Abstract
Objective: The present study was designed to investigate whether AJCC/UICC 8th edition staging system precisely differentiated patients with different prognosis of gastric cancer (GC). Methods: There were 540 GC cases included in this study. Stratification was done according to the 7th and 8th AJCC/UICC tumor-node-metastasis (TNM) staging systems. Detailed comparison was conducted between two editions in terms of the sub-classification of pN3 stage, redefinitions of stage III, homogeneity, discrimination power, predictive accuracy, and complexity. Results: Compared to the 7th edition, the 8th TNM staging system performed better by incorporating pN3a and pN3b into the final stage of GC (P<0.001), had better stage grouping homogeneity (P<0.001), prognostic value (area under the curve, AUC-value was 0.809), and comparable discrimination power. Conclusions: AJCC 8th TNM staging system showed improved efficiency in GC prognosis.
Keywords: Gastric cancer, prognosis, staging system, AJCC
Introduction
Gastric cancer (GC) remains the fourth most common cancer worldwide and the second leading cause of cancer-related deaths, with approximately one million new cases every year [1-3]. The tumor-node-metastasis (TNM) staging system has long been the standardized benchmark for classifying patients with GC, defining prognosis, and determining the best treatment approaches at a population level [4,5]. Accompanied by the increased knowledge of GC biological behaviors [6,7], and the global landscape of the clinically GC signature, periodic reasonable revisions of TNM staging system were made by the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC) [8,9]. With the anatomic extent of GC as the foundation, relevant biologic and molecular markers should be expanded as complementary to further define stage groups and to make staging more efficient for prognostication [10,11]. In addition, studies worldwide to validate changes between two contiguous editions of TNM staging system were integrated by AJCC to promulgate best staging practices.
The currently implemented AJCC 7th staging system in GC incorporated several major revisions to the 6th edition, including refinement in the definitions of pT and pN categories and stage grouping [8]. Subsequent studies to validate these changes showed inconsistent results, indicating AJCC 7th staging system either inferior to/no better than [12] or superior to AJCC 6th staging system [9]. Our previous study proved that the AJCC 7th staging system represented advancement for better prediction of GC clinical outcomes [13]. Nonetheless, AJCC 7th edition is still not the most optimal staging system in some aspects, such as the resected number of regional lymph nodes with histological metastasis (pN status), the pN3 sub-classification [14-16], the rationality of current stage grouping and the inclusion criteria of GC population used for incorporation [17].
Under the background abovementioned, the AJCC 8th TNM staging system for GC has been published in 2016 [18] based on the results of the International Gastric Cancer Association (IGCA) staging project, in which 25,411 eligible GC cases were collected retrospectively from 59 institutions in 15 countries [19]. Notably, there were 21,555 (84.8%) eligible cases submitted from Japan and Korea, and 1627 (6.4%) eligible cases from other Asian countries, including only 979 (3.9%) eligible cases from 3 Chinese institutions. Compared with AJCC 7th staging system, patients with pN3a and pN3b showed distinct prognosis. By introducing pN3a and pN3b into a cluster analysis in the final stage, AJCC 8th stage grouping was established and major changes have been developed among stage III subgroups. However, the rationale behind the proposed changes remains unclear for clinical applications in China. The revision work of the AJCC 8th staging system is not yet entirely over.
In order to validate whether the AJCC staging system promulgate the best update and improvement through this new edition, the comparison between AJCC 8th and AJCC 7th TNM staging systems was performed in this study. The subdivision and inclusion of pN3a and pN3b into final TNM stage grouping, redefinitions of stage III, homogeneity, discrimination power, predictive accuracy, and complexity were evaluated stepwise, thereby elucidating which TNM staging system was superior in the prediction of the prognosis of GC.
Patients and methods
Ethics statement
All patients provided written informed consent for their information to be stored in the hospital database; and we obtained separate consent for use of research. Study approval was obtained from independent ethics committees from Zhongnan Hospital of Wuhan University. The study was undertaken in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
Study population and follow-up
The records of patients who underwent surgical resection of GC from December 2002 to February 2011 were reviewed. Major demographic and clinic-pathological characteristics were retrieved from the established clinical database of Peng et al. [13]. The tumor type, histologic grade, depth of invasion (pT stage), number of lymph nodes retrieved, number of lymph nodes with metastases (pN stage), and distant metastasis (pM stage) were re-confirmed histologically. Staging groups of all patients in this study were determined according to the AJCC 8th and AJCC 7th TNM staging systems. Overall survival (OS), defined as the duration from operation to GC-related death or last follow-up, was used for prognosis evaluation. The primary endpoint of this study was OS, and patients alive at the last follow-up were recorded as censored events.
Comparison between the 8th and 7th AJCC staging systems
Detailed comparison was conducted between two editions using Kaplan-Meier method and receiver operating characteristic curve (ROC) analysis, including the sub-classification of pN3 stage, redefinitions of stage III, homogeneity, discrimination power, predictive accuracy, and complexity.
Statistical analysis
Statistical analyses were carried out with SPSS version 20.0 (SPSS Institute, Chicago, IL). The median OS was determined using the Kaplan-Meier method, and the log-rank test was used to determine significance. Receiver operating characteristic (ROC) curve analysis was used to determine the predictive value of the parameters. Two sided P<0.05 was considered as statistically significant.
Results
Study population and TNM stage migrations
A total of 540 patients were included in this study, detailed information about patients’ demographics, clinicopathological characteristics was extracted from the established clinical database of Peng et al. [11]. In AJCC 8th TNM staging system, there were 27 subgroups and 9 groups including 0 (n=0), IA (n=22), IB (n=62), II A (n=19), IIB (n=73), IIIA (n=185), IIIB (n=89), IIIC (n=43), and IV (n=47).
The definition of AJCC 7th and AJCC 8th TNM staging systems was depicted in Figure 1. Theoretically, restaging was occurred in stage IIB and III of AJCC 7th TNM staging system, patients in distinct 3 subgroups (T1N3bM0, T2N3bM0, and T3N3bM0) would be upstaged, and 4 subgroups (T4aN2M0, T4bN0M0, T4aN3aM0, and T4bN2M0) would be downstaged. Actually, no patient was staged into T1N3bM0 and T3N3bM0. Compared with AJCC 7th staging system, AJCC 8th staging system led to a restaging of 176 patients (32.6%), including 175 patients (32.4%) downstaged and only 1 patient (0.2%) upstaged. The percentage of patients with stage IIIA was 15.6% in AJCC 7th staging system, and increased to 34.3% in AJCC 8th staging system. In AJCC 7th staging system, the percentage of patients with stage IIIB and IIIC was 21.7% and 21.5%, respectively. In AJCC 8th staging system, the percentage of patients with stage IIIB and IIIC was decreased to 16.5% and 8.0%, respectively. Detailed information about stage migrations and the distribution of 540 patients was shown in Figure 1. Deep analyses were focused on the changes highlighted by the blue dashed rectangle.
Figure 1.
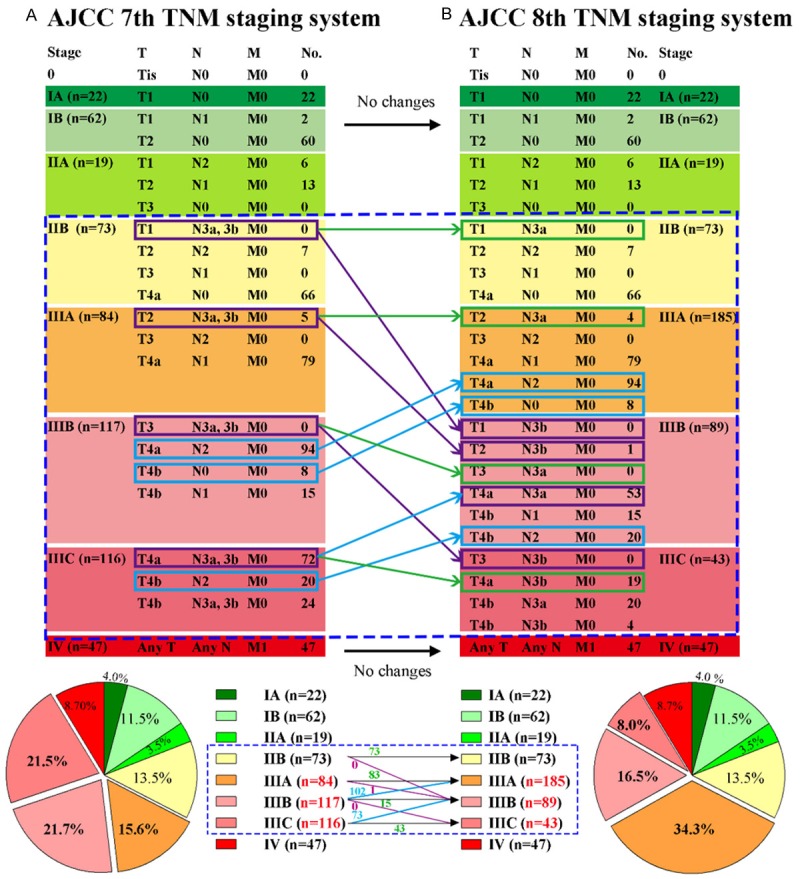
Definitions, patients’ distribution, and stage migrations in AJCC 7th and AJCC 8th TNM staging systems. A: AJCC 7th TNM staging system. B: AJCC 8th TNM staging system. According to the definition, there were no changes in stage IA, IB, IIA, and IV from AJCC 7th staging system to 8th staging system. Stage migrations were occurred in subgroups highlighted in the blue dashed rectangle, including stage IIB, IIIA, IIIB, and IIIC. Exact patients number was also shown. On the whole, the percentage of patients with stage IIIA, IIIB, and IIIC was 15.6%, 21.7%, and 21.5% in AJCC 7th staging system, and changed to 34.3%, 16.5%, and 8.0% in AJCC 8th staging system, respectively. Purple-framed categories were upstaged GC cases. Blue-framed categories were downstaged GC cases. Green-framed categories were unchanged GC cases.
pN3 classifications from AJCC7 to AJCC8 TNM staging system
In AJCC 8th staging system, pN3a and pN3b were staged independently in the final TNM staging system. Out of 540 patients, there were 115 (21.3%) patients with pN3, including 88 (16.3%) patients with pN3a and 27 (5.0%) patients with pN3b, distributing in stage IIIA (n=4), IIIB (n=54), IIIC (n=43), and IV (n=14). The median overall survival (OS) of patients with pN3b was worse than that of patients with pN3a, the difference was statistically significant (Figure 2A, P<0.001). Therefore, it was rational to classify pN3 into pN3a and pN3b subgroups. In particular, there were 72 (13.3%) patients with pN3 in stage IIIC (T4aN3M0) of AJCC 7th staging system. These patients were subdivided into stage IIIB (T4aN3aM0, n=53) and stage IIIC (T4aN3bM0, n=19) of AJCC 8th staging system. The median OS of patients with T4aN3bM0 subgroup was worse than that of patients with T4aN3aM0 subgroup, the difference was statistically significant (Figure 2B, P<0.001).
Figure 2.
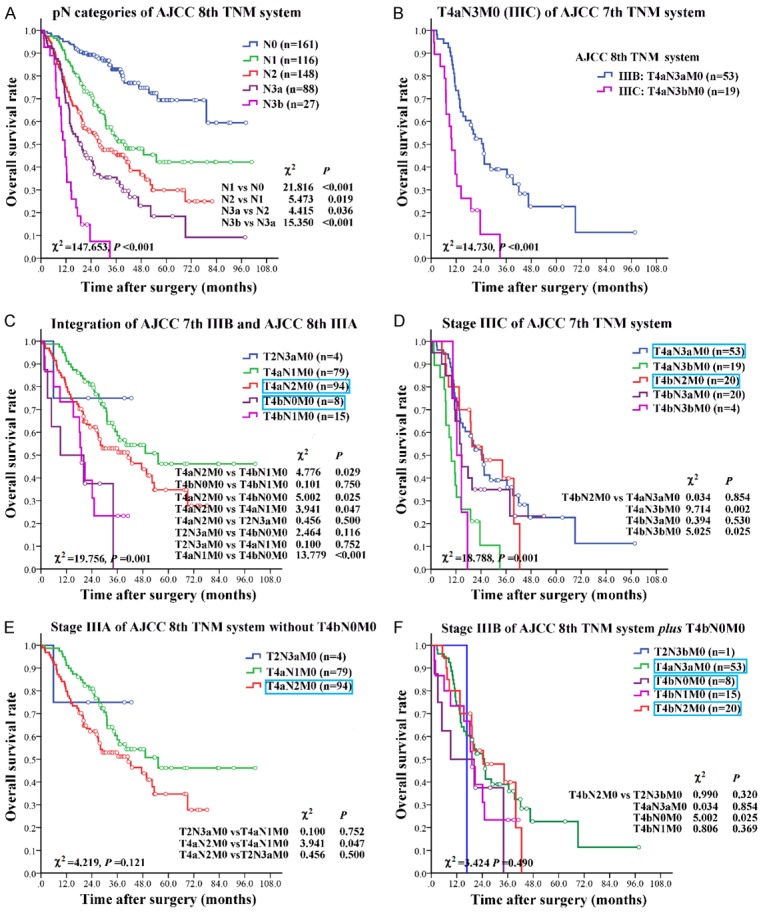
Correlations between pN categories with OS, and redefinitions of 3 subgroups in stage III. A: The correlation between pN and OS in AJCC 8th TNM staging system. AJCC 8th staging system performed well in discriminating patients with different pN status (P<0.001). Survival decreased in a stepwise fashion with increasing pN. The median OS of patients with pN3b was worse than that of patients with pN3a, the differences was statistically significant (P<0.001). B: The T4aN3M0 (IIIC) subgroup of AJCC 7th staging system was restaged into T4aN3aM0 (IIIB) and T4aN3bM0 (IIIC) of AJCC 8th staging system. C: Integration of AJCC 7th IIIB and AJCC 8th IIIA. T4aN2M0 and T4bN0M0 subgroups were downstaged from AJCC 7th IIIB into AJCC 8th IIIA. D: Stage IIIC of 7th TNM system. Subgroup T4bN2M0 was downstaged from stage IIIC of AJCC 7th system into stage IIIB of AJCC 8th system. E: Stage IIIA of AJCC 8th TNM system without T4bN0M0. No heterogeneity existed within this category. F: Stage IIIB of AJCC 8th TNM system plus T4bN0M0. No heterogeneity existed within this category. Detailed data of subgroup analysis was shown at the lower right corner of each part. Blue-framed categories were downstaged GC cases.
Homogeneity analysis and redefinitions of 3 subgroups in stage III
Except for the restaging changes caused by the subdivision of pN3a and pN3b abovementioned, there were 2 subgroups (T4bN0M0, T4aN2M0) in stage IIIB and 1 subgroup in stage IIIC (T4bN2M0) of AJCC 7th staging system restaged directly in AJCC 8th staging system.
In stage IIIB of AJCC 7th staging system, the heterogeneity was detected among the subgroups (P=0.016). The median OS of patients with T4aN2M0 (n=94) was longer than others (T4bN0M0, T4bN1M0), the difference was statically significant (Figure 2C, P value was 0.025 and 0.029, respectively). The difference in OS between subgroups of T4bN0M0 and T4bN1M0 was not statistically significant (P=0.750). Both of T4aN2M0 and T4bN0M0 were downstaged into stage IIIA of AJCC 8th staging system. Among the 4 subgroups, the median OS of patients with T4bN0M0 was shorter than others significantly (Figure 2C).
In stage IIIC of AJCC 7th staging system, the heterogeneity was detected among the subgroups (P=0.001). The median OS of patients with T4bN2M0 (n=20) was better than others (T4aN3aM0, T4aN3bM0, T4bN3aM0, and T4bN3bM0), the difference was statically significant (P-value was 0.854, 0.002, 0.530, and 0.025, respectively) (Figure 2D). The subgroup T4bN2M0 was downstaged into stage IIIB of AJCC 8th staging system, and the heterogeneity did not exist in the 4 subgroups of stage IIIB of AJCC 8th staging system (Figure 2D, P=0.611).
If subgroup T4bN0M0 was not downstaged to stage IIIA and retained in stage IIIB of AJCC 8th staging system, the heterogeneity would not exist in the adjusted 3 subgroups of stage IIIA (Figure 2E, P=0.121), and adjusted 5 subgroups of stage IIIB (Figure 2F, P=0.490).
On the whole, the homogeneity of 8th TNM staging system was assessed, and the heterogeneity was existed in 2 of 9, and 1 of 9 stage groups in AJCC 7th and 8th staging systems, respectively (Table 1).
Table 1.
The homogeneity analysis of AJCC 8th TNM staging system
| Classfication | T | N | M | No. of patients (%) | No. of events | 3-year survival rate (%) | 5-year survival rate (%) | Log-rank χ2 value | P |
|---|---|---|---|---|---|---|---|---|---|
| 0 | Tis | N0 | M0 | 0 | |||||
| IA | T1 | N0 | M0 | 22 (4.1) | 1 | 95.45 | 95.45 | ||
| IB | 62 (11.5) | 5 | 93.59 | 87.55 | 0.386 | 0.534 | |||
| T1 | N1 | M0 | 2 (0.4) | 0 | 100 | 100 | |||
| T2 | N0 | M0 | 60 (11.1) | 5 | 93.30 | 86.86 | |||
| IIA | 19 (3.5) | 7 | 61.18 | 50.05 | 0.007 | 0.931 | |||
| T1 | N2 | M0 | 6 (1.1) | 2 | 63.63 | 63.63 | |||
| T2 | N1 | M0 | 13 (2.4) | 5 | 60.58 | 45.43 | |||
| T3 | N0 | M0 | 0 | ||||||
| IIB | 73 (13.5) | 15 | 85.39 | 61.78 | 1.545 | 0.214 | |||
| T1 | N3a | M0 | 0 | ||||||
| T2 | N2 | M0 | 7 (1.3) | 0 | 100 | 100 | |||
| T3 | N1 | M0 | 0 | ||||||
| T4a | N0 | M0 | 66 (12.2) | 15 | 84.04 | 58.80 | |||
| IIIA | 185 (34.3) | 90 | 53.88 | 38.79 | 12.993 | 0.005 | |||
| T2 | N3a | M0 | 4 (0.7) | 1 | 75.0 | 0 | |||
| T3 | N2 | M0 | 0 | ||||||
| T4a | N1 | M0 | 79 (14.7) | 33 | 59.45 | 46.59 | |||
| T4a | N2 | M0 | 94 (17.5) | 50 | 51.75 | 33.94 | |||
| T4b | N0 | M0 | 8 (1.4) | 6 | 11.90 | 0 | |||
| IIIB | 89 (16.5) | 61 | 34.73 | 20.36 | 1.818 | 0.611 | |||
| T1 | N3b | M0 | 0 | ||||||
| T2 | N3b | M0 | 1 (0.2) | 1 | 0 | 0 | |||
| T3 | N3a | M0 | 0 | ||||||
| T4a | N3a | M0 | 53 (9.8) | 36 | 37.28 | 22.37 | |||
| T4b | N1 | M0 | 15 (2.8) | 11 | 23.05 | 23.05 | |||
| T4b | N2 | M0 | 20 (3.7) | 13 | 39.11 | 13.04 | |||
| IIIC | 43 (7.9) | 35 | 16.30 | 9.78 | 4.673 | 0.097 | |||
| T3 | N3b | M0 | 0 | ||||||
| T4a | N3b | M0 | 19 (3.5) | 17 | 0 | 0 | |||
| T4b | N3a | M0 | 20 (3.7) | 14 | 32.5 | 19.5 | |||
| T4b | N3b | M0 | 4 (0.7) | 4 | 0 | 0 | |||
| IV | Any T, N | M1 | 47 (8.7) | 45 | 5.91 | 0 | |||
| Overall | 540 (100) | 259 | |||||||
0 in italic indicates the subgroup that no patients survive after the second and fourth year after surgery. P-value in bold indicates that the survival rate of the subgroup was significantly different.
Discrimination power of AJCC 8th TNM staging system
The median OS of 540 GC patients was 40.83 (95% CI: 32.88-48.78) months. Discrimination power in terms of OS within the two staging systems was analyzed. Since changes only occurred within stage III, when classified into four major stages, AJCC 8th staging system was the same as AJCC 7th staging system. The 5-year survival rate for stage I, stage II, stage III, and stage IV was 88.89%, 59.93%, 29.45%, and 0.00%, respectively, the difference was statistically significant (Figure 3A, P<0.001).
Figure 3.
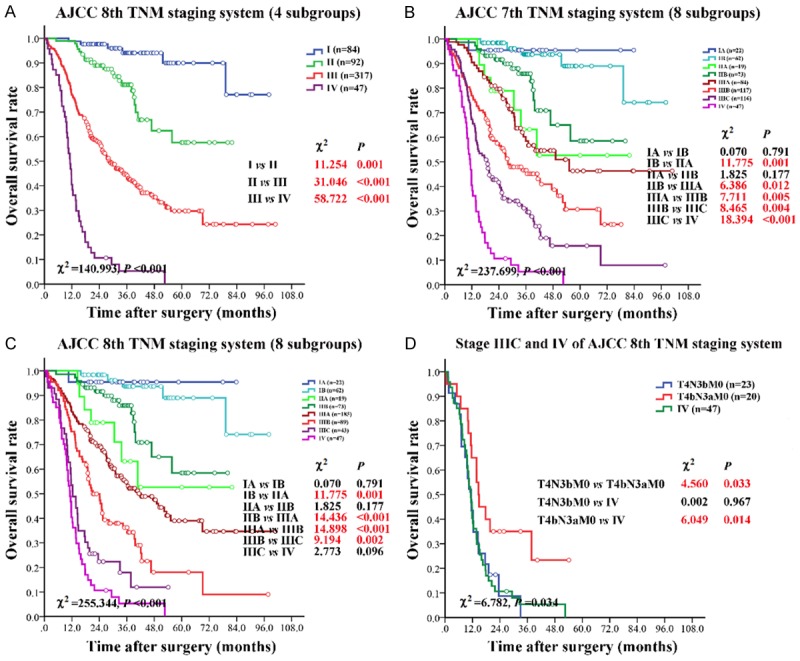
Survival analyses according to AJCC 7th and AJCC 8th TNM staging systems. A: AJCC 8th TNM staging system (4 subgroups). The AJCC 8th staging system was the same as AJCC 7th staging system when classified into four major stages. B: AJCC 7th TNM staging system (8 subgroups). C: AJCC 8th TNM staging system (8 subgroups). D: Survival comparison of stage IIIC and IV in AJCC 8th staging system. T4N3bM0 and T4bN3aM0 were compared with stage IV of AJCC 8th staging system. Detailed subgroup analysis was shown at the lower right corner of each part. P-value in bold red indicated that the survival rate of the subgroup was significantly different.
The difference in OS was not statistically significant between stage IA versus stage IB (P=0.791) and stage IIA versus stage IIB (P=0.177) in AJCC 7th staging system (Figure 3B). The Kaplan-Meier OS curves by AJCC 8th staging system showed statistically significant differences for stage IB versus stage IIA (P=0.001), stage IIB versus stage IIIA (P<0.001), stage IIIA versus stage IIIB (P<0.001), and stage IIIB versus stage IIIC (P=0.002), but not for stage IA versus stage IB (P=0.791) and stage IIA versus stage IIB (P=0.177), and stage IIIC versus stage IV (Figure 3C, P=0.096). Overall, 2 out of 7, 3 out of 7 adjacent subgroups were not statistically discriminated in AJCC 7th and AJCC 8th staging systems.
In stage IIIC of AJCC 8th TNM staging system, the subgroups (T4aN3bM0, T4bN3bM0, and T4bN3aM0) could be classified as T4(a,b)N3bM0 (n=23) and T4bN3aM0 (n=20). The median OS of T4bN3aM0 was longer than T4N3bM0 and stage IV, the difference was statistically significant (P-value was 0.033 and 0.014, respectively). The difference in OS was not statistically significant between T4N3bM0 and stage IV (Figure 3D), which indicated that the OS of patients in T4N3b was comparable to stage IV.
Comparison between AJCC 7th and 8th TNM staging systems
AJCC 7th staging system has 22 subgroups, while AJCC 8th staging system has 27 subgroups, adding 5 additional subgroups. Therefore, AJCC 8th staging system may minimize the conciseness and quickness for oncology clinicians.
Predictive value of pN classification, AJCC 6th, AJCC 7th, and AJCC 8th TNM staging systems were further studied by ROC analysis. All of the adopted factors predicted death with good accuracy (P<0.05 for all). Among the tested factors, AJCC7 pN classification was the weakest risk factor for death (AUC-value was 0.727). The AJCC 8th TNM staging system (27 subgroups) was best to predict the clinical outcomes of GC patients compared to other classifications (Figure 4). Prognostic values of all the factors were listed in Table 2.
Figure 4.
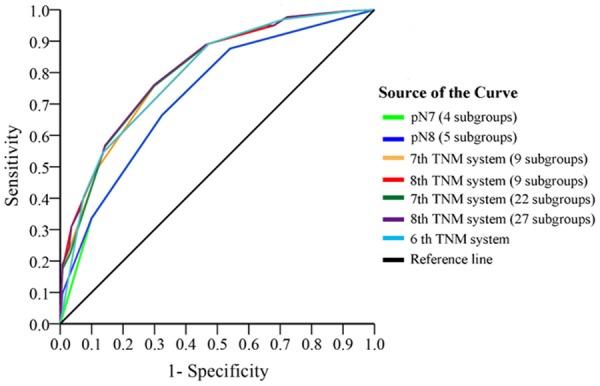
Predictive values of pN classification, AJCC 6th, 7th, and 8th TNM staging systems. The predictive value of AJCC 7th pN classification (bright green curve) was weakest. The 27 subgroups of AJCC 8th TNM staging system (purple curve) was best to predict the clinical outcomes of GC patients compared to other classifications.
Table 2.
Prognostic value of factors assessed in ROC analyses
| Factors | Area under curve (AUC) | 95% CI | Std. error | P | |
|---|---|---|---|---|---|
|
| |||||
| Lower | Upper | ||||
| pN7 (4 subgroups) | 0.727 | 0.684 | 0.769 | 0.022 | <0.001 |
| pN8 (5 subgroups) | 0.730 | 0.688 | 0.772 | 0.021 | <0.001 |
| 7th TNM system (9 subgroups) | 0.800 | 0.764 | 0.837 | 0.019 | <0.001 |
| 8th TNM system (9 subgroups) | 0.799 | 0.762 | 0.835 | 0.019 | <0.001 |
| 7th TNM system (22 subgroups) | 0.803 | 0.767 | 0.839 | 0.019 | <0.001 |
| 8th TNM system (27 subgroups) | 0.809 | 0.773 | 0.844 | 0.018 | <0.001 |
| 6th TNM system | 0.793 | 0.756 | 0.830 | 0.019 | <0.001 |
ROC, Receiver operating characteristic; CI, confidence interval.
Discussion
The AJCC TNM staging system is the global standard to evaluate GC in different institutions [4]. Recently, stage migrations from AJCC 7th staging system to AJCC 8th staging system have been showed, which resulted from either sub-classification of pN3a and pN3b categories, or redefinitions of stage III. The rationality of these revisions remains masked, and the overall performance of AJCC 8th TNM staging system needs further evidence.
Although proactive efforts were carried out by various studies to validate the advantages of pN classification of the AJCC 7th staging system; the merged pN3 (a/b) classification in AJCC 7th staging system decreased the discrimination power [20,21]. In the revised AJCC 8th staging system, pN3a and pN3b was included in the final stage grouping independently. Herein, the new pN classification was verified to indicate the difference of OS very well. However, many other studies have proposed that additional minor modifications of the well-established pN categories might improve the predictive value of pN classification. Some studies suggested that pN category should be redefined by new stratification criteria [22]. For instance, incorporation of pN0 with insufficient number of regional lymph nodes into pN1 improved the prognosis accuracy [16]. New classification systems like lymph nodes ratio (LNR) [23] and the log odds of positive lymph nodes (LODDS) [24] were also proved to be effective for GC assessment.
The other major revision of AJCC 8th staging system was that 7 of the 27 subgroups have different definitions from their counterparts in AJCC 7th staging system, mostly in subgroups of stage III [19]. Our data supported that it was rationale for T4aN2M0 and T4bN2M0 to be downstaged in terms of the homogeneity. But T4bN0M0 might be suitable to retain in stage IIIB. Inadequate eligible cases and limited constituent ratio could result in selection bias. In this study, there were 84 (15.6%) GC patients with T4b status, and only 8 (1.5%) patients with T4bN0M0 from our database. While compared with other status of anatomic depth of tumor invasion, the data of T4b was 1.7%, 1.4%, 3.3% and 3.4% in Japan, Korea, other Asian, and Western countries [19]. Few patients were diagnosed without lymph node metastasis when cancer cells have been found out in serosa (pT4) in China [25]. Therefore, the heterogeneity of pT4 status was existed, especially between China and other countries [26]. More clinical data should be validated by a large multi-institutional international database. In addition, subgroup analysis showed that survival difference was not significant between adjacent stage IIIC and IV in AJCC 8th staging system, mainly due to T4N3bM0 of stage IIIC. Therefore, pN status was critical to impact GC prognosis combined with pT4. Considering no survival difference between T4N3bM0 and AnyTAnyNM1, there is no need for sub-classification of pN3 into more advanced lymph node status. Moreover, whether T4N3bM0 should be upstaged into stage IV in next revision prompted new questions.
We then evaluate the overall performance of AJCC 8th staging system with reference to several benchmarks. First, patients within the same stage group should have only small survival differences [27,28]. In this study, the heterogeneity existed in 2 of 9, and 1 of 9 stage groups in AJCC 7th and 8th staging systems, respectively. Second, there should be discrimination between stage groups, patients in different stage groups should have larger survival differences [29]. Based on the distribution changes, AJCC 8th staging system widened the distance between the survival curves, thus better stratified the survival probabilities. Overall, 2 out of 7, and 3 out of 7 subgroups could not statistically discriminated by AJCC 7th and 8th staging systems, which was elucidated in the previous paragraph. Third, patients with a higher stage should have a worse survival, thus reaching good predictive accuracy [30]. On the whole, the predictive accuracy was better in AJCC 8th staging system with 27 subgroups. Although the prognostic value of AJCC 8th staging system has been approved, its complexity might be criticized. AJCC 8th staging system has five additional subgroups, which may be not simple and intuitive in clinical practice.
The goal of AJCC 8th staging system was to establish an accurate prognostic classification based on sufficient surgical and pathological information. To access this aim, the AJCC 8th staging system should reflect GC patients’ prognosis across the global spectrum. GC shows large geographic differences in incidence and mortality [17,31], more than 40 percent GC patients were diagnosed in China and about 70 percent new patients were advanced GC [32]. However, the demographics constituent ratio of the data supporting the AJCC 8th staging system was mostly submitted from Japan and Korea (21,555 cases, 84.8%), and only 979 (3.9%) eligible cases was from China [19]. The data was not comprehensively representative, which ignored the demographic properties of GC in China. The rigorous exclusion and inclusion criteria of AJCC 8th revision have rejected many Chinese patients. The available patients for the development of 8th edition were from multiple large, well-designed, and well-conducted national and international studies in appropriate patient populations, with appropriate endpoints and appropriate treatments. Other GC registries and databases in China were relatively inferior to those databases.
On the other hand, AJCC 8th staging system should accept the concept of molecular classification at a clinically relevant level. It is widely believed that TNM staging system will be heightened by incorporation of biological markers, and the new molecular classification schema will complement traditional anatomic staging, histological typing, and grading [33]. Human epidermal growth factor receptor 2 (HER2) heterogeneity has been validated to be one of the most important molecular markers for GC and correlated with OS [34,35]. The clinical significance of intratumoral HER2 heterogeneity was demonstrated by a multicenter large-scale study [36]. Other studies focused on vascular endothelial growth factor (VEGF) [37] had also provided therapeutic target and significance for GC. Thus further attempts in this era of precision molecular pathology were needed to build the important bridge from a “population-based” to a more “personalized” approach to patient classification [18,38].
Finally, our categorization revealed that AJCC 8th staging system was superior to AJCC 7th staging system for the following reasons: (i) The pN3a and pN3b were separately incorporated into stage grouping and verified significant prognosis difference. (ii) In the homogeneity analyses, AJCC 8th staging system had better performance. (iii) Although survival difference of stage IIIC and IV was not significant, AJCC 8th staging system was more powerful in discrimination analyses by chi-square test. (iv) The slightly increased complexity of AJCC 8th staging system was offset by improved prognostic accuracy. We acknowledge several limitations in this study. GC patients in China were mostly in advanced stages at diagnosis [32], and metastatic lymph node is less frequently involved in early gastric cancer [39]. As a result, GC cases with T1N3, T2N3, and even T3N3 were rare in our sample population [26]. Some stage migrations were failed to evaluate for the lack of adequate cases.
Conclusions
AJCC 8th TNM staging system represents advancement in pN category, staging homogeneity, discrimination power, prognostication and reproducibility for prediction of prognosis of GC. Taking epidemiological characteristics of GC cases into consideration, the next revision of TNM staging system should be improved by including more clinical data from China.
Acknowledgements
This work was supported by Science Fund of the National Natural Science Foundation of China (No. 81401515; 81230031), and the Fundamental Research Funds for the Central Universities of Ministry of Education of China (No. 2042014kf0096). This work was also funded by “351 talent project (Luojia Young Scholars)” of Wuhan University (413100002).
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330–1344. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Wittekind C. The development of the TNM classification of gastric cancer. Pathol Int. 2015;65:399–403. doi: 10.1111/pin.12306. [DOI] [PubMed] [Google Scholar]
- 5.De Marco C, Biondi A, Ricci R. N staging: the role of the pathologist. Transl Gastroenterol Hepatol. 2017;2:10. doi: 10.21037/tgh.2017.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Geng X, Li Y. Milky spots: omental functional units and hotbeds for peritoneal cancer metastasis. Tumour Biol. 2016;37:5715–5726. doi: 10.1007/s13277-016-4887-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Yoshikawa T, Bonam K, Sue-Ling HM, Taguri M, Morita S, Tsuburaya A, Hayden JD, Grabsch HI. The superiority of the seventh edition of the TNM classification depends on the overall survival of the patient cohort: comparative analysis of the sixth and seventh TNM editions in patients with gastric cancer from Japan and the United Kingdom. Cancer. 2013;119:1330–1337. doi: 10.1002/cncr.27928. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng CW, Tian Q, Yang GF, Fang M, Zhang ZL, Peng J, Li Y, Pang DW. Quantum-dots based simultaneous detection of multiple biomarkers of tumor stromal features to predict clinical outcomes in gastric cancer. Biomaterials. 2012;33:5742–5752. doi: 10.1016/j.biomaterials.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 12.Yoon HM, Ryu KW, Nam BH, Cho SJ, Park SR, Lee JY, Lee JH, Kook MC, Choi IJ, Kim YW. Is the new seventh AJCC/UICC staging system appropriate for patients with gastric cancer? J Am Coll Surg. 2012;214:88–96. doi: 10.1016/j.jamcollsurg.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Peng CW, Wang LW, Zeng WJ, Yang XJ, Li Y. Evaluation of the staging systems for gastric cancer. J Surg Oncol. 2013;108:93–105. doi: 10.1002/jso.23360. [DOI] [PubMed] [Google Scholar]
- 14.Deng J, Zhang R, Pan Y, Wang B, Wu L, Jiao X, Bao T, Hao X, Liang H. Comparison of the staging of regional lymph nodes using the sixth and seventh editions of the tumor-node-metastasis (TNM) classification system for the evaluation of overall survival in gastric cancer patients: findings of a case-control analysis involving a single institution in China. Surgery. 2014;156:64–74. doi: 10.1016/j.surg.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Jun KH, Lee JS, Kim JH, Kim JJ, Chin HM, Park SM. The rationality of N3 classification in the 7th edition of the International Union Against Cancer TNM staging system for gastric adenocarcinomas: a case-control study. Int J Surg. 2014;12:893–896. doi: 10.1016/j.ijsu.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Li B, Li Y, Wang W, Qiu H, Seeruttun SR, Fang C, Chen Y, Liang Y, Li W, Chen Y, Sun X, Guan Y, Zhan Y, Zhou Z. Incorporation of N0 stage with insufficient numbers of lymph nodes into N1 stage in the seventh edition of the TNM classification improves prediction of prognosis in gastric cancer: results of a single-institution study of 1258 Chinese patients. Ann Surg Oncol. 2016;23:142–148. doi: 10.1245/s10434-015-4578-0. [DOI] [PubMed] [Google Scholar]
- 17.Markar SR, Karthikesalingam A, Jackson D, Hanna GB. Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: comparison between West and East. Ann Surg Oncol. 2013;20:2328–2338. doi: 10.1245/s10434-012-2862-9. [DOI] [PubMed] [Google Scholar]
- 18.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 19.Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, Yamamoto Y, Ohashi Y. Proposal of a new stage grouping of gastric cancer for TNM classification: international gastric cancer association staging project. Gastric Cancer. 2017;20:217–225. doi: 10.1007/s10120-016-0601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Ha TK, Kwon SJ. Evaluation of the 7th AJCC TNM staging system in point of lymph node classification. J Gastric Cancer. 2011;11:94–100. doi: 10.5230/jgc.2011.11.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrelli D, Morgagni P, de Manzoni G, Coniglio A, Marchet A, Saragoni L, Tiberio G, Roviello F Italian Research Group for Gastric Cancer (IRGGC) Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: analysis of a large series from specialized Western centers. Ann Surg. 2012;255:486–491. doi: 10.1097/SLA.0b013e3182389b1a. [DOI] [PubMed] [Google Scholar]
- 22.Zhou R, Zhang J, Sun H, Liao Y, Liao W. Comparison of three lymph node classifications for survival prediction in distant metastatic gastric cancer. Int J Surg. 2016;35:165–171. doi: 10.1016/j.ijsu.2016.09.096. [DOI] [PubMed] [Google Scholar]
- 23.Hwang SH, Kim HI, Song JS, Lee MH, Kwon SJ, Kim MG. The ratio-based N staging system can more accurately reflect the prognosis of T4 gastric cancer patients with D2 lymphadenectomy compared with the 7th American joint committee on cancer/union for international cancer control staging system. J Gastric Cancer. 2016;16:207–214. doi: 10.5230/jgc.2016.16.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Z, Xu Y, Li de M, Wang ZN, Zhu GL, Huang BJ, Li K, Xu HM. Log odds of positive lymph nodes: a novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer. 2010;116:2571–2580. doi: 10.1002/cncr.24989. [DOI] [PubMed] [Google Scholar]
- 25.Wang HH, Li K, Xu H, Sun Z, Wang ZN, Xu HM. Improvement of T stage precision by integration of surgical and pathological staging in radically resected stage pT3-pT4b gastric cancer. Oncotarget. 2017;8:46506–46513. doi: 10.18632/oncotarget.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang HH, Huang JY, Wang ZN, Sun Z, Li K, Xu HM. Macroscopic serosal classification as a prognostic index in radically resected stage pT3-pT4b gastric cancer. Ann Surg Oncol. 2016;23:149–155. doi: 10.1245/s10434-015-4656-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Niu Z, Zhou Y, Cao S. A comparison between the seventh and sixth editions of the American joint committee on cancer/international union against classification of gastric cancer. Ann Surg. 2013;257:81–86. doi: 10.1097/SLA.0b013e31825eff3f. [DOI] [PubMed] [Google Scholar]
- 28.Strong VE, D’Amico TA, Kleinberg L, Ajani J. Impact of the 7th Edition AJCC staging classification on the NCCN clinical practice guidelines in oncology for gastric and esophageal cancers. J Natl Compr Canc Netw. 2013;11:60–66. doi: 10.6004/jnccn.2013.0009. [DOI] [PubMed] [Google Scholar]
- 29.Reim D, Loos M, Vogl F, Novotny A, Schuster T, Langer R, Becker K, Höfler H, Siveke J, Bassermann F, Friess H, Schuhmacher C. Prognostic implications of the seventh edition of the international union against cancer classification for patients with gastric cancer: the Western experience of patients treated in a single-center European institution. J. Clin. Oncol. 2013;31:263–271. doi: 10.1200/JCO.2012.44.4315. [DOI] [PubMed] [Google Scholar]
- 30.Dikken JL, van de Velde CJ, Gönen M, Verheij M, Brennan MF, Coit DG. The new American joint committee on cancer/international union against cancer staging system for adenocarcinoma of the stomach: increased complexity without clear improvement in predictive accuracy. Ann Surg Oncol. 2012;19:2443–2451. doi: 10.1245/s10434-012-2403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papenfuss WA, Kukar M, Oxenberg J, Attwood K, Nurkin S, Malhotra U, Wilkinson NW. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol. 2014;21:3008–3014. doi: 10.1245/s10434-014-3664-z. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Zheng R, Zuo T, Zeng H, Zhang S, He J. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28:1–11. doi: 10.3978/j.issn.1000-9604.2016.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10:643–655. doi: 10.1038/nrclinonc.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, Tsuji A, Omuro Y, Li J, Wang JW, Miwa H, Qin SK, Chung IJ, Yeh KH, Feng JF, Mukaiyama A, Kobayashi M, Ohtsu A, Bang YJ. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J. Clin. Oncol. 2014;32:2039–2049. doi: 10.1200/JCO.2013.53.6136. [DOI] [PubMed] [Google Scholar]
- 35.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 36.Kurokawa Y, Matsuura N, Kimura Y, Adachi S, Fujita J, Imamura H, Kobayashi K, Yokoyama Y, Shaker MN, Takiguchi S, Mori M, Doki Y. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer. 2015;18:691–697. doi: 10.1007/s10120-014-0430-7. [DOI] [PubMed] [Google Scholar]
- 37.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 38.Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 39.Huang B, Zheng X, Wang Z, Wang M, Dong Y, Zhao B, Xu H. Prognostic significance of the number of metastatic lymph nodes: is UICC/TNM node classification perfectly suitable for early gastric cancer? Ann Surg Oncol. 2009;16:61–67. doi: 10.1245/s10434-008-0193-7. [DOI] [PubMed] [Google Scholar]


