ABSTRACT
Staphylococcus aureus is responsible for a significant amount of devastating disease. Its ability to colonize the host and cause infection is supported by a variety of proteins that are dependent on the cofactor heme. Heme is a porphyrin used broadly across kingdoms and is synthesized de novo from common cellular precursors and iron. While heme is critical to bacterial physiology, it is also toxic in high concentrations, requiring that organisms encode regulatory processes to control heme homeostasis. In this work, we describe a posttranscriptional regulatory strategy in S. aureus heme biosynthesis. The first committed enzyme in the S. aureus heme biosynthetic pathway, glutamyl-tRNA reductase (GtrR), is regulated by heme abundance and the integral membrane protein HemX. GtrR abundance increases dramatically in response to heme deficiency, suggesting a mechanism by which S. aureus responds to the need to increase heme synthesis. Additionally, HemX is required to maintain low levels of GtrR in heme-proficient cells, and inactivation of hemX leads to increased heme synthesis. Excess heme synthesis in a ΔhemX mutant activates the staphylococcal heme stress response, suggesting that regulation of heme synthesis is critical to reduce self-imposed heme toxicity. Analysis of diverse organisms indicates that HemX is widely conserved among heme-synthesizing bacteria, suggesting that HemX is a common factor involved in the regulation of GtrR abundance. Together, this work demonstrates that S. aureus regulates heme synthesis by modulating GtrR abundance in response to heme deficiency and through the activity of the broadly conserved HemX.
KEYWORDS: Staphylococcus aureus, heme, tetrapyrroles
IMPORTANCE
Staphylococcus aureus is a leading cause of skin and soft tissue infections, endocarditis, bacteremia, and osteomyelitis, making it a critical health care concern. Development of new antimicrobials against S. aureus requires knowledge of the physiology that supports this organism’s pathogenesis. One component of staphylococcal physiology that contributes to growth and virulence is heme. Heme is a widely utilized cofactor that enables diverse chemical reactions across many enzyme families. S. aureus relies on many critical heme-dependent proteins and is sensitive to excess heme toxicity, suggesting S. aureus must maintain proper intracellular heme homeostasis. Because S. aureus provides heme for heme-dependent enzymes via synthesis from common precursors, we hypothesized that regulation of heme synthesis is one mechanism to maintain heme homeostasis. In this study, we identify that S. aureus posttranscriptionally regulates heme synthesis by restraining abundance of the first heme biosynthetic enzyme, GtrR, via heme and the broadly conserved membrane protein HemX.
INTRODUCTION
The tetrapyrrole cofactor heme is critical to the physiology of organisms from humans to bacteria. Heme is composed of a porphyrin ring complexed to iron at its center, making it an excellent redox-active moiety for a variety of enzymes. Across kingdoms, heme is used to shuttle electrons in the respiratory chain and is also required for the function of many critical proteins, including nitric oxide synthase, catalase, and hemoglobin. To satisfy the cellular need for heme, most heme-dependent organisms synthesize heme de novo from simple and abundant precursors.
The versatility of heme as a cofactor is based on its reactivity, which also results in its toxicity at high concentrations. Excess heme can cause damage to cellular macromolecules, and the redox cycling of heme-iron produces reactive oxygen species via Fenton chemistry (1). Bacteria encode a variety of mechanisms to resist heme toxicity (1), but the most important of these strategies may be the prevention of self-imposed toxicity by regulating endogenous heme synthesis. A variety of transcriptional and posttranscriptional strategies have evolved to regulate heme synthesis centered around providing sufficient heme to occupy hemoproteins while preventing excess heme synthesis to limit unnecessary consumption of substrates and preclude toxicity.
In this study, we sought to uncover regulatory pathways controlling heme synthesis in the human pathogen Staphylococcus aureus. S. aureus is a Gram-positive bacterium that causes a variety of devastating diseases, including skin and soft tissue infections, osteomyelitis, endocarditis, and bacteremia (2). S. aureus, as a facultative anaerobe, generates energy through aerobic respiration, anaerobic respiration, or fermentation. The final step in aerobic respiration is reduction of oxygen to water, which S. aureus performs with either of the heme-dependent QoxABCD or CydAB terminal oxidases (3, 4). Although a great deal is known about heme synthesis, heme utilization, and heme toxicity in S. aureus, no heme synthesis regulatory pathway has been identified in this organism. S. aureus encodes the newly appreciated coproporphyrin-dependent heme synthesis pathway to populate its hemoproteins (5, 6). These include the terminal oxidases, catalase, and bacterial nitric oxide synthase, all of which contribute to growth, protection from host defenses, and pathogenesis (3, 7–9). Under conditions of excess exogenous heme, the heme stress response in S. aureus is activated by the heme-sensing two-component system HssRS, which regulates the transcription of a putative efflux pump, HrtAB. This system is critical for growth and survival in toxic concentrations of heme and modulates pathogenesis in a murine model of disease (10). Sensing or regulatory pathways that connect heme synthesis with heme availability, hemoprotein abundance, or HssRS activation have not been identified.
S. aureus synthesizes δ-aminolevulinic acid (ALA), the first dedicated and universal precursor for protoheme synthesis, via the conversion of glutamyl-tRNA to glutamate-1-semialdehyde by glutamyl-tRNA reductase (GtrR) and subsequent production of ALA by glutamate-1-semialdehyde 2,1-aminomutase (11–13). Uroporphyrinogen is the precursor to heme, siroheme, and other tetrapyrroles, and the stepwise transformation of ALA to uroporphyrinogen comprises the core of the synthesis pathway. The pathway from uroporphyrinogen to heme was historically considered to be universally conserved for all organisms. However, the field’s understanding of bacterial heme synthesis has undergone a revolution as recent studies uncovered diversity in bacterial strategies to convert uroporphyrinogen to heme (reviewed in reference 14). Gram-positive bacteria proceed through a coproporphyrin-dependent branch (5, 6, 15) that is unique from the classic protoporphyrin-dependent branch in humans and many Gram-negative model organisms.
In this work, we identify GtrR abundance as a critical regulator of S. aureus heme synthesis. GtrR is posttranscriptionally maintained at low abundance in heme-proficient cells by the membrane protein HemX, but levels increase when S. aureus is deprived of heme. Without HemX regulation, GtrR abundance increases, which results in the concomitant increase in flux through the heme synthesis pathway and accumulation of heme. This excess heme synthesis activates HssRS and disrupts iron homeostasis. Together, this report reveals that S. aureus regulates heme synthesis by modulating GtrR abundance via intracellular heme levels and the widely conserved HemX.
RESULTS
GtrR abundance increases specifically in response to heme deficiency.
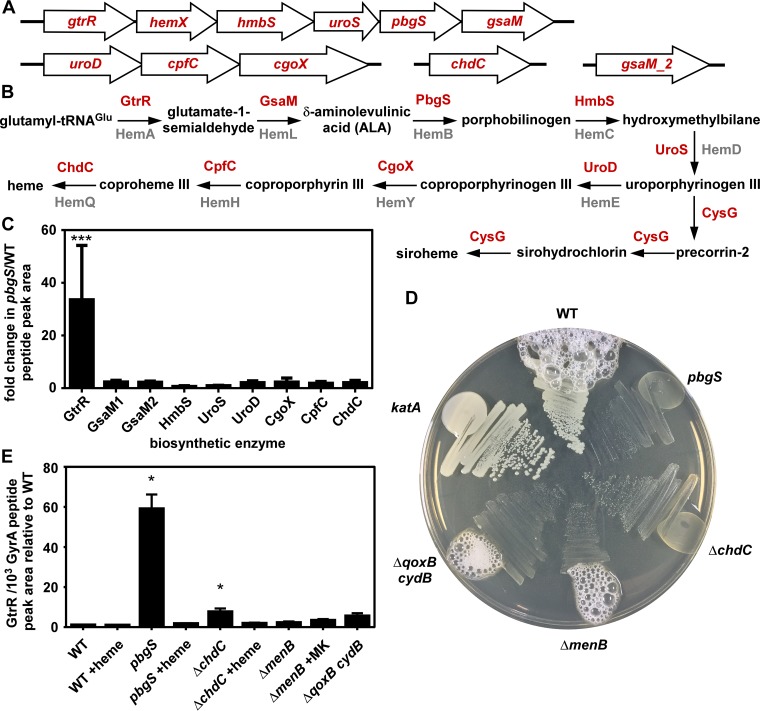
To identify key steps in the regulation of heme synthesis (Fig. 1A and B), we measured the abundance of each biosynthetic enzyme by liquid chromatography-multiple reaction monitoring-tandem mass spectrometry (LC-MRM-MS/MS). This technique allows for quantification with high resolution of even very-low-abundance cellular proteins (16). We hypothesized that comparing the S. aureus wild type (WT) to a strain incapable of synthesizing heme (pbgS mutant) (Fig. 1A and B) would allow the identification of specific steps in heme synthesis that respond to cellular heme content, directly or indirectly. Abundance of GtrR is approximately 30-fold higher in the pbgS mutant relative to the WT, while the abundances of all other biosynthetic enzymes are nearly unchanged (Fig. 1C). In WT cells, GtrR abundance is low relative to other heme synthesis enzymes (see Fig. S1 in the supplemental material). The pbgS mutant is a heme auxotroph and therefore adopts the respiration-deficient small-colony variant (SCV) phenotype. SCVs arise as the result of inactivation of respiration via inactivation of heme synthesis, the terminal oxidases, or the electron carrier menaquinone, and SCVs have a dramatically different physiology than respiration-proficient cells (17). Therefore, we sought to determine whether the increase in GtrR abundance in the pbgS mutant was the result of heme deficiency or a general defect in respiration. To confirm that the menaquinone auxotroph SCV ΔmenB strain and the ΔqoxB cydB strain lacking both terminal cytochrome oxidases synthesize heme despite being unable to respire, each strain was streaked onto agar and assessed for catalase activity (Fig. 1D). Activity of the heme-dependent catalase KatA leads to the production of oxygen bubbles when hydrogen peroxide is added. The ΔmenB and ΔqoxB cydB mutants produce bubbles, demonstrating that these SCVs synthesize heme and are not heme auxotrophs. We measured GtrR abundance in a variety of SCV strains by LC-MRM-MS/MS. GtrR abundance increases relative to the WT only in pbgS and ΔchdC strains (Fig. 1B and E), which are heme auxotroph SCVs (Fig. 1D). When chemically complemented with heme, GtrR abundance returned to WT levels for both strains. GtrR levels do not increase in the ΔmenB or ΔqoxB cydB strain. Together these data demonstrate that the abundance of GtrR is low in heme-proficient cells but increases specifically in response to heme deficiency.
FIG 1 .
Heme deficiency increases GtrR abundance. (A) The genes encoding heme biosynthesis enzymes are located at four chromosomal loci. (B) An overview of the S. aureus heme and siroheme biosynthetic pathway. In red are the updated enzyme names set forth by Dailey and colleagues (14), which correspond to the previously used gene locus names in gray. (C) The abundance of each biosynthetic enzyme was measured by LC-MRM-MS/MS and quantified by integrated chromatogram peak areas. Graphed is the ratio of each enzyme’s abundance in a strain lacking pbgS relative to WT S. aureus; the data are the average from a single experiment performed in biological triplicate with standard deviation shown. Statistical significance was determined using a one-way analysis of variance (ANOVA) with Dunnett’s correction for multiple comparisons, using a reference value of 1.0. ***, P < 0.001. (D) The S. aureus strains listed were streaked onto rich agar medium plates, and after growth, hydrogen peroxide was added at the perimeter of each streak. (E) The abundance of GtrR was measured by LC-MRM-MS/MS in S. aureus strains treated with vehicle, heme, or menaquinone (MK). The data are the average from a single experiment performed in biological triplicate with standard deviation shown. Statistical significance was determined using a one-way ANOVA with Dunnett’s correction for multiple comparisons, comparing GtrR abundance for each condition relative to the WT. *, P < 0.05.
GtrR abundance is uniquely low among heme synthesis enzymes. Shown is the abundance of each heme synthesis enzyme as measured by LC-MRM-MS/MS in WT cells. The data are the average from a single experiment performed in biological triplicate with standard deviation shown. Download FIG S1, TIF file, 0.9 MB (914.5KB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
HemX controls GtrR abundance in heme-proficient cells to regulate heme synthesis.
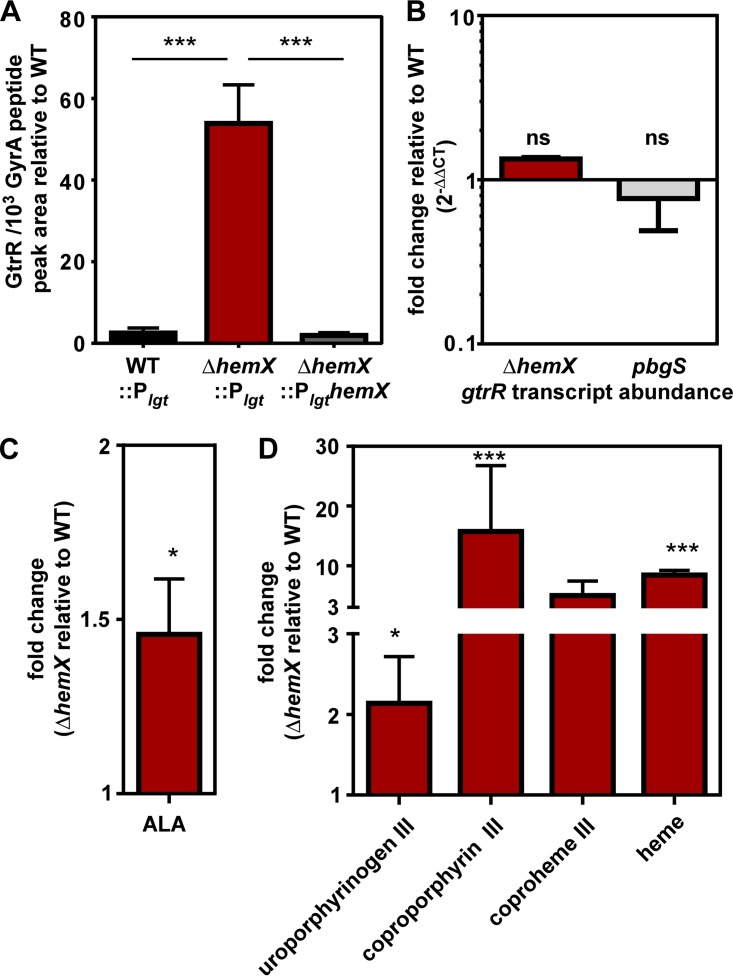
Among both Gram-negative and Gram-positive bacteria, regulation of GtrR abundance is a common feature of heme synthesis regulation pathways (18–20). In the model organism Bacillus subtilis, which is also a member of the Firmicutes phylum, GtrR abundance is impacted by the membrane protein HemX through an unknown mechanism (18, 21). While S. aureus is in the same Bacillales order as B. subtilis, S. aureus heme homeostasis is distinct because of its access to host heme and its resistance to heme toxicity mediated by HssRS. Both B. subtilis and S. aureus carry an operon comprised of gtrR-hemX-hmbS-uroS-pgbS-gsaM (formerly hemAXCBDL) (22, 23). We therefore hypothesized that in S. aureus, HemX also impacts GtrR abundance in heme-proficient cells. We created an in-frame unmarked deletion of hemX and integrated either pJC1111 Plgt or PlgthemX at a neutral site in the chromosome (24). GtrR abundance was measured by LC-MRM-MS/MS and is increased in the ΔhemX::Plgt strain relative to WT::Plgt (Fig. 2A). The phenotype can be complemented when hemX is provided in cis, showing that it is the result of deletion of hemX and not other effects of disrupting the operon. These data are consistent with the hypothesis that HemX regulates GtrR abundance in heme-proficient cells (18, 21).
FIG 2 .
HemX regulates heme synthesis by maintaining low levels of GtrR in heme-proficient cells. (A) The abundance of GtrR was measured by LC-MRM-MS/MS in multiple S. aureus strains. The data are the average from a single experiment performed in biological triplicate with standard deviation shown. Statistical significance was determined using a one-way ANOVA with Dunnett’s correction for multiple comparisons, comparing GtrR abundance for each strain relative to the ΔhemX::Plgt strain. ***, P < 0.001. (B) Steady-state transcript abundance of gtrR mRNA isolated from mid-exponential growth of S. aureus strains was measured by qRT-PCR and is graphed as fold change relative to the WT. Data are combined from two independent experiments in biological triplicate with standard deviation shown. “ns” indicates no significance by one-way ANOVA with Dunnett’s correction for multiple comparisons, comparing fold change of the pbgS and ΔhemX strains to the WT. (C) δ-Aminolevulinic acid (ALA) abundance was measured in S. aureus strains by a spectrophotometric quantification. Graphed is the fold change of ALA in the ΔhemX mutant relative to the WT, with data combined from two independent experiments with three biological replicates with standard error of the mean shown. (D) Uroporphyrinogen III (detected as uroporphyin III), coproporphyrin III, coproheme III, and heme were quantified by LC-qTOF-MS. Graphed is the fold change of metabolite abundance in the ΔhemX mutant relative to the WT, from a single experiment performed in biological triplicate with standard error of the mean shown. For panels C and D, statistical significance was determined with Student’s t test comparing the ΔhemX mutant to the WT before data transformation to fold change. *, P < 0.05; ***, P < 0.0001.
We next sought to determine whether the increase in GtrR at the protein level in both the pbgS and ΔhemX strains is the result of an increase in mRNA transcript abundance of gtrR. Therefore, the pbgS and ΔhemX strains were grown to the mid-exponential phase, and RNA was isolated, converted to cDNA, and quantified by quantitative PCR (qPCR) (Fig. 2B). The steady-state mRNA abundance of gtrR transcript is unchanged in the ΔhemX or pbgS strain relative to the WT, suggesting that the increase in GtrR abundance in these strains is not the result of a transcriptional change. Additionally, the increase in GtrR levels in the pbgS strain is not affected by the insertion of ermB to interrupt the pbgS gene, which is upstream of gsaM in the operon; there is no change in the transcript abundance of gsaM in the pbgS strain relative to the WT (see Fig. S2 in the supplemental material).
The pbgS allele is not polar on gsaM transcription. Steady-state transcript abundance of gsaM mRNA isolated from mid-exponential growth of S. aureus strains was measured by qRT-PCR and is graphed as fold change relative to the WT. Data are combined from two independent experiments in biological triplicate with standard deviation shown. “ns” indicates no significance by one-way ANOVA with Dunnett’s correction for multiple comparisons, comparing fold change of the pbgS mutant to the WT. Download FIG S2, TIF file, 0.3 MB (330.1KB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We hypothesized that the increase in GtrR observed in the ΔhemX strain would increase the amount of heme synthesized by increasing abundance of the heme precursors downstream of GtrR. As glutamate-1-semialdehyde is unstable and can convert to δ-aminolevulinic acid (ALA) in the absence of enzyme (25), we measured ALA abundance via a colorimetric method. ALA abundance increases approximately 50% in the ΔhemX strain relative to the WT (Fig. 2C). We subsequently sought to determine the impact of increased ALA availability on downstream heme intermediates and heme abundance. Total cellular porphryins were extracted from the WT and ΔhemX strains and analyzed by quantitative exact-mass liquid chromatography-quadrupole time of flight mass spectrometry (LC-qTOF-MS); total extracted ion chromatograms for porphyrins that were observed above the limits of detection are shown in Fig. S3 in the supplemental material, where a dramatic change in porphyrin levels is visible. Based on standard curves for individual porphyrins (including porphobilinogen, uroporphyrins I and III, coproporphyrins I and III, coproheme III, protoporphyrin IX, and heme b) and enumeration of colony-forming units, absolute quantifications were obtained and referenced per cell for each porphyrin molecule; data are presented in Fig. 2D in terms of fold change relative to the WT. As shown in Fig. 2D; the ΔhemX strain exhibits increased abundance of uroporphyrin III, coproporphyrin III, coproheme III, and heme b relative to the WT. Notably, because samples were prepared aerobically, the metabolite uroporphyrinogen III was detected as uroporphyrin III, in which its methylene bridge carbons have spontaneously oxidized in air; likewise, any coproporphyrinogen III that might have been present would be detected as the oxidation product, coproporphyrin III, which is also the product of the enzyme CgoX (Fig. 1). Hydroxymethylbilane spontaneously cyclizes to uroporphyrinogen I, which is decarboxylated by UroD to coproporphyrinogen I. The absence of uroporphyrin or copropophyrin I isomers indicates that hydroxymethylbilane did not accrue in the ΔhemX mutant. We hypothesize that, in the presence of excess GtrR, the initial step of the pathway may no longer be rate limiting. This may allow other subsequent steps in the pathway to become partly rate limiting, leading to the observed pattern of metabolite accumulation. Finally, the increase in heme abundance in the ΔhemX strain observed by LC-qTOF-MS is complemented when hemX is provided in cis from a neutral site in the chromosome, as measured by the pyridine hemochromagen method (see Fig. S4A in the supplemental material). Together, these data demonstrate that inactivation of hemX results in increased GtrR abundance, which increases abundances of both early- and late-pathway biosynthetic precursors and cellular heme. Therefore, dysregulation of GtrR alone is sufficient to disrupt heme homeostasis.
Representative extracted ion chromatograms of extracted porphyrins of (A) the S. aureus WT and (B) the ΔhemX mutant; each is quantified and shown in Fig. 2. Chromatograms for porphyrins above the limits of detection (250 nM) are shown. Download FIG S3, TIF file, 2.7 MB (2.7MB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Excess heme and resistance to heme toxicity in the ΔhemX mutant can be complemented. (A) Heme abundance was quantified using a pyridine hemochromagen assay in S. aureus strains. Data are combined from three independent experiments with four biological replicates with standard error of the mean shown. Statistical significance was determined by a one-way ANOVA with Tukey’s correction for multiple comparisons, comparing each strain against the others. **, P < 0.005. (B) Growth as measured by optical density (600 nm) was monitored over time for S. aureus strains in medium containing 0 or 10 µM heme. Strains were grown overnight to the stationary phase in medium alone before inoculation of the growth curve. The data are the average of the means from three independent experiments each in biological triplicate with standard error of the mean shown. (C) Growth as measured by optical density (600 nm) was monitored over time for S. aureus strains in medium containing chloramphenicol and 0 or 12.5 µM heme. Strains were grown overnight to stationary phase in medium alone before inoculation of the growth curve. The data are means from three biological replicates with standard error of the mean shown from a single experiment, representative of at least three independent experiments. Download FIG S4, TIF file, 6.8 MB (7MB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Excess endogenous heme synthesis in the ΔhemX mutant activates the heme stress response.
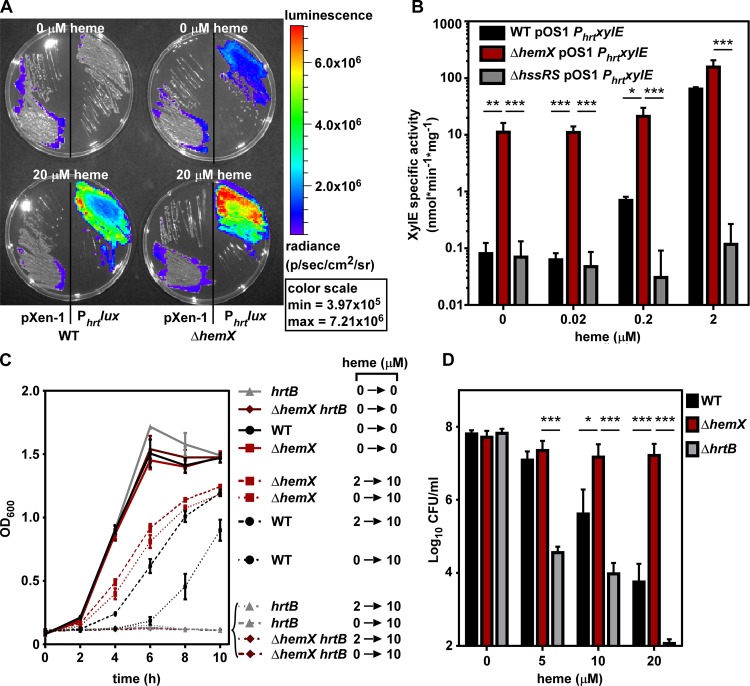
The unregulated GtrR abundance in the ΔhemX mutant results in greater cellular heme levels (Fig. 2D), which we hypothesized would activate the heme sensor system HssRS in the absence of exogenous heme, leading to transcriptional induction of the hrtAB efflux pump. The WT and ΔhemX mutant strains were transformed with plasmids containing the luminescence-producing operon luxABCDE cloned from Photorhabdus luminescens without a promoter (pXen-1) or controlled by the HssRS-regulated promoter Phrt. Phrt promoter activity, visualized by luminescent imaging, shows that HssRS is activated in the ΔhemX strain in the absence of exogenous heme, whereas HssRS is not activated in the WT (Fig. 3A). The Phrtlux activity in WT becomes apparent when 20 µM exogenous heme is added to the agar medium, and luminescence depends on the heme-responsive Phrt. To more quantitatively measure Phrt activity as a readout of HssRS activation by endogenous heme, we transformed WT, ΔhemX, and ΔhssRS strains with the pOS1 PhrtxylE plasmid. These strains report Phrt activity with the production of the XylE catechol oxidase enzyme, which can be quantified spectrophotometrically from cell lysate. Data in Fig. 3B demonstrate that in the absence of exogenous heme, Phrt is induced in the ΔhemX strain. Phrt activity does increase in the WT and ΔhemX strains in a dose-dependent manner as exogenous heme is added, but Phrt activity remains higher in the ΔhemX mutant than the WT at all tested heme concentrations. Additionally, XylE activity in this system is dependent on the HssRS two-component system. Taken together, these data suggest that excess endogenous heme synthesized in the ΔhemX strain is sufficient to activate the HssRS two-component system.
FIG 3 .
Excess heme synthesis in the ΔhemX mutant activates the heme stress response. (A) Bioluminescence was imaged on agar medium plates containing vehicle or heme onto which strains were streaked. All four plates were imaged simultaneously, and luminescence was converted to a heat map with the scale shown on the right. (B) XylE catechol oxidase activity was measured in S. aureus strains after growth in vehicle or increasing concentrations of heme. The data are the average from three independent experiments each in biological triplicate with standard deviation shown. Statistical significance was determined using a two-way ANOVA with Tukey’s correction for multiple comparisons, comparing log-transformed data for the ΔhemX pOS1 PhrtxylE strain at each heme concentration to that of each other strain. *, P < 0.01; **, P < 0.001; ***, P < 0.0001. (C) Growth as measured by OD600 was monitored over time for S. aureus strains in medium containing either vehicle or 10 µM heme. Prior to the measured growth, the strains were pregrown to the stationary phase in medium containing vehicle or 2 µM heme. The data are the average of the means from at least three independent experiments each in biological triplicate with standard error of the mean shown. (D) Viable bacteria from S. aureus strains were enumerated after incubation for 2 h in medium containing vehicle or increasing amounts of heme. The data are the average of the means from three independent experiments each in biological triplicate with standard error of the mean shown. The y axis is set to the limit of detection. Statistical significance was determined using a two-way ANOVA with Tukey’s correction for multiple comparisons, comparing log-transformed data for the WT and ΔhrtB strains to the ΔhemX mutant at each heme concentration. *, P < 0.01; ***, P < 0.0001.
We next hypothesized that the intermediate levels of HssRS activation in the ΔhemX mutant, in the absence of exogenous heme (Fig. 3A and B), would be sufficient to preadapt the ΔhemX mutant to heme toxicity. As the HssRS-HrtAB heme stress response provides resistance to heme toxicity, pretreatment with subtoxic concentrations of heme adapts S. aureus to subsequent growth in toxic concentrations of heme by activating HssRS and increasing the abundance of HrtAB (10). The WT grown in 10 µM heme without preadaptation has a severe growth defect evident by a 6-h lag time (Fig. 3C). When preadapted in 2 µM heme, the WT demonstrates a reduced lag time and greater overall growth, albeit at a lower rate and yield than when grown without heme. In contrast, the ΔhemX mutant grown in 10 µM heme with or without preadaptation exhibits increased growth compared to the WT. The enhanced growth of the ΔhemX mutant in 10 µM heme is dependent on the HrtAB efflux system, as the ΔhemX hrtB strain does not grow in 10 µM heme (Fig. 3C). Additionally, preadaptation of the ΔhemX strain in this assay can be complemented by providing hemX in the chromosome (Fig. S4B). Similarly, the ΔgtrR-hemX pOS1 PlgtgtrR strain is resistant to heme toxicity, but becomes sensitive again when hemX is introduced on the plasmid (Fig. S4C). This is further evidence that HemX control of GtrR is not transcriptional, as HemX exerts its effect independent of the native gtrR promoter and ribosome binding site in this assay. Further, the ΔhemX mutant is resistant to the bactericidal effects of acute heme toxicity, compared to a 4-log reduction in viable WT cells after 2 h in the presence of 20 µM heme (Fig. 3D). In sum, these data demonstrate that increased cellular heme in the ΔhemX mutant is sufficient to activate HssRS and cause expression of HrtAB, which leads to resistance to heme toxicity.
Excess heme synthesis disrupts iron homeostasis.
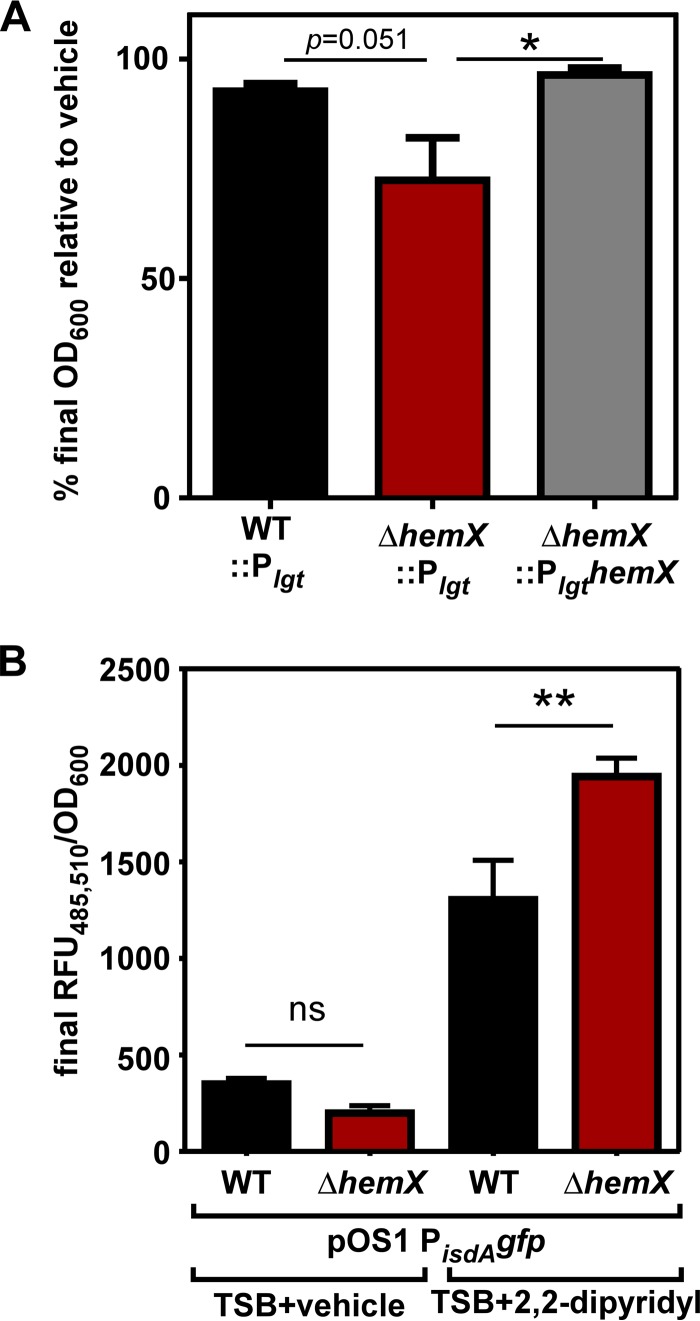
Considering that every molecule of heme contains an atom of iron, we hypothesized that unregulated heme synthesis in the ΔhemX mutant would consume high levels of iron and alter iron homeostasis. To test this hypothesis, growth in minimal medium containing the iron chelator EDDHA [ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid)] was compared to growth in minimal medium alone. As shown in Fig. 4A, the ΔhemX::Plgt strain demonstrates reduced total yield after growth for 24 h relative to WT::Plgt and the complemented ΔhemX::PlgthemX strain. To corroborate this finding, we assessed promoter activity using a PisdAgfp reporter plasmid. PisdA is controlled by the ferric uptake regulator (Fur) and is derepressed under iron-depleted conditions (26). Data in Fig. 4B show that after growth in rich medium with an alternative iron chelator, 2,2-dipyridyl, the ΔhemX pOS1 PisdAgfp strain has enhanced PisdA activity relative to WT pOS1 PisdAgfp. These data suggest excess heme synthesis depletes the cell of available iron.
FIG 4 .
Unregulated heme synthesis alters iron homeostasis. (A) Growth was measured in minimal medium containing vehicle or 1 µM iron chelator EDDHA. Graphed is the final growth as measured by the OD600 for each S. aureus strain in medium containing EDDHA relative to vehicle. The data are the average of the means from five independent experiments each in at least biological triplicate with standard error of the mean shown. Statistical significance was determined using a one-way ANOVA with Dunnett’s correction for multiple comparisons, comparing the ΔhemX::Plgt strain to each other strain. *, P < 0.05. (B) The activity of the iron limitation-responsive promoter PisdA was measured by recording fluorescence intensity over time in rich medium containing vehicle or the iron chelator 2,2-dipyridyl. The data are the average of the means from three independent experiments each in biological triplicate with standard error of the mean shown. Statistical significance was determined using a one-way ANOVA with Sidak’s correction for multiple comparisons, comparing data for the WT and ΔhemX mutant under each condition. **, P < 0.01; ns, not significant.
Inactivation of HemX reduces GtrR abundance in heme deficiency.
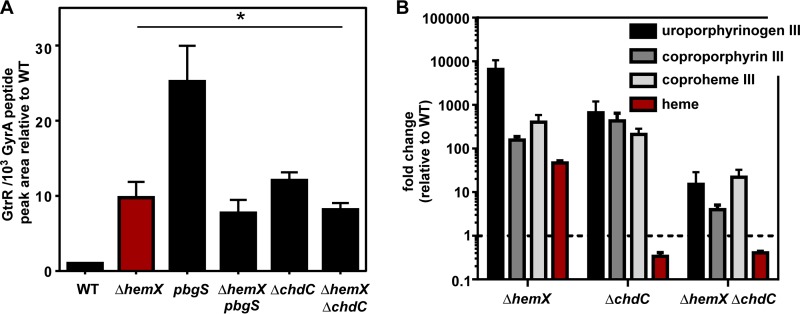
Based on the observations that HemX and cellular heme both impact GtrR abundance, we hypothesized that measuring GtrR abundance in a strain lacking hemX and unable to synthesize heme would uncover the nature of the relationship between HemX, heme, and GtrR. Surprisingly, GtrR abundance in the ΔhemX pbgS strain is unchanged from that in the ΔhemX strain and lower than that in the pbgS strain (Fig. 5A), and this effect is not the result of a change in gtrR transcription in the ΔhemX pbgS strain relative to the pbgS strain (see Fig. S5 in the supplemental material). Similarly, the ΔhemX ΔchdC mutant has lower levels of GtrR than the ΔchdC mutant (Fig. 5A). To corroborate these findings, we measured total cellular porphyrins by LC-qTOF-MS as in Fig. 2; total extracted ion chromatograms are shown in Fig. S6 in the supplemental material. Consistent with the abundance of GtrR, porphyrin intermediates are drastically increased in the ΔhemX mutant relative to the WT. The ΔchdC mutant demonstrates intermediate buildup through coproheme because of elevated GtrR levels but is unable to convert coproheme to heme (Fig. 1A). As expected, based on the reduced GtrR abundance shown in Fig. 5A, the porphyin intermediates are at lower levels in the ΔhemX ΔchdC mutant relative to the ΔhemX or ΔchdC mutant. These data suggest that heme and HemX do not independently and directly repress GtrR levels, because if so, removal of both would likely have an additive effect on GtrR abundance. Instead the relationship between HemX, heme synthesis, and GtrR levels is still unclear. However, the data are consistent with a model whereby the increase in GtrR levels in heme-deficient strains is dependent on the activity of HemX.
FIG 5 .
Inactivation of HemX reduces GtrR abundance in heme-deficient strains. (A) The abundance of GtrR was measured by LC-MRM-MS/MS in multiple S. aureus strains. The data are the average from a single experiment performed in biological triplicate with standard deviation shown. Statistical significance was determined using a one-way ANOVA with Dunnett’s correction for multiple comparisons, comparing GtrR abundance for each strain relative to WT. *, P < 0.05. (B) Uroporphyrinogen III (detected as uroporphyin III), coproporphyrin III, coproheme III, and heme were quantified by LC-qTOF-MS. Graphed is the fold change of metabolite abundance in each mutant relative to the WT from a single experiment performed in biological triplicate with standard error of the mean shown.
gtrR transcription is unchanged in the ΔhemX and ΔhemX pbgS strains compared to the pbgS strain. Steady-state transcript abundance of gtrR mRNA isolated from mid-exponential growth of S. aureus strains was measured by qRT-PCR and is graphed as fold change relative to the WT. Data are combined from two independent experiments in biological triplicate with standard deviation shown. “ns” indicates no significance by one-way ANOVA with Dunnett’s correction for multiple comparisons, comparing fold change of the ΔhemX mutant and ΔhemX pbgS strains to the pbgS strain. Download FIG S5, TIF file, 0.7 MB (780.1KB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Representative extracted ion chromatograms of extracted porphyrins of (A) the S. aureus WT and (B) the ΔhemX, (C) ΔchdC, and (D) ΔhemX ΔchdC strains. Each is quantified and shown in Fig. 5. Chromatograms for porphyrins above the limits of detection (250 nM) are shown. Download FIG S6, TIF file, 3.1 MB (3.2MB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Siroheme synthesis impacts GtrR levels under conditions of nitrite reduction.
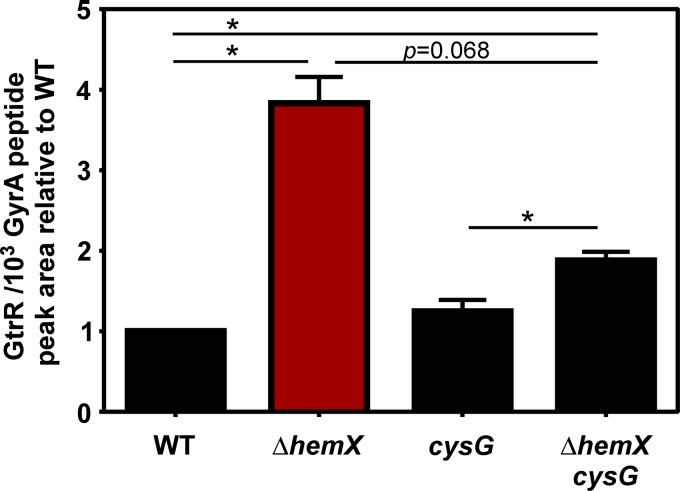
The increase in GtrR levels identified in the ΔhemX mutant likely impacts siroheme synthesis, as the cofactor siroheme is synthesized in S. aureus from the shared uroporphyrinogen III intermediate (14) (Fig. 1A). We therefore hypothesized that siroheme levels might also affect GtrR abundance. In the experiments presented thus far, in which S. aureus is grown aerobically, siroheme has likely not been synthesized. The siroheme synthesis and siroheme-dependent nitrite reductase genes are transcribed primarily under anaerobic conditions (27). To therefore test the role of siroheme, we first identified conditions under which siroheme synthesis via CysG and siroheme-dependent nitrite reduction by the NirD nitrite reductase were important for growth. As demonstrated in Fig. S7 in the supplemental material, when grown anaerobically, the growth of WT is enhanced when the terminal electron acceptor nitrate is provided. Mutants lacking cysG or nirD cannot grow to WT levels when nitrate is provided, suggesting that WT cells synthesize siroheme and utilize it in NirD. It is thought that under these conditions, the anaerobic nitrate reductase will reduce nitrate to nitrite, followed by NirD-dependent reduction of nitrite. Deletion of hemX does not overtly impact nitrite reduction, as the ΔhemX strain grows well in nitrate, the ΔhemX nirD strain phenocopies the nirD strain, and the ΔhemX cysG strain phenocopies the cysG strain. Therefore, GtrR abundance was measured by LC-MRM-MS/MS after growth in tryptic soy broth (TSB) containing nitrate (Fig. 6). The strain lacking cysG, which can make heme but not siroheme, does not demonstrate elevated GtrR levels. However, the ΔhemX cysG strain has reduced levels compared to the ΔhemX strain, suggesting that siroheme synthesis could impact GtrR regulation.
FIG 6 .
Siroheme synthesis impacts GtrR levels under conditions of nitrite utilization. GtrR was measured by LC-MRM-MS/MS in multiple S. aureus strains grown anaerobically with NO3 provided as the terminal electron acceptor. The data are the average from a single experiment performed in biological triplicate with standard deviation shown. Statistical significance was determined using a one-way ANOVA with Dunnett’s correction for multiple comparisons, comparing GtrR abundance for each strain relative to the WT. *, P < 0.05.
Nitrite reductase and the cofactor siroheme are required for full growth with nitrate as an alternative terminal electron acceptor. Shown is growth anaerobically as measured by optical density (600 nm) monitored over time for S. aureus strains in medium containing 0 or 40 mM NO3. Strains were grown overnight to the stationary phase in medium alone before inoculation of the growth curve. The data are means from three independent experiments with at least three biological replicates. Download FIG S7, TIF file, 4.1 MB (4.2MB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
HemX co-occurs with capacity for heme biosynthesis, and the corresponding genes often colocalize on the chromosome.
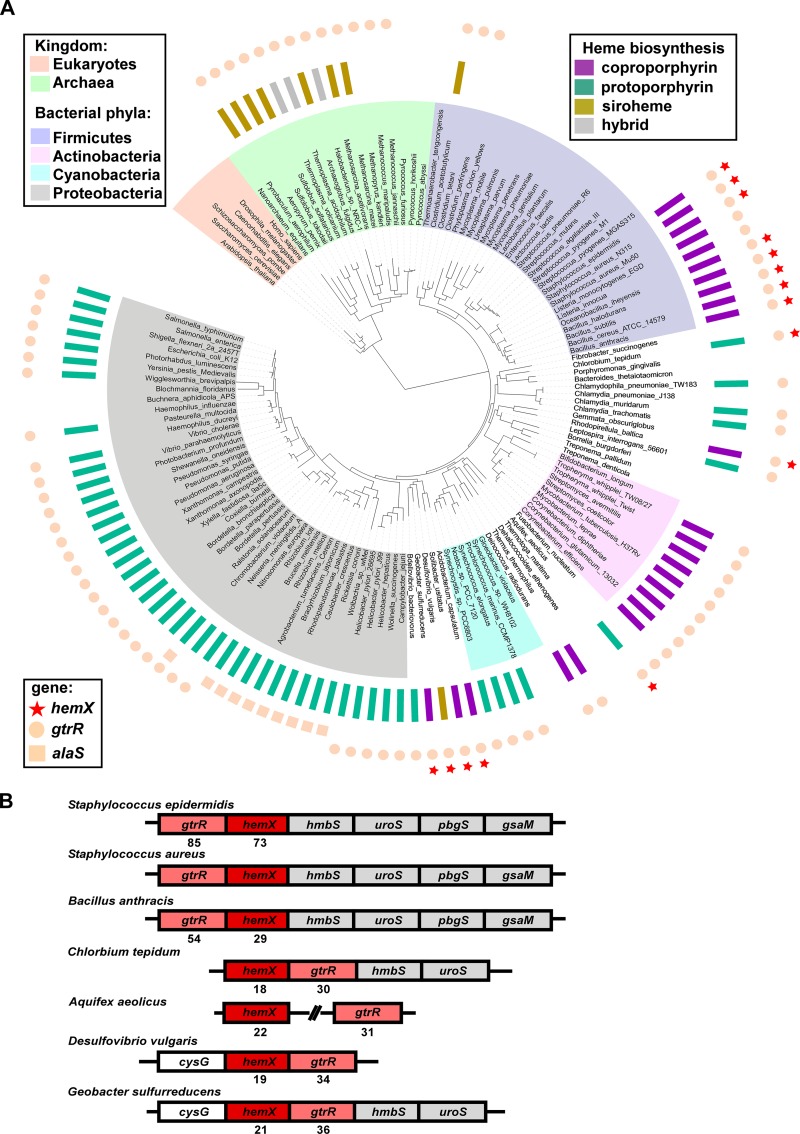
We hypothesized that B. subtilis HemX and S. aureus HemX might represent only a subset of HemX homologues that exist across bacterial phyla and function to regulate heme synthesis. Diverse genomes from 978 organisms (924 bacterial and 54 archaeal) were analyzed for the presence of hemX. Of these, 113 encode HemX; representative members of this analysis are shown in Fig. 7A. These newly identified homologues expand past the Bacillales order, of which representative HemX homologues were previously identified and shown to share function (13). HemX appears to represent an ancient protein family, as it is present in some of the evolutionarily oldest taxa, including Firmicutes, Aquificae, and Planctomycetes. The distribution of hemX strongly correlates with the capacity for de novo heme synthesis, as hemX never occurs in a genome without gtrR, and hemX never occurs without the capacity for de novo heme synthesis. This correlation holds across the microbial kingdom, where hemX never occurs in taxa lacking heme biosynthesis genes (within any representatives with sequenced genomes now available): e.g., Thermotogae, Fusobacteria, and Mollicutes. Additionally, the distribution of hemX among the members of the Firmicutes phylum supports this correlation; hemX is present largely in Bacillales but does not occur in Lactobacillales and only rarely in Clostridia (in 2 out 91 genomes analyzed), which is consistent with the frequent capacity for heme synthesis in Bacillales relative to Lactobacillales and Clostridia. Notably, the genomic co-occurrence of hemX and gtrR holds true in organisms that synthesize heme via any of the 3 heme biosynthetic pathways identified to date: the coproporphyrin-dependent, siroheme-dependent, or classic protoporphyrin-dependent route (Fig. 7A).
FIG 7 .
hemX is conserved across bacterial phyla and invariably co-occurs (A) and colocalizes (B) with gtrR. (A) The occurrence of hemX (stars), gtrR (circles), and alaS (squares) homologues (outermost rings) was mapped onto the tree of life (53). The pathway by which protoheme is synthesized in each of the analyzed organisms is presented in the middle ring as follows (adapted from reference 14): the classic protoporphyrin-dependent pathway (teal), coproporphyrin-dependent path (purple), or siroheme-dependent path (gold). Gray rectangles mark the organisms that contain unusual combinations of genes normally involved in different pathways for protoheme synthesis (hybrid paths [14]). The absence of a rectangle in the middle ring indicates the absence of any known route for protoheme synthesis in an organism. Likewise, the absence of a circle (gtrR) or square (alaS) in the outermost ring shows the inability of an organism to produce tetrapyrroles of any kind. Note that hemX does not occur in such organisms. (B) The immediate genomic neighborhood of the hemX gene in seven representative genomes, with ClustalW alignment scores for HemX and GtrR for each organism relative to S. aureus.
Interestingly, HemX is more commonly found in organisms that encode the ability to synthesize both heme and siroheme (see Fig. S8A in the supplemental material) than in organisms that synthesize heme and not siroheme. This suggests that HemX likely impacts siroheme synthesis as a consequence of affecting GtrR abundance by increasing abundance of uroporphyrinogen III, the final shared biosynthetic intermediate. The co-occurrence of hemX, gtrR, and cysG is also consistent with our findings in Fig. 6 that siroheme synthesis impacts GtrR abundance.
hemX co-occurs with heme synthesis and shares conserved secondary structure and residues. (A) The genomes shown in Fig. 7 were analyzed for the capacity to synthesize heme or both heme and siroheme, as well as the presence of hemX. (B) Alignment of HemX for each of the seven representative organisms, with predicted transmembrane domains in yellow, conserved residues in red, and moderately conserved residues marked with “:” to show conservation among strongly similar amino acids or “.” to show conservation among weakly similar amino acids. Download FIG S8, TIF file, 45.3 MB (46.4MB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Next we examined the genomic context of hemX homologues across 113 organisms carrying hemX, as genes associated with the same pathway or area of metabolism tend to colocalize in prokaryotic genomes (28). In 106 (94%) out of these 113 genomes, the hemX and gtrR genes are adjacent and likely cotranscribed, which is a very strong indicator of their functional association. Seven representatives of these organisms are shown in Fig. 7B, highlighting the common genomic context of hemX, gtrR, and other genes involved in uroporphyrinogen synthesis (hmbS, uroS, pbgS, and gsaM) and siroheme synthesis (cysG).
HemX homologues share predicted membrane topology and residues.
Comparative genome analysis identified several contextual characteristics of HemX homologues. We therefore investigated the sequences of representative HemX homologs. A multiple-sequence alignment revealed relatively low overall identity among HemX sequences (Fig. 7B); however, the alignment presented in Fig. S8B shows that each HemX homologue shares the same predicted eight-transmembrane-domain topology with N- and C-termini predicted to be extracytoplasmic. Additionally, these divergent homologues share four conserved residues, all in predicted transmembrane domains. Taken together with comparative genome analysis, identification of HemX across bacteria uncovered a strong correlation between gtrR, hemX, and de novo heme synthesis, suggesting that HemX control of GtrR to modulate heme synthesis is a common regulatory strategy among bacteria.
DISCUSSION
In this report, we identify GtrR abundance as a critical regulator of S. aureus heme biosynthesis. GtrR catalyzes the initial step in the heme biosynthetic pathway and is maintained at low levels in WT cells proficient for heme biosynthesis, but specifically increases in response to heme deficiency (Fig. 1B). In this study, we used heme auxotrophs to stimulate production of GtrR (Fig. 1D), but we would predict that in particular niches during infection, an increase in heme synthesis is required and GtrR abundance increases to accommodate this need. Host-imposed nitrosative stress, oxidative stress, and hypoxia, for example, all cause S. aureus to increase expression of heme-dependent cytochrome oxidases, catalase, and nitric oxide synthase (29–31).
The drastic difference in GtrR enrichment between the heme auxotroph pbgS and ΔchdC mutants (Fig. 1D and 5A), with deficits in genes at the beginning and end of the heme biosynthetic pathway, respectively, suggests that GtrR abundance during heme deficiency could be impacted by mechanisms other than heme availability. This is an interesting observation in light of the comparative genome analysis of hemX, which suggests that HemX could impact siroheme synthesis as well as heme synthesis. It is possible that GtrR abundance is impacted by differences in heme or siroheme abundance as well as abundance in earlier precursor levels, explaining the difference in GtrR levels in the pbgS strain relative to the ΔchdC strain.
In addition to the impact of heme deficiency on GtrR, we also identify HemX as a key regulator of GtrR in heme-proficient cells (Fig. 2A). Our broad genomic analysis has identified HemX homologues across bacterial phyla, suggesting that HemX control of heme synthesis via GtrR is a conserved strategy (Fig. 7; Fig. S8). This finding is consistent with the model set forth by Hederstedt and colleagues (18, 21); in both B. subtilis and now S. aureus, it appears that HemX regulates GtrR abundance posttranscriptionally (Fig. 2B) through an as-yet-undefined mechanism. B. subtilis HemX is sufficient to affect GtrR abundance when both are expressed ectopically in Escherichia coli (18); however, the contribution of heme or conserved E. coli proteins in this system is unclear, making it challenging to conclude if HemX directly interacts with GtrR. Together, our findings are consistent with a model whereby GtrR is regulated by heme abundance and HemX through a multiprotein mechanism. Our finding that GtrR abundance is reduced in the ΔhemX pbgS strain relative to the pbgS strain and not increased supports this model: heme and HemX both do not directly repress GtrR levels (Fig. 5A). In Salmonella strains, which do not encode HemX, GtrR is regulated by N-terminal proteolysis by ClpAP and Lon proteases to keep levels low (32). Additionally, Salmonella GtrR binds excess heme through a Cys-170 residue (33). Mutagenesis of the N-terminus degradation sequence or heme binding cysteine disrupts regulation, and it has been proposed that heme-bound GtrR but not apo-GtrR is a substrate for the proteases (32, 34). The mechanism by which Salmonella GtrR is regulated by proteases via its N terminus and heme binding is not fully understood, and these regulatory amino acids are not conserved in S. aureus GtrR. Likewise, further work is needed to dissect the unique regulatory effects of heme and HemX on GtrR levels and the potential involvement of proteolysis in this process in S. aureus.
In the absence of HemX, S. aureus synthesizes excess heme. The increase in heme synthesis disrupts intracellular iron homeostasis (Fig. 4), which could additionally disrupt the expression of the staphyloferrin B siderophore biosynthetic genes, which were recently identified to be under the control of a transcriptional activator that is inactive when bound to heme (35). This altered iron homeostasis would likely reduce the function of many important Fe-S cluster-containing enzymes critical to staphylococcal physiology. Additionally, excess heme synthesized in the ΔhemX mutant activates the heme stress response (Fig. 3). While activation of the HssRS two-component system was first recognized as the result of exogenous heme, our findings add to a growing body of literature that supports a model whereby endogenous heme and exogenous heme both contribute to HssRS activation and heme toxicity. We have previously identified small molecule activators of heme synthesis that increase intracellular heme and activate HssRS (36–38), adding to our genetic evidence presented in this work that endogenous heme activates HssRS. Here, the increase in endogenous heme in the ΔhemX mutant is not toxic because of the HssRS-HrtAB detoxification response. Rather, endogenous heme activation of HssRS provides resistance to heme toxicity through preadaptation and expression of hrtAB (Fig. 3C and D). The impact of inactivation of hemX on the fitness of pathogens that do not encode the HssRS-HrtAB system would offer insight into whether dysregulated heme synthesis is sufficient to induce heme toxicity from within.
This study found that regulation of GtrR abundance is sufficient to regulate total heme synthesis (Fig. 2), consistent with multiple reports that ALA formation is a critical rate-limiting step in heme synthesis (14). Indeed, regulation of ALA synthesis via control of either GtrR or ALAS has emerged as a theme across kingdoms. In metazoans, two ALAS isoforms exist and are impacted by heme (reviewed in reference 39). In the plant model organism Arabidopsis thaliana, ALA synthesis is regulated by degradation of GluTR via Clp proteolysis from the N terminus as well as stabilization and activation of a regulatory binding protein (40, 41). In Gram-negative model organisms, as mentioned above, GtrR abundance is regulated by heme and proteolysis (32). Our findings extend this paradigm further into the Firmicutes phylum of bacteria.
The specific mechanism by which HemX impacts GtrR abundance remains undefined. HemX is annotated as a member of the cytochrome c assembly protein family (Pfam accession no. PF01578), suggesting that it may be involved in heme binding and trafficking at the membrane. However, S. aureus does not encode c-type cytochromes. The capacity of HemX to bind heme has not been experimentally validated, but excess heme does accumulate in the membrane (42), which is suggestive of a potential role for membrane-localized heme reservoirs or chaperones. The limited regions of HemX predicted to be cytoplasmic suggest that protein-protein interactions likely occur between other membrane proteins, but no HemX-interacting partners have been identified to date. Additionally, GtrR residues that impact regulation by either heme or HemX are unknown, but would offer information as to the regulatory steps between heme, HemX, and GtrR, which appear to be complex. Although heme-dependent inhibition of S. aureus heme synthesis was first proposed in 1962 (43), the impact of HemX and heme on GtrR abundance continues to warrant further investigation.
MATERIALS AND METHODS
Bacterial strains and reagents.
Bacterial strains (Table 1), plasmids (see Table S1 in the supplemental material), and primers (Table S1) are listed in the specified table. S. aureus strains were grown routinely on tryptic soy agar (TSA) or broth (TSB) supplemented with 10 µg/ml chloramphenicol or 10 µg/ml erythromycin when necessary. When used, heme (hemin chloride) was used at the concentrations noted. Heme was prepared fresh at 10 mM in 0.1 M NaOH; for experiments in which heme was used, an equal volume of 0.1 M NaOH was used for all conditions. E. coli strains were grown on lysogeny broth (LB) or LB agar (LBA), supplemented with 50 µg/ml carbenicillin when necessary. For growth in liquid medium, an Innova44 incubator shaking at 180 rpm was used. For standard cultures of 4 to 5 ml, 15-ml round-bottomed polypropylene tubes with aeration lids were used, at a 45° angle in the incubator. For cloning and mutagenesis in plasmids, all constructs were confirmed by sequencing (GeneWiz). Unless noted otherwise, all chemicals are from Sigma. All molecular biology reagents were from New England Biolabs (NEB) and used according to the manufacturer’s instructions, unless otherwise noted. Phusion 2X Hi-fidelity master mix was used for all PCRs for cloning. As necessary, plasmids were transformed by electroporation from E. coli into the S. aureus cloning intermediate strain RN4220 before isolation and subsequent electroporation into the final S. aureus strains.
TABLE 1 .
S. aureus strains used in this study
| Strain | Genotype | Description | Source or reference |
|---|---|---|---|
| Newman | WT | Wild-type, methicillin-sensitive clinical isolate | 57 |
| Newman | pbgS | pbgS (NWMN_1562) gene interrupted with erythromycin resistance gene ermB by homologous recombination, transduced into Newman | 3 |
| Newman | ΔchdC | In-frame unmarked deletion of chdC (NWMN_0550) generated by allelic exchange | 58 |
| Newman | ΔhemX ΔchdC | In-frame unmarked deletion of chdC (NWMN_0550) generated by allelic exchange in ΔhemX strain | This work |
| Newman | ΔmenB | In-frame unmarked deletion of menB generated by allelic exchange | 59 |
| Newman | ΔqoxB cydB | In-frame unmarked deletion of qoxB and cydB::Tn | 3 |
| JE2 | katA | katA::Tn (NE1366) | BEI (46) |
| Newman | katA | katA::Tn (NE1366), transduced into Newman | This work |
| Newman | ΔhemX | In-frame unmarked deletion of hemX (NWMN_1565) | This work |
| RN9011 | RN4220 carrying pRN7023 integrase plasmid | 24 | |
| RN9011 | attC::Plgt | pJC1111 Plgt integrated into chromosome at attC locus | This work |
| RN9011 | attC::PlgthemX | pJC1111 PlgthemX integrated into chromosome at attC locus | This work |
| Newman | ΔhemX attC::Plgt | pJC1111 Plgt integrated into chromosome at attC locus | This work |
| Newman | ΔhemX attC::PlgthemX | pJC1111 PlgthemX integrated into chromosome at attC locus | This work |
| Newman | attC::Plgt | pJC1111 Plgt integrated into chromosome at attC locus | This work |
| Newman | ΔhemX pbgS | In-frame unmarked deletion of hemX (NWMN_1565) in pbgS strain | This work |
| Newman | ΔhssRS | In-frame unmarked deletion of hssRS generated by allelic exchange | 60 |
| Newman | ΔhrtB | In-frame unmarked deletion of hrtB generated by allelic exchange | 61 |
| Newman | hrtB | hrtB::Tn(PhiNE01762) | 10 |
| Newman | ΔhemX hrtB | hrtB::Tn(PhiNE01762) allele transduced to ΔhemX strain | This work |
| JE2 | cysG | cysG::Tn (NE1931; SAUSA300_2553::Tn) | BEI (46) |
| Newman | cysG | cysG::Tn (NE1931) transduced into Newman | This work |
| Newman | ΔhemX cysG | cysG::Tn (NE1931) transduced into Newman ΔhemX | This work |
| JE2 | nirD | nirD::Tn (NE1279) | BEI (46) |
| Newman | nirD | nirD::Tn (NE1279) transduced into Newman | This work |
| Newman | ΔhemX nirD | nirD::Tn (NE1279) transduced into Newman ΔhemX | This work |
| Newman | ΔgtrR-hemX | In-frame unmarked deletion of gtrR and hemX (NWMN_1565-1566) generated by single allelic exchange | This work |
| RN4220 | Restriction-deficient cloning intermediate strain | 62 |
Plasmids and primers used in this study. Download TABLE S1, DOCX file, 0.1 MB (14.1KB, docx) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(i) Deletion of genes by allelic exchange.
Deletion of hemX and chdC was performed by allelic exchange as described in reference 44 with some modifications. The pKOR1 plasmids containing ~1-kb homologous regions flanking upstream and downstream of the gene to be deleted were prepared using NEB Hi-Fi assembly according to manufacturer’s suggestions. The pKOR1 backbone was amplified by PCR using JC291/292, which produces a linear product not including the attB recombination sites. The ~1-kb flanking regions were amplified from the S. aureus Newman genomic DNA. Deletions were confirmed by PCR using isolated genomic DNA and complemented by providing the gene in cis or trans. Additional details are found in Text S1 in the supplemental material.
Supplemental materials and methods and references. Download TEXT S1, DOCX file, 0.1 MB (20.9KB, docx) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(ii) hemX chromosomal integration.
Chromosomal complementation was performed by cloning Plgt or PlgthemX into pJC1111. Plgt was PCR amplified from pOS1 Plgt using JC158/229 and subsequently cloned into the multiple cloning site of pJC1111 after restriction digestion with SalI and BamHI. hemX was cloned into pOS1 Plgt by amplifying hemX flanked by NdeI and BamHI sites from S. aureus Newman genomic DNA using primers JC157/155 and ligated (T4 ligase) into the multiple cloning site of pOS1 Plgt after restriction digestion with NdeI and BamHI. PlgthemX was amplified from pOS1 PlgthemX using JC158/155 and subsequently cloned into the SalI and BamHI sites of pJC1111 after restriction digestion with SalI and BamHI. pJC1111 Plgt and pJC1111 PlgthemX were integrated into the chromosome of strain RN9011 as described previously (24) and then transduced into the S. aureus Newman WT or ΔhemX mutant as noted. Transductions of pJC111 loci were performed with φ85 as described in reference 45, with some modifications: after incubation of donor phage with recipient strains and washing with sodium citrate, cells were allowed to recover for 4 h in TSB with 40 mM sodium citrate at 37°C with shaking and plated to TSA containing 0.15 mM cadmium chloride.
(iii) Transduction of transposon library alleles.
For transduction of transposon library alleles, the katA::Tn (NE1366), cysG::Tn (NE1931), nirD::Tn (NE1279), and hrtB::Tn (PhiNE01762) transposon alleles were transduced to the S. aureus Newman and ΔhemX strains as listed in Table 1 as described previously (45) using bacteriophage φ85; alleles were confirmed by an inverse-PCR method and Sanger sequencing (46).
Catalase activity.
To assess catalase activity, strains were grown for 16 h in TSB, and then 50 µl of each culture was spotted onto a TSA plate and streaked for isolation. After 24 h of growth at 37°C, 50 µl of 30% H2O2 was added to each strain and immediately imaged.
LC-MRM-MS/MS.
Strains were streaked onto TSA and grown for 24 h at 37°C. Cultures were started from single colonies in 5 ml of RPMI plus 1% Casamino Acids and grown at 37°C for 15 h. Overnight cultures were subcultured 1:100 into RPMI plus 1% Casamino Acids and grown until mid-exponential phase. For small-colony variants without chemical complementation, overnight cultures were subcultured 1:25. For conditions under which heme was added, 2 µM was used; for menaquinone, 12.5 µM menaquinone-vitamin K2 was used.
For anaerobic experiments, a Coy (Grass Lake, MI) anaerobic chamber was used, filled with a mixture of 90% nitrogen, 5% carbon dioxide, and 5% hydrogen gases, and hydrogen levels were monitored to ensure a minimum 2% hydrogen concentration. Palladium catalysts (Coy) were used to remove any residual oxygen by reaction with hydrogen. A Coy static incubator was maintained at 37°C. Solutions and plasticware were allowed to equilibrate for >24 h inside the glove box before use. For anaerobic samples, strains were streaked onto TSA and grown aerobically for 24 h at 37°C. Cultures were started from single colonies in 5 ml of anaerobic TSB and grown at 37°C for 15 h. Overnight cultures were subcultured 1:100 into anaerobic TSB containing 40 mM sodium nitrate and grown until the mid-exponential phase. Protein was collected, tryptically digested, and subjected to LC-MRM-MS/MS as described in Text S1 in the supplemental material.
Anaerobic growth curves.
The S. aureus Newman WT, ΔhemX, cysG, ΔhemX cysG, nirD, and ΔhemX nirD strains were streaked onto TSA and grown aerobically for 24 h at 37°C. Cultures were started from single colonies in 3 ml of anaerobic TSB and grown at 37°C for 15 h. Overnight cultures were subcultured 1:200 in round-bottomed 96-well plates with 200 μl of anaerobic TSB containing 40 mM sodium nitrate or an equal volume of sterile water and covered with Breathe-Easy gas-permeable seal (Sigma). Growth was monitored by optical density (OD) over time in a BioTek Synergy H1.
Quantitative reverse transcriptase PCR.
Strains were streaked onto TSA and grown at 24 h at 37°C. Cultures were started from single colonies in 5 ml of RPMI plus 1% Casamino Acids and grown at 37°C for 15 h. Overnight cultures were subcultured 1:100 (WT and ΔhemX mutant) or 1:25 (pbgS ΔhemX pgbS strain) into RPMI plus 1% Casamino acids and grown until the mid-exponential phase. An equal volume of ice-cold acetone-ethanol was added, and the mixture was stored at −80°C. RNA was isolated using Tri reagent and chloroform and precipitated with isopropanol. Isolated RNAs were treated with DNase I (Thermo) according to the manufacturer’s instructions, and RNA was reisolated using Qiagen RNeasy kit. cDNA was synthesized from 2 µg of RNA by incubation with Moloney murine leukemia virus (MMLV) reverse transcriptase (Thermo), using transcript-specific primers (JC83/84 for gyrA, JC53/54 for gtrR, and JC55/56 for gsaM). Quantitative PCR (qPCR) was performed using SYBR green (Thermo) according to the manufacturer’s instructions, using primers JC81/82 for gyrA, HS1/2 for gtrR, and HS for gsaM. Transcript abundance was quantified using the threshold cycle (ΔΔCT) method after normalization to gyrA abundance.
ALA quantification.
ALA quantification was modified from reference 47. The S. aureus WT and ΔhemX mutant strains were streaked onto TSA and grown for 18 h at 37°C. Single colonies were used to start 5-ml cultures in TSB and grown for 12 h at 37°C, then 1 ml was inoculated into 100 ml of TSB in a 250-ml Erlenmeyer flask and grown at 37°C for 14 h. The cell wall was removed by incubation in TSM (100 mM Tris-Cl, pH 7, 500 mM sucrose, 10 mM MgCl2) plus 40 µg/ml lysostaphin and incubated at 37°C for 45 min. Protoplasts were collected by centrifugation and resuspended in 1 ml 10% trichloroacetic acid (TCA). Samples were incubated on ice and intermittently lysed by sonication. The soluble fraction was collected by centrifugation and neutralized to pH 7 with 6 M NaOH, then added to a Dowex 1x-4 resin in column converted to the acetate form before use. In this form, the column retains porphobilinogen but allows ALA to flow through. Six hundred microliters of flowthrough was added to 200 µl of 8% acetyl acetone in 2 M sodium acetate buffer, incubated for 15 min at 90°C to form the pyrrolic condensation product, and cooled to room temperature. Five hundred microliters of sample was added to 500 µl of modified Ehrlich’s reagent and incubated for 10 min at room temperature, and the resulting absorbance was measured at 552 and 650 nm in a Cary 50 Bio UV-visible (UV-Vis) spectrophotometer. The relative concentration of ALA was calculated based on an extinction coefficient of 7.2 × 10−4 M−1 cm−1.
LC-qTOF-MS porphyrin quantification.
Porphyrins were extracted from the S. aureus WT and ΔhemX strains grown to the stationary phase and analyzed by LC-qTOF-MS described in detail in Text S1 in the supplemental material.
Pyridine hemochromagen quantification.
Strains were streaked onto TSA and grown for 18 h at 37°C. Single colonies were used to start 5-ml cultures of TSB and grown at 37°C for 10 h. Sixty microliters of each culture was added to 6 ml of TSB and grown for 16 h at 37°C. Cells were collected by centrifugation, and the cell wall was removed by incubation in 20 mM potassium phosphate buffer (pH 7.4) containing 20 µg of lysostaphin for 45 min at 37°C. Samples were lysed by sonication, and unbroken cells were collected by centrifugation. Four hundred fifty microliters of the soluble supernatant was added to 450 µl of 0.2 M NaOH containing 40% pyridine and 500 µM potassium ferricyanide. Absorbance was measured in a Cary 50 Bio UV-Vis spectrophotometer from 540 to 590 nm. Ten microliters of 0.5 M sodium dithionite prepared in 0.5 M NaOH was added to samples, the mixture was incubated for 5 min, and absorbance (A) was measured again from 540 to 590 nm. Heme quantity is calculated using ΔA = (A557 reduced − A557 oxidized) − (A575 reduced − A575 oxidized) and an extinction coefficient of 32.4 mM−1 cm−1.
Bioluminescent reporter assay.
The S. aureus WT and ΔhemX strain with pXen-1 or PhrtluxABCDE were streaked onto TSA-chloramphenicol prepared with 0 or 20 µM heme. After 18 h, the plates were imaged using a Xenogen IVIS 2000.
XylE reporter assay.
XylE abundance in cellular lysate was assessed spectrophotometrically by measuring formation of 2-hydroxymuconic acid from catechol after growth in TSB containing chloramphenicol and 0 to 2 µM heme, as described previously (10).
Heme killing assay.
S. aureus WT, ΔhemX, and ΔhrtB strains were streaked onto TSA and grown for 24 h at 37°C. Single colonies were used to start 5-ml cultures of TSB and grown at 37°C for 14 h. Two microliters of each culture was added to 148 µl of TSB containing different concentrations of heme in a 96-well round-bottomed plate and incubated at 37°C for 2 h. Samples were serially diluted in phosphate-buffered saline (PBS) and plated to TSA for CFU enumeration after 24 h of growth at 37°C.
Heme toxicity growth curves.
Strains were streaked onto TSA and grown for 24 h at 37°C. Single colonies were used to start 5 ml cultures of TSB and grown for 16 h at 37°C containing 0 or 2 µM heme as noted. One microliter of each culture was added to 199 µl of medium containing 0 or 10 µM heme, as noted, in a 96-well round-bottomed plate, and growth was monitored over time at 37°C by measuring the optical density at 600 nm (OD600) in a BioTek Synergy2 spectrophotometer and analyzed with BioTek Gen5 software.
Growth in minimal medium.
Chemically defined media (CDM) supplemented with 5 mg/ml glucose was prepared as previously described (48), with the exception that iron was not added. Strains were streaked onto TSA and grown for 24 h at 37°C. Single colonies were used to start 5-ml cultures in TSB and grown for 14 h at 37°C. Cells were collected by centrifugation, washed in PBS twice, and then resuspended in 5 ml of PBS. One microliter was added to 199 µl of CDM containing 1 µM ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid) (EDDHA; LGC Standards) or an equal volume of 0.1 M NaOH (vehicle) in a 96-well round-bottomed plate. Growth was monitored for 24 h with shaking at 37°C in a BioTek EPOCH2 spectrophotometer and analyzed with BioTek Gen5 software.
pOS1 PisdAgfp reporter assay.
The S. aureus WT pOS1 PisdAgfp and ΔhemX pOS1 PisdAgfp strains were streaked onto TSA-chloramphenicol and grown for 14 h at 37°C. Single colonies were used to inoculate 5-ml cultures of TSB-chloramphenicol and grown at 37°C for 8.5 h. One microliter of each culture was used to inoculate 199 µl of TSB-chloramphenicol containing 1 mM 2,2-dipyridyl or an equal volume of ethanol (vehicle). Growth was monitored over the course of 16 h by measuring OD600 as well as relative fluorescence at 485 nm (excitation) and 510 nm (emission) in a BioTek Cytation5 spectrophotometer and analyzed with BioTek Gen5 software.
Comparative genome analysis.
With over 100,000 prokaryotic genomes currently available in public databases and many more in the pipelines (http://www.genomesonline.org), it is not practical or possible to perform meaningful comparative analysis on all of them simultaneously. Thus, a set of diverse representative prokaryotic genomes have been developed in the SEED database as follows. The algorithm for computing molecular operational taxonomic units (OTU) based on DNA barcode data (49, 50) was used to group ~12,600 prokaryotic genomes available in the SEED database in October 2013 into about 1,000 taxon groups. One or two representative genomes (rarely three) for each OTU were selected based on the largest amount of published experimental data and the highest level of research interest within the scientific community. The resultant collection of 982 diverse genomes (928 eubacterial and 54 archaeal) creates a manageable set that accurately represents the immense diversity of the over 12,000 prokaryotic organisms with sequenced genomes. Importantly, it is not skewed by an overabundance of genomes for a few microbial genera (medically or industrially important), such as Enterobacteriaceae, streptococci, mycobacteria, etc.
The HemX protein family was exhaustively annotated for this set of 982 representative microbial genomes in the SEED database (51). Contextual associations for this family were predicted based on the patterns of co-occurrence and/or colocalization of its members with other protein families using the set of tools for comparative genome analysis available in SEED (52) within the functional and genomic contexts provided by the subsystem “Heme Biosynthesis: protoporphyrin-, coproporphyrin- and siroheme-dependent pathways” (http://pubseed.theseed.org//SubsysEditor.cgi?page=ShowSubsystem&subsystem=Heme_Biosynthesis%3A_protoporphyrin-%2C_coproporphyrin-_and_siroheme-dependent_pathways). Phylogenetic distribution of the HemX protein family was mapped onto the tree of life (53), and protoheme biosynthetic pathway analysis was adapted from reference 14.
HemX multiple sequence alignment and topology prediction.
The HemX multiple sequence alignment was KEGG ClustalW (http://www.genome.jp [accessed March 2017]) (54) using Staphylococcus aureus strain Newman, Staphylococcus epidermidis strain ATCC 12228, Bacillus anthracis strain Sterne, Chlorobium tepidum strain TLS, Aquifex aeolicus strain VF5, Desulfovfibrio vulgaris strain DP4, and Geobacter sulfurreducens strain PCA. The transmembrane domains depicted were predicted by MEMSAT3 (http://bioinf.cs.ucl.ac.uk; accessed March 2017) as described previously (55). All models were confirmed using TMHMM 2.0 (http://www.cbs.dtu.dk [accessed March 2017]) (56), and predictions were matched across prediction servers, with the exception of Chlorobium tepidum, which TMHMM2.0 predicts to have seven rather than eight transmembrane domains.
Statistical analysis.
All data analysis and statistical tests were performed using GraphPad Prism 6 software. Replicate numbers and statistical tests for each experiment are listed in the figure legends.
ACKNOWLEDGMENTS
We thank Lars Hederstedt (Lund University) and members of the Skaar laboratory for their critical evaluation of the manuscript. We thank Victor Torres (New York University) for the gift of strains and plasmids. We thank Neal Hammer and Matt Surdel for transduction of katA::Tn and nirD::Tn, respectively. We thank Hayes McDonald and the Vanderbilt Mass Spectrometry Research Core for assistance with mass spectrometry experiments. Strains NE1279, NE1366, and NE1931 were obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) for distribution by BEI Resources, NIAID, NIH (Nebraska Transposon Mutant Library [NTML] Screening Array, NR-48501).
This work was funded by National Institutes of Health F31AI126662 (J.E.C.), T32GM065086 (J.E.C.), T32HL094296 (C.M.G.), R01AI0690233 (E.P.S.), R01GM090260 (J.L.D.), and R21DK114607 (J.L.D.).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Citation Choby JE, Grunenwald CM, Celis AI, Gerdes SY, DuBois JL, Skaar EP. 2018. Staphylococcus aureus HemX modulates glutamyl-tRNA reductase abundance to regulate heme biosynthesis. mBio 9:e02287-17. https://doi.org/10.1128/mBio.02287-17.
REFERENCES
- 1.Choby JE, Skaar EP. 2016. Heme synthesis and acquisition in bacterial pathogens. J Mol Biol 428:3408–3428. doi: 10.1016/j.jmb.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core surveillance (ABCs) MRSA Investigators . 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 3.Hammer ND, Reniere ML, Cassat JE, Zhang Y, Hirsch AO, Hood MI, Skaar EP. 2013. Two heme-dependent terminal oxidases power Staphylococcus aureus organ-specific colonization of the vertebrate host. mBio 4:e00241-13. doi: 10.1128/mBio.00241-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammer ND, Schurig-Briccio LA, Gerdes SY, Gennis RB, Skaar EP. 2016. CtaM is required for menaquinol oxidase aa3 function in Staphylococcus aureus. mBio 7:e00823-16. doi: 10.1128/mBio.00823-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dailey HA, Gerdes S, Dailey TA, Burch JS, Phillips JD. 2015. Noncanonical coproporphyrin-dependent bacterial heme biosynthesis pathway that does not use protoporphyrin. Proc Natl Acad Sci U S A 112:2210–2215. doi: 10.1073/pnas.1416285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lobo SA, Scott A, Videira MA, Winpenny D, Gardner M, Palmer MJ, Schroeder S, Lawrence AD, Parkinson T, Warren MJ, Saraiva LM. 2015. Staphylococcus aureus haem biosynthesis: characterisation of the enzymes involved in final steps of the pathway. Mol Microbiol 97:472–487. doi: 10.1111/mmi.13041. [DOI] [PubMed] [Google Scholar]
- 7.Cosgrove K, Coutts G, Jonsson IM, Tarkowski A, Kokai-Kun JF, Mond JJ, Foster SJ. 2007. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J Bacteriol 189:1025–1035. doi: 10.1128/JB.01524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Sorge NM, Beasley FC, Gusarov I, Gonzalez DJ, von Köckritz-Blickwede M, Anik S, Borkowski AW, Dorrestein PC, Nudler E, Nizet V. 2013. Methicillin-resistant Staphylococcus aureus bacterial nitric-oxide synthase affects antibiotic sensitivity and skin abscess development. J Biol Chem 288:6417–6426. doi: 10.1074/jbc.M112.448738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mogen AB, Carroll RK, James KL, Lima G, Silva D, Culver JA, Petucci C, Shaw LN, Rice KC. 2017. Staphylococcus aureus nitric oxide synthase (saNOS) modulates aerobic respiratory metabolism and cell physiology. Mol Microbiol 105:139–157. doi: 10.1111/mmi.13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres VJ, Stauff DL, Pishchany G, Bezbradica JS, Gordy LE, Iturregui J, Anderson KL, Dunman PM, Joyce S, Skaar EP. 2007. A Staphylococcus aureus regulatory system that responds to host heme and modulates virulence. Cell Host Microbe 1:109–119. doi: 10.1016/j.chom.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beale SI, Castelfranco PA. 1973. 14C incorporation from exogenous compounds into δ-aminolevulinic acid by greening cucumber cotyledons. Biochem Biophys Res Commun 52:143–149. doi: 10.1016/0006-291X(73)90966-2. [DOI] [PubMed] [Google Scholar]
- 12.Schön A, Krupp G, Gough S, Berry-Lowe S, Kannangara CG, Söll D. 1986. The RNA required in the first step of chlorophyll biosynthesis is a chloroplast glutamate tRNA. Nature 322:281–284. doi: 10.1038/322281a0. [DOI] [PubMed] [Google Scholar]
- 13.Moser J, Schubert WD, Beier V, Bringemeier I, Jahn D, Heinz DW. 2001. V-shaped structure of glutamyl-tRNA reductase, the first enzyme of tRNA-dependent tetrapyrrole biosynthesis. EMBO J 20:6583–6590. doi: 10.1093/emboj/20.23.6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dailey HA, Dailey TA, Gerdes S, Jahn D, Jahn M, O’Brian MR, Warren MJ. 2017. Prokaryotic heme biosynthesis: multiple pathways to a common essential product. Microbiol Mol Biol Rev 81:81:e00048-16. doi: 10.1128/MMBR.00048-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansson M, Gustafsson MC, Kannangara CG, Hederstedt L. 1997. Isolated Bacillus subtilis HemY has coproporphyrinogen III to coproporphyrin III oxidase activity. Biochim Biophys Acta 1340:97–104. doi: 10.1016/S0167-4838(97)00030-7. [DOI] [PubMed] [Google Scholar]
- 16.Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP. 2003. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci U S A 100:6940–6945. doi: 10.1073/pnas.0832254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 18.Schröder I, Johansson P, Rutberg L, Hederstedt L. 1994. The hemX gene of the Bacillus subtilis hemAXCDBL operon encodes a membrane protein, negatively affecting the steady-state cellular concentration of HemA (glutamyl-tRNA reductase). Microbiology 140:731–740. doi: 10.1099/00221287-140-4-731. [DOI] [PubMed] [Google Scholar]
- 19.Wang LY, Brown L, Elliott M, Elliott T. 1997. Regulation of heme biosynthesis in Salmonella typhimurium: activity of glutamyl-tRNA reductase (HemA) is greatly elevated during heme limitation by a mechanism which increases abundance of the protein. J Bacteriol 179:2907–2914. doi: 10.1128/jb.179.9.2907-2914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bibb LA, Kunkle CA, Schmitt MP. 2007. The ChrA-ChrS and HrrA-HrrS signal transduction systems are required for activation of the hmuO promoter and repression of the hemA promoter in Corynebacterium diphtheriae. Infect Immun 75:2421–2431. doi: 10.1128/IAI.01821-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson P, Hederstedt L. 1999. Organization of genes for tetrapyrrole biosynthesis in Gram-positive bacteria. Microbiology 145:529–538. doi: 10.1099/13500872-145-3-529. [DOI] [PubMed] [Google Scholar]
- 22.Hansson M, Rutberg L, Schröder I, Hederstedt L. 1991. The Bacillus subtilis hemAXCDBL gene cluster, which encodes enzymes of the biosynthetic pathway from glutamate to uroporphyrinogen III. J Bacteriol 173:2590–2599. doi: 10.1128/jb.173.8.2590-2599.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schröder I, Hederstedt L, Kannangara CG, Gough P. 1992. Glutamyl-tRNA reductase activity in Bacillus subtilis is dependent on the hemA gene product. Biochem J 281:843–850. doi: 10.1042/bj2810843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Yoong P, Ram G, Torres VJ, Novick RP. 2014. Single-copy vectors for integration at the SaPI1 attachment site for Staphylococcus aureus. Plasmid 76:1–7. doi: 10.1016/j.plasmid.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoober JK, Kahn A, Ash DE, Gough S, Kannangara CG. 1988. Biosynthesis of delta-aminolevulinate in greening barley leaves. IX. Structure of the substrate, mode of gabaculine inhibition, and the catalytic mechanism of glutamate 1-semialdehyde aminotransferase. Carlsberg Res Commun 53:11–25. doi: 10.1007/BF02908411. [DOI] [PubMed] [Google Scholar]
- 26.Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O. 2003. Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299:906–909. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- 27.Schlag S, Fuchs S, Nerz C, Gaupp R, Engelmann S, Liebeke M, Lalk M, Hecker M, Götz F. 2008. Characterization of the oxygen-responsive NreABC regulon of Staphylococcus aureus. J Bacteriol 190:7847–7858. doi: 10.1128/JB.00905-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overbeek R, Fonstein M, D’Souza M, Pusch GD, Maltsev N. 1999. The use of gene clusters to infer functional coupling. Proc Natl Acad Sci U S A 96:2896–2901. doi: 10.1073/pnas.96.6.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang W, Small DA, Toghrol F, Bentley WE. 2006. Global transcriptome analysis of Staphylococcus aureus response to hydrogen peroxide. J Bacteriol 188:1648–1659. doi: 10.1128/JB.188.4.1648-1659.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horsburgh MJ, Clements MO, Crossley H, Ingham E, Foster SJ. 2001. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect Immun 69:3744–3754. doi: 10.1128/IAI.69.6.3744-3754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinkel TL, Roux CM, Dunman PM, Fang FC. 2013. The Staphylococcus aureus SrrAB two-component system promotes resistance to nitrosative stress and hypoxia. mBio 4:e00696-13. doi: 10.1128/mBio.00696-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Elliott M, Elliott T. 1999. Conditional stability of the HemA protein (glutamyl-tRNA reductase) regulates heme biosynthesis in Salmonella typhimurium. J Bacteriol 181:1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones AM, Elliott T. 2010. A purified mutant HemA protein from Salmonella enterica serovar Typhimurium lacks bound heme and is defective for heme-mediated regulation in vivo. FEMS Microbiol Lett 307:41–47. doi: 10.1111/j.1574-6968.2010.01967.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Wilson S, Elliott T. 1999. A mutant HemA protein with positive charge close to the N terminus is stabilized against heme-regulated proteolysis in Salmonella typhimurium. J Bacteriol 181:6033–6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laakso HA, Marolda CL, Pinter TB, Stillman MJ, Heinrichs DE. 2016. A heme-responsive regulator controls synthesis of staphyloferrin B in Staphylococcus aureus. J Biol Chem 291:29–40. doi: 10.1074/jbc.M115.696625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutter BF, Mike LA, Reid PR, Chong KM, Ramos-Hunter SJ, Skaar EP, Sulikowski GA. 2016. Decoupling activation of heme biosynthesis from anaerobic toxicity in a molecule active in Staphylococcus aureus. ACS Chem Biol 11:1354–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mike LA, Dutter BF, Stauff DL, Moore JL, Vitko NP, Aranmolate O, Kehl-Fie TE, Sullivan S, Reid PR, DuBois JL, Richardson AR, Caprioli RM, Sulikowski GA, Skaar EP. 2013. Activation of heme biosynthesis by a small molecule that is toxic to fermenting Staphylococcus aureus. Proc Natl Acad Sci U S A 110:8206–8211. doi: 10.1073/pnas.1303674110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Surdel MC, Horvath DJ Jr, Lojek LJ, Fullen AR, Simpson J, Dutter BF, Salleng KJ, Ford JB, Jenkins JL, Nagarajan R, Teixeira PL, Albertolle M, Georgiev IS, Jansen ED, Sulikowski GA, Lacy DB, Dailey HA, Skaar EP. 2017. Antibacterial photosensitization through activation of coproporphyrinogen oxidase. Proc Natl Acad Sci U S A 114:E6652–E6659. doi: 10.1073/pnas.1700469114 pii: 201700469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Girvan HM, Munro AW. 2013. Heme sensor proteins. J Biol Chem 288:13194–13203. doi: 10.1074/jbc.R112.422642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao A, Fang Y, Chen X, Zhao S, Dong W, Lin Y, Gong W, Liu L. 2014. Crystal structure of Arabidopsis glutamyl-tRNA reductase in complex with its stimulator protein. Proc Natl Acad Sci U S A 111:6630–6635. doi: 10.1073/pnas.1400166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apitz J, Nishimura K, Schmied J, Wolf A, Hedtke B, van Wijk KJ, Grimm B. 2016. Posttranslational control of ALA synthesis includes GluTR degradation by Clp protease and stabilization by GluTR-binding protein. Plant Physiol 170:2040–2051. doi: 10.1104/pp.15.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. 2004. Iron-source preference of Staphylococcus aureus infections. Science 305:1626–1628. doi: 10.1126/science.1099930. [DOI] [PubMed] [Google Scholar]
- 43.Jensen J. 1962. The effect of heme on tetrapyrrol synthesis in a heme requiring Staphylococcus aureus. Biochem Biophys Res Commun 8:271–277. doi: 10.1016/0006-291X(62)90276-0. [DOI] [PubMed] [Google Scholar]
- 44.Bae T, Schneewind O. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Choby JE, Mike LA, Mashruwala AA, Dutter BF, Dunman PM, Sulikowski GA, Boyd JM, Skaar EP. 2016. A small-molecule inhibitor of iron-sulfur cluster assembly uncovers a link between virulence regulation and metabolism in Staphylococcus aureus. Cell Chem Biol 23:1351–1361. doi: 10.1016/j.chembiol.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kardon JR, Yien YY, Huston NC, Branco DS, Hildick-Smith GJ, Rhee KY, Paw BH, Baker TA. 2015. Mitochondrial ClpX activates a key enzyme for heme biosynthesis and erythropoiesis. Cell 161:858–867. doi: 10.1016/j.cell.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vitko NP, Richardson AR. 2013. Laboratory maintenance of methicillin-resistant Staphylococcus aureus (MRSA). Curr Protoc Microbiol Chapter 9:Unit 9C.2. doi: 10.1002/9780471729259.mc09c02s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blaxter M, Mann J, Chapman T, Thomas F, Whitton C, Floyd R, Abebe E. 2005. Defining operational taxonomic units using DNA barcode data. Philos Trans R Soc Lond B Biol Sci 360:1935–1943. doi: 10.1098/rstb.2005.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones M, Ghoorah A, Blaxter M. 2011. jMOTU and Taxonerator: turning DNA barcode sequences into annotated operational taxonomic units. PLoS One 6:e19259. doi: 10.1371/journal.pone.0019259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Overbeek R, Begley T, Butler RM, Choudhuri JV, Chuang HY, Cohoon M, de Crécy-Lagard V, Diaz N, Disz T, Edwards R, Fonstein M, Frank ED, Gerdes S, Glass EM, Goesmann A, Hanson A, Iwata-Reuyl D, Jensen R, Jamshidi N, Krause L, Kubal M, Larsen N, Linke B, McHardy AC, Meyer F, Neuweger H, Olsen G, Olson R, Osterman A, Portnoy V, Pusch GD, Rodionov DA, Rückert C, Steiner J, Stevens R, Thiele I, Vassieva O, Ye Y, Zagnitko O, Vonstein V. 2005. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res 33:5691–5702. doi: 10.1093/nar/gki866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2014. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ciccarelli FD, Doerks T, von Mering C, Creevey CJ, Snel B, Bork P. 2006. Toward automatic reconstruction of a highly resolved tree of life. Science 311:1283–1287. doi: 10.1126/science.1123061. [DOI] [PubMed] [Google Scholar]
- 54.Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones DT. 2007. Improving the accuracy of transmembrane protein topology prediction using evolutionary information. Bioinformatics 23:538–544. doi: 10.1093/bioinformatics/btl677. [DOI] [PubMed] [Google Scholar]
- 56.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 57.Duthie ES, Lorenz LL. 1952. Staphylococcal coagulase: mode of action and antigenicity. J Gen Microbiol 6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 58.Mayfield JA, Hammer ND, Kurker RC, Chen TK, Ojha S, Skaar EP, DuBois JL. 2013. The chlorite dismutase (HemQ) from Staphylococcus aureus has a redox-sensitive heme and is associated with the small colony variant phenotype. J Biol Chem 288:23488–23504. doi: 10.1074/jbc.M112.442335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wakeman CA, Hammer ND, Stauff DL, Attia AS, Anzaldi LL, Dikalov SI, Calcutt MW, Skaar EP. 2012. Menaquinone biosynthesis potentiates haem toxicity in Staphylococcus aureus. Mol Microbiol 86:1376–1392. doi: 10.1111/mmi.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stauff DL, Skaar EP. 2009. Bacillus anthracis HssRS signalling to HrtAB regulates haem resistance during infection. Mol Microbiol 72:763–778. doi: 10.1111/j.1365-2958.2009.06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Attia AS, Benson MA, Stauff DL, Torres VJ, Skaar EP. 2010. Membrane damage elicits an immunomodulatory program in Staphylococcus aureus. PLoS Pathog 6:e1000802. doi: 10.1371/journal.ppat.1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kreiswirth BN, Löfdahl S, Betley MJ, O’Reilly M, Schlievert PM, Bergdoll MS, Novick RP. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GtrR abundance is uniquely low among heme synthesis enzymes. Shown is the abundance of each heme synthesis enzyme as measured by LC-MRM-MS/MS in WT cells. The data are the average from a single experiment performed in biological triplicate with standard deviation shown. Download FIG S1, TIF file, 0.9 MB (914.5KB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The pbgS allele is not polar on gsaM transcription. Steady-state transcript abundance of gsaM mRNA isolated from mid-exponential growth of S. aureus strains was measured by qRT-PCR and is graphed as fold change relative to the WT. Data are combined from two independent experiments in biological triplicate with standard deviation shown. “ns” indicates no significance by one-way ANOVA with Dunnett’s correction for multiple comparisons, comparing fold change of the pbgS mutant to the WT. Download FIG S2, TIF file, 0.3 MB (330.1KB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Representative extracted ion chromatograms of extracted porphyrins of (A) the S. aureus WT and (B) the ΔhemX mutant; each is quantified and shown in Fig. 2. Chromatograms for porphyrins above the limits of detection (250 nM) are shown. Download FIG S3, TIF file, 2.7 MB (2.7MB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Excess heme and resistance to heme toxicity in the ΔhemX mutant can be complemented. (A) Heme abundance was quantified using a pyridine hemochromagen assay in S. aureus strains. Data are combined from three independent experiments with four biological replicates with standard error of the mean shown. Statistical significance was determined by a one-way ANOVA with Tukey’s correction for multiple comparisons, comparing each strain against the others. **, P < 0.005. (B) Growth as measured by optical density (600 nm) was monitored over time for S. aureus strains in medium containing 0 or 10 µM heme. Strains were grown overnight to the stationary phase in medium alone before inoculation of the growth curve. The data are the average of the means from three independent experiments each in biological triplicate with standard error of the mean shown. (C) Growth as measured by optical density (600 nm) was monitored over time for S. aureus strains in medium containing chloramphenicol and 0 or 12.5 µM heme. Strains were grown overnight to stationary phase in medium alone before inoculation of the growth curve. The data are means from three biological replicates with standard error of the mean shown from a single experiment, representative of at least three independent experiments. Download FIG S4, TIF file, 6.8 MB (7MB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
gtrR transcription is unchanged in the ΔhemX and ΔhemX pbgS strains compared to the pbgS strain. Steady-state transcript abundance of gtrR mRNA isolated from mid-exponential growth of S. aureus strains was measured by qRT-PCR and is graphed as fold change relative to the WT. Data are combined from two independent experiments in biological triplicate with standard deviation shown. “ns” indicates no significance by one-way ANOVA with Dunnett’s correction for multiple comparisons, comparing fold change of the ΔhemX mutant and ΔhemX pbgS strains to the pbgS strain. Download FIG S5, TIF file, 0.7 MB (780.1KB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Representative extracted ion chromatograms of extracted porphyrins of (A) the S. aureus WT and (B) the ΔhemX, (C) ΔchdC, and (D) ΔhemX ΔchdC strains. Each is quantified and shown in Fig. 5. Chromatograms for porphyrins above the limits of detection (250 nM) are shown. Download FIG S6, TIF file, 3.1 MB (3.2MB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Nitrite reductase and the cofactor siroheme are required for full growth with nitrate as an alternative terminal electron acceptor. Shown is growth anaerobically as measured by optical density (600 nm) monitored over time for S. aureus strains in medium containing 0 or 40 mM NO3. Strains were grown overnight to the stationary phase in medium alone before inoculation of the growth curve. The data are means from three independent experiments with at least three biological replicates. Download FIG S7, TIF file, 4.1 MB (4.2MB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
hemX co-occurs with heme synthesis and shares conserved secondary structure and residues. (A) The genomes shown in Fig. 7 were analyzed for the capacity to synthesize heme or both heme and siroheme, as well as the presence of hemX. (B) Alignment of HemX for each of the seven representative organisms, with predicted transmembrane domains in yellow, conserved residues in red, and moderately conserved residues marked with “:” to show conservation among strongly similar amino acids or “.” to show conservation among weakly similar amino acids. Download FIG S8, TIF file, 45.3 MB (46.4MB, tif) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Plasmids and primers used in this study. Download TABLE S1, DOCX file, 0.1 MB (14.1KB, docx) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Supplemental materials and methods and references. Download TEXT S1, DOCX file, 0.1 MB (20.9KB, docx) .
Copyright © 2018 Choby et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.