Abstract
miR-132, a microRNA, has been reported to be down-regulated in several human cancers and is related with tumor progression; however, its function in non-small cell lung cancer (NSCLC) progression remains unclear. This study aimed to investigate the putative role of miR-132 in the metastasis of NSCLC. We determined the function of miR-132 in the migration and invasion of a NSCLC cell line in vitro using a miR-132 inhibitor and mimic. Our results showed overexpression of miR-132 significantly inhibited the migration and invasion of NSCLC cells in vitro. We then identified USP9X as a potential target of miR-132, and demonstrated miR-132 could regulate the expression of USP9X at both the mRNA and protein level. miR-132 could directly bind to the 3’ untranslated region (3’-UTR) of USP9X. Inhibition of USP9X by its inhibitor WP1130 reduced the migration and invasion of NSCLC cells. Furthermore, USP9X inhibition also reversed the increased migration and invasion mediated by miR-132 inhibition. We found USP9X inhibition up-regulated expression of the epithelial-mesenchymal transition (EMT) marker E-cadherin, but down-regulated vimentin expression. A similar effect was seen with miR-132 overexpression, while the opposite effect occurred with miR-132 knockdown. USP9X inhibition reversed the miR-132 inhibitor-induced vimentin up-regulation and E-cadherin down-regulation. Taken together, these results indicate miR-132 prohibits the migration and invasion of NSCLC cells via targeting USP9X-induced EMT. Our data provides further evidence for the critical role of miR-132 and USP9X in regulating cell invasion and migration of NSCLC.
Keywords: Epithelial-mesenchymal transition, non-small cell lung cancer, miR-132, USP9X, invasion and migration
Introduction
Lung cancer is a leading cause of cancer-related mortality worldwide [1]. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer. With the development of genomics, the complex molecular mechanisms underlying NSCLC carcinogenesis and progression have been uncovered to a certain extent, and several molecular targets (e.g. EGFR and C-met) have been used to treat NSCLC [2]. However, the development of NSCLC is a complicated process, and it is essential to explore more novel and specific markers for NSCLC to improve therapeutic strategies and clinical outcomes.
MicroRNAs (miRNAs) are highly conserved, endogenous non-coding RNAs of ~22 nucleotides in size, which can regulate target gene expression at the post-transcriptional level by inhibiting transcription or degrading mRNA [3]. miRNAs have been implicated in variety of human disease, particular in cancer [4-8]. Indeed, miRNAs are involved in a variety of biological processes in cancer, including tumor initiation, progression, cell invasion, and metastasis [9].
miR-132 is a known tumor-related miRNA; however, it appears to play different roles in various tumor types [10]. For example, miR-132 was reported to be up-regulated and function as a promoter in cancers such as colorectal cancer [11] and pancreatic cancer [12]; while it is down-regulated and functions as a repressor in hepatocellular carcinoma [13], prostate cancer [14], and breast cancer [15]. In NSCLC, miR-132 was previously reported to act as a repressor by inhibiting cell proliferation, apoptosis, migration, and invasion [16-20]. In particular, previous studies showed miR-132 could suppress the migration and invasion of NSCLC cells through targeting ZEB2 and SOX4 [17,18]. Although numerous genes have already been shown to be targets of miR-132, each miRNA is predicted to target more than 100 genes on average. In addition, one miRNA may regulate several targets in an individual cancer [21]. Therefore, the molecular mechanisms and functional targets of miR-132 in NSCLC required further investigation.
Ubiquitin-specific peptidase 9, X-linked (USP9X), a member of the ubiquitin-specific peptidase (USP) family, is involved in multiple physiological pathways by deubiquitinating and stabilizing its substrates [22]. Increasing evidence suggests that USP9X is significantly elevated in multiple human cancers, including breast cancer [23], pancreatic cancer [24], liver cancer [25], and lung cancer [26]. Overexpression of USP9X was shown to be closely related to tumor proliferation, apoptosis, chemo-resistance, and metastasis [22]. In addition, elevated expression of USP9X was shown to correlate with poor prognosis in NSCLC [26]. Moreover, evidence suggests USP9X can deubiquitinate MCL1, an anti-apoptotic protein, to inhibit cell apoptosis in NSCLC [27]. However, little is known about the role of USP9X in NSCLC cell migration and invasion.
In the present study, we aimed to investigate the putative role of miR-132 in metastasis of NSCLC.
Materials and methods
Cell culture and transfection
The human NSCLC cell lines A549 and NCI-H1299 were obtained from the Chinese Academy of Science Cell Bank (Shanghai, China), and were cultured in RMPI 1640 medium containing 10% fetal bovine serum (FBS; Gibco, CA, USA) at 37°C in a humidified atmosphere of 5% CO2. For transfection, cells were cultured to 80% confluence and transfected with recombinant eukaryotic vector and empty vector using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s instructions. The USP9X inhibitor WP1130 was obtained from Selleck (S2243, Texas, USA) and dissolved in DMSO for this research.
RNA oligoribonucleotides and transfection
The miR-132 mimic, inhibitor, and negative control siRNA were synthesized by GenePharma (Suzhou, China). The transfection was conducted using Lipofectamine 2000 (Invitrogen, CA, USA) according to manufacturer’s instructions.
Cell migration assay
The migration ability was determined using a wound-healing assay. The cells were plated into 6-well plates at a density of 3 × 105, and then transfected with miR-132 mimic or inhibitor or control. Alternatively, cells were cultured in medium with WP1130 at a concentration of 0.625 μM for A549 or 1.25 μM for NCI-H1299. After 24 h, the cells were wounded with a sterile plastic 100-μL micropipette tip; the wounded cells were then washed with PBS and cultured in serum-free medium. The width of the wound was measured at 0 h, 24 h, and 48 h. Three different views were photographed under a phase-contrast inverted microscope.
Cell invasion assay
Basement membrane invasion assays were performed using Transwell plates (Corning, NY). Cells were transfected with miR-132 mimic or inhibitor or control. Alternatively, cells were cultured in medium with WP1130 at a concentration of 0.625 μM for A549 or 1.25 μM for NCI-H1299. After 24 h, the transfected cells were trypsinized and resuspended at a density of 2 × 105 cells in 200 μL serum free RPMI 1640 medium and placed into the upper chambers (8-mm pore size) coated with matrigel (BD Bioscience, Bedford, MA, USA). The lower chambers were filled with 600 μL complete medium with 10% FBS. For cells incubated with WP1130, WP1130 was added to both the upper and lower chambers. After incubation for 24 h at 37°C, non-invading cells on the upper surface of the inserts were removed with a cotton swab. The invaded cells on the lower surface of the inserts were fixed and stained with 0.1% crystal violet, and five random fields were counted and photographed at 200 × magnification.
Cell viability assay
The cell viability examination was using a Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Japan) according to the manufacturer’s instructions. Cell viability was measured under different concentrations of WP1130 (0, 0.625, 1.25, 2.5, 5, 10 μM). An MRX II microplate reader (Dynex, Chantilly, VA, USA) was used to measure the OD value at the 450 nm.
Quantitative real-time PCR (qRT-PCR)
Total RNA including miRNAs was extracted using the TRIZOL reagent (Invitrogen) according to manufacturer’s instructions. Total RNA (0.5 μg) was reverse transcribed into cDNA. SYBR Green PCR Master mix (Takara, Japan) was used to determine the mRNA level of miR-132 and USP9X (USP9X: Forward 5’-CAATGGATAGATCGCTTTATA-3’; Reverse 5’-CTTCTTGCCATGGCCTTAAAT-3’, miR-132: TAACAGTCTACAGCCATGGTCG; GAPDH: Forward 5’-CGGAGTCAACGGATTTGGTCGTAT-3’; Reverse 5’-AGCCTTCTCCATGGTGGTGAAGAC-3’). All reactions were performed in triplicate. The relative expression of miR-132 and USP9X was respectively normalized to the internal reference U6 and GAPDH. Data were analyzed using the 2ΔΔCt method.
Western blot
Lung cancer cell lines with different treatment were seeded at a density of 2 × 105 cells/well in 6-well plates. Cells were then lysed in RIPA lysis buffer with 1 mM PMSF (Beyotime, Shanghai, China) for 1 h on ice. Sample proteins were quantified using a Pierce BCA protein assay (Thermo Scientific, Waltham, MA). Equal amounts of proteins were separated by SDS-PAGE, transferred to PVDF membranes, and probed with the antibody of interest. The concentration for the primary antibodies Ecadherin, vimentin, and GAPDH (CST, Danvers, MA, USA) was 1:1000; the concentration of the corresponding secondary antibody (CST, HRP-conjugated) was 1:2000.
Statistical analysis
SPSS17.0 software was used for statistical analysis. The experimental data were expressed as mean ± standard deviation (SD), and analyzed by a two-tailed student’s T test. Statistical significance was accepted if P < 0.05.
Results
miR-132 overexpression inhibits the migration and invasion of NSCLC cells in vitro
To demonstrate the role of miR-132 on the aggressiveness of NSCLC cells in vitro, we used a wound-healing assay and a transwell assay. The wound-healing assay showed overexpression of miR-132 in NSCLC cells (using an miR-132 mimic) significantly prohibited cell migration compared to cells transfected with a control or miR-132 inhibitor (Figure 1A and 1B). Moreover, the transwell assay demonstrated overexpression of miR-132 in NSCLC cells significantly decreased the invasion ability compared to the control and miR-132 inhibitor group (Figure 1C). Together, these results indicate miR-132 inhibits the migration and invasion of NSCLC cells in vitro.
Figure 1.
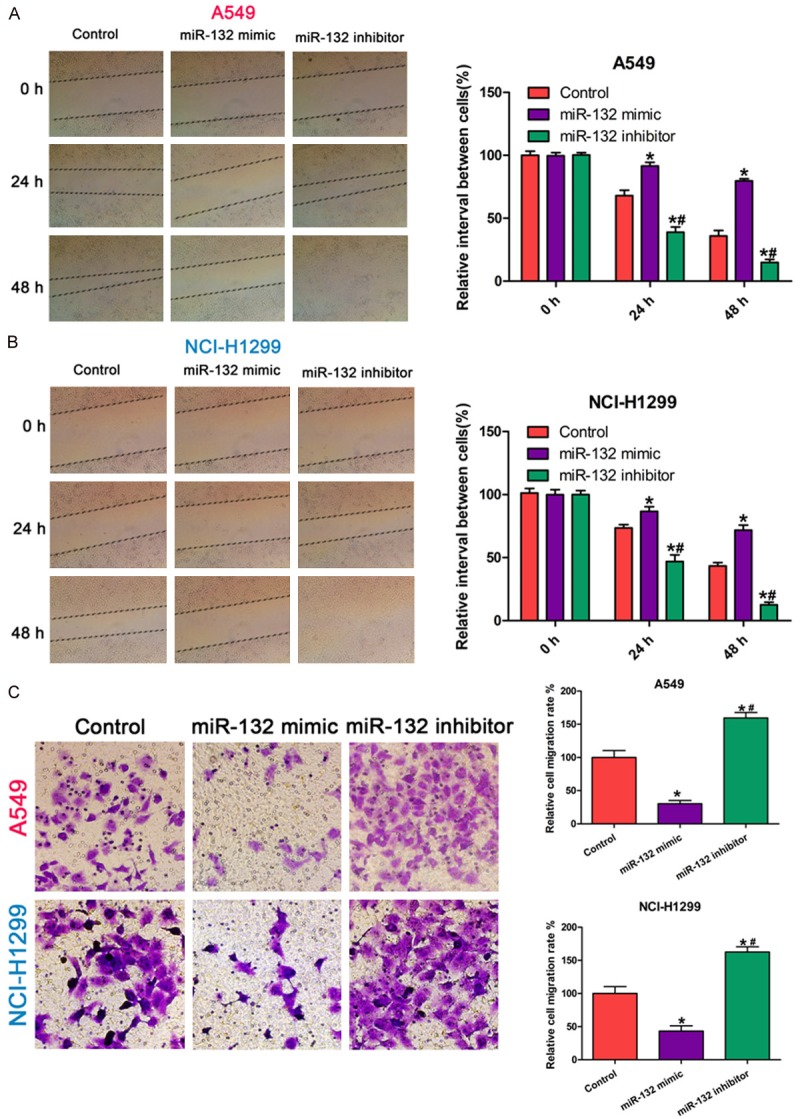
miR-132 inhibits lung cancer cell migration and invasion. A, B: Migration ability of A549 and NCI-H1299 cells transfected with the miR-132 mimic, inhibitor, or negative control using a wound-healing assay. C: Invasion ability of A549 and NCI-H1299 cells transfected with the miR-132 mimic, inhibitor, or negative control using a transwell assay. All results were obtained from three independent experiments. The migration or invasion cell number in each group was normalized to the control (* P < 0.05) or miR-132 mimics (# P < 0.05).
miR-132 directly inhibits the expression of USP9X via binding its 3’-UTR in NSCLC cells
To explore how miR-132 suppresses the migration and invasion of NSCLC cells, we predicted the potential target genes of miR-132 in NSCLC cells using the miRanda, PicTar, and TargetScan databases. Among them, we found an unreported candidate gene USP9X harbored a potential miR-132 binding site (Figure 2A). Further study showed that a negative correlation between miR-132 and USP9X expression in NSCLC cells (Figure 2B-D). Moreover, miR-132 overexpression in NSCLC cells led to a decreased USP9X expression at both the mRNA and protein level, while miR-132 inhibition showed the opposite results (Figure 2E-G). Therefore, our results demonstrate that USP9X is a potential target gene of miR-132 in NSCLC cells.
Figure 2.
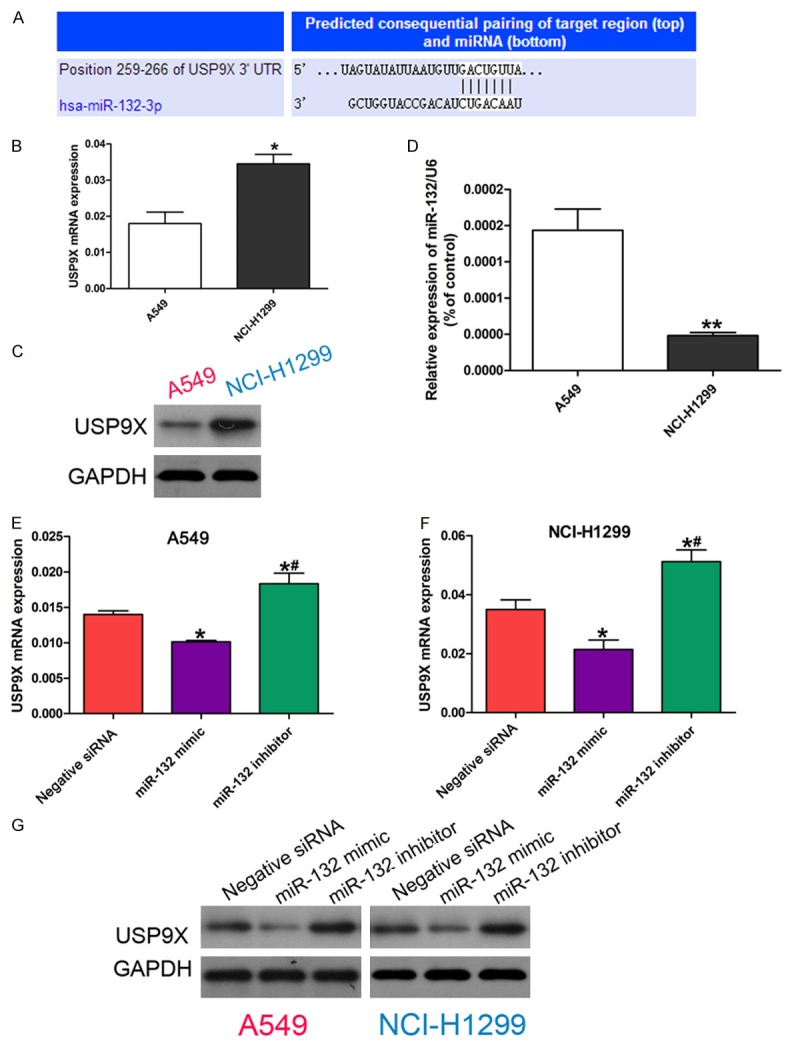
miR-132 directly inhibits the expression of USP9X via binding its 3’UTR. A: The predicted miR-132 binding site in the 3’UTR of USP9X by microRNA database. B: mRNA expression of USP9X in A549 and NCI-H1299 cells by quantitative RT-PCR. * P < 0.05 vs A549. C: Protein expression of USP9X in A549 and NCIH-1299 cells determined by western blot. D: Relative expression of miR-132 in A549 and NCI-H1299 cells by quantitative RT-PCR. ** P < 0.01 vs A549. E, F: mRNA expression of USP9X in cells transfected withnegative control siRNA, or miR-132 mimic, or miR-132 inhibitor by quantitative RT-PCR. The data in each group was normalized to the control (* P < 0.05) or miR-132 mimic (# P < 0.05). G: Protein expression of USP9X in cells transfected with negative control siRNA, or miR-132 mimic, or miR-132 inhibitor determined by western blot.
Inhibition of USP9X suppresses the migration and invasion of NSCLC cells in vitro
Since USP9X is a potential target gene of miR-132, we investigated whether USP9X contributes to the migration and invasion of NSCLC cells by using WP1130, a specific inhibitor of USP9X, to inactivate USP9X [25,28]. First, we showed WP1130 inhibited the cell viability of NSCLC cells in a concentration dependent manner (Figure 3A and 3B). For subsequent experiments, we chose to use a WP1130 concentration of 0.625 μM for A549 and 1.25 μM for NCI-H1299, which were the maximum concentrations tolerated by the two NSCLC cell lines. Using the wound-healing assay, we found that inhibition of USP9X in NSCLC cells significantly prohibited cell migration compared to the control group (Figure 3C and 3D). Moreover, the transwell assay showed that inhibition of USP9X in NSCLC cells significantly decreased the invasion ability compared to the control group (Figure 3E). These results indicate USP9X regulates the migration and invasion of NSCLC cells in vitro.
Figure 3.
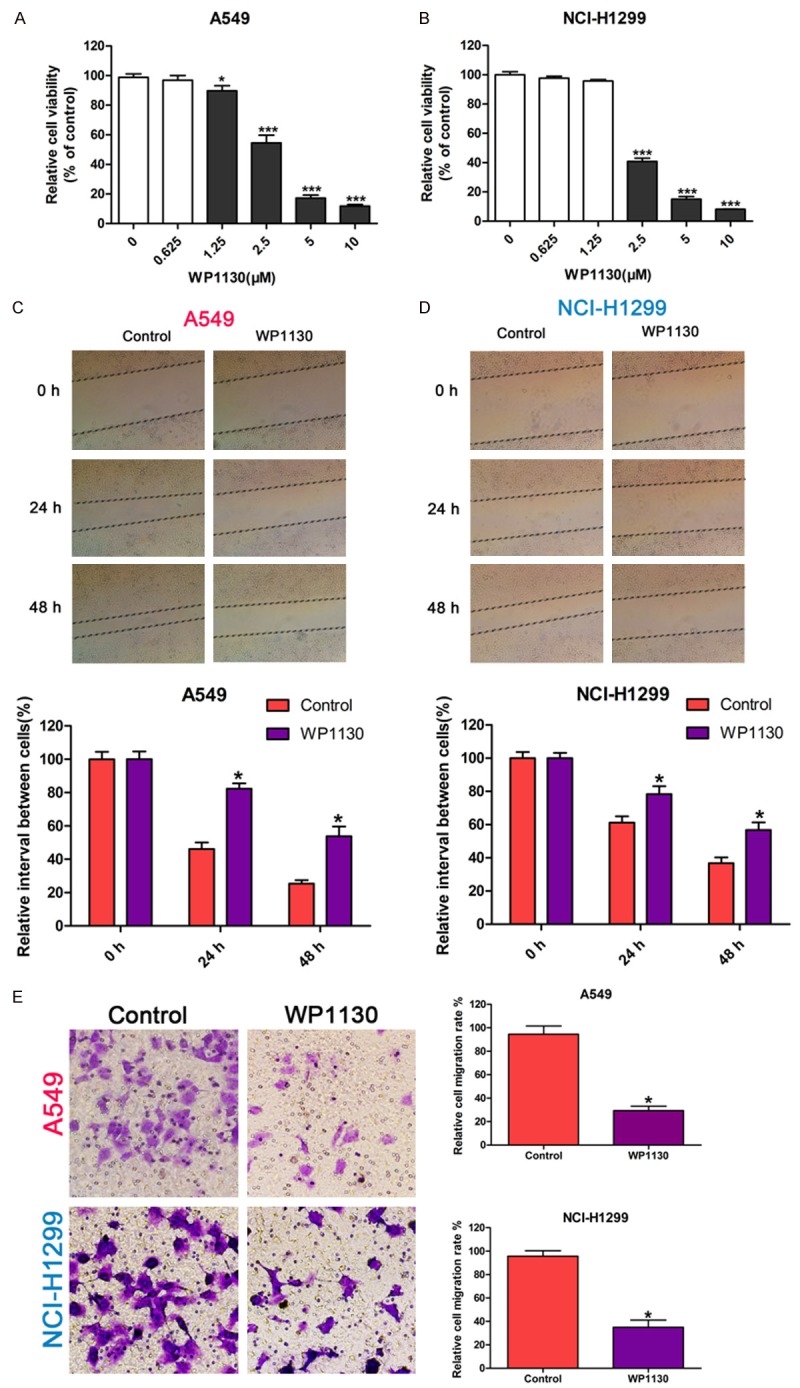
USP9X inhibition prohibits lung cancer cell migration and invasion. A, B: The effect of WP1130 on the cell viability of A549 and NCI-H1299 cells assayed by CCK-8. C, D: The effect of WP1130 on the migration ability of A549 and NCI-H1299 cells determined by the wound-healing assay. The cells were cultured in medium with WP1130 at a concentration of 0.625 μM for A549 or 1.25 μM for NCI-H1299; the width of the wound was measured at 0 h, 24 h, and 48 h. E: The effect of WP1130 on the invasion ability of A549 and NCI-H1299 cells by the transwell assay. The cells were cultured in medium with WP1130 at a concentration of 0.625 μM for A549 or 1.25 μM for NCI-H1299 for 24 h. The data in each group was normalized to the control (* P < 0.05, *** P < 0.001).
USP9X is involved in the miR-132 mediated regulation of migration and invasion of NSCLC cells
Next we investigated whether the functional effect of miR-132 on NSCLC cells was dependent on USP9X. The migration and invasion abilities of NSCLC cells treated with both WP1130 and a miR-132 inhibitor were examined. As shown in Figure 4A and 4B, there was no difference in cell migration ability between the WP1130 group and the combined WP1130/miR-132 inhibitor group, indicating that USP9X inhibition abolished the promoting effect of the miR-132 inhibitor on NSCLC cell migration. In parallel, USP9X inhibition reversed the pro-invasion role of the miR-132 inhibitor in NSCLC cells (Figure 4C). Taken together, these results indicate USP9X is involved in miR-132 mediated regulation of the migration and invasion of NSCLC cells.
Figure 4.
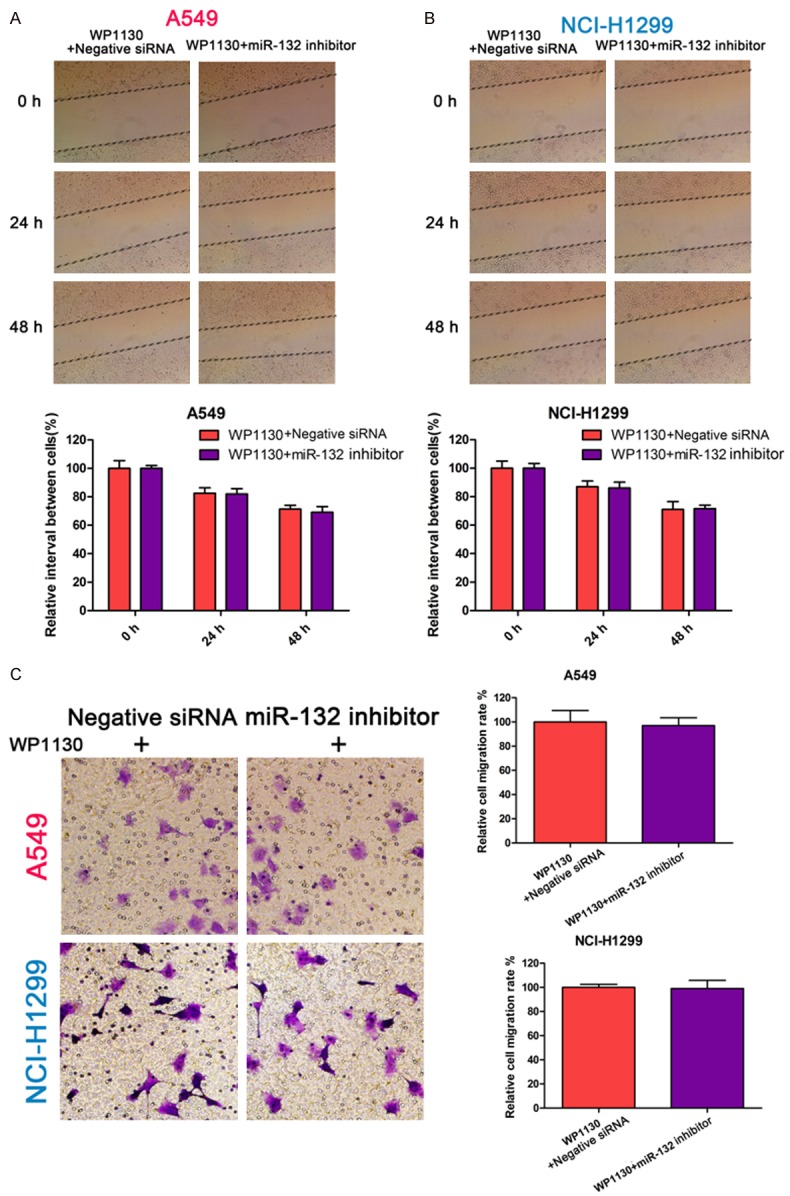
USP9X inhibition reverses the pro-invasion role of the miR-132 inhibitor. A, B: The migration ability of A549 and NCI-H1299 cells pre-transfected with miR-132 inhibitor or control, and cultured with WP1130 (at a concentration of 0.625 μM for A549 or 1.25 μM for NCI-H1299) determined using the wound-healing assay. The width of the wound was measured at 0 h, 24 h, 48 h. C: The invasion ability of A549 and NCI-H1299 cells pre-transfected with miR-132 inhibitor or control, and cultured with WP1130 (at a concentration of 0.625 μM for A549 or 1.25 μM for NCI-H1299) determined using the transwell assay.
Epithelial-mesenchymal transition is involved in the miR-132 and USP9X mediated regulation of the migration and invasion of NSCLC cells
To further explore the molecular mechanism underlying the miR-132 and USP9X mediated regulation of the migration and invasion of NSCLC cells, we examined the expression of the epithelial-mesenchymal transition (EMT) related molecular markers E-cadherin and vimentin. As shown in Figure 5A, inhibition of USP9X up-regulated the epidermal marker E-cadherin, but down-regulated the mesenchymal marker vimentin. As shown in Figure 5B, overexpression of miR-132 showed the same effect on E-cadherin and vimentin expression with USP9X inhibition. On the contrary, miR-132 knockdown promoted the expression of vimentin and inhibited the expression of E-cadherin, thus enhancing the EMT alteration in NSCLC cells. However, inhibition of USP9X reversed the pro-EMT role of miR-132 knockdown (Figure 5C). Collectively, our results indicate that EMT is involved in the miR-132 and USP9 mediated regulation of the migration and invasion in NSCLC cells, this further proves that the function of miR-132 on the regulation of NSCLC cell migration and invasion depends on USP9X.
Figure 5.
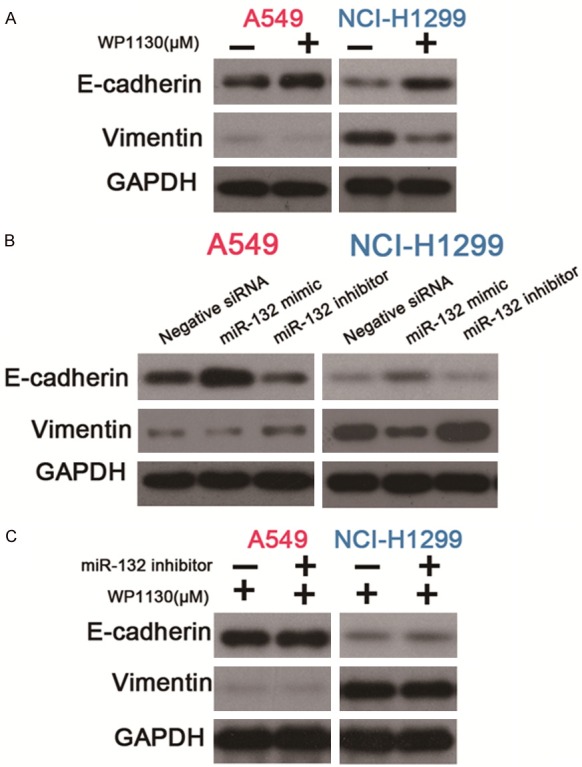
miR-132 suppresses EMT in NSCLC cells through targeting USP9X. A: Protein expression of E-cadherin or vimentin in A549 and NCI-H1299 cells cultured with WP1130 (at a concentration of 0.625 μM for A549 or 1.25 μM for NCI-H1299) determined by western blot. B: Protein expression of E-cadherin or vimentin in A549 and NCI-H1299 cells transfected with negative control siRNA, or miR-132 mimic, or miR-132 inhibitor, as determined by western blot. C: Protein expression of E-cadherin or vimentin in A549 and NCI-H1299 cells pre-transfected with miR-132 inhibitor or control, which were cultured with WP1130 (at a concentration of 0.625 μM for A549 or 1.25 μM for NCI-H1299) as determined by western blot.
Discussion
The mortality and morbidity of NSCLC is increasing [1]. It is therefore essential to investigate the molecular mechanisms underlying the development of NSCLC, and to explore novel molecular targets to enable accurate diagnosis and treatment. In the present study, we found miR-132 overexpression could inhibit the migration and invasion of NSCLC cells in vitro. We also demonstrated USP9X was a novel target gene of miR-132. We then showed miR-132 and USP9X regulate the migration and invasion of NSCLC by affecting EMT. To the best of our knowledge, this is the first study to show USP9X is a target gene of miR-132 in NSCLC.
MiR-132 has been shown to play diverse functions in cancer pathogenesis and progression. It has been reported to be down-regulated and function as a repressor in several cancers including hepatocellular carcinoma [29], osteosarcoma [30], and prostate cancer [14]. Meanwhile, it was shown to function as an oncogene in colorectal cancer [11], squamous cell carcinoma of the tongue [31], and pancreatic cancer [12], among others. In NSCLC, miR-132 was shown to be down-regulated, and the down-regulation of miR-132 correlated with poor prognosis [10]. In addition, previous studies found miR-132 could suppress the migration and invasion of lung cancer cells via targeting ZEB2 and SOX4 [17,18]. Our present study further confirmed the inhibitory role of miR-132 on NSCLC cell migration and invasion.
To unravel the mechanism behind the inhibitory effects of miR-132 in lung cancer, we firstly proved that USP9X was a functional target of miR-132. USP9X is involved in the regulation of several cancer-related biological functions such as cell adhesion, apoptosis, migration, and invasion. USP9X has been implicated as both an oncogene and tumor suppressor, depending on the type and stage of cancer [22]. Indeed, one of the best described oncogenic functions of USP9X is its interaction with the anti-apoptotic protein MCL1 [32]. Meanwhile, USP9X can also act as tumor suppressor via interacting with Kras [33,34]. In NSCLC, USP9X was reported to be elevated and correlate with poor prognosis [26]. However, the role of USP9X in NSCLC cell migration and invasion was incompletely disclosed. In the present study, we proved USP9X inhibition could prohibit NSCLC cell migration and invasion, and showed USP9X could regulate EMT in lung cancer.
In conclusion, we further explored the role of miR-132 in the migration and invasion of NSCLC. Our findings indicate miR-132 prohibits the migration and invasion of NSCLC cells through targeting USP9X, and that EMT is involved in miR-132 mediated cell migration and invasion. Our data provide new insight into the mechanism responsible for the metastasis of human NSCLC. In particular, miR-132 and USP9X may serve as novel therapeutic candidates in the treatment of NSCLC.
Acknowledgements
This study was supported by National Natural Science Foundation (NO.81501830), Zhejiang Natural Science Foundation (NO.LY16H160040, 2017C33178, 2017C33187), Medicine and Health Project of Zhejiang Province (No.2015RCB028).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Zhang G, Wang H, Ma Z. [Recent advances and prospect of advanced non-small cell lung cancer targeted therapy: focus on small molecular tyrosine kinase inhibitors] . Zhongguo Fei Ai Za Zhi. 2017;20:278–286. doi: 10.3779/j.issn.1009-3419.2017.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan L, Yu JT, Hu N, Tan L. Non-coding RNAs in Alzheimer’s disease. Molecular Neurobiology. 2013;47:382–393. doi: 10.1007/s12035-012-8359-5. [DOI] [PubMed] [Google Scholar]
- 5.Shen J, Stass SA, Jiang F. MicroRNAs as potential biomarkers in human solid tumors. Cancer Lett. 2013;329:125–136. doi: 10.1016/j.canlet.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koutsis G, Siasos G, Spengos K. The emerging role of microRNA in stroke. Curr Top Med Chem. 2013;13:1573–1588. doi: 10.2174/15680266113139990106. [DOI] [PubMed] [Google Scholar]
- 7.Sayed D, Abdellatif M. AKT-ing via microRNA. Cell Cycle. 2010;9:3213–3217. doi: 10.4161/cc.9.16.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meiri E, Levy A, Benjamin H, Ben-David M, Cohen L, Dov A, Dromi N, Elyakim E, Yerushalmi N, Zion O, Lithwick-Yanai G, Sitbon E. Discovery of microRNAs and other small RNAs in solid tumors. Nucleic Acids Res. 2010;38:6234–6246. doi: 10.1093/nar/gkq376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Yan S, Pei C, Cui Y. Decreased microRNA-132 and its function in human non-small cell lung cancer. Mol Med Rep. 2015;11:3601–3608. doi: 10.3892/mmr.2015.3222. [DOI] [PubMed] [Google Scholar]
- 11.Qin J, Ke J, Xu J, Wang F, Zhou Y, Jiang Y, Wang Z. Downregulation of microRNA-132 by DNA hypermethylation is associated with cell invasion in colorectal cancer. Onco Targets Ther. 2015;8:3639–3648. doi: 10.2147/OTT.S91560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JK, Henry JC, Jiang J, Esau C, Gusev Y, Lerner MR, Postier RG, Brackett DJ, Schmittgen TD. miR-132 and miR-212 are increased in pancreatic cancer and target the retinoblastoma tumor suppressor. Biochem Biophys Res Commun. 2011;406:518–523. doi: 10.1016/j.bbrc.2011.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Tang W, Li R, He R, Gan T, Luo Y, Chen G, Rong M. Downregulation of microRNA-132 indicates progression in hepatocellular carcinoma. Exp Ther Med. 2016;12:2095–2101. doi: 10.3892/etm.2016.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Formosa A, Lena AM, Markert EK, Cortelli S, Miano R, Mauriello A, Croce N, Vandesompele J, Mestdagh P, Finazzi-Agro E, Levine AJ, Melino G, Bernardini S, Candi E. DNA methylation silences miR-132 in prostate cancer. Oncogene. 2013;32:127–134. doi: 10.1038/onc.2012.14. [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZG, Chen WX, Wu YH, Liang HF, Zhang BX. MiR-132 prohibits proliferation, invasion, migration, and metastasis in breast cancer by targeting HN1. Biochem Biophys Res Commun. 2014;454:109–114. doi: 10.1016/j.bbrc.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Chen X, Chen L, Ma Y, Zhou L, Qi Q, Liu Y, Zhang S, Luo J, Zhou X. Upregulation of the miR-212/132 cluster suppresses proliferation of human lung cancer cells. Oncol Rep. 2015;33:705–712. doi: 10.3892/or.2014.3637. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Zu L, Wang Y, Wang M, Chen P, Zhou Q. miR-132 inhibits lung cancer cell migration and invasion by targeting SOX4. J Thorac Dis. 2015;7:1563–1569. doi: 10.3978/j.issn.2072-1439.2015.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You J, Li Y, Fang N, Liu B, Zu L, Chang R, Li X, Zhou Q. MiR-132 suppresses the migration and invasion of lung cancer cells via targeting the EMT regulator ZEB2. PLoS One. 2014;9:e91827. doi: 10.1371/journal.pone.0091827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang B, Lu L, Zhang X, Ye W, Wu J, Xi Q, Zhang X. Hsa-miR-132 regulates apoptosis in non-small cell lung cancer independent of acetylcholinesterase. J Mol Neurosci. 2014;53:335–344. doi: 10.1007/s12031-013-0136-z. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JX, Zhai JF, Yang XT, Wang J. MicroRNA-132 inhibits migration, invasion and epithelial-mesenchymal transition by regulating TGFbeta1/Smad2 in human non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2016;20:3793–3801. [PubMed] [Google Scholar]
- 21.Brodersen P, Voinnet O. Revisiting the principles of microRNA target recognition and mode of action. Nat Rev Mol Cell Biol. 2009;10:141–148. doi: 10.1038/nrm2619. [DOI] [PubMed] [Google Scholar]
- 22.Murtaza M, Jolly LA, Gecz J, Wood SA. La FAM fatale: USP9X in development and disease. Cell Mol Life Sci. 2015;72:2075–2089. doi: 10.1007/s00018-015-1851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Song N, Liu L, Liu X, Ding X, Song X, Yang S, Shan L, Zhou X, Su D, Wang Y, Zhang Q, Cao C, Ma S, Yu N, Yang F, Wang Y, Yao Z, Shang Y, Shi L. USP9X regulates centrosome duplication and promotes breast carcinogenesis. Nat Commun. 2017;8:14866. doi: 10.1038/ncomms14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenhill C. Pancreatic cancer: USP9X can be used to predict pancreatic cancer outcomes. Nat Rev Gastroenterol Hepatol. 2012;9:302. doi: 10.1038/nrgastro.2012.92. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Chen W, Liang C, Chen BW, Zhi X, Zhang S, Zheng X, Bai X, Liang T. WP1130 increases doxorubicin sensitivity in hepatocellular carcinoma cells through usp9x-dependent p53 degradation. Cancer Lett. 2015;361:218–225. doi: 10.1016/j.canlet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Liu Y, Yang B, Cao H, Yang CX, Ouyang W, Zhang SM, Yang GF, Zhou FX, Zhou YF, Xie CH. Elevated expression of USP9X correlates with poor prognosis in human non-small cell lung cancer. J Thorac Dis. 2015;7:672–679. doi: 10.3978/j.issn.2072-1439.2015.04.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kushwaha D, O’Leary C, Cron KR, Deraska P, Zhu K, D’Andrea AD, Kozono D. USP9X inhibition promotes radiation-induced apoptosis in non-small cell lung cancer cells expressing mid-to-high MCL1. Cancer Biol Ther. 2015;16:392–401. doi: 10.1080/15384047.2014.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu P, Du F, Liu Y, Yao M, Zhang S, Zheng X, Zheng S. WP1130 increases cisplatin sensitivity through inhibition of usp9x in estrogen receptor-negative breast cancer cells. Am J Transl Res. 2017;9:1783–1791. [PMC free article] [PubMed] [Google Scholar]
- 29.Liu K, Li X, Cao Y, Ge Y, Wang J, Shi B. MiR-132 inhibits cell proliferation, invasion and migration of hepatocellular carcinoma by targeting PIK3R3. Int J Oncol. 2015;47:1585–1593. doi: 10.3892/ijo.2015.3112. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Xu G, Shen F, Kang Y. miR-132 targeting cyclin E1 suppresses cell proliferation in osteosarcoma cells. Tumour Biol. 2014;35:4859–4865. doi: 10.1007/s13277-014-1637-2. [DOI] [PubMed] [Google Scholar]
- 31.Lian R, Lu B, Jiao L, Li S, Wang H, Miao W, Yu W. MiR-132 plays an oncogenic role in laryngeal squamous cell carcinoma by targeting FOXO1 and activating the PI3K/AKT pathway. Eur J Pharmacol. 2016;792:1–6. doi: 10.1016/j.ejphar.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, Maecker H, O’Rourke K, Bazan F, Eastham-Anderson J, Yue P, Dornan D, Huang DC, Dixit VM. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463:103–107. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Mancera PA, Rust AG, van der Weyden L, Kristiansen G, Li A, Sarver AL, Silverstein KA, Grutzmann R, Aust D, Rummele P, Knosel T, Herd C, Stemple DL, Kettleborough R, Brosnan JA, Li A, Morgan R, Knight S, Yu J, Stegeman S, Collier LS, ten Hoeve JJ, de Ridder J, Klein AP, Goggins M, Hruban RH, Chang DK, Biankin AV, Grimmond SM Australian Pancreatic Cancer Genome Initiative. Wessels LF, Wood SA, Iacobuzio-Donahue CA, Pilarsky C, Largaespada DA, Adams DJ, Tuveson DA. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature. 2012;486:266–270. doi: 10.1038/nature11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox JL, Wilder PJ, Wuebben EL, Ouellette MM, Hollingsworth MA, Rizzino A. Context-dependent function of the deubiquitinating enzyme USP9X in pancreatic ductal adenocarcinoma. Cancer Biol Ther. 2014;15:1042–1052. doi: 10.4161/cbt.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]


