Abstract
The aim of this trial was to assess the efficacy of polarized polychromatic noncoherent light (Bioptron light) in the treatment of Carpal Tunnel Syndrome (CTS) in pregnancy. An uncontrolled experimental study was conducted in pregnant patients with CTS who visited our clinic from January 2006 to January 2010. Bioptron light (480–3400 nm; 95% polarization; 40 mW/cm2; and 2.4 J/cm2) was administered perpendicular to the carpal tunnel area. The irradiation time for each session was 6 min at an operating distance of 5–10 cm from the carpal tunnel area, twice each day, five days per week for 2 weeks. Pain and paraesthesia using a visual analogue scale (VAS) and finger pinch strength were evaluated at the end of treatment (week 2) and 1-month (week 6) after the end of treatment. The Student'sttest was used and p values < 0.05 were accepted as statistically significant. 46 patients participated in the study. The mean age of subjects was 27.6 years (range 22–37). The patient population had a mean duration of CTS of 2.3 months (range 1–4). All patients were in the third trimester. Pain and paraesthesia decreased at the end of treatment and at the 1-month follow-up, whereas the finger pinch strength increased at the end of treatment and at the 1-month follow-up. In conclusion, the results of the present study suggest that Bioptron light is a reliable, safe, and effective treatment option in pregnant patients with CTS. Controlled clinical trials are needed to establish the absolute and relative effectiveness of this intervention.
Keywords: Bioptron Light, carpal tunnel syndrome, pregnancy
Introduction
Carpal Tunnel Syndrome (CTS) is a compression neuropathy of the median nerve at the level of the carpal tunnel and is by far the most common of all peripheral nerve entrapments 1). The CTS patient often presents with symptoms of nocturnal pain and numbness, weakness, or clumsiness in holding small objects as well as paraesthesia in the median nerve distribution of the hand 2, 3).
CTS is a common pregnancy complication. The true prevalence is unknown, but has been reported to be as high as 62% 4). CTS commonly presents during the third trimester, but can occur during the first or second trimester 4). Hormonal fluctuations, fluid accumulation with tendency to oedemas, nerve hypersensitivity and glucose level fluctuations are factors that predispose pregnant women to the development of symptoms 5). The diagnosis is relatively easy, based on history and clinical tests such as carpal tunnel compression test, Phalen test and Tinel sign 5). Electrophysiological studies measuring median nerve function are the only objective way to show the nerve deficit 1, 6, 7), but electrodiagnostic or sonographic testing provides no meaningful diagnostic aid in pregnancy 5).
Although the signs and symptoms of CTS are clear and its diagnosis is easy, to date, no ideal treatment has emerged. Many clinicians advocate a conservative approach as the treatment of choice for CTS. Physiotherapy is a conservative treatment that is usually recommended for CTS patients. A wide array of physiotherapy treatments such as electrotherapeutic and non - electrotherapeutic modalities has been recommended for the management of CTS 8). These treatments have different theoretical mechanisms of action, but all have the same aim, to reduce pain and improve function. Such a variety of treatment options suggests that the optimal treatment strategy is not known, and more research is needed to discover the most effective treatment in patients with CTS.
Low-level laser therapy (LLLT) is the form of light therapy that is usually recommended in the management of CTS 9). More recently, physiotherapists have been able to use a new modality of light therapy for the management of musculoskeletal injuries such as CTS, one known as polarized polychromatic noncoherent light (in this article, the term Bioptron light will be used). The manufacturer's explanation of how Bioptron's light works is given in Table 1 10).
Table 1: Manufacturer's explanation of how Bioptron's light works.
| Polarization: Its waves move on parallel planes. In this device, polarization reaches a degree of approximately 95%, which narrows and concentrates the beam. |
| Polychromy: Polychromatic light contains a wide range of wavelengths, including visible light and part of the infrared range. The wavelength of this device's light ranges from 480 nm to 3400 nm, making it able to stimulate a greater range of light-sensitive receptors in the skin. This electromagnetic spectrum does not contain ultraviolet radiation. |
| Incoherency: This device's light is incoherent or out-of-phase light. This means the light waves are not synchronized. |
| Low-energy: This device light has a low-energy density (fluence) of an average 2.4 J/cm2, which has biostimulative effects. This means the light can simulate various biological processes in the body in a positive way. |
However, arguments for the presence of these biochemical effects are lacking and often theoretical. Even if biochemical effects are found in laboratory models, it by no means follows that they will translate into clinically meaningful effects. The extent of clinical use of Bioptron light is not known, although novel modalities such as this are attractive to practitioners working in rehabilitation settings. To our knowledge, there have been no studies to investigate the effectiveness of Bioptron light for CTS in pregnancy. This preliminary, prospective, and open trial reports our experience with the use of Bioptron light to manage nocturnal pain and paraesthesia associated with CTS in pregnancy. The hypothesis of the study is that the Bioptron light is effective treatment approach in CTS with pregnancy.
Methods
Selection of participants
The present investigation was a preliminary, prospective and open trial carried out over a period of 4 years, and was approved by the Local Ethics Committee (018). Following the receipt of a patient information sheet and thorough discussions with the investigators, each study participant gave written informed consent.
Pregnant patients' with CTS were examined and evaluated in the Rheumatology and Rehabilitation Centre, Athens, Greece between January 2006 and January 2010. All patients lived in Athens, Greece and were native speakers of Greek. All patients were either self-referred or were referred by their physician.
All pregnant women with CTS were included in the study. Carpal tunnel syndrome was diagnosed using standard electrophysiological criteria, which were motor distal latency and sensory antidrotic nerve conduction velocity 11–13). Patients with diabetes mellitus, gestational diabetes mellitus, eclampsia, preeclampsia, thyroid disorders, arthropathies, trauma to the hand or wrist, and prior history of CTS (such as recurrence or a CTS diagnosis before pregnancy) were excluded 11–13).
Procedure
Two investigators were involved in the study: a specialist rheumatologist (IS) evaluated the patients to confirm the diagnosis of CTS and performed all assessments at baseline and at follow-up; and the primary investigator (DS) administered the treatments. All treatments were administered at the Rheumatology and Rehabilitation Centre by a qualified physiotherapist with a certificate in orthopaedic medicine on Cyriax principles (DS).
All patients were asked to avoid activities that irritated the hand and to refrain from taking analgesic medication for any condition during the course of the study. Bioptron light was administered as monotherapy and patients were given no additional treatment for CTS until the follow-up assessment. Patients were able to withdraw from the study at any stage without reason and would immediately receive the standard care (polytherapy) for CTS as provided by the clinic. Patients reporting moderate or severe symptoms 3–4 months after the end of the treatment were offered an alternative form of treatment for this condition.
Communication and interaction (verbal and nonverbal) between the therapist and patient was kept to a minimum, and behaviours sometimes used by therapists to facilitate positive treatment outcomes were purposefully avoided (for example, patients were given no indication of the potentially beneficial effects of any of the treatments given, nor were they given any feedback on their performance in the pre- and post-treatment assessments).
Bioptron light treatment intervention
Patients attended the clinic twice each day, five times per week over a 2-week period for each Bioptron light treatment (personal communication with the company). Bioptron light (Harrier Inc., USA, created in Switzerland) was administered by one of us (D.S.) as monotherapy and following the advice provided in the manufacturer's users guide. Patients sat upright with the arm placed on an adjacent bed with the elbow in extension and supination. The Bioptron light probe was held at a 90° angle 5–10 cm above the clean bare skin of the carpal tunnel area, as this is reported to achieve maximal penetration of light, for exactly 6 min (Fig. 1). A Bioptron 2 device (Harrier Inc.) was used to deliver the Bioptron light with the following output characteristics: rated power of halogen = 90 W; light wavelength = 480–3400 nm; degree of polarization = 95%; specific power density = 40 mW/cm2; and energy density = 2.4 J/cm2. A “beep” signified the end of the 6-min treatment 10) Bioptron 2 is approved by the FDA (USA), TGA Australia, EEC and carries an ISO 9001 certificate and EN 46001 as a patented medically approved product.
Fig. 1:
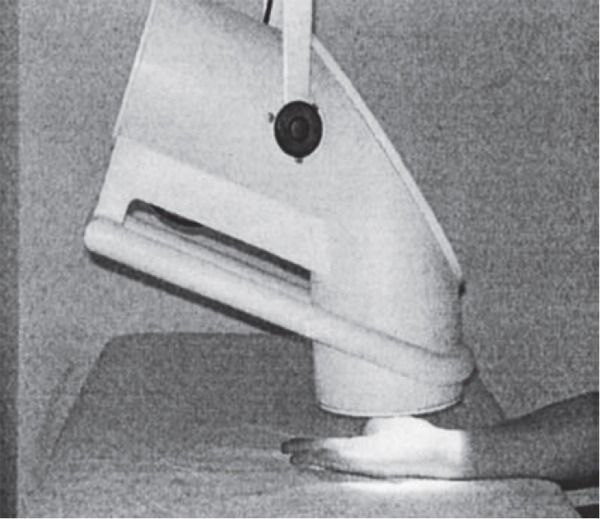
Application of Bioptron light
Outcome measures
The patients' pain and paraesthesia levels were evaluated using a visual analogue scale (VAS). Its scale ranged from 0 (no pain/no paraesthesia) to 10 (extremely severe pain/paraesthesia). In addition, finger pinch strength was measured using a Jamar dynamometer and the outcomes were recorded in kilograms. Patients were instructed to sit with their shoulders adducted and neutrally rotated, with their elbows flexed at 90°. The forearms and wrists were maintained in the neutral position for the palmar pinch 14). The finger pinch was performed three times and the mean value was used for the analysis. In order to reduce the muscle fatigue effects, 1-min intervals separated each measurement 15). Outcome measures were taken at the end of the treatment (week 2) and at the 1-month follow-up after the end of treatment (week 6) by the manager of the clinic (IS).
Statistical analysis
In the presentation of data, (1) the pre-treatment pain VAS, paraesthesia VAS and finger pinch strength parametric values were compared to the post-treatment values; (2) the pre-treatment pain VAS, paraesthesia VAS and finger pinch strength parametric values were compared to the post-treatment values in the first month; and (3) the post-treatment pain VAS, paraesthesia VAS and finger pinch strength parametric values were compared to the post treatment values in the first month using the Student's t test.
Results
Sixty-seven patients eligible for inclusion visited the clinic within the trial period. Eleven were unwilling to participate in the study, and ten did not meet the inclusion criteria described above. The other 46 patients participated in the study (Fig. 2). The mean age of subjects was 27.6 years (range 22–37). The patient population had a mean duration of CTS of 2.3 months (range 1–4). All patients were in the third trimester. No patients requested to withdraw from the study and all patients provided data at the 1-month follow-up.
Fig. 2:
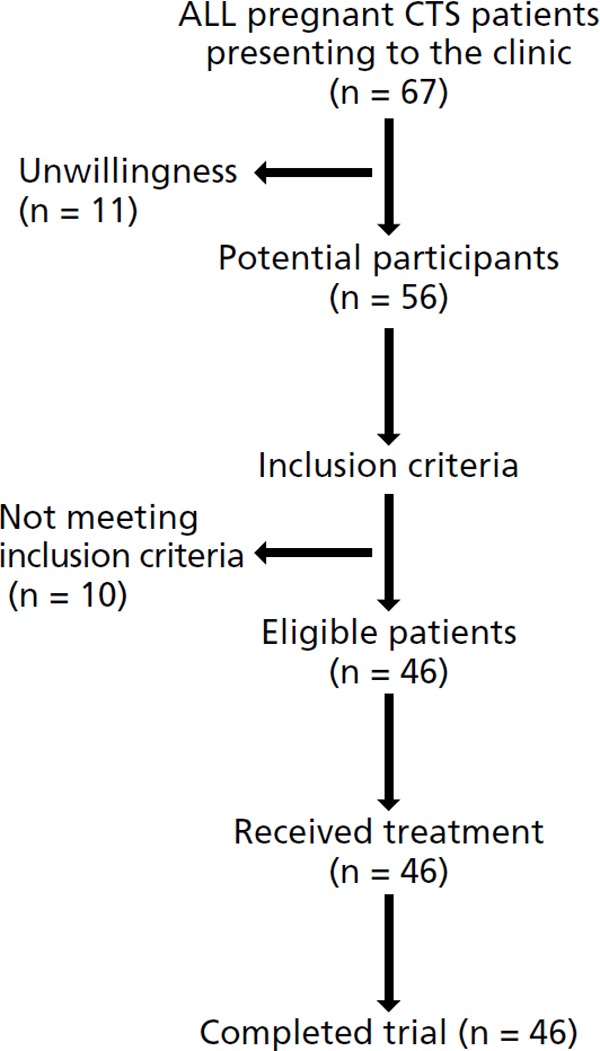
Flow chart of the study.
Pain and paraesthesia measurements
When the scores before treatment were compared to the ones after treatment, there were statistically significant decreases in pain and paraesthesia VAS evaluations after the treatment and in the first month. In the comparison of the scores after the treatment and in the first month, the VAS levels revealed a statistically significant decrease (Table 2).
Table 2: Pain and paresthesia.
| Pre-treatment | Post-treatment | pa | First month | pB | P* | |
|---|---|---|---|---|---|---|
| VAS Pain | 8.71±1.64 | 5.834±2.37 | .000 | 3.47±2.45 | .000 | .000 |
| VAS Paresthesia | 8.66±1.55 | 5.536±2.28 | .000 | 3.27±2.21 | .000 | .000 |
pα pre-treatment-post-treatment p value, pB pre-treatment-first month p value,
p* post-treatment-first month p values, VAS visual analogue scale
Finger pinch strength
The finger pinch strength measurements after the treatment and in the first post-treatment month compared to the scores before treatment showed a statistically significant improvement. The first month post-treatment measurements also showed continuing statistically significant improvement when compared to the values obtained after the treatment (Table 3).
Table 3: Finger pinch strength.
| Pre-treatment | Post-treatment | pa | First month | pB | P* | |
|---|---|---|---|---|---|---|
| Finger pinch strength | 4.22 ± 1.11 | 7.56 ± 1.39 | .000 | 9.23 ± 1.34 | .000 | .000 |
pα pre-treatment-post-treatment p value, pB pre-treatment-first month p value,
p* post-treatment-first month p values
Discussions
The aim of this study was not to explain how the Bioptron light acts but, rather, to find out whether this intervention is an effective treatment in pregnant patients with CTS so that clinicians treating pregnant patients with CTS can use it. The results of this study support the hypothesis of the study.
The present controlled clinical trial was the first study to examine the effectiveness of light therapy using Bioptron light in pregnant patients with CTS. Two pilot studies assessed the effectiveness of this treatment in acute lateral epicondylitis 16) and in carpal tunnel syndrome 17), two controlled clinical studies assessed the effectiveness of Biopton light in chronic lateral epicondylitis 18, 19) and one controlled study assessed the effectiveness of Bioptron light in acute ankle sprains 20). The most likely explanation for the lack of trials is that Bioptron light has only become recently available in the physiotherapy area. All previously reported trials found that a course of Bioptron light treatment based on manufacturers' claims may improve patients' symptoms. The findings of these six trials encourage the design of future well-designed RCTs that might produce strong evidence for the effectiveness of Bioptron light on acute and overuse injuries.
Like laser therapy, Bioptron is also a low-power light source, but differs in that it is polychromatic and incoherent rather than monochromatic and coherent. Moreover, Bioptron combines visible light at a wavelength of 480–700 nm and infrared light at a wavelength of 700–3400 nm. In contrast, low power laser contains either visible or infrared light at one specific wavelength. Several drawbacks have impaired the usefulness of low-power laser light in comparison to Bioptron light, such as high cost, high risk, required user skills, and the small diameter of the laser beam, which allows only a limited area to be treated.
The Bioptron light therapy booklet 10) states that incorrect application cannot be health hazardous but that the effects of the Bioptron light will be reduced if any of the following apply:
It is not applied to bare skin.
It is held at an operating distance of > 10 cm. The appropriate distance is 5–10 cm.
It is not held at a 90° angle from the skin. For the greater penetration depth, the device should be perpendicular to the area.
The light and skin should not be steady.
The irradiation time is < 6 minutes. The appropriate irradiation time is 6 min. Irradiation times more than > 6 min do not produce better results.
The period of treatment is < 3 times per week or < 1 month.
The data from this preliminary, prospective and open trial in pregnant patients with CTS suggests that a course of Bioptron light treatments given in 6-min twice per day five days per week for four weeks reduce the self-reported nocturnal pain and paraesthesia and increase the finger pinch strength when compared to baseline data. However, the absence of a notreatment control group means that we cannot be certain that these findings were due to the Bioptron light treatment intervention itself rather than to natural fluctuations in symptoms, resolution of the CTS, or expectation of treatment success associated with receiving a medical intervention. We also cannot discount the possibility that patients reported prolonged improvement at the 1-month follow-up to please the investigator, as there was no placebo control.
However, none of the patients wanted to discontinue Bioptron light in favor of conventional polytherapy, so we believe that symptom reduction was an actual phenomenon. For this reason, the finding that a high proportion of patients report long-term improvement with Bioptron light given as a monotherapy merits dissemination, as management of CTS is often unsatisfactory. Some patients self-manage CTS in the initial stages by reducing activities of the hands for 1 or 2 months to reduce symptoms. However, this approach is effective in < 10% of patients 11). For most patients, CTS is managed in the initial stages by conservative treatment and by surgery if conventional treatment fails 11, 13, 21).
Both the visible and infrared parts of the electromagnetic spectrum of Bioptron light can explain its mechanism of action. It is probable that Bioptron light has biostimulative effects accelerates the cellular mechanisms and improves the blood supply, but research is needed to investigate how this occurs.
It is important to mention that no side effects were reported during or after the treatment period. There is no ultraviolet light in the Bioptron spectrum, so there is no tanning or heat effect on the skin. It is not harmful to the eyes or to pregnant women. Finally, Bioptron light cannot cause cancer in any way, as the dangerous range for cancer risk is low ultraviolet light at 250 nm and the lowest Bioptron range is 400 nm.
Pregnant women in the third trimester were only included in the present trial. This happened because CTS occurs most frequently during the third semester of pregnancy 4, 5). This trial lasted 4 years because a few women mention their symptoms to doctors to receive any treatment 11). Electrodiagnostic or sonographic testing were not used in the present trial because these tests provide no meaningful diagnostic aid in pregnancy 5).
The findings of our preliminary study should encourage the design of a randomized controlled trial with sufficient power to determine the effectiveness of Bioptron light against a valid placebo and conventional laser therapy. This is needed to confirm or refute the manufacturer's claims that Bioptron light has long-term effectiveness in pregnant patients with CTS. Moreover, studies are needed to find out the analgesic effects of Bioptron light as well to investigate the role of Bioptron light treatment as physical therapy for common musculoskeletal or orthopedic conditions.
Conclusion
The efficacy of Bioptron light applied as monotherapy in the current preliminary, prospective, open clinical trial indicated a positive clinical effect on nocturnal pain relief, paraesthesia and finger pinch strength from carpal tunnel syndrome in pregnancy. Future placebo-controlled studies with adequate sample size and outcome measures of known validity are required to investigate the absolute and relative effectiveness of Bioptron light.
Conflict of interest statement The authors declare that they have no competing interest.
Role of funding source NA
Ethical statement The current study has not been sent to any journal for consideration except Lasers in Medical Science. All of the authors agreed to submit this study (Treatment of Carpal Tunnel Syndrome in pregnancy with Polarized Polychromatic Non-coherent Light (Bioptron Light): A Preliminary, Prospective, Open Clinical Trial) to Lasers in Medical Science to be published. The authors whose names appear on this submission have contributed sufficiently to the scientific work and, therefore, share collective responsibility and accountability for the results.
References
- 1: Szabo RM. (1998) Carpal tunnel syndrome as a repetitive motion disorder. Clin Orthop Relat Res 351:78-89 [PubMed] [Google Scholar]
- 2: Boscheinen-Morrin J, Conolly B. (2001) The hand: Fundamentals of therapy. 3rd edn. London, UK: Butterworth-Heinemann [Google Scholar]
- 3: Donatelli R, Wooden M. (2001) Orthopaedic physical therapy. 3rd edn. Philadelphia: Churchill Livingstone [Google Scholar]
- 4: Ablove RH, Ablove TS. (2009) Prevalence of carpal tunnel syndrome in pregnant women. WMJ 108:194-6 [PubMed] [Google Scholar]
- 5: Zyluk A. (2013) Carpal tunnel syndrome in pregnancy: a review. Pol Orthop Traumatol 78:223-7 [PubMed] [Google Scholar]
- 6: Gerr F, Letz R. (1998) The sensitivity and specificity of tests for carpal tunnel syndrome vary with the comparison subjects. J Hand Surg B 23:151-5 [DOI] [PubMed] [Google Scholar]
- 7: Johnson EW. (1993) “Diagnosis of carpal tunnel syndrome. The gold standard.” Am J Phys Med Rehab 72:1. [DOI] [PubMed] [Google Scholar]
- 8: Piazzini DB, Aprile I, Ferrara PE, et al. (2007) A systematic review of conservative treatment of carpal tunnel syndrome. Clin Rehabil 21:299-314 [DOI] [PubMed] [Google Scholar]
- 9: Barbosa RI, Fonseca MC, Rodrigues EK, et al. (2016) Efficacy of low-level laser therapy associated to orthoses for patients with carpal tunnel syndrome: A randomized single-blinded controlled trial. J Back Musculoskelet Rehabil 29:459-66 [DOI] [PubMed] [Google Scholar]
- 10: Visible polarized light information packet Bioptron 2, Harrier Inc. USA: 2002 [Google Scholar]
- 11: Sapuan J, Yam KF, Noorman MF, et al. (2012) Carpal tunnel syndrome in pregnancy - you need to ask! Singapore Med J 53:671-5 [PubMed] [Google Scholar]
- 12: Yazdanpanah P, Aramesh S, Mousavizadeh A, et al. (2012) Prevalence and severity of carpal tunnel syndrome in women. Iran J Public Health 41:105-10 [PMC free article] [PubMed] [Google Scholar]
- 13: Moghtaderi AR, Moghtaderi N, Loghmani A. (2011) Evaluating the effectiveness of local dexamethasone injection in pregnant women with carpal tunnel syndrome. J Res Med Sci 16(5):687-90 [PMC free article] [PubMed] [Google Scholar]
- 14: Chen LC, Ho CW, Sun CH, et al. (2015) Ultrasound-guided pulsed radiofrequency for carpal tunnel syndrome: A single-blinded randomized controlled study. PLoS One 10:e0129918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15: Wu YT, Ke MJ, Chou YC, et al. (2016) Effect of radial shock wave therapy for carpal tunnel syndrome: A prospective randomized, double-blind, placebo-controlled trial. J Orthop Res. 34:977-84. [DOI] [PubMed] [Google Scholar]
- 16: Stasinopoulos D. (2005) The use of polarized polychromatic non-coherent light as therapy for acute tennis elbow/lateral epicondylalgia: a pilot study. Photomed Laser Surg 23: 66 - 9 [DOI] [PubMed] [Google Scholar]
- 17: Stasinopoulos D, Stasinopoulos I, Johnson MI. (2005) Treatment of carpal tunnel syndrome with polarized polychromatic noncoherent light (Bioptron light): A preliminary, prospective, open clinical trial. Photomed Laser Surg 23:225-8 [DOI] [PubMed] [Google Scholar]
- 18: Stasinopoulos D, Stasinopoulos I, Pantelis M, Stasinopoulou K. (2009) Comparing the effects of exercise program and low-level laser therapy with exercise program and polarized polychromatic non-coherent light (bioptron light) on the treatment of lateral elbow tendinopathy. Photomed Laser Surg 27(3):513-20 [DOI] [PubMed] [Google Scholar]
- 19: Stasinopoulos D, Stasinopoulos I. (2006) Comparison of effects of Cyriax physiotherapy, a supervised exercise programme and polarized polychromatic non-coherent light (Bioptron light) for the treatment of lateral epicondylitis. Clin Rehabil 20(1):12-23 [DOI] [PubMed] [Google Scholar]
- 20: Stasinopoulos D, Papadopoulos C, Lamnisos D, Stasinopoulos I. (2017) The use of Bioptron light (polarized, polychromatic, non-coherent) therapy for the treatment of acute ankle sprains. Disabil Rehabil 39:450-457. [DOI] [PubMed] [Google Scholar]
- 21: Khosrawi S, Maghrouri R. (2012) The prevalence and severity of carpal tunnel syndrome during pregnancy. Adv Biomed Res 1:43. [DOI] [PMC free article] [PubMed] [Google Scholar]


