Abstract
Aim
We aimed in this study to assess the role of left atrial (LA), in addition to left ventricular (LV) indices, in predicting exercise capacity in patients with heart failure (HF).
Methods
This study included 88 consecutive patients (60 ± 10 years) with stable HF. LV end-diastolic and end-systolic dimensions, ejection fraction (EF), mitral and tricuspid annulus peak systolic excursion (MAPSE and TAPSE), myocardial velocities (s′, e′ and a′), LA dimensions, LA volume and LA emptying fraction were measured. A 6-min walking test (6-MWT) distance was performed on the same day of the echocardiographic examination.
Results
Patients with limited exercise performance (≤ 300 m) were older (p = 0.01), had higher NYHA functional class (p = 0.004), higher LV mass index (p = 0.003), larger LA (p = 0.002), lower LV EF (p = 0.009), larger LV end-systolic dimension (p = 0.007), higher E/A ratio (p = 0.03), reduced septal MAPSE (p < 0.001), larger LA end-systolic volume (p = 0.03), larger LA end-diastolic volume (p = 0.005) and lower LA emptying fraction (p < 0.001) compared with good performance patients. In multivariate analysis, only the LA emptying fraction [0.944 (0.898–0.993), p = 0.025] independently predicted poor exercise performance. An LA emptying fraction < 60% was 68% sensitive and 73% specific (AUC 0.73, p < 0.001) in predicting poor exercise performance.
Conclusion
In heart failure patients, the impaired LA emptying function is the best predictor of poor exercise capacity. This finding highlights the need for routine LA size and function monitoring for better optimization of medical therapy in HF.
Keywords: Heart failure, Six-minute walk test, Exercise capacity, Left atrial emptying function
1. Introduction
Heart failure (HF) is a clinical syndrome, which is becoming a major problem in public health in recent decades 1, 2. Despite many new achievements in pharmacological and non-pharmacological treatments, the morbidity and mortality associated with HF still remain high 2, 3, 4, 5, 6. Several echo-parameters were tested previously 7, 8, 9, 10, 11, 12, 13, 14, 15 for clinical outcome prediction in patients with HF. Different indices were also, proposed as predictors of survival 11, 12, 13, 14, 15, 16, 17, quality of life 11, 12, 13, 14, 15 and exercise capacity 22, 23, 24, 25, 26 in these patients. Six-minute walk test (6-MWT) has been introduced as an accurate tool for assessing exercise capacity in HF patients, being safe, simple to perform and its results can predict clinical outcomes 18, 19, 20, 21.
Left ventricular (LV) systolic function indices 22, 23 and those of global mechanical dyssynchrony 24, 25, 26 have been shown to independently predict exercise capacity in HF patients. However, the left atrial (LA) function indices and their relationship with exercise markers have not been completely tested yet in this setting. Therefore, we aimed to test LA total emptying fraction as a potential predictor of exercise capacity in HF patients in comparison with other clinical and echocardiographic parameters.
2. Methods
2.1. Study population
We studied 88 patients (mean age 60 ± 10 years, 61% female) with clinical diagnosis of HF, and New York Heart Association (NYHA) functional class I–class III. Patients were referred to the Service of Cardiology, Internal Medicine Clinic, University Clinical Centre of Kosovo, between February 2013 and November 2013. At the time of the study, all patients were on conventional medical treatment, optimized at least 2 weeks prior to enrollment, based on patient's symptoms and renal function: 84.6% were receiving ACE inhibitors or ARB, 70.5% beta-blockers, 55% diuretics, 77% aspirin and 20.5% Ca-blockers. Of the studied cohort, 19% had ischemic etiology, 65.5% hypertension, 7.14% valve disease and 8.3% unknown etiology.
Patients with clinical evidence for cardiac decompensation, limited physical activity due to factors other than cardiac symptoms (e.g. arthritis), chronic renal failure with a stage > 2 (glomerular filtration rate ≥ 89 mL/min), chronic obstructive pulmonary disease (COPD) or those with recent acute coronary syndrome, stroke or anemia, were excluded. Patients gave a written informed consent to participate in the study, which was approved by the local Ethics Committee.
2.2. Data collection
Detailed history and clinical assessment were obtained in all patients, in whom routine biochemical tests were also performed including, lipid profile, blood glucose level and kidney function tests. Estimated body mass index (BMI) was calculated from weight and height measurements. Waist and hip measurements were also made and waist/hip ratio calculated.
2.3. Echocardiographic examination
A single operator performed all echocardiographic examinations using a Philips Intelligent E-33 system with a multi-frequency transducer and harmonic imaging as appropriate. Images were obtained with the patient in the left lateral decubitus position and during quiet expiration. LV end-systole and end-diastole dimension measurements were made from the left parasternal long axis view with the M-mode cursor positioned by the tips of the mitral valve leaflets. LV volumes and EF were calculated from the apical 2 and 4 chamber views using the modified Simpson's method. MAPSE and TAPSE were studied by placing the M-mode cursor at the lateral and septal angles of the mitral annulus and the lateral angle of the tricuspid annulus.
Total amplitude of long axis motion (MAPSE or TAPSE) was measured as previously described [27] from peak inward to peak outward points. LV and right ventricular (RV) long axis myocardial velocities were also studied using Doppler myocardial imaging technique. From the apical 4-chamber view, longitudinal velocities were recorded with the pulsed wave Doppler sample volume placed at the basal part of LV lateral and septal segments as well as RV free wall. Systolic (s′) and early and late (e′ and a′) diastolic myocardial velocities were measured with the gain optimally adjusted. Mean value of the lateral and septal LV velocities was calculated.
Diastolic function of the LV and RV was assessed from filling velocities using spectral pulsed wave Doppler with the sample volume positioned at the tips of the mitral and tricuspid valve leaflets, respectively, during a brief apnea. Peak LV and RV early (E wave) and late (A wave) diastolic velocities were measured, and E/A ratios were calculated. The E/e′ ratio was calculated as the ratio between transmitral E wave and mean lateral and septal e′ wave velocities. The isovolumic relaxation time was also measured from aortic valve closure to mitral valve opening on the pulsed wave Doppler recording. LV filling pattern was considered “restrictive” when E/A ratio was > 2.0, E wave deceleration time < 140 ms and the left atrium dilated of more than 40 mm in transverse diameter [28].
Mitral regurgitation severity was assessed by color and continuous wave Doppler and was graded as mild, moderate or severe according to the relative jet area to that of the left atrium as well as the flow velocity profile, in line with the recommendations of the American Society of Echocardiography [29]. Likewise, tricuspid regurgitation was assessed by color Doppler and continuous-wave Doppler. Retrograde trans-tricuspid pressure drop > 35 mmHg was taken as an evidence for pulmonary hypertension [30]. All M-mode and Doppler recordings were made at a fast speed of 100 mm/s with a superimposed ECG (lead II).
2.4. Measurements of left atrial dimensions and function
LA diameter was measured from aortic root recordings with the M-mode cursor positioned at the level of the aortic valve leaflets. LA volumes were measured using area-length method from the apical four and two chamber views, according to the guidelines of the American Society of Echocardiography [31]. Left atrial maximum volume (LA end-systolic volume) was measured at the end of LV systole, just before the opening of the mitral valve, LA minimum volume (LA end-diastolic volume) was measured at end diastole, right after the closure of the mitral valve, and left atrial total emptying fraction (LA emptying function) was calculated automatically 31, 32.
2.5. Six-minute walk test
Within 24 h of the echocardiographic examination, a 6-MWT was performed on a level hallway surface for all patients and was administered by a specialized nurse, blinded to the results of the echocardiogram. According to the method of Gyatt et al. [33], patients were informed of the purpose and protocol of the 6-MWT, which was conducted in a standardized fashion while patients on their regular medications 34, 35. A 15-m flat, obstacle-free corridor was used, and patients were instructed to walk as far as they can, turning 180° after reaching the end of the corridor, during the allocated time of 6 min. Patients walked unaccompanied so as not to influence walking speed. At the end of the 6 min, the supervising nurse measured the total distance walked by the patient. Pulse and blood pressure were measured before and at the end of the walking test.
2.6. Statistical analysis
Data are presented as mean ± SD or proportions (% of patients). Continuous data were compared with two-tailed unpaired Student t test and discrete data with chi-square test. Correlations were tested with Pearson coefficients. Predictors of 6-MWT distance were identified with univariate analysis, and multivariate logistic regression was performed using the step-wise method. A significant difference was defined as p < 0.05 (2-tailed). Patients were divided according to their ability to walk > 300 m into good and limited exercise performance groups [36] and were compared using unpaired Student t test.
3. Results
Patients' mean age was 60 ± 10 years, and 61% were females (Table 1). The patients group as a whole exercised for a mean of 298 ± 109 m.
Table 1.
Baseline patient's data.
| Clinical data | |
|---|---|
| Sex (female, %) | 61 |
| Age (years) | 60 ± 10 |
| Smoking (%) | 21.5 |
| Diabetes (%) | 29 |
| Body mass index | 29 ± 3.5 |
| Body surface area | 1 ± 0.2 |
| Waist/hip ratio | 0.95 ± 0.5 |
| NYHA class | 1.8 ± 0.8 |
| LBBB | 19 |
| Fasting glucose (mmol/L) | 7 ± 2.9 |
| Total cholesterol (mmol/L) | 4.6 ± 1.2 |
| Triglycerides (mmol/L) | 1.7 ± 1 |
| Urea (mmol/L) | 8.8 ± 6 |
| Creatinine (mmol/L) | 93 ± 29 |
NYHA: New York Heart Association; LBBB: left bundle branch block.
3.1. Patients with limited vs. good exercise performance
Forty-five patients had good exercise, and the remaining 43 patients had limited exercise. Patients with limited exercise capacity (6-MWT < 300 m) were older (p = 0.01) and had higher creatinine level (p = 0.03), higher NYHA class (p = 0.004) and lower systolic and diastolic blood pressure (p = 0.001, for both) compared with those with good exercise performance (Table 2).
Table 2.
Comparison of clinical and biochemical data between patient's groups.
| Variable |
Good performance |
Limited performance |
P |
|---|---|---|---|
| (> 300 m distance) N = 45 |
(≤ 300 m distance) N = 43 |
||
| Age (years) | 58 ± 8.0 | 63 ± 10 | 0.01 |
| Body mass index (kg/m2) | 29 ± 3.7 | 28 ± 3.3 | 0.52 |
| Body surface are | 1.1 ± 0.3 | 1.0 ± 0.2 | 0.40 |
| Waist/hip ratio | 0.95 ± 0.5 | 0.96 ± 0.5 | 0.36 |
| SBP (mmHg) | 145 ± 24 | 128 ± 21 | 0.001 |
| DBP (mmHg) | 90 ± 13 | 81 ± 12 | 0.001 |
| NYHA class | 1.5 ± 0.8 | 2.1 ± 0.8 | 0.004 |
| Fasting glucose (mmol/L) | 6.1 ± 2.0 | 7.9 ± 3.9 | 0.05 |
| Total cholesterol (mmol/L) | 4.7 ± 1.2 | 4.6 ± 1.3 | 0.83 |
| Tryglycerides (mmol/L) | 2.0 ± 1.3 | 1.5 ± 0.7 | 0.05 |
| Urea (mmol/L) | 7.7 ± 6 | 10 ± 6 | 0.12 |
| Creatinine (mmol/L) | 82 ± 22 | 99 ± 32 | 0.03 |
Data are mean ± standard deviation. NYHA: New York Heart Association; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Patients with limited exercise performance also had higher LV mass index (p = 0.003), larger LA (p = 0.002), lower LV EF (p = 0.009), larger LV end-systolic dimension (p = 0.007), higher E/A ratio (p = 0.03), reduced septal MAPSE (p < 0.001), larger LA end-systolic volume (p = 0.03), larger LA end-diastolic volume (p = 0.005) and lower LA emptying fraction (p < 0.001) compared with good performance patients (Table 3). Nine of the 43 with limited exercise had restrictive LV filling pattern compared to 2 of 45 patients with good exercise performance, the difference of this incidence between groups was not significant.
Table 3.
Comparison of echocardiographic data between patient's groups.
| Variable |
Good performance |
Limited performance |
P |
|---|---|---|---|
| (> 300 m distance) N = 45 |
(≤ 300 m distance) N = 43 |
||
| LV dimensions and mass | |||
| LV mass index (g/m 2.7) | 50 ± 19 | 65 ± 26 | 0.003 |
| LV EDD (cm) | 5.1 ± 0.6 | 5.6 ± 1.3 | 0.036 |
| LV ESD (cm) | 3.4 ± 0.8 | 4.1 ± 1.5 | 0.007 |
| IVSd (cm) | 1.16 ± 0.3 | 1.21 ± 0.3 | 0.5 |
| LVPWd (cm) | 0.97 ± 0.1 | 1.04 ± 0.1 | 0.04 |
| EDV (ml) | 127 ± 27 | 165 ± 97 | 0.01 |
| ESV (ml) | 54 ± 34 | 93 ± 86 | 0.006 |
| LV systolic function | |||
| LV ejection fraction (%) | 59 ± 12 | 51 ± 17 | 0.009 |
| LV shortening fraction (%) | 32 ± 8.3 | 27 ± 11 | 0.01 |
| MAPSE septal (cm) | 1.2 ± 0.2 | 0.9 ± 0.3 | < 0.001 |
| Septal s′ (cm/s) | 4.8 ± 1.1 | 4.2 ± 1.2 | 0.06 |
| MAPSE lateral (cm) | 1.3 ± 0.2 | 1.3 ± 0.3 | 0.15 |
| Lateral s′ (cm) | 5.4 ± 1.3 | 5.0 ± 1.3 | 0.18 |
| E/e′ ratio | 10 ± 4.7 | 12 ± 9.0 | 0.29 |
| LV diastolic function | |||
| E wave (cm/s) | 58 ± 18 | 67 ± 24 | 0.07 |
| A wave (cm/s) | 70 ± 16 | 65 ± 26 | 0.33 |
| E/A ratio | 0.9 ± 0.4 | 1.3 ± 1.1 | 0.02 |
| E wave DT (ms) | 188 ± 46 | 160 ± 51 | 0.01 |
| Lateral e′ (cm/s) | 6.4 ± 2.4 | 7.0 ± 2.8 | 0.33 |
| Lateral a′ (cm/s) | 8.5 ± 2.9 | 6.6 ± 2.0 | 0.009 |
| Septal e′ (cm/s) | 5.8 ± 1.7 | 5.5 ± 2.3 | 0.45 |
| LA dimensions and function | |||
| LA diameter (cm) | 3.4 ± 0.7 | 4.6 ± 1.3 | 0.002 |
| LA transversal diameter (cm) | 3.9 ± 0.8 | 4.7 ± 1.4 | 0.004 |
| LA longitudinal diameter (cm) | 5.7 ± 0.8 | 6.1 ± 1.3 | 0.09 |
| LA end systolic volume (ml) | 56 ± 36 | 80 ± 62 | 0.03 |
| LA end diastolic volume (ml) | 23 ± 25 | 43 ± 40 | 0.005 |
| LA total EF (%) | 62 ± 12 | 50 ± 16 | < 0.001 |
LV: left ventricle; EDD: end-diastolic dimension; ESD: end-systolic dimension; IVSd: interventricular septum in diastole; PWd: parietal wall in diastole; s′: systolic myocardial velocity; MAPSE: mitral annular plane systolic excursion; A: atrial diastolic velocity; E: early diastolic filling velocity; LA: left atrial; LA total; EF: left atrial total emptying fraction; DT: deceleration time.
3.2. Correlation of 6-MWT distance with echo parameters
From the list of echocardiographic measurements, only LA emptying fraction (r = 0.26, p = 0.01), LV EF (r = 0.22, p = 0.03), MAPSE septal (r = 0.33, p = 0.002) and Septal s′ (r = 0.26, p = 0.02) correlated with the 6-MWT distance (Table 4).
Table 4.
Correlations of clinical and echocardiographic variables with 6-min walk distance.
| Variable | R | P |
|---|---|---|
| Clinical correlates | ||
| Age | − 0.29 | 0.01 |
| Body mass index | − 0.35 | 0.01 |
| Creatinine | − 0.06 | 0.62 |
| Echocardiographic correlates | ||
| LA total EF | 0.26 | 0.01 |
| LV ejection fraction | 0.22 | 0.03 |
| E wave velocity | − 0.09 | 0.38 |
| A wave velocity | 0.01 | 0.93 |
| E/A ratio | − 0.13 | 0.24 |
| E wave DT | 0.18 | 0.09 |
| MAPSE lateral | 0.21 | 0.06 |
| MAPSE septal | 0.33 | 0.002 |
| Septal e′ | 0.19 | 0.07 |
| Septal a′ | 0.11 | 0.32 |
| Septal s′ | 0.26 | 0.02 |
LV: left ventricle; LA total EF: left atrial total emptying fraction; E: early diastolic filling velocity; A: atrial diastolic filling velocity; E′: early diastolic myocardial velocity; MAPSE: mitral annular plane systolic excursion.
3.3. Predictors of limited 6-MWT distance
From the biochemical and clinical findings, only age (p = 0.01) and NYHA class (p = 0.007) predicted limited 6-MWT distance in univariate analysis. However, low LV EF (p = 0.01), higher LV mass index (p = 0.006), larger LV end-systolic dimension (p = 0.01) and end-diastolic dimension (p = 0.04), reduced septal MAPSE (p = 0.001), higher E/A ratio (p = 0.03), larger LA dimension (p = 0.006) and lower LA emptying fraction (p = 0.001) were univariate echocardiographic predictors of limited exercise capacity (Table 5). In multivariate analysis, only LA emptying fraction [0.944 (0.898–0.993), p = 0.025] independently predicted poor exercise performance (Table 5). An LA emptying fraction < 60% was 68% sensitive and 73% specific (AUC 0.73, p < 0.001) in predicting poor exercise performance (Fig. 1).
Table 5.
Predictors of limited exercise performance.
| Variable | Odds ratio (95% CI) | P |
|---|---|---|
| Clinical univariate predictors | ||
| Age | 1.066 (1.013–1.122) | 0.01 |
| Body mass index | 0.958 (0.841–1.092) | 0.52 |
| Body surface are | 0.410 (0.052–3.254) | 0.39 |
| NYHA class | 2.346 (1.268–4.341) | 0.007 |
| Fasting glucose | 1.214 (0.988–1.490) | 0.06 |
| Urea | 1.097 (0.970–1.240) | 0.14 |
| Creatinine | 1.026 (0.999–1.053) | 0.56 |
| Echocardiographic univariate predictors | ||
| LV mass index (g/m 2.7) | 1.039 (1.011–1.067) | 0.006 |
| LV EDD (cm) | 1.047 (1.001–1.095) | 0.04 |
| LV ESD (cm) | 1.053 (1.011–1.097) | 0.01 |
| LV ejection fraction (%) | 0.963 (0.935–0.992) | 0.01 |
| MAPSE septal (cm) | 0.043 (0.007–0.256) | 0.001 |
| Septal s′ (cm/s) | 0.707 (0.490–1.020) | 0.06 |
| MAPSE lateral (cm) | 0.317 (0.078–1.293) | 0.10 |
| Lateral s′ (cm) | 0.796 (0.570–1.111) | 0.18 |
| E wave (cm/s) | 1.018 (0.998–1.039) | 0.08 |
| A wave (cm/s) | 0.989 (0.968–1.011) | 0.32 |
| E/A ratio | 2.313 (1.047–5.111) | 0.03 |
| LA diameter (cm) | 2.223 (1.260–3.923) | 0.006 |
| LA total EF (%) | 0.936 (0.902–0.971) | < 0.001 |
| Multivariate predictors | ||
| Age | 1.036 (0.970–1.107) | 0.29 |
| Gender | 1.082 (0.303–3.859) | 0.90 |
| NYHA class | 1.673 (0.745–3.752) | 0.21 |
| LVMI (gm/2.7) | 1.013 (0.984–1.044) | 0.38 |
| LV EF | 1.019 (0.972–1.069) | 0.43 |
| MAPSE septal | 0.470 (0.043–5.113) | 0.53 |
| LA total EF | 0.944 (0.898–0.993) | 0.02 |
LV: left ventricle, EDD: end-diastolic dimension, ESD: end-systolic dimension, s′: systolic myocardial velocity, MAPSE: mitral annular plane systolic excursion, A: atrial diastolic velocity, E: early diastolic filling velocity, t-IVT: total isovolumic time, LA: left atrial, LA total EF: left atrial total emptying fraction.
Fig. 1.
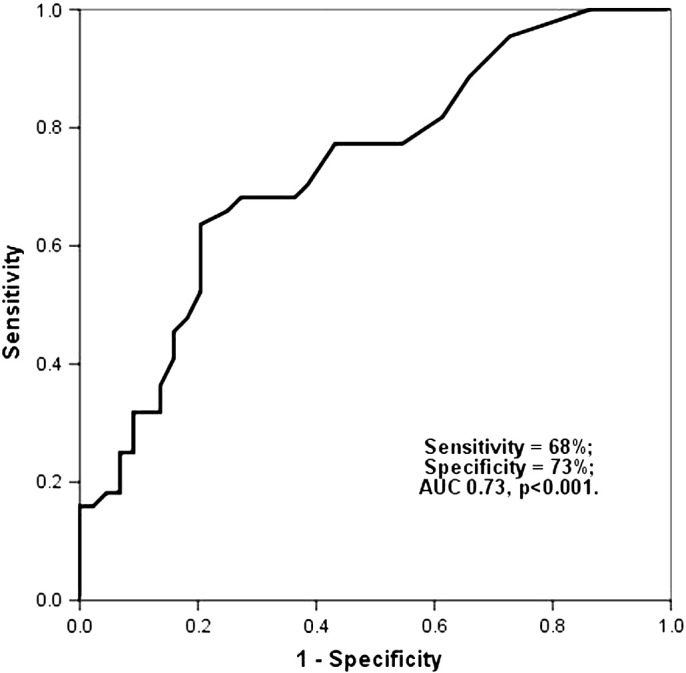
An LA total EF < 60% was 68% sensitive and 73% specific (AUC 0.73, p < 0.001) in predicting poor exercise performance.
4. Discussion
4.1. Findings
Our findings show that beyond LV EF and its longitudinal systolic function, the LA emptying function correlated with the 6-MWT distance in a group of patients with clinically stable HF. It was also the only independent predictor of limited exercise capacity, assessed by 6-MWT, in these patients.
4.2. Data interpretation
The main exercise limiting symptom in heart failure is breathlessness, for which a number of mechanisms have been identified, including compromised stroke volume and cardiac output, raised LV filling pressures, mitral regurgitation, secondary pulmonary hypertension and inadequate peripheral muscle oxygen supply and demand. In our patients, numerous parameters of cardiac function differentiated the two groups of patients according to the 6-MW distance. Obviously, LV global and segmental function were worse in patients with limited exercise capacity as were markers of filling pressures; however, the frequency of patients with restrictive filling pattern was not different between the two groups. In addition, the left atrium was significantly larger in patients with limited exercise capacity, and its function was quite disturbed, as shown by the reduced total emptying fraction with a value of < 60% predicting limited exercise. While the contribution of low LV ejection fraction to exertional breathlessness is easily understood on the basis of compromised stroke volume [36] and cardiac output, that of the LV long axis function (MAPSE) requires further explanation. LV long axis function is supported by the longitudinal myocardial fibers located subendocardially. They have been shown to contribute significantly to the overall myocardial fattening and hence contractile function as shown by thickening fraction and ejection fraction [37]. In our patients, LV long axis function indeed correlated with exercise capacity and univariately predicted poor performance. Furthermore, during exercise, the magnitude of long axis excursion normally increases in order to allow reciprocal increase in left atrial volume and consequently venous return [38]. This behavior was, again, suboptimal in our patients because of the abnormal long axis function, at rest, and the enlarged left atrium with its stiff myocardium. Finally, the left atrial emptying function proved to be the only independent predictor of limited exercise capacity. This could be explained on the basis of the chronically enlarged left atrial cavity with the potential loss of adequate contractile function, on the plateau of Frank–Starling curve 39, 40, irrespective of the severity of raised pressures. The perpetual increase of left atrial pressure secondary to raised LV end-diastolic pressure and mitral regurgitation results eventually into stretched LA myocardium with fibrosis, which limits the cavity ability to fill and empty [41]. This causes pulmonary venous hypertension and consequently breathlessness with exertion.
4.3. Clinical implications
While restrictive LV filling has been well documented as an explanation of exercise intolerance in heart failure, our findings show that impaired LA emptying function, irrespective of restrictive filling, could explain patient's exertional breathlessness. Regular incorporation of LA function assessment in heart failure follow-up protocol should assist in identifying patients who need aggressive left atrial pressure off-loading therapy to save them developing restrictive filling, which in many patients might be irreversible and is known for its poor clinical outcome [42]. Our findings show that LV EF should not be taken solely as an accurate measure of subtle functional changes in heart failure patients. Furthermore, our results are supported by Terzi S et al. [43], who demonstrated similar relationship between LA function and objective measurements of exercise capacity by VO2.
4.4. Limitations
We did not assess left atrial intrinsic myocardial function in this study, using strain and strain rate measurements. They would have shed light on explaining our findings. Our comments on raised left atrial pressure were based on Doppler findings, which are well validated, rather than direct measurements of left atrial pressures, which would have needed clinically unjustifiable invasive techniques. The modest predictive value of individual measurements might be due to the small patient's number, as well as the known heterogeneity of this syndrome.
4.5. Conclusion
In stable heart failure patients, the intrinsic left atrial function seems to be the best predictor of exercise capacity, assessed by 6-MWT distance, irrespective of the presence of restrictive filling pattern. These findings suggest a potential use of left atrial emptying fraction as a sign of early function disturbances, which might recover with optimum adjustment of left atrial pressure offloading therapy.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Footnotes
Available online 24 Apr. 2014
References
- 1.Bui A.L., Horwich T.B., Fonarow G.C. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger V.L. Epidemiology of heart failure. Circ Res. 2013;113(6):646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosterd A., Hoes A.W. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henkel D.M., Redfield M.M., Weston S.A., Gerber Y., Roger V.L. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1(2):91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedrich E.B., Böhm M. Management of end stage heart failure. Heart. 2007;93(5):626–631. doi: 10.1136/hrt.2006.098814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy D., Kenchaiah S., Larson M.G., Benjamin E.J., Kupka M.J., Ho K.K. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347(18):1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 7.Carluccio E., Dini F.L., Biagioli P., Lauciello R., Simioniuc A., Zuchi C. The ‘Echo Heart Failure Score’: an echocardiographic risk prediction score of mortality in systolic heart failure. Eur J Heart Fail. 2013;15(8):868–876. doi: 10.1093/eurjhf/hft038. [DOI] [PubMed] [Google Scholar]
- 8.Mogelvang R., Sogaard P., Pedersen S.A., Olsen N.T., Marott J.L., Schnohr P. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009;119(20):2679–2685. doi: 10.1161/CIRCULATIONAHA.108.793471. [DOI] [PubMed] [Google Scholar]
- 9.Hirata K., Hyodo E., Hozumi T., Kita R., Hirose M., Sakanoue Y. Usefulness of a combination of systolic function by left ventricular ejection fraction and diastolic function by E/E′ to predict prognosis in patients with heart failure. Am J Cardiol. 2009;103(9):1275–1279. doi: 10.1016/j.amjcard.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Doughty R.N., Klein A.L., Poppe K.K., Gamble G.D., Dini F.L., Møller J.E. Independence of restrictive filling pattern and LV ejection fraction with mortality in heart failure: an individual patient meta-analysis. Eur J Heart Fail. 2008;10(8):786–792. doi: 10.1016/j.ejheart.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Venturi F., Gianfaldoni M.L., Melina G., Cecchi A., Petix N.R., Monopoli A. Mitral effective regurgitant orifice area versus left ventricular ejection fraction as prognostic indicators in patients with dilated cardiomyopathy and heart failure. Ital Heart J. 2004;5(10):755–761. [PubMed] [Google Scholar]
- 12.Yamamoto T., Oki T., Yamada H., Tanaka H., Ishimoto T., Wakatsuki T. Prognostic value of the atrial systolic mitral annular motion velocity in patients with left ventricular systolic dysfunction. J Am Soc Echocardiogr. 2003;16(4):333–339. doi: 10.1016/s0894-7317(02)74537-9. [DOI] [PubMed] [Google Scholar]
- 13.Bajraktari G., Dini F.L., Fontanive P., Elezi S., Berisha V., Napoli A.M. Independent and incremental prognostic value of Doppler-derived left ventricular total isovolumic time in patients with systolic heart failure. Int J Cardiol. 2011;148(3):271–275. doi: 10.1016/j.ijcard.2009.09.567. [DOI] [PubMed] [Google Scholar]
- 14.Damy T., Viallet C., Lairez O., Deswarte G., Paulino A., Maison P. Comparison of four right ventricular systolic echocardiographic parameters to predict adverse outcomes in chronic heart failure. Eur J Heart Fail. 2009;11(9):818–824. doi: 10.1093/eurjhf/hfp111. [DOI] [PubMed] [Google Scholar]
- 15.Olson J.M., Samad B.A., Alam M. Prognostic value of pulse-wave tissue Doppler parameters in patients with systolic heart failure. Am J Cardiol. 2008;102(6):722–725. doi: 10.1016/j.amjcard.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 16.Elhendy A., Sozzi F., van Domburg R.T., Bax J.J., Schinkel A.F., Roelandt J.R. Effect of myocardial ischemia during dobutamine stress echocardiography on cardiac mortality in patients with heart failure secondary to ischemic cardiomyopathy. Am J Cardiol. 2005;96(4):469–473. doi: 10.1016/j.amjcard.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Dokainish H., Sengupta R., Patel R., Lakkis N. Usefulness of right ventricular tissue Doppler imaging to predict outcome in left ventricular heart failure independent of left ventricular diastolic function. Am J Cardiol. 2007;99(7):961–965. doi: 10.1016/j.amjcard.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 18.Crapo R.O., Casaburi R., Coates A.L., Enright P.L., MacIntyre N.R., McKay R.T. ATS Statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:1111–1117. [Google Scholar]
- 19.Rostagno C., Galanti G., Romano M., Chiostri G., Gensini G.F. Prognostic value of 6-minute walk corridor testing in women with mild to moderate heart failure. Ital Heart J. 2002;3(2):109–113. [PubMed] [Google Scholar]
- 20.Castel M.A., Méndez F., Tamborero D., Mont L., Magnani S., Tolosana J.M. Six-minute walking test predicts long-term cardiac death in patients who received cardiac resynchronization therapy. Europace. 2009;11(3):338–342. doi: 10.1093/europace/eun362. [DOI] [PubMed] [Google Scholar]
- 21.Boxer R., Kleppinger A., Ahmad A., Annis K., Hager D., Kenny A. The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congest Heart Fail. 2010;16(5):208–213. doi: 10.1111/j.1751-7133.2010.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trivi M., Thierer J., Kuschnir P., Acosta A., Marino J., Guglielmone R. Echocardiographic predictors of exercise capacity in patients with heart failure and systolic dysfunction: role of mitral regurgitation. Rev Esp Cardiol. 2011;64(12):1096–1099. doi: 10.1016/j.recesp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Berisha V., Bajraktari G., Dobra D., Haliti E., Bajrami R., Elezi S. Echocardiography and 6-minute walk test in left ventricular systolic dysfunction. Arq Bras Cardiol. 2009;92(2):121–134. doi: 10.1590/s0066-782x2009000200009. [DOI] [PubMed] [Google Scholar]
- 24.Bajraktari G., Batalli A., Poniku A., Ahmeti A., Olloni R., Hyseni V. Left ventricular markers of global dyssynchrony predict limited exercise capacity in heart failure, but not in patients with preserved ejection fraction. Cardiovasc Ultrasound. 2012;10(1):36. doi: 10.1186/1476-7120-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajraktari G., Elezi S., Berisha V., Lindqvist P., Rexhepaj N., Henein M.Y. Left ventricular asynchrony and raised filling pressure predict limited exercise performance assessed by 6 minute walk test. Int J Cardiol. 2011;146(3):385–389. doi: 10.1016/j.ijcard.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Daullxhiu I., Haliti E., Poniku A., Ahmeti A., Hyseni V., Olloni R. Predictors of exercise capacity in patients with chronic heart failure. J Cardiovasc Med (Hagerstown) 2011;12(3):223–225. doi: 10.2459/JCM.0b013e328343e950. [DOI] [PubMed] [Google Scholar]
- 27.Höglund C., Alam M., Thorstrand C. Atrioventricular valve plane displacement in healthy persons. An echocardiographic study. Acta Med Scand. 1988;224(6):557–562. doi: 10.1111/j.0954-6820.1988.tb19626.x. [DOI] [PubMed] [Google Scholar]
- 28.Appleton C.P., Hatle L.K., Popp R.L. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol. 1988;12(2):426–440. doi: 10.1016/0735-1097(88)90416-0. [DOI] [PubMed] [Google Scholar]
- 29.Zoghbi W.A., Enriquez-Sarano M., Foster E., Grayburn P.A., Kraft C.D., Levine R.A. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 30.Gardin J.M., Adams D.B., Douglas P.S., Feigenbaum H., Forst D.H., Fraser A.G. Recommendations for a standardized report for adult transthoracic echocardiography: a report from the American Society of Echocardiography's Nomenclature and Standards Committee and Task Force for a Standardized Echocardiography Report. J Am Soc Echocardiogr. 2002;15(3):275–290. doi: 10.1067/mje.2002.121536. [DOI] [PubMed] [Google Scholar]
- 31.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Jarnert C., Melcher A., Caidahl K., Persson H., Ryden L., Eriksson M.J. Left atrial velocity vector imaging for the detection and quantification of left ventricular diastolic function in type 2 diabetes. Eur J Heart Fail. 2008;10:1080–1087. doi: 10.1016/j.ejheart.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Guyatt G.H., Sullivan M.J., Thompson P.J., Fallen E.L., Pugsley S.O., Taylor D.W. The 6-min walk test: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 34.Guyatt G.H., Thompson P.J., Berman L.B., Sullivan M.J., Townsend M., Jones N.L. How should we measure function in patients with chronic heart and lung disease? J Chronic Dis. 1985;28:517–524. doi: 10.1016/0021-9681(85)90035-9. [DOI] [PubMed] [Google Scholar]
- 35.Chung E.S., Leon A.R., Tavazzi L., Sun J.P., Nihoyannopoulos P., Merlino J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 36.Litchfield R.L., Kerber R.E., Benge J.W., Mark A.L., Sopko J., Bhatnagar R.K. Normal exercise capacity in patients with severe left ventricular dysfunction: compensatory mechanisms. Circulation. 1982;66(1):129–134. doi: 10.1161/01.cir.66.1.129. [DOI] [PubMed] [Google Scholar]
- 37.Henein M.Y., Gibsion D.G. Abnormal subendocardial function in restrictive left ventricular disease. Br Heart J. 1994;72(3):237–242. doi: 10.1136/hrt.72.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webb-Peploe K.M., Henein M.Y., Coats A.J., Gibson D.G. Echo derived variables predicting exercise tolerance in patients with dilated and poorly functioning left ventricle. Heart. 1998;80(6):565–569. doi: 10.1136/hrt.80.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossi A., Cicoira M., Bonapace S., Golia G., Zanolla L., Franceschini L. Left atrial volume provides independent and incremental information compared with exercise tolerance parameters in patients with heart failure and left ventricular systolic dysfunction. Heart. 2007;93(11):1420–1425. doi: 10.1136/hrt.2006.101261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bajraktari G., Fontanive P., Qirko S., Elezi S., Simioniuc A., Huqi A. Independent and incremental value of severely enlarged left atrium in risk stratification of very elderly patients with chronic systolic heart failure. Congest Heart Fail. 2012;18(4):222–228. doi: 10.1111/j.1751-7133.2011.00280.x. [DOI] [PubMed] [Google Scholar]
- 41.Moe G.W., Grima E.A., Angus C., Wong N.L., Hu D.C., Howard R.J. Response of atrial natriuretic factor to acute and chronic increases of atrial pressures in experimental heart failure in dogs. Role of changes in heart rate, atrial dimension, and cardiac tissue concentration. Circulation. 1991;83(5):1780–1787. doi: 10.1161/01.cir.83.5.1780. [DOI] [PubMed] [Google Scholar]
- 42.Duncan A.M., Lim E., Gibson D.G., Henein M.Y. Effect of dobutamine stress on left ventricular filling in ischemic dilated cardiomyopathy: pathophysiology and prognostic implications. J Am Coll Cardiol. 2005;46(3):488–496. doi: 10.1016/j.jacc.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 43.Terzi S., Dayi S.U., Akbulut T., Sayar N., Bilsel T., Tangurek B. Value of left atrial function in predicting exercise capacity in heart failure with moderate to severe left ventricular systolic dysfunction. Int Heart J. 2005;46(1):123–131. doi: 10.1536/ihj.46.123. [DOI] [PubMed] [Google Scholar]


