Abstract
Background
Aortic elastic properties are determinants of left ventricular function by means of ventriculo-arterial coupling and indicators of cardiovascular risk. Aortic valve stenosis surgical replacement temporary reduces aortic function damaging vasa vasorum, while transcatheter aortic valve implantation (TAVI) does not influence it in the short term. We studied aortic distensibility, stiffness, M-mode strain and tissue strain after 6 and 12 months from TAVI.
Methods
We enrolled 15 patients with symptomatic severe aortic stenosis who underwent CoreValve prosthesis (Medtronic, Minneapolis, MN) implantation. Everyone had blood pressure measurement and echocardiography registration before TAVI and after 6 and 12 months.
Results
After TAVI NYHA class (p = 0.016), peak and mean aortic valve gradients (p < 0.001 for both) improved. Aortic distensibility increased (p = 0.032 in the first 6 months, p = 0.005 in the second 6 months, and p = 0.003 from baseline to 12 months), as well as stiffness decreased (p = 0.034; 0.090; 0.001), M-mode strain and tissue strain ameliorated (p = 0.041; 0.004; 0.004; and p = 0.013; 0.002; 0.001, respectively), tissue Doppler imaging improved (S′ wave: p = 0.289; 0.347; 0.018. E′ wave: p = 0.018; 0.113; 0.007. A′ wave: p = 0.002; 0.532; 0.001). Moreover, some left ventricular parameters improved at 6 months, such as ejection fraction (from 49 ± 16 to 57 ± 11%; p = 0.044) and diastolic interventricular septum thickness (from 14 ± 2 to 12 ± 2 mm; p = 0.010). Even systolic pulmonary artery pressure (p = 0.019) and left diastolic dysfunction grade ameliorated (p = 0.042).
Conclusions
For the first time we demonstrated that aortic elastic properties improve at 6 and 12 months after TAVI, thus influencing ventriculo-arterial coupling and ameliorating left ventricular function.
Keywords: TAVI, Strain, Doppler echocardiography, Aorta, Distensibility, Stiffness
1. Introduction
Aortic elastic properties are important determinant of left ventricular function by means of ventriculo-arterial coupling. They also influence coronary blood flow and are independent prognostic factors of cardiovascular risk 1, 2. Geometry of aorta, qualities of its wall, pressure in it, autonomic nervous system and perfusion via vasa vasorum flow: all determine aortic elastic properties 3, 4, 5. Therefore, they are altered in several pathologic conditions involving aorta and aortic valve 6, 7, 8, 9, 10. Aortic valve stenosis is a quite frequent valvular disease which could require a surgical treatment 11, 12. It could be done in open or transcatheter. Open chest surgery is still considered the gold standard for symptomatic patients, but recently transcatheter aortic valve implantation (TAVI) is an option for patients at high surgical risk 13, 14, 15, 16, 17, 18. After open surgery vasa vasorum are removed or damaged, so that various studies reported a reduction of aortic elastic properties; vice versa TAVI do not alter them 19, 20, 21. Insofar, it is reasonable to hypothesize that elastic aortic properties may remain stable or improve after the procedure. A recent work by Vavuranakis M et al. in fact found that seven days after TAVI aortic distensibility and stiffness do not change [22]. However, to date, no studies have been published with a longer follow-up (for example 6 or 12 months). In addition, none have evaluated TAVI effects on aortic elastic properties by means of M-mode strain and tissue strain of aortic wall. The aim of the present study was to evaluate aortic distensibility, stiffness, M-mode strain and tissue strain 6 and 12 months after TAVI compared with pre-procedural ones.
2. Methods
2.1. Subjects
From January 2011 to August 2011 we consecutively enrolled 15 patients with symptomatic severe aortic stenosis and left ventricular ejection fraction > 45% who underwent successful TAVI at the Cardiologic Unit of University Civil Hospital of Brescia, Italy.
Patients were treated with TAVI if the aortic valve area was < 1 cm2, if the European System for Cardiac Operative Risk Evaluation Score (EuroSCORE) [23] was > 20% or if ≥ 1 of the following criteria was met: contraindication for surgery, severely reduced pulmonary function, liver cirrhosis, or metastatic cancer.
All patients underwent TAVI procedure with a third-generation self-expanding CoreValve prosthesis (Medtronic, Minneapolis, MN). The procedure was performed at the catheterization laboratory under local anesthesia and mild sedation with fluoroscopy guidance. The prosthesis was implanted via the transfemoral approach [13]. Procedural success was defined as implantation of a functioning aortic prosthesis valve without intraprocedural mortality and with a paravalvular leak < 2.
Written informed consent was obtained from each patient after the explanation of rationale and study protocol. The investigational protocol was conformed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and was approved by the institutional committee.
The pre-procedural echocardiography acquisition was performed the same day of the procedure.
2.2. Blood pressure measurement
Blood pressure was assessed using a standard, calibrated sphygmomanometer. The mean of three sitting and standing blood pressures was recorded. The arm in which the highest sitting diastolic pressures found was the arm used for all subsequent readings throughout the study. Every effort was made to have the same staff member obtain blood pressure measurements in each individual patient, at the same time of day, using the same equipment. Systolic pressure was recorded when the initial sound is heard (Phase I of the Korotkoff sound), while diastolic pressure at the disappearance of the sound (Phase V of the Korotkoff sound). The cuff was deflated at a rate not greater than 2 mm Hg/s.
2.3. Echocardiography
Echocardiograms were done using Vivid 7 (General Electric Medical Systems, Milwaukee, WI, USA) equipment with a 3.5 MHz transducer, with the patients in the left lateral decubitus position, in accordance with the standardization of the American Society of Echocardiography [24]. Digital loops were stored on the hard disk of the echocardiograph for on-line and off-line analyses and transferred to a workstation (EchoPac; GE Health-care, Waukesha, WI, USA) for off-line analysis. Left ventricular volumes and ejection fraction were obtained by the modified biplane Simpson method. Aortic valve parameters and left ventricular diastolic function were also evaluated. All these parameters were analyzed the day of the procedure before it and 6 months later, while aortic elastic properties were evaluated even 12 months after TAVI.
All conventional and tissue Doppler imaging (TDI) measurements were taken in five consecutive cycles and the means were used for statistical comparison. Aortic size was assessed at four levels: Valsalva sinuses, sinotubular junction, tubular tract, and aortic arch at the end of diastole. Aortic elastic indexes: distensibility, and stiffness index were calculated from the echocardiographically-derived thoracic aortic diameters (mm/m2). Aortic elasticity was assessed on the basis of a 2D guided M-mode recording of systolic (AoS) and diastolic (AoD) aortic diameters, 3 cm above the aortic valve. AoD was obtained at the peak of the R wave at the simultaneously recorded ECG, and AoS was measured at the maximal anterior motion of the aortic wall. The following indexes of aortic elasticity were calculated: aortic distensibility = [2 × (AoS − AoD) / (AoD × PP)] (10− 6 × cm2 × dyn− 1); aortic stiffness index = ln(SBP / DBP) / [(AoS − AoD) / AoD] (pure number) where SBP and DBP refer to brachial systolic and diastolic blood pressure respectively, in mm Hg; pulse pressure (PP) was calculated as SBP–DBP, and ln(SBP / DBP) refers to the natural logarithm of the relative pressure [25]. Parasternal long-axis recordings of the aortic anterior wall were done with activated TDI. Two-dimensional tissue velocity images of the aortic wall were obtained at 130 ± 15 frames/s, which implies a temporal resolution of approximately 16 ms. The velocity scale was modified to avoid aliasing. A sample volume was placed in the region of interest on the anterior aortic wall (3 cm above the aortic valve at the same position as in M-mode measurements). TDI wall velocities during systole (S′), early relaxation (E′) and atrial systole (A′) were measured. Velocity data sets were analyzed off-line using dedicated software (EchoPac; GE Health-care, Waukesha, WI, USA), and peak systolic strain was measured from the resulting deformation curves.
2.4. Statistical analysis
All analyses were carried out using IBM SPSS Statistics 20 for Windows (SPSS, Inc., Chicago, IL). Continuous variables were tested for normality with Kolmogorov–Smirnov test and represented by mean ± standard deviation, while categorical variables as frequency (n) and percentage of the sample. Paired-samples t test was performed to analyze the difference between means for continuous variables between baseline, 6 months and 12 months follow-up, and χ2 test for the difference between proportions for categorical ones. For all statistical tests, probability values < 0.05 were considered significant.
3. Results
The characteristics at baseline are summarized in Table 1, Table 2. The mean age was 83 ± 5 years, 6 patients were male (40.0%) and the Logistic EuroSCORE was 28.1 ± 20.8%. Mean Body Mass Index (BMI) was 25.86 ± 4.24 kg/m2. All patients were symptomatic: 5 in New York Heart Association (NYHA) class II (33.3%), 8 in NYHA class III (53.3%) and 2 in NYHA class IV (13.4%). The peak and mean baseline transvalvular gradient were 83 ± 28 mm Hg and 49 ± 19 mm Hg, respectively. Calculated indexed aortic valve area at baseline was 0.33 ± 0.15 cm2/m2 and maximal aortic flow velocity was 4.7 ± 0.6 m/s2. Baseline dimensions of aorta were: Valsalva sinus diameter of 35 ± 4 mm, sinotubular junction diameter of 27 ± 4 mm, tubular tract diameter of 35 ± 5 mm, and aortic arch diameter of 24 ± 3 mm. At 6 months of follow-up NYHA class improved to 3 patients in NYHA class I (20.0%), 10 in NYHA class II (66.7%), and 2 in NYHA class III (13.3%) (p = 0.016), while systolic and diastolic blood pressures and heart rate remained unchanged.
Table 1.
Baseline characteristics of study populations.
| Variable | Value |
|---|---|
| Age (years) | 83 ± 5 |
| Sex (n and % of males) | 6/15 (40.0%) |
| BMI (kg/m2) | 25.86 ± 4.24 |
| BSA (m2) | 1.79 ± 0.14 |
| Logistic EuroSCORE (%) | 28.1 ± 20.8 |
| Valsalva sinuses diameter (mm) | 35 ± 4 |
| Sinotubular junction diameter (mm) | 27 ± 4 |
| Tubular tract diameter (mm) | 35 ± 5 |
| Aortic arch diameter (mm) | 24 ± 3 |
| Indexed aortic valve area (cm2/m2) | 0.33 ± 0.15 |
| Maximal aortic flow velocity (m/s) | 4.7 ± 0.6 |
BMI = Body Mass Index; BSA = Body Surface Area; EuroSCORE = European System for Cardiac Operative Risk Evaluation Score.
Table 2.
Blood pressure, heart rate and NYHA class.
| Variable | 0 months | 6 months | p |
|---|---|---|---|
| SBP (mm Hg) | 121 ± 10 | 120 ± 9 | 0.096 |
| DBP (mm Hg) | 71 ± 8 | 70 ± 9 | 0.164 |
| HR (bpm) | 74 ± 11 | 75 ± 11 | 0.670 |
| NYHA class | I: 0/15 (0.0%) | I: 3/15 (20.0%) | 0.016 |
| II: 5/15 (33.3%) | II: 10/15 (66.7%) | ||
| III: 8/15 (53.3%) | III: 2/15 (13.3%) | ||
| IV: 2/15 (13.4%) | IV: 0/15 (0.0%) |
SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; HR = Heart Rate; NYHA = New York Heart Association.
A complete study dataset was available in all patients: the echocardiographic parameters evaluated at baseline and during follow-up of 6 months are summarized in Table 3. There were significant periprocedural reductions in peak (to 19 ± 8 mm Hg; p < 0.001) and mean (to 10 ± 5 mm Hg; p < 0.001) transvalvular gradients. Left ventricular ejection fraction improved from 49 ± 16% to 57 ± 11% (p = 0.044), while end-diastolic and end-systolic volumes not significantly decreased (from 112 ± 45 to 108 ± 39 mL, p = 0.530; from 59 ± 43 to 45 ± 19 mL, p = 0.167; respectively). Indexed left ventricular mass decreased from 181.8 ± 38.4 to 166.4 ± 38.7 g/m2 without reaching statistical significance (p = 0.202), as well as tele-diastolic diameter, systolic and diastolic posterior wall thickness and systolic interventricular septum thickness not significantly improved. Only diastolic intraventricular septum thickness significantly decreases after 6 months (from 14 ± 2 to 12 ± 2 mm, p = 0.010). Finally, systolic pulmonary artery pressure was significantly reduced by the procedure (from 40 ± 12 to 28 ± 9 mm Hg, p = 0.019), but S wave on left ventricular lateral wall increased without statistical significance.
Table 3.
Echocardiographic parameters.
| Variable | 0 months | 6 months | p |
|---|---|---|---|
| Diastolic IVST (mm) | 14 ± 2 | 12 ± 2 | 0.010 |
| Systolic IVST (mm) | 16 ± 4 | 17 ± 3 | 0.812 |
| Diastolic PWT (mm) | 14 ± 3 | 12 ± 3 | 0.127 |
| Systolic PWT (mm) | 18 ± 3 | 19 ± 4 | 0.565 |
| End-diastolic diameter (mm) | 56 ± 8 | 58 ± 9 | 0.131 |
| Indexed left ventricular mass (g/m2) | 181.8 ± 38.4 | 166.4 ± 38.7 | 0.202 |
| End-diastolic volume (mL) | 112 ± 45 | 108 ± 39 | 0.530 |
| End-systolic volume (mL) | 59 ± 43 | 45 ± 19 | 0.167 |
| Ejection fraction (%) | 49 ± 16 | 57 ± 11 | 0.044 |
| Peak aortic valve gradient (mm Hg) | 83 ± 28 | 19 ± 8 | < 0.001 |
| Mean aortic valve gradient (mm Hg) | 49 ± 19 | 10 ± 5 | < 0.001 |
| E wave | 0.93 ± 0.33 | 0.82 ± 0.38 | 0.390 |
| A wave | 0.79 ± 0.40 | 0.88 ± 0.42 | 0.513 |
| E/A | 1.41 ± 0.95 | 1.28 ± 1.10 | 0.678 |
| Deceleration time | 217 ± 67 | 215 ± 39 | 0.911 |
| E′ wave | 3.00 ± 1.29 | 3.43 ± 1.40 | 0.356 |
| A′ wave | 5.64 ± 0.75 | 5.21 ± 1.58 | 0.356 |
| S′ wave | 5.67 ± 1.62 | 6.15 ± 0.76 | 0.364 |
| E/E′ | 41.81 ± 30.68 | 22.02 ± 5.28 | 0.134 |
| Systolic pulmonary artery pressure (mm Hg) | 40 ± 12 | 28 ± 9 | 0.019 |
IVST = InterVentricular Septum Thickness; PWT = Posterior Wall Thickness.
Patients who underwent TAVI showed an improvement in left ventricular diastolic function during 6 months follow-up (see Table 3). In fact, at baseline 9 patients had grade I (60.0%), 5 grade II (33.3%), and 1 grade III (6.7%), while after 6 months from TAVI procedure 3 had normal diastolic function (20.0%), 11 grade I (73.3%), and 1 grade III (6.7%) (p = 0.042). Nevertheless, mitral flow and left ventricular TDI parameters did not show statistically significant changes from baseline.
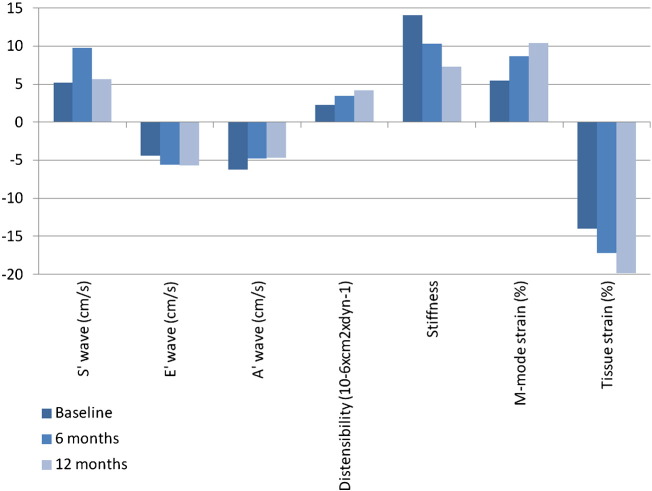
In Table 4 and Fig. 1 aortic elastic properties are resumed, reporting baseline, 6 and 12 months of follow-up parameters. TDI S′ wave significantly improved after 12 months, changing from 5.17 ± 1.37 at baseline to 5.68 ± 1.18 cm/s (p = 0.018), but not after 6 months (9.77 ± 16.70 cm/s; p value from baseline to 6 months: 0.289; p value from 6 to 12 months: 0.347). TDI E′ and A′ waves significantly improved only in the first 6 months, respectively moving from − 4.42 ± 2.55 to − 5.57 ± 2.37 cm/s (p = 0.018) and from − 6.28 ± 2.27 to − 4.76 ± 1.89 cm/s (0.002); vice versa, changes in from 6 to 12 months did not reach significance, being 12-month E′ value − 5.68 ± 2.20 cm/s (p = 0.113) and A′ value − 4.72 ± 1.78 (0.532). Therefore, TDI E′ and A′ waves improvement from baseline to 12 months was statistically significant (p = 0.007 and 0.001 respectively). Moreover, aortic distensibility showed an improvement from 2.24 ± 1.69 at baseline to 3.46 ± 3.03 at 6 months (p = 0.032) to 4.14 ± 2.96 10− 6 × cm2 × dyn− 1 at 12 months (p from 6 to 12 months = 0.005; p from baseline to 12 months = 0.018) and aortic stiffness decreased from 14.05 ± 7.13 at baseline to 10.30 ± 6.99 at 6 months (p = 0.034) and to 7.31 ± 4.55 at 12 months (p from 6 to 12 months = 0.090; p from baseline to 12 months = 0.001). In conclusion, aortic strain values significantly improved after 6 and 12 months. In particular, M-mode strain moved from 5.50 ± 4.12 at baseline to 8.67 ± 8.16 at 6 months (p = 0.041) and to 10.41 ± 8.03% at 12 months (p from 6 to 12 months and from baseline to 12 months = 0.004), while tissue strain from − 14.0 ± 9.2 at baseline to − 17.2 ± 7.5 at 6 months (p = 0.013) and to − 19.9 ± 6.3% at 12 months (p from 6 to 12 months = 0.002; p from baseline to 12 months = 0.001).
Table 4.
Aortic elastic properties.
| Variable | 0 months | p (0–6 months) | 6 months | p (6–12 months) | 12 months | p (0–12 months) |
|---|---|---|---|---|---|---|
| S′ wave (cm/s) | 5.17 ± 1.37 | 0.289 | 9.77 ± 16.70 | 0.347 | 5.68 ± 1.18 | 0.018 |
| E′ wave (cm/s) | − 4.42 ± 2.55 | 0.018 | − 5.57 ± 2.37 | 0.113 | − 5.68 ± 2.20 | 0.007 |
| A′ wave (cm/s) | − 6.28 ± 2.27 | 0.002 | − 4.76 ± 1.89 | 0.532 | − 4.72 ± 1.78 | 0.001 |
| Distensibility (10− 6 × cm2 × dyn− 1) | 2.24 ± 1.69 | 0.032 | 3.46 ± 3.03 | 0.005 | 4.14 ± 2.96 | 0.003 |
| Stiffness | 14.05 ± 7.13 | 0.034 | 10.30 ± 6.99 | 0.090 | 7.31 ± 4.55 | 0.001 |
| M-mode strain (%) | 5.50 ± 4.12 | 0.041 | 8.67 ± 8.16 | 0.004 | 10.41 ± 8.03 | 0.004 |
| Tissue strain (%) | − 14.0 ± 9.2 | 0.013 | − 17.2 ± 7.5 | 0.002 | − 19.9 ± 6.3 | 0.001 |
Fig. 1.
Aortic elastic properties (p values reported in Table 4).
Finally, we compared aortic distensibility, stiffness, M-mode strain and tissue strain variations from baseline to 6 months with them from 6 to 12 months of follow-up (Table 5). We found that there were no statistical differences between them.
Table 5.
Aortic elastic properties variations in the first and in the second 6 months.
| Variable | Delta 0–6 months | Delta 6–12 months | p |
|---|---|---|---|
| Distensibility (10− 6 × cm2 × dyn− 1) | − 1.22 ± 1.98 | − 0.68 ± 0.79 | 0.369 |
| Stiffness | 3.75 ± 6.19 | 2.99 ± 6.36 | 0.793 |
| M-mode strain (%) | − 3.17 ± 5.47 | − 1.74 ± 1.95 | 0.373 |
| Tissue strain (%) | 3.2 ± 4.4 | 2.7 ± 2.7 | 0.694 |
4. Discussion
This study confirms data reported by Vizzardi E et al. in 2012 regarding left ventricular diastolic function and mass [26]. However, to our knowledge, this is the first study considering mid-term follow-up aortic elastic properties changes (6 and 12 months) after a TAVI, and the first using M-mode strain and tissue strain of aortic wall. Recently, Vavuranakis M et al. demonstrated that 7 days after the procedure aortic distensibility and stiffness remain unchanged [22], in contrast with the early post-surgical period (open chest) in which aortic vasa vasorum are damaged and/or removed with the periaortic fat tissue and aortic function decreases 19, 20, 21. Albeit our study enrolled only 15 people with severe symptomatic aortic stenosis, it interestingly seems to demonstrate that aortic function really improves after TAVI at 6 and 12-month follow-up. In fact, distensibility increases, stiffness reduces and M-Mode and tissue strains ameliorate. These data are very useful in understanding how aortic function may influence ventriculo-arterial coupling and so cardiovascular risk. In fact, although our study seems underpowered to detect statistically significant differences in left ventricular morphology and function, some parameters instead significantly improved 6 months after TAVI, such as diastolic interventricular septum thickness, ejection fraction, systolic pulmonary artery pressure and diastolic dysfunction grade. We think that these mid-term changes could be almost partly attributed to the improving of aortic elastic properties. Further studies are needed to test this hypothesis. In fact, if why aortic function decreases after open chest surgery is easy to understand, vice versa why it improves after TAVI is really unclear. Perhaps the normalization of wall stress upon the proximal aortic root due to the drastic reduction of transvalvular gradient could be responsible of the recovery of aortic elastic properties.
The present study shows some limitations of note. First is the small number of patients, which limits the possibility of a more robust statistical analysis. Second, improved aortic elastic properties may partly reflect the improved flow across the aortic valve, and by itself, not really be at all a marker for improved cardiovascular risk.
In conclusion, what prognostic role this phenomenon could have is difficult to be explored because open chest surgery is still considered the gold standard and TAVI is only an alternative to high surgical risk patients. These findings suggest a further advantage of TAVI respect conventional surgery.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Footnotes
Every author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Available online 26 Apr. 2014
References
- 1.Arnett D.K., Evans G.W., Riley W.A. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol. 1994;140:669–682. doi: 10.1093/oxfordjournals.aje.a117315. [DOI] [PubMed] [Google Scholar]
- 2.Vlachopoulos C., Aznaouridis K., Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 3.Heistad D.D., Marcus M.L. Role of vasa vasorum in nourishment of the aorta. Blood Vessels. 1979;16:225–238. doi: 10.1159/000158209. [DOI] [PubMed] [Google Scholar]
- 4.Stefanadis C., Vlachopoulos C., Karayannacos P., Boudoulas H., Stratos C., Filippides T. Effect of vasa vasorum flow on structure and function of the aorta in experimental animals. Circulation. 1995;91:2669–2678. doi: 10.1161/01.cir.91.10.2669. [DOI] [PubMed] [Google Scholar]
- 5.Stefanadis C.I., Karayannacos P.E., Boudoulas H.K., Stratos C.G., Vlachopoulos C.V., Dontas I.A. Medial necrosis and acute alterations in aortic distensibility following removal of the vasa vasorum of canine ascending aorta. Cardiovasc Res. 1993;27:951–956. doi: 10.1093/cvr/27.6.951. [DOI] [PubMed] [Google Scholar]
- 6.O'Rourke M.F. Edinburgh' Churchill Livingstone; 1982. Arterial function in health and disease; pp. 185–224. [Google Scholar]
- 7.Giannattasio C., Mancia G. Arterial distensibility in humans. Modulating mechanisms, alterations in diseases and effects of treatment. J Hypertens. 2002;20:1889–1899. doi: 10.1097/00004872-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Stefanadis C., Stratos C., Boudoulas H., Kourouklis C., Toutouzas P. Distensibility of the ascending aorta: comparison of invasive and noninvasive techniques in healthy men and in men with coronary artery disease. Eur Heart J. 1990;11:990–996. doi: 10.1093/oxfordjournals.eurheartj.a059639. [DOI] [PubMed] [Google Scholar]
- 9.Pitsavos C., Toutouzas K., Dernellis J., Skoumas J., Skoumbourdis E., Stefanadis C. Aortic stiffness in young patients with heterozygous familial hypercholesterolemia. Am Heart J. 1998;135:604–608. doi: 10.1016/s0002-8703(98)70274-1. [DOI] [PubMed] [Google Scholar]
- 10.Vlachopoulos C., Kosmopoulou F., Panagiotakos D., Ioakeimidis N., Alexopoulos N., Pitsavos C. Smoking and caffeine have a synergistic detrimental effect on aortic stiffness and wave reflections. J Am Coll Cardiol. 2004;44:1911–1917. doi: 10.1016/j.jacc.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 11.Iung B., Baron G., Butchart E.G., Delahaye F., Gohlke-Bärwolf C., Levang O.W. A prospective survey of patients with valvular heart disease in Europe: the Euro heart survey on valvular heart disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 12.Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A., Alfieri O., Andreotti F., Antunes M.J., Barón-Esquivias G., Baumgartner H. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33:2451–2496. [Google Scholar]
- 13.Vahanian A., Alfieri O., Al-Attar N., Antunes M., Bax J., Cormier B. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2008;29:1463–1470. doi: 10.1093/eurheartj/ehn183. [DOI] [PubMed] [Google Scholar]
- 14.Figulla L., Neumann A., Figulla H.R., Kahlert P., Erbel R., Neumann T. Transcatheter aortic valve implantation: evidence on safety and efficacy compared with medical therapy. A systematic review of current literature. Clin Res Cardiol. 2011;100:265–276. doi: 10.1007/s00392-010-0268-x. [DOI] [PubMed] [Google Scholar]
- 15.Motloch L.J., Rottlaender D., Reda S., Larbig R., Bruns M., Müller-Ehmsen J. Local versus general anesthesia for transfemoral aortic valve implantation. Clin Res Cardiol. 2012;101:45–53. doi: 10.1007/s00392-011-0362-8. [DOI] [PubMed] [Google Scholar]
- 16.Puls M., Viel T., Danner B.C., Jacobshagen C., Teucher N., Hanekop G. The risk-to-benefit ratio of transcatheter aortic valve implantation in specific patient cohorts: a single-centre experience. Clin Res Cardiol. 2012;101:553–563. doi: 10.1007/s00392-012-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vavouranakis M., Vrachatis D.A., Toutouzas K.P., Chrysohoou C., Stefanadis C. “Bail out” procedures for malpositioning of aortic valve prosthesis (CoreValve) Int J Cardiol. 2010;145:154–155. doi: 10.1016/j.ijcard.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 18.Vavuranakis M., Voudris V., Vrachatis D.A., Thomopoulou S., Toutouzas K., Karavolias G. Transcatheter aortic valve implantation, patient selection process and procedure: two centres’' experience of the intervention without general anaesthesia. Hellenic J Cardiol. 2010;51:492–500. [PubMed] [Google Scholar]
- 19.Barbetseas J., Alexopoulos N., Brili S., Aggeli C., Marinakis N., Vlachopoulos C. Changes in aortic root function after valve replacement in patients with aortic stenosis. Int J Cardiol. 2006;110:74–79. doi: 10.1016/j.ijcard.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Nemes A., Galema T.W., Geleijnse M.L., Soliman O.I., Yap S.C., Anwar A.M. Aortic valve replacement for aortic stenosis is associated with improved aortic distensibility at long-term follow-up. Am Heart J. 2007;153:147–151. doi: 10.1016/j.ahj.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Nemes A., Galema T.W., Soliman O.I., Bogers A.J., ten Cate F.J., Geleijnse M.L. Improved aortic distensibility after aortic homograft root replacement at long-term follow-up. Int J Cardiol. 2009;136:216–219. doi: 10.1016/j.ijcard.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 22.Vavuranakis M., Vrachatis D.A., Boudoulas H., Papaioannou T.G., Moldovan C., Kariori M.G. Effect of transcatheter aortic valve implantation on the ascending aorta's elasticity. Clin Res Cardiol. 2012;101:895–899. doi: 10.1007/s00392-012-0473-x. [DOI] [PubMed] [Google Scholar]
- 23.Nashef S.A., Roques F., Michel P., Gauducheau E., Lemeshow S., Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 24.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A. Recommendations for chamber quantification: a report from the American Society of Echocardiography’'s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki T., Sasayama S., Yagi S.-I., Asakawa T., Hirai T. Non-invasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res. 1987;21:678–687. doi: 10.1093/cvr/21.9.678. [DOI] [PubMed] [Google Scholar]
- 26.Vizzardi E., D'Aloia A., Fiorina C., Bugatti S., Parrinello G., De Carlo M. Early regression of left ventricular mass associated with diastolic improvement after transcatheter aortic valve implantation. J Am Soc Echocardiogr. 2012;25:1091–1098. doi: 10.1016/j.echo.2012.06.010. [DOI] [PubMed] [Google Scholar]