, CD105, and CD166 but negative for CD34, CD45, CD123, and HLA-DR (Figure 1E-P). UCMSC from passages 7 and 23 showed a normal karyotype with the absence of polyploidy (Figure 2A, 2B). At day 60, no transplanted tumors were detected in nude mice subcutaneously injected with UCMSC from passages 7 and 23 (Figure 2C, 2D).
Figure 1.
Multi-differentiation of UCMSCs in vitro. A. Undifferentiated UCMSCs displayed fibroblast-like cell morphology. B. Von Kossa staining for osteogenic differentiation. C. Oil red O staining for adipogenic differentiation. D. Neurofilament M immunofluorescence staining for neurogenic differentiation. E-P. Immunophenotypic characterization of UCMSCs by flow cytometry. The cells were positive for CD49, CD90, CD29, CD271, CD73, CD105, CD166, and CD44 but negative for CD34, CD45, CD123, and HLA-DR.
Figure 2.
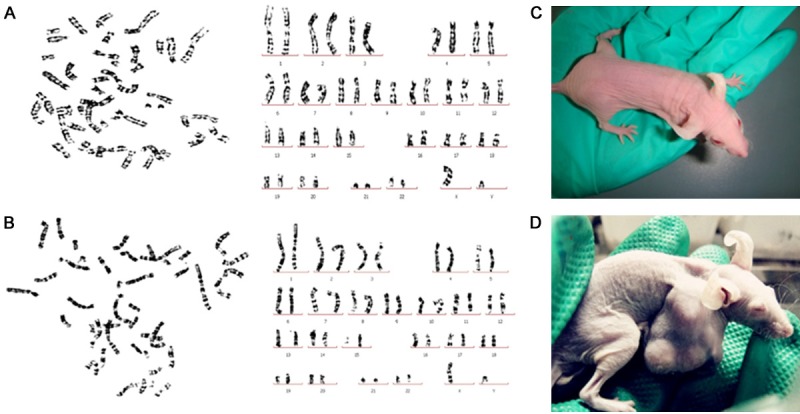
A, B. UCMSCs from passages 7 and 23 presented a normal chromosome karyotype with the absence of polyploidy. C. The representative photo of mice inoculated with UCMSC after 8 weeks, no tumor formation. D. The representative photo of mice inoculated with SPC-A-1 as the positive control.
Baseline patient data
Patients in the treatment group received seven times of UCMSCs treatments. During that period of time, they didn’t undergo other drug treatment. The patients in the control group continued those medications they had already been taking, including methylprednisolone, glucocorticoid hormones, interferon, human immunoglobulin, neurotrophic factor, and traditional Chinese medicines. However, their conditions were still progressively aggravated. The baseline patient data are listed in Table 1.
Table 1.
Baseline characteristics of the three patients
| Transplant patient 1 | Transplant patient 2 | Control patient | |
|---|---|---|---|
| Type of disease | Relapsing-remitting | Secondary progressive | Secondary progressive |
| Onset age/sex | 30/Male | 30/Female | 33/Male |
| Onset time | September, 2006 | September, 2003 | June, 2002 |
| Duration (year) | 5 | 8 | 9 |
Adverse reactions
The two patients in the treatment group received seven times of UCMSC transplantation. The observed adverse reactions were mainly dizziness, headache, skin redness, and vascular irritation. These adverse reactions mainly occurred during transplantation. Fever was the most common adverse reaction, but the conditions generally did not require any intervention. The patients’ temperature returned to normal within 36 h. Toxic reactions of UCMSC were not monitored (Table 2).
Table 2.
The procedures of cell transplantation and adverse reactions
| Times | Patient 1 | Patient 2 | ||
|---|---|---|---|---|
|
| ||||
| Cell number | Adverse reaction | Cell number | Adverse reaction | |
| 1 | 1.01×106/kg | Fever/6 h, dizziness 12 h, vascular irritation/1 wk | 2.49×105/kg | No |
| 2 | 4.28×106/kg | Headache 2 h, local skin redness/2 d, vascular irritation/1 wk | 1.13×106/kg | No |
| 3 | 2.62×106/kg | Local skin redness/2 d, vascular irritation/1 w | 1.68×106/kg | No |
| 4 | 2.31×106/kg | Dizziness/1 h, headache/6 h | 2.26×106/kg | Dizziness/1 h, headache/6 h |
| 5 | 3.17×106/kg | Fever/4 h, dizziness 12 h, vascular irritation/1 wk | 2.77×103/kg | No |
| 6 | 2.73×106/kg | Dizziness/2 h | 6.17×106/kg | Local skin redness/1 d, vascular irritation/3 d |
| 7 | 2.24×106/kg | Dizziness/1 h, headache/1 h | 2.64×106/kg | Dizziness/1 h, headache/6 h |
Improvements in symptoms and vital signs
After cell treatment, the symptoms involved in unstable walking lessened, mental state, appetite, coordination ability and balance force of patient 1 were remarkably improved. The symptoms including numbness of the right limbs, constipation of patient 2 were mitigated. At the same time, mental status and memory of patient 2 were also improved, indicating the patient’s condition was stable. However, the symptoms and vital signs of patient in control group were not improved but aggravated (Table 3).
Table 3.
Symptoms and vital signs of patients at different stages of clinical trail
| Transplant patient 1 | Transplant patient 2 | Control patient | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 2006.11a | 2008.11b | 2011.9c | 2003.9a | 2010.10b | 2011.12c | 2002.6 | 2004.9 | 2007.5 | 2011.9 | |
| Symptom | ||||||||||
| Vision | 0 | 4 | 4 | 0 | 0 | 0 | 2 | 4 | 4 | 4 |
| Leg weakness | 2 | 2 | 2 | 2 | 2 | 4 | 0 | 0 | 4 | 4 |
| Limb tremor | 0 | 4 | 4 | 0 | 4 | 4 | 0 | 0 | 4 | 4 |
| Walking instability | 4 | 2 | 2 | 2 | 4 | 4 | 4 | 2 | 6 | 6 |
| Numbness of limbs | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 4 |
| Constipation | 0 | 0 | 0 | 0 | 4 | 2 | 2 | 0 | 4 | 4 |
| Vital signs | ||||||||||
| Vision | 2 | 4 | 2 | 0 | 0 | 0 | 2 | 6 | 4 | 4 |
| Nystagmus | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 4 | 6 |
| Muscle strength↓ | 2 | 2 | 2 | 2 | 2 | 4 | 0 | 0 | 0 | 4 |
| Muscle tension↑ | 0 | 4 | 2 | 2 | 4 | 4 | 0 | 0 | 0 | 4 |
| Ataxia | 2 | 6 | 4 | 2 | 4 | 4 | 2 | 0 | 4 | 6 |
| Pathologic reflex | 4 | 6 | 4 | 2 | 6 | 6 | 0 | 0 | 4 | 6 |
Time of onset.
Before the first cell transplantation.
After the seventh cell transplantation.
0, Normal; 2, mild Kurtzke score; 4, moderate Kurtzke score; 6, severe Kurtzke score.
Evaluation of neurological function
After cell treatment, the EDSS score decreased to 2 in Patient 1 and the clinical symptoms did not become aggravated but lessened. In Patient 2, the symptoms were stable, and the EDSS score was 6.5. The EDSS score of patient in control group was 7.0, after received drug treatment, indicating the disease was continuously progressive (Figure 3).
Figure 3.
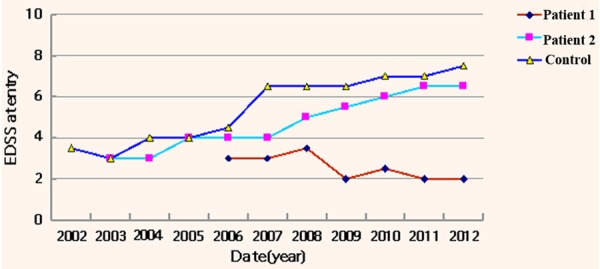
Evaluation of changes in EDSS scores in three multiple sclerosis patients at different time points. In Patient 2, the symptoms were stable, and the EDSS score was 6.5. The EDSS score of patient in control group was 7.0, after received drug treatment, indicating the disease was continuously progressive.
Evaluation of the changes of MRI value in foci after UCMSC transplantation
After patient 1 received seven times of treatments, lesions on the right side of the cerebral ventricle became pale and tended to disappear through MRI examination. In addition, the lesions on the left side were remarkably reduced and the ranges of the frontal and parietal lobes and semi-oval area were reduced. The lesion on the cervical spinal cord also became lighter (Figure 4A). After patient 2 received four times of treatments, MRI results revealed that the high signal intensity was reduced at the site next to the left ventricle and basal ganglia (Figure 4B). The MRI results from the patient in control, high signal intensity was visible in the white matter lateral to the bilateral ventricles and semi-oval area. Multiple nodular lesions were observed in the corona radiata and the site next to the lateral ventricles (Figure 4C).
Figure 4.
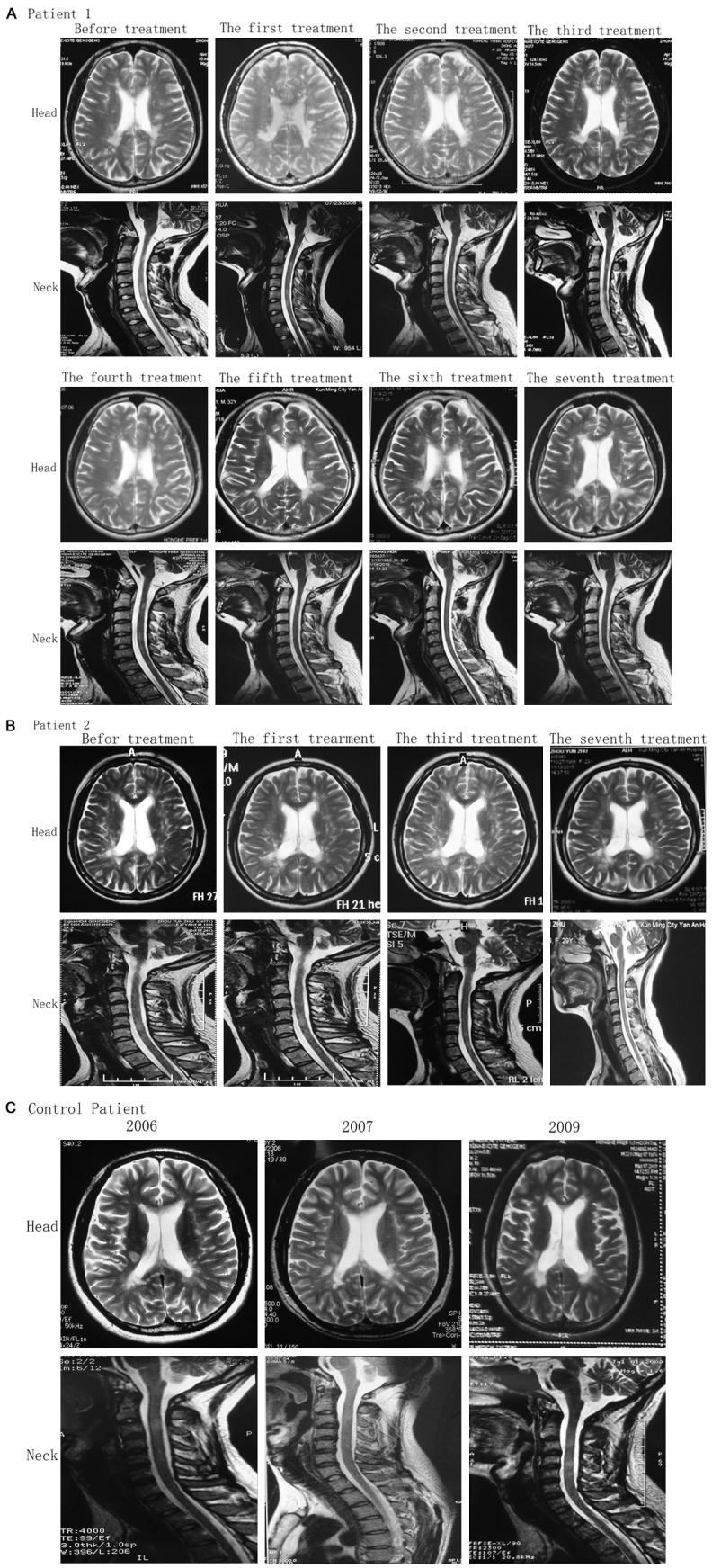
MRI images of patients in the cerebral transverse plane and sagittal cervical segment at different time points. A. Patient 1, after patient 1 received seven times of treatments, lesions on the right side of the cerebral ventricle became pale and tended to disappear through MRI examination. In addition, the lesions on the left side were remarkably reduced and the ranges of the frontal and parietal lobes and semi-oval area were reduced. The lesion on the cervical spinal cord also became lighter. B. Patient 2, MRI results revealed that the high signal intensity was reduced at the site next to the left ventricle and basal ganglia. C. Patient in the control group, high signal intensity was visible in the white matter lateral to the bilateral ventricles and semi-oval area. Multiple nodular lesions were observed in the corona radiata and the site next to the lateral ventricles.
Detection of the changes of cytokines through mRNA expression levels
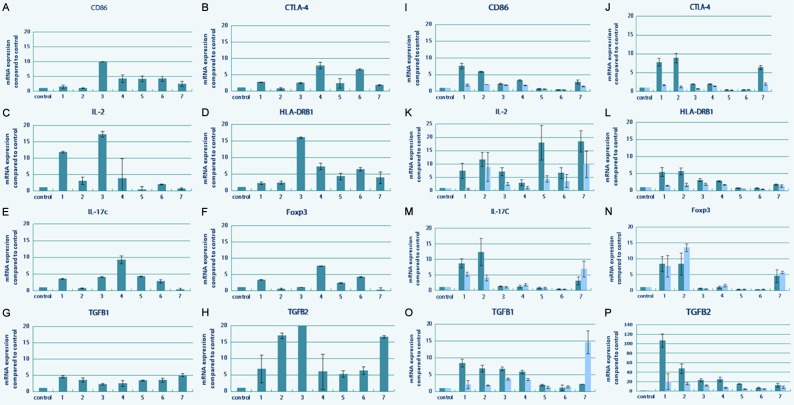
To evaluate the immunomodulatory effects of UCMSCs, peripheral blood from patients was collected on the day of transplantation and day 3 after transplantation for RNA extraction. Real-time polymerase chain reaction was utilized to determine the mRNA expression of CD86, IL-2, IL-17c, Foxp3, CTLA-4, HLA-DRB1, TGF-β1, and TGF-β2 in peripheral blood. The mRNA expression of CD86, IL-2, CTLA-4, and HLA-DRB1 were significantly reduced before and after each transplantation (P < 0.05). CD86 and CTLA-4 had similar trends during transplantation. When the patient’s condition worsened, HLA-DRB1 was highly expressed (P < 0.01). The correlation between IL-2 and the disease was not as strong as that between HLA-DRB1 and the disease. The expression of Foxp3 and IL-17c was decreased in the samples from patient in control group. Significant differences in the expression of Foxp3 and IL-17c were detected between the patients in treatment group and the control group (P < 0.01). TGF-β2 expression was clearly decreased with the progress of treatment (Figure 5).
Figure 5.
Quantitative real time PCR analyses of mRNA of CD86, IL-2, IL-17c, Foxp3, CTLA-4, HLA-DRB1, TGF-β1, and TGF-β2 genes in peripheral blood from patients were shown. Data were normalized to corresponding GAPDH expressions as internal control. The results of mRNA expressions are expressed as fold of control. Evaluation of changes of cytokines with mRNA level in Patient 1 (A-H) and Patient 2 (I-P). The mRNA levels of cytokines were monitored before each transplantation, and compared with their relative levels at the beginning of the trail, which was considered as the control.
Discussion
Multiple sclerosis is a demyelinating disease and the most common autoimmune disorder affecting the central nervous system. It frequently involves the region from the spinal cord to the white matter, gray matter, and peripheral nerves, resulting in paralysis and sensory disturbances [6]. Multiple sclerosis is characterized by recurrent attacks and mitigation [6]. Multiple sclerosis is more common in Europe and the US, with a prevalence rate of 100 to 200 per 100,000 individuals. Asia is a low-incidence area. An epidemiological investigation [7] demonstrated that the prevalence rate was 0.88 to 10 per 100,000 individuals in China, but had a upward trend. Multiple sclerosis cannot be completely cured. As the disease develops, dysfunction is gradually progressive, resulting in disability. The main principle of treatment is immunosuppression and immunomodulation, but these still cannot prevent recurrence. The proportion of patients with disease progression and the incidence of long-term disability do not reduce. Therefore, effective treatment methods with few side effects are urgently needed.
MSCs are stem cells with multiple differentiation potential. They are derived from the mesoderm and can differentiate into osteoblasts, chondrocytes, and nerve cells [8-11]. MSCs have low immunogenicity and effective immunomodulatory properties [12-14]. Previous studies have suggested that MSCs amplified in vitro can suppress the proliferation of T lymphocytes, B lymphocytes, and natural killer cells and inhibit maturation and differentiation of dendritic cells [15-17]. Animal studies have confirmed that MSCs can arrive at the site of injury through various pathways and differentiate into nerve stem cells, mature neurons, and glial cells and that they can safely and effectively improve the functional recovery of the central nervous system [11,18]. Umbilical cords can be easily harvested; the collection process is noninvasive and has no ethical issues. Therefore, UCMSCs have been deemed one of the best sources of MSCs in clinical and scientific research.
In this study, UCMSC transplantation was used to treat two patients with multiple sclerosis for a total of seven times of treatments. No obvious adverse reactions or residual pathological syndromes appeared during transplantation. The physiological examination and MRI results revealed normal indexes. Toxic reactions of the UCMSCs were not detected during the 8-year follow-up. Clinical signs and symptoms were mitigated in the two patients after transplantation. The onset frequency was compared within the same time after transplantation, and the average annual onset frequency in the transplant patients were remarkably less than before transplantation. The EDSS scores demonstrated that the clinical symptoms were mitigated in Patient 1. At the time of this writing, his symptom was stable and not progressive. After the first and second transplantations, the symptoms of Patient 2 were progressive. Therefore, we shortened the time interval and administered cell therapy. His condition was stable at the time of this writing. The MRI findings showed that the number of foci was obviously reduced after transplantation, suggesting that UCMSC transplantation promoted remyelination [5]. In vivo immunological parameters revealed that as the patient’s condition stabilized, the mRNA expression of each cytokine decreased and no noticeable inhibitory effect was detectable, indicating the immunomodulatory properties of MSCs. MSCs have low immunogenicity and exert immunomodulatory effects on the body [19]. CD86 and CTLA-4 are important co-stimulatory molecules with a negative regulating function. CD86 bound to CTLA-4 produces inhibitory signals. Termination of T-cell activation is the key link to maintaining homeostasis of lymphocytes. We found that the expression of CD86 and CTLA-4 was significantly diminished before and after MSC transplantation. Moreover, the expression of CD86 and CTLA-4 was highest before the first transplantation and during onset in Patient 2. Therefore, during disease progression, the body’s pathological immune response induces high expression of the inhibitory co-stimulatory signals. After MSCs correct the pathological immune response in vivo, the expression of CD86 and CTLA-4 decrease [20]. IL-2 is the core of the immune regulatory network [21]. In the present study, the expression of IL-2 mRNA was lower after than before transplantation. T-helper 17 (Th17) cells are a subset of CD4 T-helper cells characterized by their production of IL-17. IL-17 is considered pro-inflammatory cytokine, which is highly expressed in the serum and tissue of patients with rheumatoid arthritis [21], asthma [22], systemic lupus erythematosus [23], and multiple sclerosis [24,25]. In the present study, IL-17c expression decreased comparing with it in the control of the patient’s condition. And its expression was similar with the healthy person. These findings indicated that MSCs inhibited IL-17c expression. The CD4+CD25+ T cells are considered a group of T cells with an immune regulatory function; they are also called regulatory T cells (Tregs). The CD4+CD25+ cell population highly expressing the Foxp3 gene is the main regulatory T lymphocyte subset that exerts an inhibitory function [26]. Tregs play important roles in maintaining environmental stability in vivo by monitoring tumor immunity, inducing transplantation tolerance. In this study, Foxp3 mRNA expression decreased after UCMSC transplantation. However, Foxp3 mRNA expression was high during disease progression and low during disease remission, showing an altered trend similar to that of IL-17c. Foxp3 is an important negative regulatory factor in the body and plays an important role in immune protection. HLA-DRB1, another important predisposing gene, tends to be normal with increased times of UCMSC transplantations. Furthermore, HLA-DRB1 expression increases during onset of the disease. HLA-DRB1 expression, which is closely related to progression of the disease, can be used as a key factor to determine disease onset [27]. TGF-β2 is tightly associated with cell proliferation, differentiation, apoptosis, and immunoregulation [28]. TGF-β2 expression dramatically decreased before, during, and after transplantation in Patient 2 as the times of transplantations increased. Therefore, TGF-β2 is an important protective factor against antibody attacks.
In summary, UCMSCs play an important role in immune regulation and neural protection. UCMSCs can regulate pathological immune responses and inhibit IL-17c, HLA-DRB1, and IL-2. Foxp3 and TGF-β2 are important protective factors in the body, helping to protect against antibody attacks. The neuroprotective effect is strongly associated with the mechanism of promoting remyelination. Our findings confirm that UCMSCs have functions of immune regulation and nerve protection, indicating the feasibility of UCMSC transplantation for multiple sclerosis. However, because of the low number of cases in the present study, our results need to be validated in large clinical trials in the future.
Acknowledgements
This study was supported by the General Program of Applied Basic Research in Yunnan Province of China, No. 2013FZ227; the Joint Special Program of Applied Basic Research in Yunnan Province and Kunming Medical University of China, No. 2013FB188; the Research Organization Project of Yunnan Provincial Health and Family Planning Commission in China, No. 2014NS213; the Cooperation Project with Foreign Countries in Yunnan Province of China, No. 2013IA013.
Disclosure of conflict of interest
None.
References
- 1.Lei XD, Xu F, Li YH. The research progress of tumor necrosis factor alpha in multiple sclerosis and remyelination. Tianjin Yiyao. 2014;11:1141–1143. [Google Scholar]
- 2.Mohajeri M, Farazmand A, Mohyeddin Bonab M, Nikbin B, Minagar A. FOXP3 gene expression in multiple sclerosis patients pre- and post mesenchymal stem cell therapy. Iran J Allergy Asthma Immunol. 2011;10:155–161. [PubMed] [Google Scholar]
- 3.Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9:A387–394. doi: 10.1016/j.autrev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Yamout B, Hourani R, Salti H, El-Hajj T, Al-Kutoubi A, Herlopian A, Baz EK, Mahfouz R, Khalil-Hamdan R, Kreidieh NM, El-Sabban M, Bazarbachi A. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol. 2010;227:185–189. doi: 10.1016/j.jneuroim.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Hou ZL, Liu Y, Mao XH, Wei CY, Meng MY, Liu YH, Zhuyun Yang Z, Zhu H, Short M, Bernard C, Xiao ZC. Transplantation of umbilical cord and bone marrow-derived mesenchymal stem cells in a patient with relapsing-remitting multiple sclerosis. Cell Adh Migr. 2013;7:404–407. doi: 10.4161/cam.26941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9:A387–394. doi: 10.1016/j.autrev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A International Society for Cellular Therapy. Clarification of the nomenclature for MSC: the international society for cellular therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 8.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 9.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 10.Anghileri E, Marconi S, Pignatelli A, Cifelli P, Galié M, Sbarbati A, Krampera M, Belluzzi O, Bonetti B. Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem Cells Dev. 2008;17:909–916. doi: 10.1089/scd.2007.0197. [DOI] [PubMed] [Google Scholar]
- 11.Kassis I, Grigoriadis N, Gowda-Kurkalli B, Mizrachi-Kol R, Ben-Hur T, Slavin S, Abramsky O, Karussis D. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Arch Neurol. 2008;65:753–761. doi: 10.1001/archneur.65.6.753. [DOI] [PubMed] [Google Scholar]
- 12.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 13.Gordon D, Pavlovska G, Uney JB, Wraith DC, Scolding NJ. Human mesenchymal stem cells infiltrate the spinal cord, reduce demyelination, and localize to white matter lesions in experimental autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 2010;69:1087–1095. doi: 10.1097/NEN.0b013e3181f97392. [DOI] [PubMed] [Google Scholar]
- 14.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 15.Kearns-Jonker M, Dai W, Kloner RA. Stem cells for the treatment of heart failure. Curr Opin Mol Ther. 2010;12:432–441. [PubMed] [Google Scholar]
- 16.Sun L, Zhang T, Lan X, Du G. Effects of stem cell therapy on left ventricular remodeling after acute myocardial infarction: a meta-analysis. Clin Cardiol. 2010;33:296–302. doi: 10.1002/clc.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koninckx R, Daniëls A, Windmolders S, Carlotti F, Mees U, Steels P, Rummens JL, Hendrikx M, Hensen K. Mesenchymal stem cells or cardiac progenitors for cardiac repair? A comparative study. Cell Mol Life Sci. 2011;68:2141–2156. doi: 10.1007/s00018-010-0560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 19.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 20.Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S, Frassoni F, Locatelli F. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- 21.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 22.Kohno M, Tsutsumi A, Matsui H, Sugihara M, Suzuki T, Mamura M, Goto D, Matsumoto I, Ito S, Suguro T, Sumida T. Interleukin-17 gene expression in patients with rheumatoid arthritis. Mod Rheumatol. 2008;18:15–22. doi: 10.1007/s10165-007-0015-y. [DOI] [PubMed] [Google Scholar]
- 23.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Fransson ME, Liljenfeldt LS, Fagius J, Tötterman TH, Loskog AS. The T-cell pool is anergized in patients with multiple sclerosis in remission. Immunology. 2009;126:92–101. doi: 10.1111/j.1365-2567.2008.02881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fransson ME, Liljenfeldt LS, Fagius J, Tötterman TH, Loskog AS. The T-cell pool is anergized in patients with multiple sclerosis in remission. Immunology. 2009;126:92–101. doi: 10.1111/j.1365-2567.2008.02881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 2004;6:745–51. doi: 10.1016/j.micinf.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Sawcer S, Hellenthal G, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–9. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]