Abstract
Purpose: To assess the appropriate dose of sodium nitroprusside for establishing acute retinal photoreceptor degeneration models in rabbits. Methods: Sodium nitroprusside (SNP) was delivered intravitreously. Sixteen New Zealand White rabbits are divided into four groups randomly: 0.1 mM, 0.25 mM, 0.5 mM SNP intravitreal injection group (experimental groups), and normal saline intravitreal injection group (control group). Assessments included weight, anterior segment photography, fundus photography, Hematoxylin-eosin staining, immunofluorescence, multi-focal electroretinogram (mfERG) and pupillary direct light reflex were performed at baseline and day 28 after injection. The spectral domain optical coherence tomography (SD-OCT) and full field electroretinogram (fERG) were performed at baseline and day 1, 3, 7, 14, 21 and 28 after injection. Results: No complications and no significant different in weight were found among all groups. No obvious change was found by slit lamp and fundus photography after injection in all groups. In SD-OCT exams, a time-dependent and dose-dependent injury of photoreceptor was found in SNP injection groups (P<0.05). The thickness of inner nuclear and plexiform layer was significantly decreased in 0.5 mM group. HE staining and immunofluorescence present the photoreceptor damage at the posterior pole (0.1, 0.25, 0.5 mM groups) and periphery (0.5 mM group). fERG and mfERG showed significant dose-dependent responses depression in SNP injection groups (P<0.05). The pupillary direct light reflex in SNP groups declined significantly at day 28th than pre-injection (P<0.05). Conclusions: Sodium nitroprusside of 0.1 mM and 0.25 mM can lead to monolayer photoreceptor degeneration at posterior pole in rabbits and the lesion is stable at 1 month after SNP injection.
Keywords: Animal model, photoreceptor degeneration, sodium nitroprusside, retinitis pigmentosa
Introduction
Retinitis pigmentosa (RP) is a kind of hereditary retinal disease that feature degeneration of rod and cone photoreceptors. All over the world, more than 1 million individuals were affected [1] and most patients are legally blind by age 40 years because of severely constricted visual fields. However, there is currently no cure or effective therapy for the treatment of RP. Electronic Retinal prostheses that elicit phosphenes by stimulating the remaining retinal neurons have been studied as potential tools to restore vision in these patients [2-4]. Stem cells also offer unprecedented opportunities for the development of strategies geared toward the treatment of retinal degenerative diseases [5-7]. Having a suitable and easy-made animal model is of overriding importance in preclinical testing of any potential treatment.
Rodent models such as Royal College of Surgeons (RCS) rat and Rho-/- mice are the most widely used models of RP because these small mammals are easy to manage and generate for investigations [8-10]. But the complete injury of photoreceptors may need 2 to 12 months [8,11,12]. Additionally, more and more larger animals were used in recent years like rabbits, monkeys and so forth because in these animals, surgical treatments such as subretinal injection of cells for replacement therapy and implantation of intraocular devices are easily performed [7,13-16]. Of these models, only monkeys have macula which is an important area for vision due to the high density of cone photoreceptors. However, it is hard to study the pathophysiology of RP in monkeys due to handling and breeding difficulties. In contrast, rabbits are easy to breed and handle. What’s more, rabbits are known to have a visual streak where the photoreceptor density is highest. Therefore, rabbits are very useful for studying retinal degeneration diseases and testing new therapeutic interventions [13,17-24].
Several chemically induced animal models develop retinal degeneration faster than gene knockout models. The mammalian eyes are highly sensitive to toxic substances. Acute retina injury model like sodium iodate or iodoacetic acid intravenous model have the disadvantage of systemic complications and high mortality. On the other hand, sodium nitroprusside (SNP), which is used as a vasodilator to reduce blood pressure, can target photoreceptor cells specifically by intravitreal injection, and it may be a good candidate for the induction of retinal damage [24].
To circumvent side effects of retinal degeneration-induced chemicals, our study established a safe, acute and easily made RP model by SNP intravitreal injection for the study of visual function recovery such as stem cell-based therapy, retinal prostheses and pharmaceutical therapy. The suitable SNP dose, success rate, retinal damage features and complications of the model were detailed assessed. Time-dependent changes of photoreceptors in morphology and visual functions were also investigated by SD-OCT, fERG and mfERG.
Methods
Experimental animals
This study was conducted in accordance with the Association Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. All animal experiments were conducted with the approval of the Animal Research Committee, Zhongshan Ophthalmic Center, Sun Yat-sen University.
Sixteen male New Zealand White rabbits (32 eyes) aged 8 weeks were used for experiments and were randomly assigned to 4 groups included 1 control group and 3 experiment groups. Sodium nitroprusside (Shuanghe, China) was dissolved in saline and protected from light. The rabbits were anesthetized by the intramuscular injection of Xylazine (33 mg/kg) and 3% pentobarbital sodium (0.5 ml/kg). After proparacaine hydrochloride (Alcaine, Alcon, USA) was applied topically to the eye, 100 μl of various concentrations (0.1 mM, 0.25 mM, 0.5 mM) of SNP solution was injected intravitreally through the ora serrata with a 30-gauge needle in different groups. The control group was received an intravitreal injection with saline only. The weight of rabbits was recorded before SNP injection and 1 month after SNP injection. Criteria for the photoreceptor degeneration model successfully built were as follows: (1) Without local and systemic serious complications, (2) Inner nuclear layer, inner plexiform layer and retinal ganglion cell keep the structure clear and organized, (3) Function of photoreceptors were declined permanently. Rabbits which not meet the criteria were excluded.
Morphological evaluations
The morphological evaluations included anterior segment photograph, fundus photograph, spectral domain optical coherence tomography (SD-OCT) and pathological section. Anterior segment photograph was taken by a slit-lamp camera system (YZ5J, 66VT, China; EOS60D, Canon, Japan) before SNP injection and 1 month after SNP injection. Conjunctiva, cornea, anterior chamber, iris, pupil and lens were observed. The morphological changes of retina, optic nerve head and vessels before and after SNP injection can be found on fundus photographs which was collected by fundus camera (TRC-50DX, Topcon, Japan).
To measure the variation of total retinal thickness at posterior pole, a volume scan image was obtained by SD-OCT (Spectralis OCT, Heidelberg, Germany) before SNP injection and at day 1, 3, 7, 14, 21 and 28 after SNP injection. The mean total retinal thickness (internal limiting membrane to Bruch’s membrane) was measured within a circle 6 mm in diameter which is right below the optical nerve head and covered the visual streaks. Four weeks after the SNP injection, rabbit eyes were enucleated, fixed overnight in 4% paraformaldehyde, and then embedded in paraffin. Five micrometre-thick sections of the visual streak in retina were cut along the horizontal meridian and stained with haematoxylin and eosin. For immunofluorescence, the samples were cut along the horizontal meridian with 30 micrometre-thick after embedded in OCT overnight. Then permeabilized with 0.1% Triton X-100/PBS (1X; 140 mM NaCl, 10 mM KCl, 8 mM Na2HPO4, 2 mM KH2PO4, pH 7.4; Thermo Scientific, Rockford, IL) and blocked in 10% donkey serum albumin for 1 hour. Incubated with primary antibodies recoverin (rabbit, 1:500, Millipore, AB5585) overnight at 4°C. The next day, the samples were washed three times with PBS and subsequently incubated with Alexa Fluor 555 labeled secondary antibody (1:500, Invitrogen) for 60 min at room temperature in the dark. After washing three times with PBS, the samples were counterstained with 4’,6-diamidino-2-phenylindole (DAPI, 1 μg/ml; Molecular Probes, Carlsbad, CA). Fluorescent images were acquired using a laser scanning microscope (LSM 510; Carl Zeiss, Thornwood, NY).
Functional evaluations
The functional evaluations included full field electroretinogram (fERG), multi-focal electroretinogram (mfERG) and direct light reflex examination. fERG was recorded with the Roland electrophysiological instrument (Roland Consult, Germany). All rabbits underwent fERG 7 days prior to intravitreal injection, and at day 1, 3, 7, 14, 21, 28 post-injection. The examinations were conducted according to the standards of the International Society for Clinical Electrophysiology of Vision (ISCEV): (1) Dark-adapted 0.01 ERG (a rod-driven response of on-bipolar cells), (2) Dark-adapted 3 ERG (combined responses arising from photoreceptors and bipolar cells of both the rod and cone systems; rod dominated), (3) Dark-adapted 10 ERG (combined response with enhanced a-waves reflecting photoreceptor function), (4) Dark-adapted oscillatory potentials (responses primarily from amacrine cells), (5) Light-adapted 3 ERG (responses of the cone system; a-waves arise from cone photoreceptors and cone Off-bipolar cells; the b-wave comes from On- and Off-cone bipolar cells), (6) Light-adapted 30 Hz flicker ERG (a sensitive cone-pathway-driven response) [25]. The pupil was dilated with 0.25% tropicamide to a size of 8-9 mm. The animals were dark adapted for 30 min. A gold loop corneal electrode was applied to the topically anesthetized cornea of both eyes, and a subcutaneous reference electrode was connected to the temporal side of external orbit rim. A ground electrode was attached to the tail. The recordings were repeated at each stimulus intensity to check for reproducibility.
The results of mf-ERG were assessed by the Roland electrophysiological instrument (Roland Consult, Germany) and followed the standards of ISCEV [26]. The pupil was dilated with 0.25% tropicamide to a size of 8-9 mm. The animals were kept in normal room light (120 lux) 1 hour before examination. The fundus of the rabbit eye was visualized using an infrared camera so that the stimulus pattern was consistently placed in the same recording area each time. At the end of each recording, a fundus photograph derived from the infrared detection system was taken to document the correct fundus position.
The direct pupillary light reflex (DPLR) examination performed following normal room light adaptation for 1 hour, unanesthetized rabbit were held with the eye facing an infrared camera and taken a photo which represent the normal size of pupil. Then the eye was subjected to a light guide from a 5-W lamp. Pupil area was measured 1.9 s after stimulus offset (this latency was consistently on the downslope of the initial fast phase of the pupil constriction curve) and was normalized to prestimulus pupil area for comparisons between individuals. Image J (National Institutes of Health, USA) was used to measure the pupil area. In each group, DPLR were tested before and 4 weeks after SNP injection.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (GraphPad Software, San Diego, CA). The unpaired t-test was used to compare the differences in weight, mfERG amplitude, pupil area change ratio between pre- and post-injection among all groups. The paired t-test was used to compare the change in fERG amplitude and OCT retinal thickness between different examination time points. Results are expressed as the mean ± standard deviation (SD). The criterion for statistical significance was P<0.05.
Results
A rabbit from 0.1 mM SNP group which retina damaged too light and a rabbit from 0.25 mM SNP group which retina damaged too much were excluded. The successful rate of modeling is 83.33%. No local and systemic adverse reactions were found in the SNP intravitreal injection groups. There is no significantly differences among the 4 groups (P>0.05) in weights which is the most basic indicator of health condition (Figure 1A).
Figure 1.
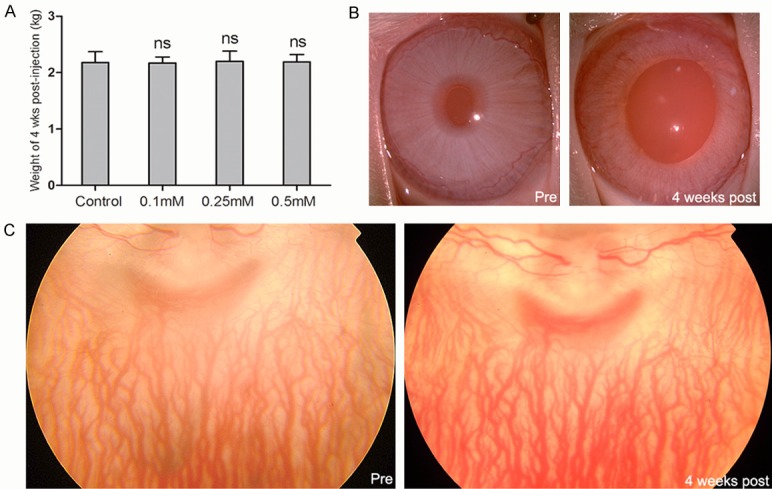
The SNP did not cause evident local and systemic adverse effect on rabbits 4th week after SNP intravitreal injection. A. The weight of rabbits at day 28 post-injection. There is no significant difference between control group and SNP injection groups. B. The slit lamp photography of rabbits pre-injection and 4th week post-injection. There is no side effect of SNP found in the cornea, iris and lens except larger pupil appearance. C. The fundus photography of rabbits pre-injection and 4th week post-injection with clear refractive media. No obvious morphological change was found by fundus photography. ns: no significance.
The slit lamp photography showed no obvious changes in conjunctiva, cornea, anterior chamber, iris, and lens between pre-injection and 4th week post-injection in both SNP injection group and control group (Figure 1B). Meanwhile, fundus photography of rabbits pre- and post-injection showed no obvious changes in all groups too (Figure 1C).
In the SNP injection group, SD-OCT outcome exhibited a lot of changes. Obvious outer nuclear layer (ONL) edema was observed at 1st day after SNP administration (Figure 2A), and the thickness of retina increased significantly in SNP treated groups because of the ONL edema. At 3rd day, the ONL edema wholly or in part subsided and presented a rapid photoreceptor damage process until 14th day. After 14th day, significant lesions of ONL continued in high dose groups (0.25 mM, 0.5 mM), but with relatively slow pace. At 28th day, the ONL is nearly all damaged or disappeared in all the three SNP treated groups (P<0.001), and showed no significant decrease than day 21. Although the structure of the inner nuclear layer (INL) and inner plexiform layer (IPL) in 0.5 mM group was still clear, a significantly damaged (P<0.05) was found throughout the whole observation period while the other 3 groups were stay the same (Figure 2A-E).
Figure 2.
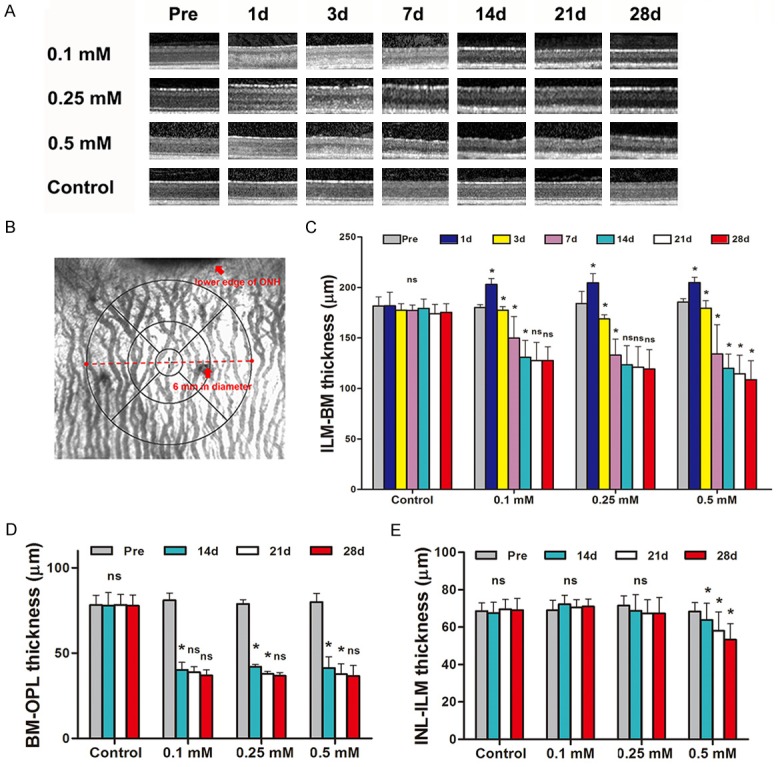
SD-OCT showed gradual decrease of retina thickness of rabbits after SNP treatment. A. Example of SD-OCT images of posterior pole of rabbits’ retina over 28-day observation period. The retinal edemas were observed at D1 post-injection, but soon decreased at D3 day post-injection, and then continue to decrease over time till D28. B. Example of fundus images showing the scope of retinal thickness at posterior pole measured by SD-OCT. C. The thickness from Bruch membrane (BM) to internal limiting membrane (ILM) were significantly decreased over 28-day period in 0.1 mM, 0.25 mM, 0.5 mM SNP groups while the control group has remained stable. Dose-dependent significantly differences were found among 0.1 mM, 0.25 mM, 0.5 mM SNP groups. D. The thickness from Bruch membrane (BM) to outer plexiform layer (OPL) were significantly decreased over 28-day period in 0.1 mM, 0.25 mM, 0.5 mM SNP groups and no differences was found among the three SNP groups. E. The thickness from inner nuclear layer (INL) to internal limiting membrane (ILM) were significantly decreased over 28-day period in 0.5 mM SNP groups and remained stable in 0.1 mM and 0.25 mM SNP group and control group. ns: no significance; *: P<0.05; All the ns and *represent the results compare with the previous time point.
In SNP injection groups, hematoxylin-eosin staining at the posterior pole of retina 4th week post-injection presented an obvious damage in ONL which was consistent with the results of SD-OCT. However, no obvious periphery retina damage was found in 0.1 mM and 0.25 mM group but in 0.5 mM group (Figure 3A). The expression of photoreceptor cell marker recoverin was not observed at the posterior pole of retina in SNP groups but prominently expressed in control group and periphery retina of 0.1 mM and 0.25 mM groups. 0.5 mM group showed a negative expression of recoverin in periphery retina which is in accord with the results of hematoxylin-eosin staining (Figure 3B).
Figure 3.
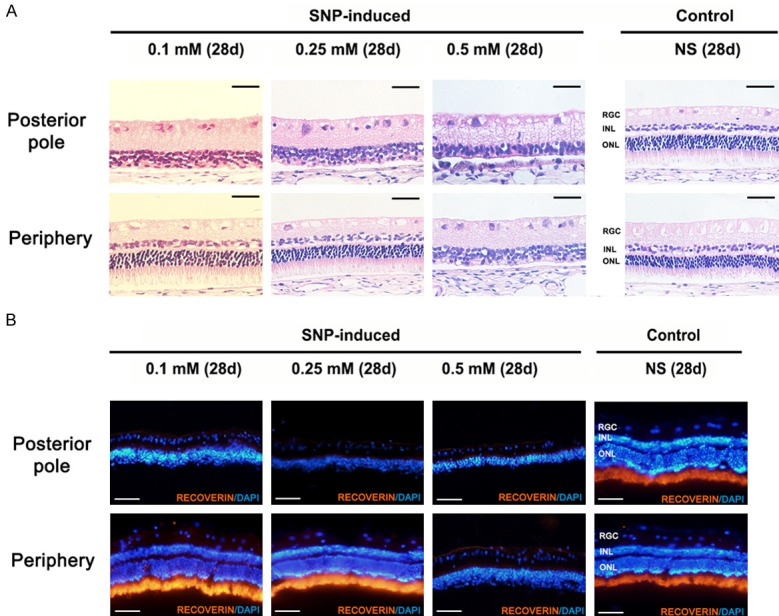
Hematoxylin-eosin staining and immunofluorescence staining of the retina in New Zealand White rabbits. A. Hematoxylin-eosin staining of retinas at posterior pole and periphery day 28 post-injection. The outer nuclear layer and outer plexiform layer were obviously damaged at posterior pole of retina in three SNP groups and at periphery retina in 0.5 mM group while no obvious damage was found at periphery retina in 0.1 mM, 0.25 mM group and control group. Scale bar: 50 µm. B. Immunofluorescence staining of retinas with photoreceptor marker recoverin at posterior pole and periphery day 28 post-injection. The expression of photoreceptor cell marker recoverin was not observed at the posterior pole of retina in SNP groups and periphery retina in 0.5 mM group but prominently expressed in control group and periphery retina of 0.1 mM and 0.25 mM groups. Scale bar: 100 µm.
The mixed cone and rod response decreased 1 day after injection than before in SNP injection groups (P<0.05) and control group (P=0.058), and SNP injection groups declined more than control group (P<0.05) (Figure 4). The response in control group returned to pre-injection levels at 3rd day after injection. In 0.1 mM group and 0.25 mM group, a certain level of rebounded reaction was observed at 14th day whereas 0.5 mM group remained low response all the time. At 28th day, all SNP groups presented lower response than control group (P<0.05) and significantly decreased than pre-injection (P<0.05). In SNP injection groups, the decline degree of response is in accord with the SNP dosage as same as OCT outcome.
Figure 4.
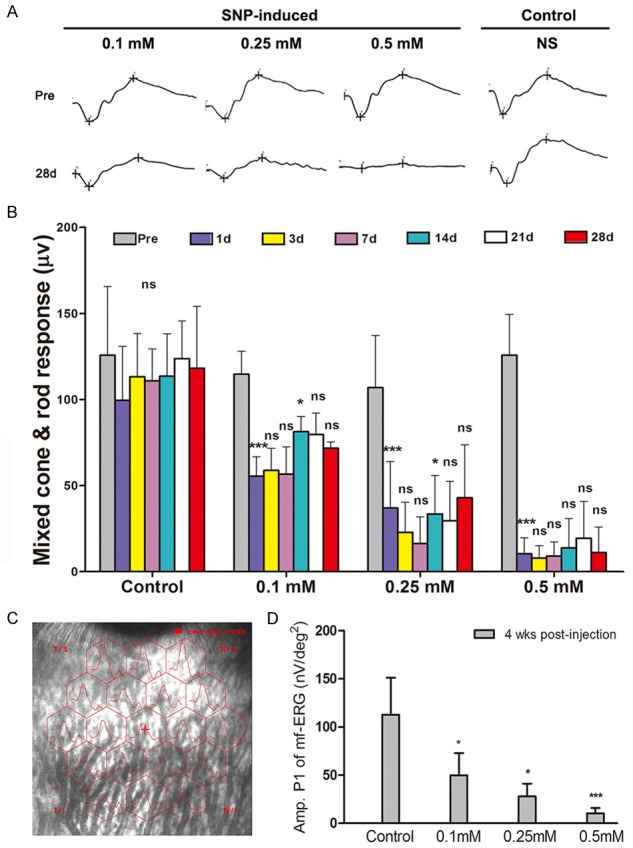
Time-dependent changes in fERG and mfERG of the retina in New Zealand White rabbits. (A) Sample waveform of control group, 0.1 mM SNP injection group, 0.25 mM SNP injection group, and 0.5 mM SNP injection group pre- and 28 days post-injection. (B) The mixed cone & rod response change among control group, 0.1 mM SNP injection group, 0.25 mM SNP injection group, and 0.5 mM SNP injection group pre- and post-injection. The amplitude of mixed cone & rod response showed significantly dose-dependent decrease after SNP injection. (C) The sample graph of mf-ERG shows the specific site in the mfERG test. (D) The comparison of P1 amplitude among control group, 0.1 mM SNP injection group, 0.25 mM SNP injection group, and 0.5 mM SNP injection group at day 28 post-injection. The P1 amplitude showed significantly dose-dependent decrease after SNP injection. ns: no significance; *: P<0.05; **: P<0.01; ***: P<0.001; The ns and *in (B) part represent the results compare with the previous time point.
In mf-ERG, SNP injection groups presented a marked decrease at 28th day after injection (P<0.05). Furthermore, 0.5 mM SNP group showed a more serious decrease than 0.25 mM SNP group (P<0.05) (Figure 4). The rates of change in ratios of pupil area after and before stimulus raised in all SNP injection groups but not control group (P<0.05). In addition, 0.5 mM group presented significant larger ratio than 0.1 mM group and 0.25 mM group while no significant difference was found between 0.1 mM group and 0.25 mM group (Figure 5).
Figure 5.
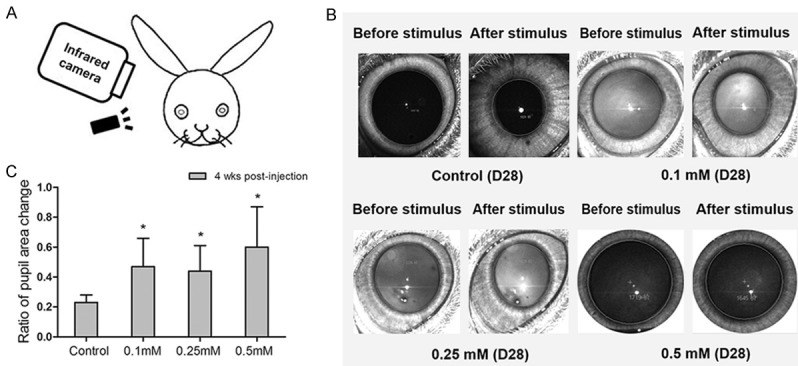
The pupillary direct light reflex change in New Zealand White rabbits before and after SNP injection. A. The illustration of pupillary direct light reflex in New Zealand White rabbits. B. The sample graphs of pupillary direct light reflex from control group, 0.1 mM SNP injection group, 0.25 mM SNP injection group, and 0.5 mM SNP injection group at day 28 after injection. C. The comparison of rate of pupil size change among control group, 0.1 mM SNP injection group, 0.25 mM SNP injection group and 0.5 mM SNP injection group pre- and 28 days post-injection. The ratio of pupil size in SNP injection groups were significantly larger than control group. ns: no significance. *: P<0.05.
Discussion
Photoreceptor cell death is the major hallmark of retinitis pigmentosa, although the mechanisms leading to photoreceptor apoptosis remain poorly understood. We observed preferred loss of the photoreceptor layer in the rabbits after the intravitreal injection of sodium nitroprusside (SNP), with no local and systemic adverse reactions, low-cost and shortly injury time (1 month) which overcome shortcomings of high mortality in chemicals intravenous injection model and slowly degeneration course in transgenic model or natural RP model. In addition, the extent of retinal damage was dependent on the concentration of SNP injected. The significant morphologic and visual functional changes appeared in all the three concentrations but with different lesion level which may be helpful to fulfill different requirements of experiments.
Sodium nitroprusside (SNP) is known to release NO primarily through photochemical reactions [27]. Peroxynitrite might be the major factor in the acute photoreceptor degeneration induced by NO, and the primary target of NO toxicity tend to be the outer retinal layers [24]. In our study, even low dose applications of SNP (0.1 mM) induced degeneration in the outer retinal layers, whereas no change was found in conjunctiva, cornea, iris and lens among all the three SNP injection groups. Furthermore, no obvious exudation, hemorrhage, intraocular inflammation reaction was observed after injection, and the weight went up steadily in experimental groups. The reasons of the two cases failed possibly because the different individual sensitivity to SNP or the diffusion and convection produced by the aqueous humor flow through the vitreous [28]. The diffusion may also be responsible for the different extent of damage between posterior pole and periphery retina among groups with various SNP dose. The SNP model of photoreceptor cell damage may represent a useful experimental tool for several reasons, such as modeling by a single intravitreal injection, short modeling time, and modulation of photoreceptor damage degree by various SNP dose.
In SD-OCT examinations, photoreceptor lesions were observed on the first day of SNP delivery which is in agreement with the results of other researchers who have taken intravenous sodium iodate delivery [29,30]. However, some studies used different delivery routes and the pathologic changes in target layer were reported on days 3 and 7 after injection [31,32]. In contrast to the control group, our study regarding the retina showed that the thickness reduction occurred and was more significant at high dose than at low dose, and the lesion tend to be stable at days 28 after SNP injection which is consistent with the findings showed by Machalinska et al [29]. Similar results have also been reported by Enzmann et al and Hamid et al [30,33]. What’s more, we demonstrated that 0.1 mM and 0.25 mM SNP could lead to monolayer photoreceptor degeneration whereas a significant lesion of INL and IPL was found in 0.5 mM SNP treated group. This result suggested that the SNP concentration of 0.1 mM and 0.25 mM could be better to establish the monolayer photoreceptor degeneration model instead of 0.5 mM.
We used three different visual functional tests in this study, the full field electroretinogram (FERG), multi-focal electroretinogram (mfERG) and direct light reflex. There were significant cone & rod responses depression on day 1 in experimental groups as same as Hamid’s [33]. One of the interesting findings in this study is that a transient response reduction was observed at day 1 after injection in control group which were different with Hamid’s results [33]. It is possible that liquid intravitreal injection affected intraocular microenvironment led to a transient decrease in cone & rod response whereas injected via the retro-bulbar venous plexus in Hamid’s study did not cause the effect. Although responses were depressed in SNP groups overall, we observed a slight but non-significant temporary return of responses toward baseline values around day 14, which again significantly decreased on day 21. This observation might have resulted from partial recovery of injured photoreceptor cells [34]. The decrease degrees of fERG and mfERG varied significantly among the three SNP injection groups and were consistent with the damage degrees of retina. This could be due to peripheral residual photoreceptor in low dose group still possessed partial function of sensing light whereas there is no residual photoreceptor at both periphery and post pole of retina in 0.5 mM SNP injection group. The use of the direct light reflex examination could provide evidence not only that the photoreceptor is capable of detecting light, but also that signals are being transmit to central nervous system targets [35]. The results of direct pupillary light reflex exams reflected that the degree of photoreceptor damage is more severe in 0.5 mM group than the other two SNP groups. Interestingly, no difference was found between 0.1 mM group and 0.25 mM group. It may because direct pupillary light reflex exams are not sensitive enough to determine the differences between the two group which both have residual photoreceptors at periphery.
This study has some limitations. The area imaged with SD-OCT was quite restricted such that the degeneration in retinas obtained with SD-OCT did not always correlate with the total retinal function. Furthermore, a larger size of samples and longer follow-up time could get more accurate data.
In conclusion, we developed and evaluated a reproducible, quantitative, easily made and low-cost photoreceptor degeneration rabbits model which is induced by SNP without systemic adverse effects. The SNP concentration of 0.1 mM and 0.25 mM could induce monolayer photoreceptor degeneration at posterior pole in 1 month.
Acknowledgements
This study was supported by Grant 81430009 from National Natural Science Foundation of China, 2014B020225001, 2017B030314025, 2017B020230003 from The Science and Technology Planning Projects of Guangdong Province, Grant 81570874 from National Natural Science Foundation of China, 2016YFC1101103 & 2017YFA0104101 from National Key Research and Development Program of China. The funding organizations had no role in the design or conduct of this research.
Disclosure of conflict of interest
None.
References
- 1.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 2.Lorach H, Goetz G, Mandel Y, Lei X, Galambos L, Kamins TI, Mathieson K, Huie P, Dalal R, Harris JS, Palanker D. Performance of photovoltaic arrays in-vivo and characteristics of prosthetic vision in animals with retinal degeneration. Vision Res. 2015;111:142–148. doi: 10.1016/j.visres.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Cruz L, Dorn JD, Humayun MS, Dagnelie G, Handa J, Barale PO, Sahel JA, Stanga PE, Hafezi F, Safran AB, Salzmann J, Santos A, Birch D, Spencer R, Cideciyan AV, de Juan E, Duncan JL, Eliott D, Fawzi A, Olmos de Koo LC, Ho AC, Brown G, Haller J, Regillo C, Del Priore LV, Arditi A, Greenberg RJ Argus II Study Group. Five-year safety and performance results from the Argus II retinal prosthesis system clinical trial. Ophthalmology. 2016;123:2248–2254. doi: 10.1016/j.ophtha.2016.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohmann TK, Kanda H, Morimoto T, Endo T, Miyoshi T, Nishida K, Kamei M, Walter P, Fujikado T. Surgical feasibility and biocompatibility of wide-field dual-array suprachoroidal-transretinal stimulation prosthesis in middle-sized animals. Graefes Arch Clin Exp Ophthalmol. 2016;254:661–673. doi: 10.1007/s00417-015-3104-1. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 6.Song WK, Park KM, Kim HJ, Lee JH, Choi J, Chong SY, Shim SH, Del Priore LV, Lanza R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Rep. 2015;4:860–872. doi: 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirai H, Mandai M, Matsushita K, Kuwahara A, Yonemura S, Nakano T, Assawachananont J, Kimura T, Saito K, Terasaki H, Eiraku M, Sasai Y, Takahashi M. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci U S A. 2016;113:E81–90. doi: 10.1073/pnas.1512590113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- 9.Lund RD, Wang S, Klimanskaya I, Holmes T, Ramos-Kelsey R, Lu B, Girman S, Bischoff N, Sauve Y, Lanza R. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8:189–199. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- 10.Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, Lanza R, Lund R. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27:2126–2135. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- 11.Sanyal S, De Ruiter A, Hawkins RK. Development and degeneration of retina in rds mutant mice: light microscopy. J Comp Neurol. 1980;194:193–207. doi: 10.1002/cne.901940110. [DOI] [PubMed] [Google Scholar]
- 12.Neuhardt T, May CA, Wilsch C, Eichhorn M, Lütjen-Drecoll E. Morphological changes of retinal pigment epithelium and choroid in rd-mice. Exp Eye Res. 1999;68:75–83. doi: 10.1006/exer.1998.0589. [DOI] [PubMed] [Google Scholar]
- 13.Zhu L, Jang GF, Jastrzebska B, Filipek S, Pearce-Kelling SE, Aguirre GD, Stenkamp RE, Acland GM, Palczewski K. A naturally occurring mutation of the opsin gene (T4R) in dogs affects glycosylation and stability of the G protein-coupled receptor. J Biol Chem. 2004;279:53828–53839. doi: 10.1074/jbc.M408472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rah H, Maggs DJ, Blankenship TN, Narfstrom K, Lyons LA. Early-onset, autosomal recessive, progressive retinal atrophy in Persian cats. Invest Ophthalmol Vis Sci. 2005;46:1742–1747. doi: 10.1167/iovs.04-1019. [DOI] [PubMed] [Google Scholar]
- 15.Li SY, Yin ZQ, Chen SJ, Chen LF, Liu Y. Rescue from light-induced retinal degeneration by human fetal retinal transplantation in minipigs. Curr Eye Res. 2009;34:523–535. doi: 10.1080/02713680902936148. [DOI] [PubMed] [Google Scholar]
- 16.Nishida K, Kamei M, Kondo M, Sakaguchi H, Suzuki M, Fujikado T, Tano Y. Efficacy of suprachoroidal-transretinal stimulation in a rabbit model of retinal degeneration. Invest Ophthalmol Vis Sci. 2010;51:2263–2268. doi: 10.1167/iovs.09-4120. [DOI] [PubMed] [Google Scholar]
- 17.Ashburn FS Jr, Pilkerton AR, Rao NA, Marak GE. The effects of iodate and iodoacetate on the retinal adhesion. Invest Ophthalmol Vis Sci. 1980;19:1427–1432. [PubMed] [Google Scholar]
- 18.Ogino H, Ito M, Matsumoto K, Yagyu S, Tsuda H, Hirono I, Wild CP, Montesano R. Retinal degeneration induced by N-methyl-N-nitrosourea and detection of 7-methyldeoxyguanosine in the rat retina. Toxicol Pathol. 1993;21:21–25. doi: 10.1177/019262339302100103. [DOI] [PubMed] [Google Scholar]
- 19.Peng YW, Hao Y, Petters RM, Wong F. Ectopic synaptogenesis in the mammalian retina caused by rod photoreceptor-specific mutations. Nat Neurosci. 2000;3:1121–1127. doi: 10.1038/80639. [DOI] [PubMed] [Google Scholar]
- 20.Winkler BS, Sauer MW, Starnes CA. Modulation of the Pasteur effect in retinal cells: implications for understanding compensatory metabolic mechanisms. Exp Eye Res. 2003;76:715–723. doi: 10.1016/s0014-4835(03)00052-6. [DOI] [PubMed] [Google Scholar]
- 21.Liang L, Katagiri Y, Franco LM, Yamauchi Y, Enzmann V, Kaplan HJ, Sandell JH. Long-term cellular and regional specificity of the photoreceptor toxin, iodoacetic acid (IAA), in the rabbit retina. Vis Neurosci. 2008;25:167–177. doi: 10.1017/S0952523808080401. [DOI] [PubMed] [Google Scholar]
- 22.Kondo M, Sakai T, Komeima K, Kurimoto Y, Ueno S, Nishizawa Y, Usukura J, Fujikado T, Tano Y, Terasaki H. Generation of a transgenic rabbit model of retinal degeneration. Invest Ophthalmol Vis Sci. 2009;50:1371–1377. doi: 10.1167/iovs.08-2863. [DOI] [PubMed] [Google Scholar]
- 23.Amirpour N, Karamali F, Rabiee F, Rezaei L, Esfandiari E, Razavi S, Dehghani A, Razmju H, Nasr-Esfahani MH, Baharvand H. Differentiation of human embryonic stem cell-derived retinal progenitors into retinal cells by Sonic hedgehog and/or retinal pigmented epithelium and transplantation into the subretinal space of sodium iodate-injected rabbits. Stem Cells Dev. 2012;21:42–53. doi: 10.1089/scd.2011.0073. [DOI] [PubMed] [Google Scholar]
- 24.Isago H, Sugano E, Murayama N, Tamai M, Tomita H. Establishment of monocular-limited photoreceptor degeneration models in rabbits. BMC Ophthalmol. 2013;13:19. doi: 10.1186/1471-2415-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M. ISCEV Standard for full-field clinical electroretinography (2015 update) Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 26.Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, Marmor MF, McCulloch DL, Palmowski-Wolfe AM International Society For Clinical Electrophysiology of Vision. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition) Doc Ophthalmol. 2012;124:1–13. doi: 10.1007/s10633-011-9296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rochelle LG, Kruszyna H, Kruszyna R, Barchowsky A, Wilcox DE, Smith RP. Bioactivation of nitroprusside by porcine endothelial cells. Toxicol Appl Pharmacol. 1994;128:123–128. doi: 10.1006/taap.1994.1189. [DOI] [PubMed] [Google Scholar]
- 28.Jooybar E, Abdekhodaie MJ, Farhadi F, Cheng YL. Computational modeling of drug distribution in the posterior segment of the eye: effects of device variables and positions. Math Biosci. 2014;255:11–20. doi: 10.1016/j.mbs.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Machalinska A, Lubinski W, Klos P, Kawa M, Baumert B, Penkala K, Grzegrzolka R, Karczewicz D, Wiszniewska B, Machalinski B. Sodium iodate selectively injuries the posterior pole of the retina in a dose-dependent manner: morphological and electrophysiological study. Neurochem Res. 2010;35:1819–1827. doi: 10.1007/s11064-010-0248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enzmann V, Row BW, Yamauchi Y, Kheirandish L, Gozal D, Kaplan HJ, McCall MA. Behavioral and anatomical abnormalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. Exp Eye Res. 2006;82:441–448. doi: 10.1016/j.exer.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Iacovelli J, Spencer C, Saint-Geniez M. Direct effect of sodium iodate on neurosensory retina. Invest Ophthalmol Vis Sci. 2014;55:1941–1953. doi: 10.1167/iovs.13-13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho BJ, Seo JM, Yu HG, Chung H. Monocular retinal degeneration induced by intravitreal injection of sodium iodate in rabbit eyes. Jpn J Ophthalmol. 2016;60:226–237. doi: 10.1007/s10384-016-0429-1. [DOI] [PubMed] [Google Scholar]
- 33.Kadkhodaeian HA, Tiraihi T, Daftarian N, Ahmadieh H, Ziaei H, Taheri T. Histological and electrophysiological changes in the retinal pigment epithelium after injection of sodium iodate in the orbital venus plexus of pigmented rats. J Ophthalmic Vis Res. 2016;11:70–77. doi: 10.4103/2008-322X.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adachi-Usami E, Mizota A, Ikeda H, Hanawa T, Kimura T. Transient increase of b-wave in the mouse retina after sodium iodate injection. Invest Ophthalmol Vis Sci. 1992;33:3109–3113. [PubMed] [Google Scholar]
- 35.Singh MS, Charbel Issa P, Butler R, Martin C, Lipinski DM, Sekaran S, Barnard AR, MacLaren RE. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc Natl Acad Sci U S A. 2013;110:1101–1106. doi: 10.1073/pnas.1119416110. [DOI] [PMC free article] [PubMed] [Google Scholar]


