Abstract
Premature infants are at an increased risk of developing cognitive and motor handicaps due to chronic hypoxia. Although the current therapies have reduced the incidence of these handicaps, untoward side effects abound. Using a murine model of sublethal hypoxia, we demonstrated reduction in several transcription factors that modulate expression of genes known to be involved in several neural functions. We demonstrate the induction of these genes by minocycline, a tetracycline antibiotic with noncanonical functions, in both in vitro and in vivo studies. Specifically, there was induction of genes, including Sox10, Hif1a, Hif2a, Birc5, Yap1, Epo, Bdnf, Notch1 (cleaved), Pcna, Mag, Mobp, Plp1, synapsin, Adgra2, Pecam1, and reduction in activation of caspase 3, all known to affect proliferation, apoptosis, synaptic transmission, and nerve transmission. Minocycline treatment of mouse pups reared under sublethal hypoxic conditions resulted in improvement in open field testing parameters. These studies demonstrate beneficial effects of minocycline treatment following hypoxic insult, document up-regulation of several genes associated with improved cognitive function, and support the possibility of minocycline as a potential therapeutic target in the treatment of neurodevelopmental handicaps observed in the very premature newborn population. Additionally, these studies may aid in further interpretation of the effects of minocycline in the treatment trials and animal model studies of fragile X syndrome and multiple sclerosis.
Of the >4 million infants born in the United States every year, 1% to 2% are born very premature, resulting in increased risk of motor and cognitive neurodevelopmental handicaps. Despite continued advancements in perinatal care, preterm birth results in significant cognitive and motor disabilities. Although clinical improvement has been documented over time in this infant population, neonatal illness and severe intraventricular hemorrhage have increased, resulting in an increase of severely compromised patients that require care in neonatal intensive care units.1, 2, 3, 4, 5, 6, 7
The deleterious effects of low oxygen in the perinatal period are likely the consequences of altered neural differentiation, synaptogenesis, and loss of neurons, glia, and their progenitor cells due to excessive apoptosis.1, 2, 3, 4, 5, 8, 9, 10, 11, 12, 13 A possible explanation for the variable recovery from hypoxic insult observed in this population may be variable responses in the neurogenic zones of the brain, the subventricular zone (SVZ), and the subgranular zone.14, 15, 16, 17, 18, 19, 20, 21, 22
Recently, we have documented significant differences in mRNA expression in C57BL/6 and CD1 SVZ harvested under normoxic and hypoxic conditions as well as differences between C57BL/6 and CD1 SVZ under both conditions.23 Specifically, we noted differences in expression of gene sets associated with Sox10-mediated neural functions, proliferation, and apoptosis between these strains. The cellular and tissue responses of these two strains to hypoxic insult were postulated to explain their differential cognitive and motor responsiveness and provide a potential therapeutic approach to ameliorating the variable adverse responses of this human premature infant population.23
In this report, we investigate the use of minocycline, a tetracycline antibiotic with known noncanonical properties, as a modulator of Sox10 expression in neural stem cells (NSC)24, 25, 26, 27, 28, 29, 30 and as a modulator of behaviors in our sublethal hypoxia murine model of premature delivery. Specifically, we have found that minocycline treatment differentially up-regulates expression of several genes, including Sox10, in hypoxic C57BL/6 NSC cultures in a dose-specific fashion. In addition to up-regulating Sox10,23 minocycline treatment elicits induction of hypoxia-inducible factor (HIF)-1α14, 19 (transcription factors), erythropoietin (EPO),31 and brain-derived neurotrophic factor (BDNF)18 (factors associated with survival and proliferation), phospho (p)AKT,14, 32 and cleaved notch-119, 33 (pathway components associated with survival and proliferation), yes-associated protein 1 (YAP)34 and survivin35, 36 (pathway components associated with proliferation and inhibition of apoptosis), G-protein coupled receptor (GPR)124 (ADGRA2)37 and CD31 (PECAM1)38 (microvascular endothelial cell markers), myelin associated glycoprotein (MAG), myelin-associated oligodendrocyte basic protein (MOBP), and proteolipid protein (PLP)13, 23 (components of myelin), and synapsin13 (a component of synapses), while exhibiting a decrease in cleaved caspase 318 in cultured secondary neurospheres, the SVZ, corpus callosum, cortical fiber tracts, and the hippocampus. Lastly, minocycline-treated pups exhibit increased center time and distance in open field testing and decreased total turns compared to untreated pups in the free swim test.
These studies illustrate the potential of minocycline as a therapeutic agent in the treatment of the neurodevelopmental handicaps observed in the very premature newborn population.
Materials and Methods
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.39 The protocol was approved by the Institutional Animal Care and Use Committee of Yale University (Protocol Number: 07366). All efforts were made to minimize suffering.
Reagents
Antibody directed against Sox10 was purchased from Abgent (San Diego, CA); antibodies directed against HIF-1α, HIF-2α, PLP, and GPR124 were purchased from Novus Biologicals (Littleton, CO); antibodies directed against proliferating cell nuclear antigen (PCNA), cleaved Notch1, cleaved caspase 3, synapsin, Sox2, survivin, and YAP were purchased from Cell Signaling Technology (Danvers, MA); antibodies directed against MAG and MOBP were purchased from Santa Cruz Biotechnology (Dallas, TX); antibodies directed against β-actin, Ki-67, and glial fibrillary acidic protein (GFAP) were purchased from Abcam (Cambridge, MA); antibodies directed against nestin were purchased from Millipore (Darmstadt, Germany). Antibodies directed against murine CD31 ecto-domain (affinity purified SL-4) were raised in rabbits and purified as described previously.40 Antibodies directed against EPO and BDNF, and secondary antibodies used in Western blots [donkey anti-rabbit IgG (sc-2313) and donkey anti-mouse IgG (sc-2318), which were conjugated to horseradish peroxidase] were purchased from Santa Cruz Biotechnology. Secondary antibodies used for immunofluorescence were Alexa Fluor 488- or 594-conjugated goat anti-rabbit IgG (H+L), goat anti-mouse IgG (H+L), or goat anti-rat IgG (H+L), purchased from Life Technologies (Carlsbad, CA). All antibodies were used at a 1:1000 dilution for Western blots and 1:100 or 1:200 dilutions for immunofluorescence. Minocycline was purchased from Sigma-Aldrich Co. (Cat #M9511; St. Louis, MO), dissolved in sterile phosphate-buffered saline (PBS), and used at 1, 5, 10, and 50 μg/mL for tissue culture. When used to treat mouse pups between the ages of P3 and P20, minocycline at a concentration of 150 mg/L was added to sterile 5% sucrose (vehicle) water and given ad libitum to the nursing dams as described.41 Control litters received sterile 5% sucrose water or plain water ad libitum as a vehicle control.
Cells
Neural progenitor cells (NSC) were isolated from P1 C57BL/6 and CD1 pups and were cultured as neurospheres or dispersed cells as previously described.14, 18, 19, 23, 42
Hypoxic Treatment
Cells
NSC neurosphere cultures were cultured in either 20% O2 or 5% O2. Standard tissue culture conditions are usually set at 20% O2, a hyperoxic environment. As previously described,14, 18, 43 we took advantage of cells' ability to sense changes from their normoxic set point by placing them into a reduced oxygen environment (5% O2), mimicking hypoxia.14 We have previously demonstrated that reducing the percentage of oxygen from 20% to 10% or 5%, or from 10% to 5% results in HIF-1α induction.14 NSC neurosphere cultures were cultured in 20% O2 or 5% O2 environments for 3 days in the absence or presence of a range of minocycline concentrations.
Animals
Timed-pregnant litters and associated dams were kept in normoxic conditions (20% O2) from birth [postnatal day 0 (P0)] until P3. Each cage contained both a CD1 and a C57BL/6 dam and pups. (CD1 dams were used as foster dams as C57BL/6 dams are known to be poor mothers.) The combined litters in each cage were culled to 10 pups typically containing 7 to 8 C57BL/6 pups and the balance (2 to 3 pups) being CD1 pups. At P3, one-half of the minocycline-treated and untreated control litters were placed in a hypoxia chamber at 10% O2 from P3 to P11, whereas the other half were maintained in normoxic (20% O2) conditions as previously described.14, 18 This period of brain development in mice corresponds to approximately 23 weeks of gestation to full term in the humans.13, 14, 44 At P11, the litters were removed from the hypoxic environment and placed in ambient oxygen conditions (20% O2). At P20, the minocycline treatment was discontinued, and all litters were provided with sterile 5% sucrose water or plain sterile water ad libitum.
Behavioral Studies
At P20, the pups were tested in an open field activity apparatus, followed by testing in the free swim test at P40.
Open Field Activity
Twenty-five P20 hypoxic C57BL/6 mice (13 males and 12 females consisting of 8 vehicle-only males and 5 minocycline-treated males, and 3 vehicle-only females and 9 minocycline-treated females) and a second group consisting of 26 normoxic and 16 hypoxic mice [21 males and 21 females, consisting of 9 untreated animals (5 males and 4 females); 13 vehicle-only treated animals (7 males and 6 females); and 20 minocycline-treated mice (9 males and 11 females)] were evaluated for spontaneous open field behavior. Mice were placed in a Plexiglas-enclosed open field (25 × 25 × 40 cm) equipped with infrared photo beams coupled to a computer running TruScan software version 2.06-USB (Coulbourn Instruments, Whitehall, PA) to automatically record movements within the field. Activity was monitored during a single 15-minute session and measures of total distance moved (centimeters per 3 minutes), the mean velocity of movements (coordinate changing movements), the amount of time without movement (rest time; seconds per 3 minutes), margin time (time within 3.8 cm of the chamber wall), and center time (time central to the area within 3.8 cm of the chamber wall) were recorded. Data were binned into three 3-minute intervals and were analyzed using a multiple analysis of variance test with repeated measures. Statistical significance (determined by two-way analysis of variance) were ascribed to the data that achieved P < 0.05 expressed as means ± SD using the StatView program version 5 (SAS Institute, Cary, NC) in the Excel:mac 2011 statistical package version 14.3.8 on a Macintosh G5 computer (Apple, Cupertino, CA).
Free Swim Test
At P40, the mice were assessed using the free swim task as previously described.14 This test assesses behavioral laterality and cerebral asymmetries, and is particularly sensitive to the organization and integrity of the corpus callosum linking the two hemispheres.45, 46, 47 Mice were tested for 5 minutes in each of three test sessions spaced approximately 48 hours apart. In each session, the mouse was placed in the center of a tank of water with a depth of 33 cm and measuring 33 cm in diameter. The swimming activity of the mouse was videotaped, and the amount of movement in the clockwise and counterclockwise directions, changes in the direction of swimming, and the consistency of preferred swimming direction across sessions was analyzed in 30-degree increments. Each swimming activity of 30 degrees was scored as a turn in a given direction (clockwise or counterclockwise). Statistical significance (determined by two-way analysis of variance) was ascribed to the data that achieved P < 0.05 as expressed as means ± SD using the StatView program in the Excel statistical package on a Macintosh G5 computer.
Biochemical and Morphological Studies
At P20, P40, and P70, three pups from each condition were anesthetized, perfused with sterile PBS, and their brains removed. The harvested brains were weighed and total cerebral hemispheres or dissected SVZ tissues were prepared for Western blotting following lysis as previously described.19
Western Blot Analysis
SVZ regions were dissected out of the brains of pups from each condition and homogenized in lysis buffer. Alternatively, whole brains devoid of brainstem and olfactory bulbs were harvested following PBS perfusion, rinsed, and then homogenized in lysis buffer as described.14, 18, 19, 42 All data obtained from Western blot analyses were reported as averages of three independent analyses. Statistical significance (determined using two-way analysis of variance) was ascribed to the data that achieved P < 0.05 (expressed as means ± SD) using the StatView statistical package on a Macintosh G5 computer. Specifically, two-way analysis of variance was used to evaluate the statistical significance of comparisons of Western blots of normoxic and reduced oxygen–treated NSC cultures with each other in the absence and presence of a concentration range of minocycline. Significance levels for these Western blots are listed in Supplemental Table S1. Two-way analysis of variance was also used in examination of Western blots of SVZ and cerebral tissues isolated from P20, P40, and P70 pups reared under normoxic and reduced oxygen levels in the absence and presence of minocycline.
Immunofluorescence
At P20, P40, and P70, three pups from each condition were anesthetized and then perfused with sterile PBS followed by 4% paraformaldehyde and sterile PBS. Brains were then placed in 10% to 20% sucrose overnight, placed on chucks with OCT, and sectioned at 6 to 10 μm on a Reichert microtome as previously described.14, 18, 19, 42 Sections of cerebrum and SVZ were stained for nestin, Ki-67, PCNA, EPO, BDNF, GFAP, synapsin, survivin, YAP, and phospho-YAP, myelin, cleaved caspase 3, GRP124, and CD31. Tissue sections were analyzed using an Olympus IX 71 inverted fluorescence/phase and bright field microscope (Olympus, Tokyo, Japan) equipped with an Optronics (Goleta, CA) Microfire camera and Pictureframe version 3.00.30 software. Photoshop CS6 (Adobe Systems, San Jose, CA) and InDesign CS6 (Adobe Systems) were used to generate publication micrographs as previously described.48, 49
Results
Minocycline Treatment Affects Proliferation and Apoptosis of C57BL/6 Neurosphere Cultures
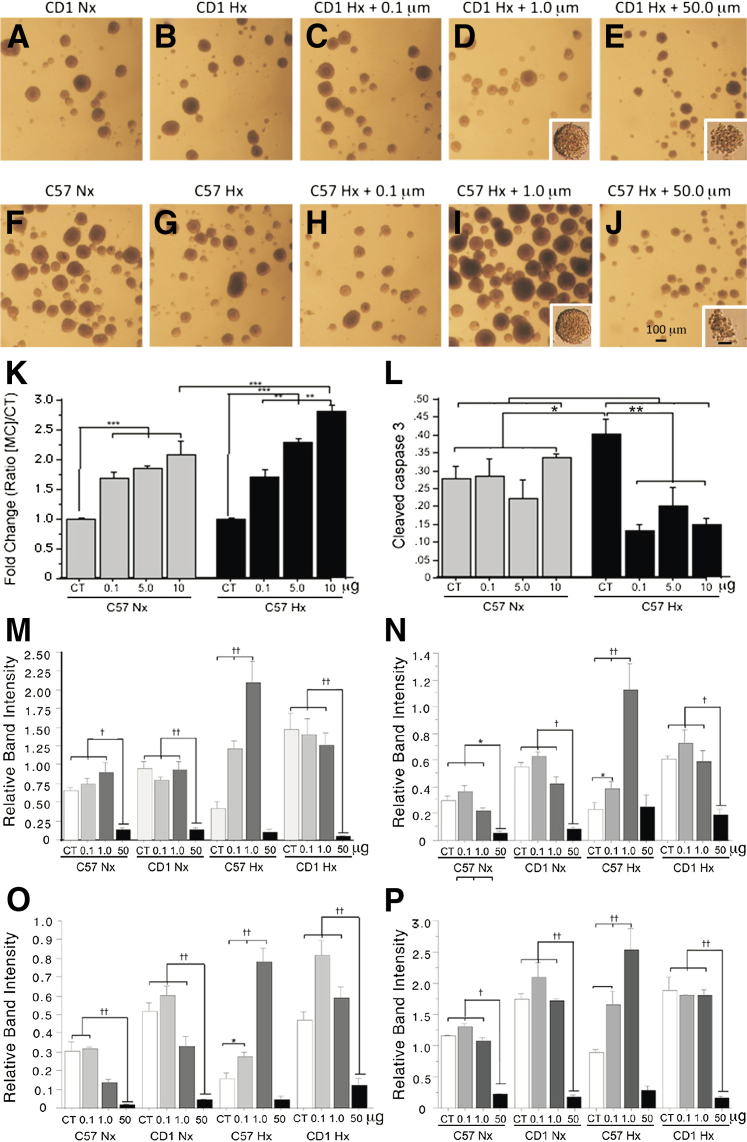
As observed, minocycline treatment of CD1 and C57BL/6 secondary neurosphere cultures elicited a dose-responsive increase in neurosphere numbers in C57BL/6 NSC under reduced oxygen conditions at 0.1 and 1.0 μmol/L minocycline. By contrast, CD1 NSC culture proliferations were unaffected (Figure 1, A–D and F–I) except at 50 μmol/L minocycline, where they appeared similar to the C57 NSC. Specifically, at 50 μmol/L minocycline, neurospheres of both strains appeared smaller and less cohesive, and the cells appeared apoptotic (Figure 1, E and J). Quantitation of C57BL/6 neurosphere number and cleaved caspase 3 revealed increases in neurosphere numbers in response to minocycline in normoxic (20% O2) and reduced oxygen (5% O2) conditions, with higher neurosphere numbers noted in reduced oxygen conditions at a minocycline concentration of 10.0 μmol/L (Figure 1K). Cleaved caspase 3 levels were unchanged in untreated and minocycline-treated normoxic conditions, whereas levels were higher in reduced oxygen conditions in untreated cultures but significantly lower in reduced oxygen minocycline-treated conditions (Figure 1L). A complete listing of the significance values comparing all of the conditions is listed in Supplemental Table S1.
Figure 1.
Minocycline treatment elicits proliferation and reduces caspase 3 activation of reduced oxygen-cultured secondary neurosphere neural stem cell (NSC) cultures and increases in Sox10, HIF-1α, PCNA, and cleaved notch1 expression in reduced oxygen-cultured (HX) C57BL/6 NSC compared to normoxic (NX) cultures in a dose-specific fashion. Representative low-power micrographs illustrate the lack of responsiveness of CD1 secondary neurosphere cultures to reduced oxygen (10%) (A–D); whereas treatment with minocycline shows the increased number of neurospheres noted in hypoxic neurospheres cultures of C57BL/6 after treatment of 0.1 and 1.0 μmol/L minocycline (F–I). E and J and insets illustrate the loss of cohesiveness and crenated morphology of CD1 and C57BL/6 secondary neurospheres and cells after treatment with 50 μmol/L minocycline. Quantitation of neurospheres numbers (K) and cleaved caspase 3 (L) in cultures of C57BL/6 secondary neurospheres cultured under normoxic (20% O2) and reduced oxygen (10% O2) conditions, treated with 0.1, 5.0, and 10.0 μmol/L minocycline. Quantitation of Western blots of normoxic (20% O2) and reduced oxygen (10% O2) cultured C57BL/6 and CD1 NSC illustrating the differential induction of Sox10 (M), HIF-1α (N), cleaved notch1 (O), and PCNA (P) in the absence and presence of 0.1, 1.0, and 50 μmol/L minocycline. Data are expressed as means ± SD. n = 3. ∗P < 0.05, ∗∗P < 0.005, and ∗∗∗P < 0.0005; †P < 0.01, ††P < 0.001. CT, control added; HIF, hypoxia-inducible factor; MC, minocycline; NSC, neural stem cell; PCNA, proliferating cell nuclear antigen.
Minocycline Treatment Elicits Changes in Sox10, HIF-1α, Cleaved Notch1, and PCNA Levels in a Strain-Dependent Manner
A dose-responsive increase in Sox10 expression in C57BL/6 NSC under reduced oxygen conditions (5%) at 0.1 and 1.0 μmol/L minocycline was noted. By contrast, no appreciable effects in normoxic (20% O2) and reduced oxygen levels (5% O2) of CD1 NSC cultures were noted at 0.1 and 1.0 μmol/L minocycline. Interestingly, dramatic decreases in cell numbers of both strains were noted at 50 μmol/L minocycline in both normoxic and reduced oxygen conditions (Figure 1M) (not shown). Minocycline treatment of CD1 and C57BL/6 secondary neurosphere cultures elicited a dose-responsive increase in HIF-1α, Sox10, and PCNA expression in C57BL/6 NSC under reduced oxygen conditions, while eliciting no significant effects in CD1 NSC cultures.
At 50 mmol/L minocycline, both strains exhibited dramatic reductions in HIF-1α and PCNA expression (Figure 1, N and P). Unlike its effects on the expression of Sox10, HIF-1α, and PCNA, minocycline treatment of CD1 and C57BL/6 secondary neurosphere cultures elicited a dose-responsive increase in cleaved notch1 expression in both C57BL/6 and CD1 NSC under hypoxic conditions, albeit at different dose ranges. Specifically, at 1.0 μmol/L, C57BL/6 NSC cultures exhibited increased expression of cleaved notch1, whereas at both 0.1 and 1.0 μmol/L minocycline, CD1 NSC cultures exhibited increased cleaved notch1 expression (Figure 1O). Similar to our observations with Sox10, HIF-1α and PCNA, both CD1 and C57BL/6 NSC exhibited dramatically reduced expression of cleaved notch1 expression at 50 μmol/L minocycline. A complete listing of the significance values comparing the Western blot data for all of the conditions illustrated in Figure 1, M–P, are presented in Supplemental Tables S2–S5. Additionally, Supplemental Figure S1 is a composite micrograph of representative autoradiographs documenting our findings selected from the more than 1100 Western blots comprising the data accrued in our analysis of Western blots.
In addition to illustrating the effects of minocycline using relative band intensities, we also illustrated its effects by using ratios of the expression of each protein following minocycline treatment divided by the expression of each protein without minocycline treatment. The ratios of minocycline-treated divided by untreated expression levels increased from 1.0 only in the reduced oxygen, minocycline-treated C57BL6/B NSC (Supplemental Figure S2). Dramatic decreases in ratios were noted at the 50 μmol/L minocycline concentration in all four protein determinations (Supplemental Figure S2).
In light of our previous data demonstrating no significant changes in Sox10 or genes associated with neural processes in CD1 pups23 and confirmatory results in neurosphere cultures illustrated in Figure 1, we proceeded with continued testing of C57BL/6 pups.
Minocycline Treatment of Nursing C57BL/6 Pups Elicits Increases in Expression of Proteins Associated with Proliferation, Apoptosis Inhibition, and Neural and Vascular Processes in the SVZ and Cerebrum
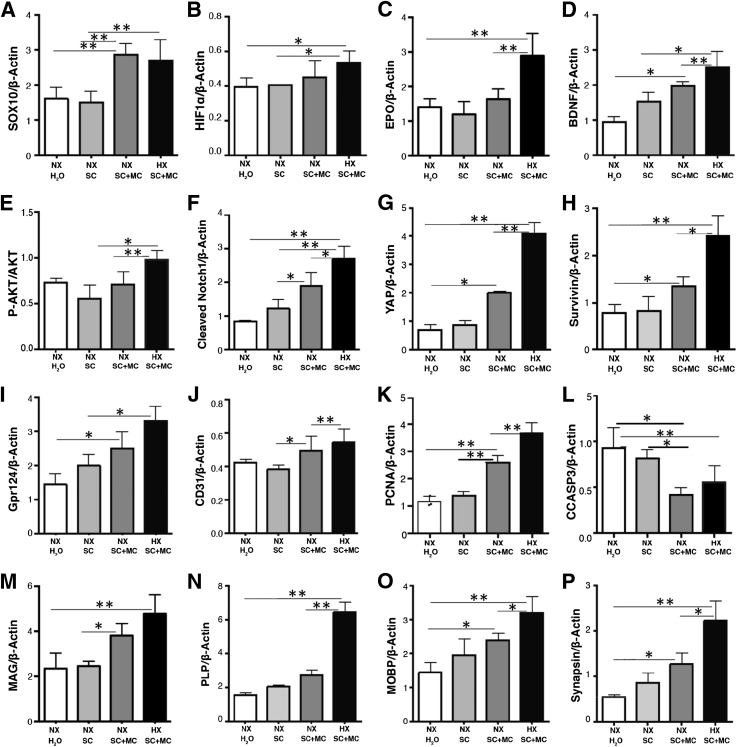
When analyzed by Western blotting at P20, minocycline treatment of reduced oxygen–reared C57BL/6 pups elicited statistically significant increases in Sox10, HIF-1α, EPO, BDNF, pAKT, cleaved notch1, YAP, survivin, GPR124, CD31, PCNA, MAG, MOBP, PLP, and synapsin, while exhibiting a decrease in cleaved caspase 3 in isolated SVZ tissues compared to vehicle-only treated pups (Figure 2, A–P).
Figure 2.
Western blot analysis of postnatal day 20 (P20) isolated SVZ tissues harvested from normoxia-reared nursing pups treated with either plain water, 5% sucrose (vehicle), or minocycline and reduced oxygen–reared nursing pups treated with either plain water, 5% sucrose (vehicle), or minocycline. Minocycline treatment of hypoxic-reared nursing C57BL/6 pups elicits statistically significant increases in Sox10 (A), HIF-1α (B), EPO (C), BDNF (D), phospho-AKT (E), cleaved notch1 (F), YAP (G), survivin (H), GPR124 (I), CD31 (J), PCNA (K), MAG (M), PLP (N), MOBP (O), and synapsin (P), and decreases in CCASP3(L) compared to vehicle-only and normoxia-reared pups. Data are expressed as means ± SD. n = 3. ∗P < 0.05, ∗∗P < 0.005. CCASP3, cleaved caspase 3; EPO, erythropoietin; GPR, G-protein coupled receptor; H2O, water-treated; HIF, hypoxia-inducible factor; HX, hypoxic; MAG, myelin associated glycoprotein; MC, minocycline-treated; MOBP, myelin-associated oligodendrocyte basic protein; NX, normoxic; PCNA, proliferating cell nuclear antigen; PLP, proteolipid protein; SC, sucrose (vehicle); SVZ, subventricular zone; YAP, yes-associated protein 1.
There were no detected differences between water-fed and 5% sucrose-fed animals in any of the proteins analyzed at the P20 time point. Interestingly, minocycline treatment also raised Sox10 expression to the same levels in normoxic and reduced oxygen conditions (Figure 2A). Additionally, BDNF, cleaved notch1, YAP, survivin, GPR124, CD31, PCNA, MAG, MOBP, and synapsin levels were also noted to be increased following minocycline treatment under both normoxic and reduced oxygen conditions (Figure 2, D–K, M, O, and P, respectively). In addition, Sox10 levels (Figure 2A), GPR124, and CD31 (Figure 2, I and J) levels were increased in pups receiving vehicle-treated water (5% sucrose) and minocycline-treated water under normoxic conditions as well. BDNF, YAP (Figure 2, D and G), and MOBP (Figure 2O) levels were increased in pups receiving minocycline-treated water under normoxic conditions also. A complete listing of the significance values comparing the Western blot data for all of the conditions illustrated in Figure 2 are listed in Supplemental Table S6.
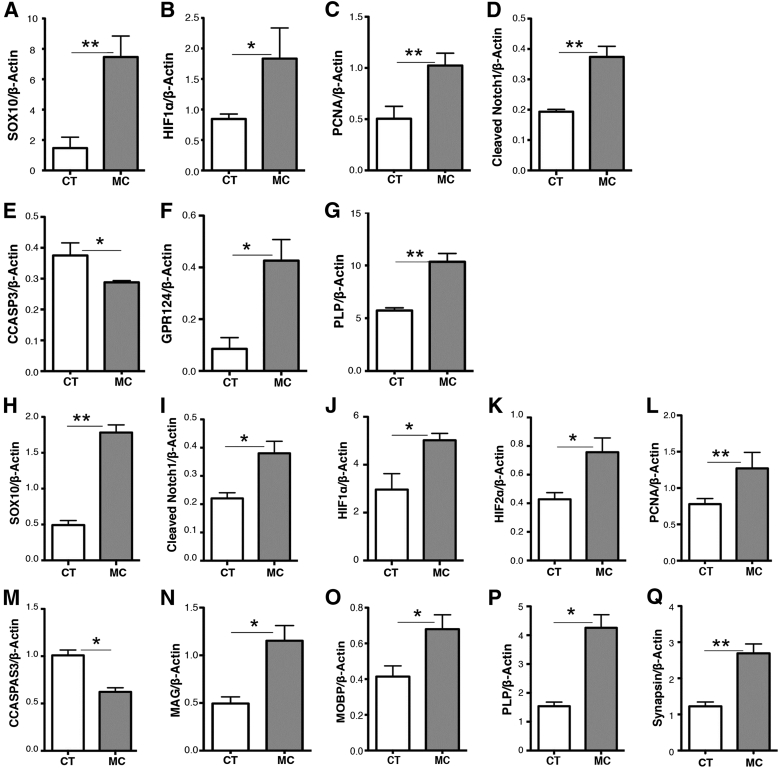
When analyzed by Western blotting at P40, minocycline treatment of normoxic and reduced oxygen–reared C57BL/6 pups also revealed statistically significant increases in Sox10, HIF-1α, EPO, BDNF, pAKT, cleaved notch1, YAP, Survivin, PCNA, MAG, MOBP, PLP, synapsin, Gpr 124, and CD31 in isolated SVZ tissues compared to vehicle-only treated pups. P40-harvested SVZ tissues of minocycline-treated, reduced oxygen–reared C57BL/6 pups also exhibited a decrease in cleaved caspase 3 (Supplemental Figure S3, A–P). As observed in the P20 Western blot data, minocycline treatment also raised Sox10 expression to the same levels in normoxic and reduced oxygen conditions (Supplemental Figure S3A). BDNF, YAP, Gpr 124, CD31, MOBP, and synapsin levels were also noted to be increased following minocycline treatment in both normoxic and reduced oxygen conditions (Supplemental Figure S3, D, E, G, I, J, and O, respectively). A complete listing of the significance values comparing the Western blot data of all of the conditions illustrated in Supplemental Figure S3 are listed in Supplemental Table S7.
At P70, similar changes were noted when comparing Western blot data from reduced oxygen-reared minocycline-treated pups with normoxic vehicle-only (CT) treated pups (Figure 3, A–G). Specifically, Sox10, HIF-1α, PCNA, cleaved notch-1, Gpr 124, and PLP were still elevated and cleaved caspase 3 levels decreased compared to normoxic vehicle-only treated pups. In addition to our findings in our analysis of SVZ tissues at P70, we analyzed whole cerebral tissue lysates harvested from P70 reduced oxygen–reared minocycline-treated and normoxic vehicle-only mice. Similar to our findings in SVZ tissues, we observed increases in Sox10, cleaved notch1, HIF-1α, and 2α, PCNA, MAG, MOBP, PLP, and synapsin (Figure 3, H–L and N–Q) and decreases in cleaved caspase 3 (Figure 3M) in Western blot analyses.
Figure 3.
Western blot analysis of postnatal day 70 (P70) isolated SVZ tissues harvested from reduced oxygen–reared nursing pups treated with either 5% sucrose (vehicle), or minocycline. A–G: Similar to the changes noted in SVZ tissues at P20 and P40, Sox10 (A), HIF-1α (B), PCNA (C), cleaved notch1 (D), CCASPAS3 (E), GPR124 (G), and PLP (G) are still elevated in the minocycline-treated pups compared to the vehicle-only treated pups. Changes noted in the cerebral cortex mirror those noted in the SVZ tissues at P20 and P40. Specifically, significant increases are noted in Sox10 (H), cleaved notch1 (I), HIF-1α (J), HIF-2α (K), PCNA (L), MAG (N), MOBP (O), PLP (P), and synapsin (Q) and decreases in CCASPAS3 (M) in minocycline treated pups. Data are expressed as means ± SD. n = 3. ∗P < 0.05, ∗∗P < 0.005. CCASPAS3, cleaved caspase 3; CT, control; GPR, G-protein coupled receptor; HIF, hypoxia-inducible factor; IB, immunoblot; MAG, myelin associated glycoprotein; MC, minocycline-treated; MOBP, myelin-associated oligodendrocyte basic protein; PCNA, proliferating cell nuclear antigen; PLP, proteolipid protein; SVZ, subventricular zone.
Immunofluorescence Analyses Confirms Increases in Nestin, Ki-67, PCNA, EPO, BDNF, GFAP, Synapsin, Survivin, YAP, CD31, and Myelin and Decreases in Cleaved Caspase 3 in Minocycline-Treated C57BL/6 Pups at P20 and P70
To confirm our tissue culture and Western blot analyses, we performed immunofluorescence analysis of SVZ tissues and cerebral tissues harvested from the P20 and P70 water-only, vehicle-only, and minocycline-treated pups.
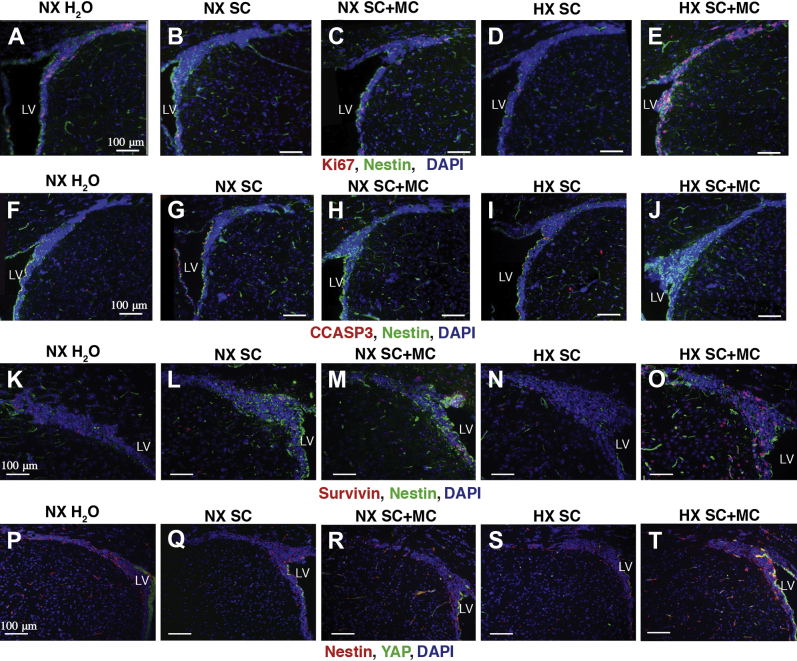
At P20, SVZ tissue harvested from reduced oxygen–reared, minocycline-treated pups exhibited increased NSC proliferation as evidenced by increased Ki-67 and nestin colocalization compared to normoxic water-treated, vehicle (5% sucrose)-treated, and reduced oxygen–reared vehicle-treated pups (Figure 4). Interestingly, normoxia-reared, minocycline-treated pups also exhibited increased proliferation, albeit at a decreased level compared to the reduced oxygen–reared, minocycline-treated pups (Figure 4, A–E), Conversely, reduced NSC cleaved caspase 3 activation was observed in reduced oxygen–reared minocycline-treated pups. Again, normoxia-reared, minocycline-treated pups also exhibited decreased caspase 3 activation, albeit at a lesser level compared to the reduced oxygen–reared, minocycline-treated pups (Figure 4, F–J). Consistent with the increase noted in Ki-67 staining in NSC, increased survivin–nestin colocalization in the SVZ was observed in the reduced oxygen–reared, minocycline-treated pups (Figure 4, K–O). In addition, increased SVZ nuclear YAP–nestin colocalization in NSC was also observed in the reduced oxygen–reared, minocycline-treated pups compared to the other conditions (Figure 4, P–T).
Figure 4.
Immunofluorescence analysis of postnatal day 20 (P20) isolated SVZ tissues harvested from normoxia-reared nursing pups treated with either plain water, 5% sucrose (vehicle), or minocycline, and reduced oxygen–reared nursing pups treated with either plain water, 5% sucrose (vehicle), or minocycline. A–E: Immunofluorescence analyses of P20 NX H2O, NX SC, NX SC+MC, HX SC, and HX SC+MC pups reveals increased NSC proliferation as evidenced by increased Ki-67 and nestin colocalization compared to normoxic water-treated, vehicle-treated, minocycline-treated, and reduced-oxygen vehicle-treated pups. By contrast, reduced NSC CCASP3 activation is observed in hypoxic minocycline-treated pups (F–J). Consistent with the decreased CCASP3 activation and the increase noted in Ki-67 expression in NSC, increased nuclear survivin (K–O) and YAP (P–T) colocalizations in NSC were observed in reduced oxygen, minocycline-treated pups compared to the other conditions. n = 3. Scale bars: 100 μm. CCASP3, cleaved caspase 3; HX SC, hypoxic vehicle-treated; HX SC+MC, minocycline-treated; LV, lateral ventricle; NSC, neural stem cell; NX H2O, normoxic water-treated; NX SC, vehicle (5% sucrose)-treated; NX SC+MC, minocycline-treated; SVZ, subventricular zone; YAP, yes-associated protein 1.
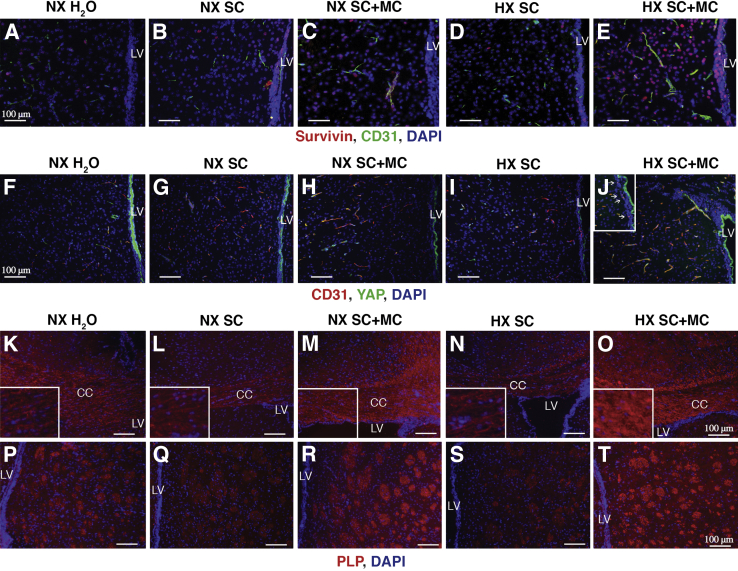
SVZ tissue harvested at P20 from reduced oxygen–reared, minocycline-treated pups exhibited increased microvascular endothelial cell proliferation as evidenced by increased survivin and CD31 colocalization compared to normoxic water-treated, vehicle (5% sucrose)-treated, and normoxic minocycline-treated and hypoxic vehicle-treated pups (Figure 5, A–E), Consistent with these findings, we again observed modestly increased YAP colocalization with CD31 in microvascular endothelial cells in the subventricular region area and throughout the vessel segments in hypoxic minocycline-treated pups (Figure 5, F–J).
Figure 5.
Postnatal day 20 (P20) immunofluorescence analysis of survivin and YAP-isolated SVZ (A–J), CC (K–O), and cortical fiber (P–T) tissues harvested from normoxia-reared nursing pups treated with either plain water, 5% sucrose (vehicle), or minocycline, and reduced oxygen–reared nursing pups treated with either plain water, 5% sucrose (vehicle), or minocycline at P20. A–J: Immunofluorescence analyses of P20 NX H2O, NX SC, NX SC+MC, HX SC, and HX SC+MC pups reveal increased endothelial survivin (A–F) and YAP (F–J) expression as evidenced by increased survivin and CD31 colocalization and YAP and CD31 colocalization in reduced oxygen, minocycline-treated pups compared to NX H2O, NX SC, and HX SC+MC pups. White arrows in J, inset, denote YAP–CD31 endothelial cell colocalization. K–T: Immunofluorescence analyses of P20 NX H2O, NX SC, NX SC+MC, HX SC, and HX SC+MC pups reveals increased PLP expression in the CC (K–O) and cortical fiber tracks (P–T) in reduced oxygen minocycline-treated pups compared to NX H2O, NX SC, and hypoxic vehicle-treated pups. Pups reared in normoxic conditions treated with minocycline exhibit increased PLP expression compared to NX H2O–treated, and NX SC–treated pups. n = 3. Scale bars: 100 μm. CC, corpus callosum; HX SC, reduced oxygen, vehicle-treated; HX SC+MC, minocycline-treated; LV, lateral ventricle; NX H2O, normoxic water-treated; NX SC, vehicle (5% sucrose)-treated; NX SC+MC, minocycline-treated; PLP, proteolipid protein; SVZ, subventricular zone; YAP, yes-associated protein 1.
To ascertain the effects of minocycline on myelination at P20, we used anti-PLP to assess relative PLP expression levels in the corpus callosum and myelinated fiber tracts in the cortex, respectively. We compared staining intensities in normoxic water-treated, vehicle (5% sucrose)-treated, and minocycline-treated pups and reduced oxygen–reared vehicle-treated and minocycline-treated pups. Increased labeling intensities of PLP in the corpus callosum of pups reared in reduced oxygen and treated with minocycline compared to pups receiving vehicle alone and pups reared in normoxia, receiving plain water, vehicle alone, or minocycline was observed (Figure 5, K–O). Pups raised in normoxia and treated with minocycline exhibited modestly increased PLP labeling intensities compared to pups treated with plain water or vehicle only, but less labeling compared to reduced oxygen–reared pups receiving minocycline.
Similar labeling intensities were noted when staining myelinated fiber tracts in the cortex using anti-PLP. Specifically, increased labeling intensities of PLP in the cortical fiber tracts of pups reared in reduced oxygen and treated with minocycline compared to pups receiving vehicle alone and pups reared in normoxia, receiving plain water, vehicle alone, or minocycline were noted (Figure 5, P–T). Also, as noted in stains of the corpus callosum, pups reared in normoxia and treated with minocycline exhibited modestly increased PLP labeling intensities compared to pups treated with plain water or vehicle only, but less labeling intensities compared to reduced oxygen–reared pups receiving minocycline.
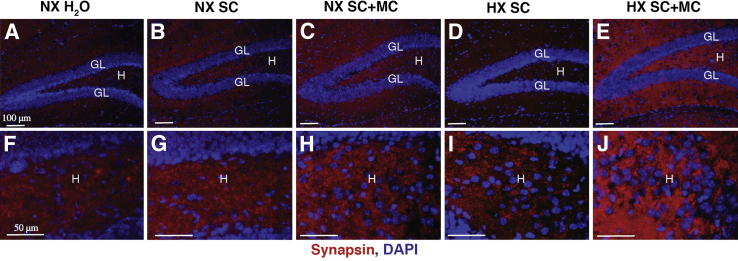
Increased punctate labeling intensities of synapsin in the hilus region of the hippocampus, bounded by the granular layer of P20 pups reared in reduced oxygen and treated with minocycline compared to pups receiving vehicle alone and pups reared in normoxia, receiving plain water, vehicle alone, or minocycline was observed (Figure 6, A–E). Higher-power micrographs illustrating labeling in the hilus regions of the hippocampus of pups reared in hypoxia and treated with either vehicle alone or minocycline revealed significant increases in punctate labeling patterns in the minocycline-treated pups, indicative of increased synapse formation (Figure 6, F–J).
Figure 6.
Immunofluorescence analysis of postnatal day 20 (P20) isolated hippocampal tissues harvested from normoxia-reared nursing pups treated with either plain water, 5% sucrose (vehicle), or minocycline, and hypoxia-reared nursing pups treated with either plain water, 5% sucrose (vehicle), or minocycline. Immunofluorescence analyses of P20 NX H2O, NX SC, NX SC+MC, HX SC, and HX SC+MC pups reveals increased synapsin expression in the hilus region of the hippocampus (A–J) in hypoxic, minocycline-treated pups compared to NX H2O, NX SC, and HX SC–treated. Pups reared in normoxic conditions treated with minocycline exhibit increased synapsin expression compared to NX H2O and NX SC–treated pups. n = 3. Scale bars: 100 μm (A–E); 50 μm (F–J). GL, granular layer; H, hilus region of the hippocampus; HX SC, hypoxic vehicle-treated; HX SC+MC, minocycline-treated; NX H2O, normoxic water-treated; NX SC, vehicle (5% sucrose)-treated; NX SC+MC, minocycline-treated.
Immunofluorescence analyses of P70 vehicle-only and minocycline-treated C57 pup SVZ and cerebral tissues revealed increased SVZ proliferation; increased SVZ endothelial cell proliferation, and decreased SVZ apoptosis of neural progenitors; and increased PLP expression in corpus callosum and myelinated fiber tracts, and increased expression of synapsin in the hippocampus of minocycline-treated pups (Supplemental Figure S4). Frozen sections of the SVZ zone of vehicle-only and minocycline-treated P70 pups revealed increased proliferation of neural progenitors, labeled bright pink due to colocalization of nestin and Ki-67, in the minocycline-treated pups (Supplemental Figure S4, A and B). By contrast, apoptosis (denoted by colocalization of nestin and cleaved caspase 3) was significantly decreased in minocycline-treated pups (Supplemental Figure S4, C and D). Sections of the SVZ zone of vehicle-only and minocycline-treated pups revealed increased proliferation of SVZ microvascular endothelial cells, labeled bright yellow due to colocalization of CD31 and PCNA, in the minocycline-treated pups (Supplemental Figure S4, E and F). Sections of the corpus callosum and cortex of vehicle-only and minocycline-treated pups revealed increased expression of PLP in the corpus callosum (Supplemental Figure S4, G–J) and myelinated fiber tracts in the cortex (Supplemental Figure S2, K and L) in the minocycline-treated pups compared to the vehicle-only treated pups.
Sections of the hippocampus of vehicle-only and minocycline-treated pups revealed increased punctate expression of synapsin in the hilus region of the dentate gyrus bounded by the granular layer in the minocycline-treated pups compared to the vehicle-only treated pups (Supplemental Figure S4, M–R).
Interestingly, brain weight determinations at P70 revealed increased average brain weights in the minocycline-treated pups compared to vehicle-only treated pups. Specifically, reduced-oxygen, vehicle-treated pups had an average brain weight of 0.44 g, whereas reduced-oxygen, minocycline-treated pups had an average brain weight of 0.48 g (n = 8, P < 0.005).
Taken in aggregate, these data are consistent with our biochemical and behavioral data (below).
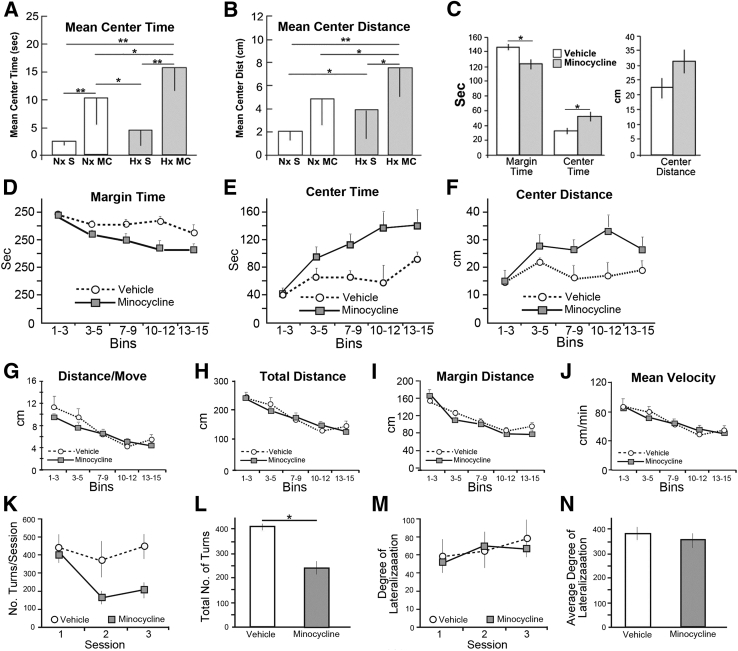
Reduced Oxygen–Reared, Minocycline-Treated C57BL/6 Pups Exhibit Increased Center Time and Distance in Open Field Testing and Decreased Turns in Free Swim Testing Compared to Untreated Pups
To investigate whether minocycline treatment had any effect on the behavior of pups reared in the reduced oxygen environment (10% O2) from P3 to P11, we monitored open field activity at P20. We compared vehicle-only and minocycline-treated pups reared under normoxic and reduced oxygen conditions from P3 to P11 in a cohort of C57BL/6 mice consisting of normoxic-reared, vehicle-only, minocycline-treated, reduced oxygen–reared vehicle-only, and minocycline-treated P20 C57BL/6 pups. Minocycline-treated, reduced oxygen–reared pups exhibited significantly greater center time (Figure 7A) and center distance (Figure 7B) compared to hypoxic-reared vehicle-only treated pups, and normoxic-reared minocycline-treated pups exhibited greater center time compared to normoxic-reared vehicle-only treated pups (Figure 7A). When we compared a cohort composed of reduced oxygen–reared vehicle-only to minocycline-treated (P20 pups, we again observed significant differences in margin time and center time and a trend in center distance in minocycline-treated pups compared with vehicle-only treated pups (Figure 7C). Further, plots of days from which these aggregate values were calculated also revealed the differences between the vehicle-only and minocycline-treated pups, with minocycline treatment reducing margin time (Figure 7D) and increasing center time (Figure 7E) and center distance (Figure 7F). When distance per move, total distance, margin distance, and mean velocity (Figure 7, G–J) were assessed, no appreciable differences were noted.
Figure 7.
A–N: Postnatal day 20 (P20) open field and P40 free swim activities of hypoxic-reared minocycline-treated and vehicle-only treated C57BL/6 pups. A and B: In a cohort of 33 pups (15 males and 18 females) consisting of eight Nx S, nine Nx MC, five Hx S, and 11 Hx MC pups, P20 open field activities were assessed. Hx MC–reared pups exhibit greater center time (A) and center distance (B) compared to Hx S treated pups. B: Nx MC pups exhibit greater center time compared to Nx S treated pups. C–N: In a second smaller cohort composed of 11 vehicle-only and 14 minocycline-treated pups (12 female and 13 male; total 25 pups), P20 open field activities were assessed. Hx MC pups (shaded boxes) exhibit statistically less margin time and greater center time and a trend toward greater center distance compared to the Nx S (open boxes). D–F: P20 open field activities of Hx MC pups (open squares and dashed lines) exhibit statistically less margin time (D) and greater center time (E) and center distance (F) compared to the vehicle-treated cohorts (open circles and dotted lines). When the values in each bin were summed and averaged, the changes in mean margin and center times are statistically significant (P < 0.05), whereas the differences in mean center distance exhibits a trend toward greater center distance in the minocycline-treated cohort (C). G–J: No appreciable differences between the vehicle-only and minocycline-treated cohorts are noted when distance or movement (G), total distance (H), margin distance (I), and mean velocity (J) are assessed. K–N: Hx MC (gray squares and dashed lines) and vehicle-only treated (open circles and dotted lines) C57BL/6 pups exhibit distinct behaviors in the free swim test at P40. Minocycline-treated pups exhibit decreases in total turns compared to vehicle-only treated pups when the three timed sessions (K) and the total numbers of turns (L) are plotted. By contrast, minocycline- and vehicle-only treated pups exhibit no appreciable differences in degree of lateralization when the three timed sessions (M) and the average degree of lateralization (N) are plotted. Data are expressed as means ± SD. ∗P ≤ 0.05, ∗∗P ≤ 0.005. Hx MC, hypoxic minocycline-treated; Hx S, hypoxic vehicle-only; Nx MC, normoxic minocycline-treated; Nx S, normoxic vehicle-only.
In addition, the free swim test at P40 revealed that reduced oxygen–reared minocycline-treated P40 pups exhibited decreased total turns compared to vehicle-only treated pups (Figure 7, K and L). By contrast, reduced oxygen–reared minocycline-treated and vehicle-only treated P40 pups exhibited no appreciable differences in degree of lateralization (Figure 7, M and N).
Discussion
Treatments including prenatal maternal steroids, postnatal administration of indomethacin and other drugs,50 oxygen supplementation,51 the administration of thyroxine,52 and epidermal growth factor receptor (EGFR) stimulation following the hypoxic insult53 in the premature newborn have and may reduce some of the untoward effects of premature delivery. Although encouraging and having potential as therapeutic agents, these treatments may have side effects. For example, detrimental effects of transient intranasal EGF treatment due to increased EGFR activation in other organs, specifically the kidney during this period of development, should be considered.54, 55
In a recent study using our murine model of chronic sublethal hypoxia in the premature newborn, we began to elucidate the global nature of responsiveness to reduced-oxygen insult using an unbiased transcriptome approach.23 The identification of a particular transcription factor, Sox10, which we found to modulate genes important in several aspects of proliferation, apoptosis, neural development, and maintenance, was decreased in reduced oxygen–reared C57BL/6 pups, but not in CD1 pups.23 The lack of reduction of Sox10 observed in CD1 pup SVZ coupled with the lack of down-regulation of genes known to be downstream of this transcription factor, several of which are known to be associated with increased proliferation, decreased apoptosis, increased myelination and synapse formation, and improvement of cognitive function, are consistent with the notion that maintenance of appropriate levels of Sox10 may be beneficial in blunting or ameliorating untoward effects of chronic sublethal hypoxia in the very premature newborn.23 This and data illustrated in Figures 1 and 2 led us to further investigate the effects of minocycline on normoxic and reduced oxygen–reared C57BL/6 pups that we have shown to be prone to cognitive handicaps following chronic sublethal hypoxia.14
Here, we investigated the use of minocycline, a tetracycline antibiotic with known noncanonical properties including inhibition of MMPs, scavenging reactive oxygen species, inhibiting PARP, inhibiting the mitochondrion calcium uniporter, down-regulating cytokine expression, and up-regulating SOD isoforms, as a modulator of Sox10 expression in NSC,24, 25, 26, 27, 28, 29, 30 as a potential modulator of behaviors in our sublethal hypoxia murine model of premature delivery. Our analysis of cultured NSC, SVZ tissues, and whole cerebral tissues harvested from P20, P40, and P70 mice revealed increases in Sox10 and several of the proteins known to be downstream of this transcription factor, several of which are known to be associated with increased proliferation, decreased apoptosis, increased myelination and synapse formation, and improvement of cognitive function.23
Minocycline Treatment Up-Regulates Sox10 Expression and Selected Genes Associated with Neuronal and Vascular Responses
Specifically, we found that minocycline treatment differentially up-regulates Sox10 expression in hypoxic C57BL/6 NSC cultures in a dose-dependent fashion. Additionally, in our murine model of chronic sublethal hypoxia in the premature newborn, transient minocycline treatment elicits up-regulation of a number of genes including SOX10, HIF1A, EPO, and BDNF, all of which have been found to be associated with responses of neuronal and vascular cells to hypoxic insult.28, 56, 57, 58, 59, 60, 61 Additionally, several genes associated with proliferation, cell survival, and neuronal differentiation including YAP, survivin, cleaved notch1, PCNA, pAKT, and genes associated with myelination (MAG, MOBP, PLP) and angiogenesis [GPR124 (ADGRA2) and CD31 (PECAM1)] were increased, whereas cleaved caspase 3 levels were decreased, consistent with increases in proliferation and reduction of apoptosis in NSC and facilitating vascularization during recovery. Further, brain weights of minocycline-treated pups were noted to be heavier than vehicle-alone treated pups.
Minocycline Treatment Results in Increased Survival, Proliferation, Decreased Apoptosis, and Increased and Improved Cognitive Outcome
Our findings of increased minocycline-mediated Sox1023 and HIF-1α,14, 18, 19 as well as YAP and survivin induction62, 63 are consistent with an emerging dynamic interaction among these important modulators of the hypoxic response.34 Specifically, minocycline-induced Sox10 and HIF-1α (and their downstream signaling pathways14, 18, 19) and myelination-associated genes,23 also up-regulate YAP and survivin, both of which also positively influence each other's expression,63 increasing proliferation and decreasing apoptosis.63 Furthermore, investigators have recently found that hypoxia-induced SIAH2, an E3 ligase, destabilizes LATS2, reducing Hippo pathway activation, increasing YAP signaling, and affecting proliferation and apoptosis.34 They also determined that YAP complexes with HIF-1α in the nucleus, stabilizing HIF-mediated signaling, affecting survival, proliferation, and apoptosis.34 In addition, investigators found that SIAH2 reduces prolyl hydroxylase domain (PHD) enzyme activities, reducing HIF-1α degradation. Given these data, we postulate that minocycline-mediated induction of Sox10, HIF-1α, YAP, and survivin elicits increased survival and proliferation, and decreased apoptosis following hypoxic insult and also increased myelination, resulting in an improved cognitive outcome (Supplemental Figure S5).
Immunofluorescence Localization Studies Confirm Increased Protein Levels
In addition, our immunofluorescence data confirmed our biochemical data. Specifically, both SVZ NSC and microvascular endothelial cells exhibited increases in proliferation in response to minocycline. This coupled increase in both cell types is consistent with our previous studies that documented coupled increases in both cell types in in vivo and in vitro studies following hypoxic (reduced oxygen) insult.14, 15, 19, 42 Additionally, the up-regulation of myelin-associated genes is consistent with our behavioral studies.
Minocycline-Treated Pups Are Less Anxious and More Exploratory Compared to Vehicle-Only Treated Controls
Consistent with our biochemical and immunofluorescence analyses, the results of the behavioral analysis examining open field activity and spatial memory using the free swim test suggest that there are substantial differences in the impact of minocycline treatment on reduced oxygen–reared C57BL/6 pups compared to vehicle-only treated pups. Specifically, minocycline-treated pups exhibited decreased margin time and increased center time and distance in open field testing when tested at P20, suggesting that the minocycline-treated treated pups were less anxious and more exploratory compared to vehicle-only controls. Free swim testing at P40 revealed no differences in responsiveness to minocycline treatment, when degree of lateralization was assessed. By contrast, minocycline-treated pups exhibited decreased total turns compared to vehicle-only treated pups in the free swim test.
Minocycline-Treated Pups Exhibit Decreased Average and Total Number of Turns Compared to Vehicle-Only Treated Pups, Affecting Emotional Responses and/or Activity Levels of the Pups, Resulting in Improved Cognitive Behavior
Studies using the free swimming test have indicated that variations in rotational preference and lateralization for this task are linked to patterns of callosal connectivity and behavioral lateralization of the cerebral hemispheres. Additionally, the earlier the age at which callosal alteration occurs, the greater the impact it has on long-term laterality46; evidence has been presented showing that cerebral lateralization is protective in the very prematurely born.65 Our analysis revealed that although the degree of lateralization was not affected following minocycline treatment, the average and total number of turns in minocycline-treated pups was decreased following minocycline treatment compared to vehicle-only treated pups. The effect of minocycline treatment on total turns in the C57BL/6 pups may reflect an effect on the emotional responses and/or activity levels of the pups not apparent in the untreated pups, which is consistent with the significant differences noted in margin time, center time, and a trend in center distance in minocycline-treated pups, with minocycline treatment reducing margin time and increasing center time and center distance.
These studies i) demonstrate the beneficial effects of minocycline treatment following hypoxic insult and add to the growing literature of this repurposed therapeutic as a beneficial agent in promoting neuroprotection, neuronal remodeling, and improving neuronal behaviors64, 66, 67; ii) document the up-regulation of Sox10, HIF1A, and several of the genes associated with proliferation and apoptosis (survivin and YAP), myelination in neurogenic zones and improved cognitive function; and iii) support the possibility of minocycline as a potential transient therapeutic drug in the treatment of the neurodevelopmental handicaps observed in the very premature newborn population. In addition, these studies may aid in the further interpretation of minocycline's effects in the ongoing treatment trials and animal model studies of fragile X syndrome and multiple sclerosis.28, 29, 41 As with other potential treatments proposed in the treatment of chronic sublethal hypoxia and its sequel in the premature newborn, additional investigations are warranted to elucidate potential untoward effects during this critical state of development.68, 69, 70
Footnotes
Supported in part by US Public Health Service NIH grants PO1-NS00344738 (J.A.M. and M.S.), R01HD062007, a Reed Foundation Fellowship and an unrestricted gift from Joseph and Lucille Madri (J.A.M.), a grant-in-aid from Japan Society for the Promotion of Science (JSPS) research fellowship for young scientists (No. 23-1154), a JSPS postdoctoral fellowship for research abroad (No. 513) and the Uehara Memorial Foundation postdoctoral fellowship for study abroad (No. 201230024) (M.T.), and a Yale University Clinical and Translational Science award BUL1TR000142 (M.K.).
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2015.05.016.
Supplemental Data
Representative autoradiograph images of C57BL/6 and CD1 neural stem cell (NSC) lysates blotted with anti-cleaved caspase 3, anti-Sox10, anti–HIF-1α, anti–cleaved notch1, anti-PCNA, and anti–β-actin, and C57/BL6 and CD1 postnatal day 20 (P20) and P40 SVZ tissue lysates blotted with anti-Sox10, anti–HIF-1α, anti-EPO, anti-BDNF, anti–phospo-Akt, anti-Akt, anti–cleaved notch1, anti-YAP, anti-survivin, anti-GPR124, anti-CD31, anti-PCNA, anti-cleaved caspase 3, anti-MAG, anti-PLP, anti-MOBP, anti-synapsin, and anti–β-actin. BDNF, brain-derived neurotrophic factor; CT, control; EPO, erythropoietin; GPR, G-protein coupled receptor; HIF, hypoxia-inducible factor; HX, hypoxic; MAG, myelin associated glycoprotein; MOBP, myelin-associated oligodendrocyte basic protein; NX, normoxic; PCNA, proliferating cell nuclear antigen; PLP, proteolipid protein; SVZ, subventricular zone; YAP, yes-associated protein 1.
Ratios of control/control [CT/CT] and [minocycline]/control ([MC]/CT) for normoxic- and reduced oxygen-cultured C57BL/6 and CD1 neural stem cells (NSC). Using the data accrued in our Western analysis of normoxic- and reduced oxygen–cultured C57BL/6 and CD1 NSC illustrated in Figure 1, we illustrated the effects of minocycline using ratios of the expression of each protein following minocycline treatment divided by the expression of each protein without minocycline treatment. The ratios of minocycline-treated divided by untreated expression levels increases from 1.0 only in the reduced oxygen, minocycline-treated C57BL6/B NSC. Additionally, dramatic decreases in ratios are noted at 50 mmol/L minocycline in all four protein determinations. (A, Sox10; B, HIF-1α; C, cleaved notch1; D, PCNA). HIF, hypoxia-inducible factor; HX, hypoxic; NX, normoxic; PCNA, proliferating cell nuclear antigen.
Western blot analysis of postnatal day 40 (P40) isolated subventricular zone (SVZ) tissues harvested from normoxia-reared nursing pups treated with either plain water, 5% sucrose (vehicle), or minocycline, and reduced oxygen–reared nursing pups treated with either plain water, 5% sucrose (vehicle), or minocycline. Similar to our results at the P20 time point, minocycline treatment of hypoxic-reared nursing C57BL/6 pups elicits statistically significant increases in Sox10 (A), HIF-1α (B), EPO (C), BDNF (D), phospho-AKT (E), cleaved notch1 (F), YAP (G), survivin (H), GPR124 (I), CD31 (J), PCNA (K), MAG (M), PLP (N), MOBP (O), and synapsin (P) and decreases in CCASP3 (L) compared to vehicle-only and normoxia-reared pups. Specifically, SVZ tissues of minocycline-treated, hypoxic-reared C57BL/6 pups exhibited increases in expression of Sox10, HIF-1α, HIF-2α, EPO, BDNF, phospho-AKT, PCNA, cleaved notch1 (proliferation markers); GPR124 and CD31 (microvascular endothelial cell markers); MAG, MOBP, and PLP (components of myelin); and synapsin (a component of synapses), while exhibiting a decrease in CCASP3 (an apoptosis marker). Data are expressed as means ± SD. n = 3. ∗P < 0.05, ∗∗P < 0.005, and ∗∗∗P < 0.0005. BDNF, brain-derived neurotrophic factor; CCASP3, cleaved caspase 3. EPO, erythropoietin; GPR, G-protein coupled receptor; HIF, hypoxia-inducible factor; HX, hypoxic; MAG, myelin associated glycoprotein; MC, minocycline-treated; MOBP, myelin-associated oligodendrocyte basic protein; NX, normoxic; PCNA, proliferating cell nuclear antigen; PLP, proteolipid protein; SC, sucrose (vehicle); SVZ, subventricular zone; YAP, yes-associated protein 1.
Immunofluorescence analyses of postnatal day 70 (P70) vehicle-only and minocycline-treated C57 pup SVZ and cerebral tissues reveals increased SVZ proliferation and decreased SVZ apoptosis of neural progenitors; increased PLP expression in CC and myelinated fiber tracts and increased expression of synapsin in the hippocampus of minocycline-treated pups. A and B: Frozen sections of the SVZ zone of vehicle-only and minocycline-treated pups revealed increased proliferation of neural progenitors, labeled bright pink due to colocalization of nestin and Ki-67, in the minocycline-treated pups. C and D: By contrast, apoptosis (denoted by colocalization of nestin and cleaved caspase 3) is significantly decreased in minocycline-treated pups. E and F: Frozen sections of the SVZ zone of vehicle-only and minocycline-treated pups reveal increased proliferation of SVZ microvascular endothelial cells, labeled bright yellow due to colocalization of CD31 and PCNA, in the minocycline-treated pups. Nestin is indicated by green fluorescence, Ki-67 and cleaved caspase 3 by red fluorescence, CD13 by red fluorescence, PCNA by green fluorescence, and DAPI by blue fluorescence. Frozen sections of the corpus callosum and cortex of vehicle-only and minocycline-treated pups reveal increased expression of PLP in the corpus callosum (G–J) and myelinated fiber tracts in the cortex (K and L) in the minocycline-treated pups compared to the vehicle-only treated pups. PLP is indicated by red fluorescence, DAPI by blue fluorescence. Frozen sections of the hippocampus of vehicle-only and minocycline-treated pups reveal increased punctate expression of synapsin in the hilus region (H) of the dentate gyrus bounded by the GL in the minocycline-treated pups compared to the vehicle-only treated pups (M–R). Synapsin is indicated by red fluorescence, DAPI by blue fluorescence. CC, corpus callosum; GL, granular layer; LV, lateral ventricle; PCNA, proliferating cell nuclear antigen; PLP, proteolipid protein; SVZ, subventricular zone.
Working model of a minocycline-induced signaling cascade comprising the induction of Sox10 and HIF-1α (and their downstream signaling pathways and myelination-associated genes); the up-regulation of YAP and survivin, both of which positively influence each other's expression, increasing proliferation and decreasing apoptosis; hypoxia-induced SIAH2, an E3 ligase, destabilizing PHDs and LATS2, reducing Hippo pathway activation, increasing YAP signaling, affecting proliferation and apoptosis; and YAP complexing with HIF-1α in the nucleus, stabilizing HIF-mediated signaling, affecting survival, proliferation, and apoptosis and ultimately leading to improved behaviors. Dashed lines denote data derived from Ma et al.34 The overall scheme was derived from the current data and previous studies.14, 18, 19, 23, 34, 35, 36, 42, 62, 63, 64 HIF, hypoxia-inducible factor; PHD, prolyl hydroxylase domain; YAP, yes-associated protein 1.
References
- 1.Wilson-Costello D., Friedman H., Minich N., Fanaroff A.A., Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115:997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 2.Tyson J.E., Saigal S. Outcomes for extremely low-birth-weight infants: disappointing news. JAMA. 2005;294:371–373. doi: 10.1001/jama.294.3.371. [DOI] [PubMed] [Google Scholar]
- 3.Saigal S., Stoskopf B., Streiner D., Boyle M., Pinelli J., Paneth N., Goddeeris J. Transition of extremely low-birth-weight infants from adolescence to young adulthood: comparison with normal birth-weight controls. JAMA. 2006;295:667–675. doi: 10.1001/jama.295.6.667. [DOI] [PubMed] [Google Scholar]
- 4.Saigal S., Doyle L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 5.Hack M., Flannery D.J., Schluchter M., Cartar L., Borawski E., Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 6.Lee A.C., Kozuki N., Blencowe H., Vos T., Bahalim A., Darmstadt G.L., Niermeyer S., Ellis M., Robertson N.J., Cousens S., Lawn J.E. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74:50–72. doi: 10.1038/pr.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blencowe H., Lee A., Cousens S., Bahalim A., Narwal R., Zhong N., Chou D., Say L., Modi N., Katz J., Vos T., Marlow N., Lawn J.E. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res. 2013;74(Suppl 1):17–34. doi: 10.1038/pr.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schafer R.J., Lacadie C., Vohr B., Kesler S.R., Katz K.H., Schneider K.C., Pugh K.R., Makuch R.W., Reiss A.L., Constable R.T., Ment L.R. Alterations in functional connectivity for language in prematurely-born adolescents. Brain. 2009;132:661–670. doi: 10.1093/brain/awn353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ment L.R., Constable R.T. Injury and recovery in the developing brain: evidence from functional MRI studies of prematurely born children. Nat Clin Pract Neurol. 2007;3:558–571. doi: 10.1038/ncpneuro0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassan H., Feldman H.A., Limperopoulos C., Benson C.B., Ringer S.A., Veracruz E., Soul J.S., Volpe J.J., du Plessis A.J. Periventricular hemorrhagic infarction: risk factors and neonatal outcome. Pediatr Neurol. 2006;35:85–92. doi: 10.1016/j.pediatrneurol.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Paul D.A., Leef K.H., Locke R.G., Bartoshesky L., Walrath J., Stefano J.L. Increasing illness severity in very low birth weight infants over a 9-year period. BMC Pediatr. 2006;6:2. doi: 10.1186/1471-2431-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke R.W. Preterm mortality and morbidity over 25 years. Arch Dis Child Fetal Neonatal Ed. 2006;91:F293–F294. doi: 10.1136/adc.2005.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curristin S.M., Cao A., Stewart W.B., Zhang H., Madri J.A., Morrow J.S., Ment L.R. Disrupted synaptic development in the hypoxic newborn brain. Proc Natl Acad Sci U S A. 2002;99:15729–15734. doi: 10.1073/pnas.232568799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q., Liu J., Michaud M., Schwartz M.L., Madri J.A. Strain differences in behavioral and cellular responses to perinatal hypoxia and relationships to neural stem cell survival and self-renewal: modeling the neurovascular niche. Am J Pathol. 2009;175:2133–2146. doi: 10.2353/ajpath.2009.090354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madri J.A. Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol. 2009;60:95–104. [PubMed] [Google Scholar]
- 16.Ward N.L., Lamanna J.C. The neurovascular unit and its growth factors: coordinated response in the vascular and nervous systems. Neurol Res. 2004;26:870–883. doi: 10.1179/016164104X3798. [DOI] [PubMed] [Google Scholar]
- 17.Sheldon R.A., Sedik C., Ferriero D.M. Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Res. 1998;810:114–122. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- 18.Li Q., Michaud M., Stewart W., Schwartz M., Madri J.A. Modeling the neurovascular niche: murine strain differences mimic the range of responses to chronic hypoxia in the premature newborn. J Neurosci Res. 2008;86:1227–1242. doi: 10.1002/jnr.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q., Michaud M., Canosa S., Kuo A., Madri J.A. GSK-3β: a signaling pathway node modulating neural stem cell and endothelial cell interactions. Angiogenesis. 2011;14:173–185. doi: 10.1007/s10456-011-9201-9. [DOI] [PubMed] [Google Scholar]
- 20.Keane T.M., Goodstadt L., Danecek P., White M.A., Wong K., Yalcin B. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–294. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yalcin B., Wong K., Agam A., Goodson M., Keane T.M., Gan X., Nellåker C., Goodstadt L., Nicod J., Bhomra A., Hernandez-Pliego P., Whitley H., Cleak J., Dutton R., Janowitz D., Mott R., Adams D.J., Flint J. Sequence-based characterization of structural variation in the mouse genome. Nature. 2011;477:326–329. doi: 10.1038/nature10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwemer C.F., Song M.Y., Carello K.A., D'Alecy L.G. Strain differences in response to acute hypoxia: CD-1 versus C57BL/6J mice. J Appl Physiol. 2007;102:286–293. doi: 10.1152/japplphysiol.00536.2006. [DOI] [PubMed] [Google Scholar]
- 23.Li Q., Canosa S., Flynn K., Michaud M., Krauthammer M., Madri J.A. Modeling the neurovascular niche: unbiased transcriptome analysis of the murine subventricular zone in response to hypoxic insult. PLoS One. 2013;8:e76265. doi: 10.1371/journal.pone.0076265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffina M.O., Ceballosc G., Villarreal F.J. Tetracycline compounds with non-antimicrobial organ protective properties: possible mechanisms of action. Pharmacol Res. 2011;63:102–107. doi: 10.1016/j.phrs.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrido-Mesa N., Zarzuelo A., Gálvez A.J. What is behind the non-antibiotic properties of minocycline? Pharmacol Res. 2013;67:18–30. doi: 10.1016/j.phrs.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Daniele Orsucci D., Mancuso M., Filosto M., Siciliano G. Tetracyclines and neuromuscular disorders. Curr Neuropharmacology. 2012;10:134–148. doi: 10.2174/157015912800604498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H.-S., Suh Y.-H. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196:168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 28.Bilousova T.V., Dansie L., Ngo M., Aye J., Charles J.R., Ethell D.W., Ethell I.M. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2013;46:94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- 29.Paribello C., Tao L., Folino A., Berry-Kravis E., Tranfaglia M., Ethell I.M., Ethell D.W. Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol. 2010;10:91. doi: 10.1186/1471-2377-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitz T., Krabbe G., Weikert G., Scheuer T., Matheus F., Wang Y., Mueller S., Kettenmann H., Matyash V., Bührer C., Endesfelder S. Minocycline protects the immature white matter against hyperoxia. Exp Neurol. 2014;254:153–165. doi: 10.1016/j.expneurol.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Weidemann A., Kerdiles Y.M., Knaup K.X., Rafie C.A., Boutin A.T., Stockmann C., Takeda N., Scadeng M., Shih A.Y., Haase V.H., Simon M.C., Kleinfeld D., Johnson R.S. The glial cell response is an essential component of hypoxia-induced erythropoiesis in mice. J Clin Invest. 2009;119:3373–3383. doi: 10.1172/JCI39378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow J., Ogunshola O., Fan S.Y., Li Y., Ment L.R., Madri J.A. Astrocyte-derived VEGF mediates survival and tube stabilization of hypoxic brain microvascular endothelial cells in vitro. Brain Res Dev Brain Res. 2001;130:123–132. doi: 10.1016/s0165-3806(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 33.Karsan A. The role of notch in modeling and maintaining the vasculature. Can J Physiol Pharmacol. 2005;83:14–23. doi: 10.1139/y04-125. [DOI] [PubMed] [Google Scholar]
- 34.Ma B., Chen Y., Chen L., Cheng H., Mu C., Li J., Gao R., Zhou C., Cao L., Liu J., Zhu Y., Chen Q., Wu S. Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat Cell Biol. 2015;17:95–103. doi: 10.1038/ncb3073. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Xia Z.L., Chen L.B. HIF-1-α and survivin involved in the anti-apoptotic effect of 2ME2 after global ischemia in rats. Neurol Res. 2011;33:583–592. doi: 10.1179/1743132810Y.0000000013. [DOI] [PubMed] [Google Scholar]
- 36.Mormile R., Vittori G., Vitale R., Squarcia U. Birth asphyxia and hypothermia therapy: is survivin the orchestrator of recovery? J Pediatr Endocrinol Metab. 2012;25:1045–1046. doi: 10.1515/jpem-2012-0232. [DOI] [PubMed] [Google Scholar]
- 37.Engelhardt B., Liebner S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. 2014;355:687–699. doi: 10.1007/s00441-014-1811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ilan N., Madri J.A. PECAM-1: old friend, new partners. Curr Opin Cell Biol. 2003;15:515–524. doi: 10.1016/s0955-0674(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 39.Committee for the Update of the Guide for the Care and Use of Laboratory Animals . National Academies Press; Washington, DC: 2011. National Research Council: Guide for the Care and Use of Laboratory Animals: Eighth Edition. [Google Scholar]
- 40.Pinter E., Barreuther M., Lu T., Imhof B.A., Madri J.A. Platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) tyrosine phosphorylation state changes during vasculogenesis in the murine conceptus. Am J Pathol. 1997;150:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 41.Rotschafer S.E., Trujillo M.S., Dansie L.E., Ethell I.M., Razak K.A. Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of Fragile X Syndrome. Brain Res. 2012;1439:7–14. doi: 10.1016/j.brainres.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 42.Li Q., Ford M.C., Lavik E.B., Madri J.A. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res. 2006;84:1656–1668. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- 43.Khanna S., Roy S., Maurer M., Ratan R., Sen C.K. Oxygen-sensitive reset of hypoxia-inducible factor transactivation response: prolyl hydroxylases tune the biological normoxic set point. Free Radic Biol Med. 2006;40:2147–2154. doi: 10.1016/j.freeradbiomed.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fagel D.M., Ganat Y., Silbereis J., Ebbitt T., Stewart W., Zhang H., Ment L.R., Vaccarino F.M. Cortical neurogenesis enhanced by chronic perinatal hypoxia. Exp Neurol. 2005;199:77–91. doi: 10.1016/j.expneurol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Filgueiras C.C., Manhaes A.C. Effects of callosal agenesis on rotational side preference of BALB/cCF mice in the free swimming test. Behav Brain Res. 2004;155:13–25. doi: 10.1016/j.bbr.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 46.Manhaes A.C., Abreu-Villaca Y., Schmidt S.L., Filgueiras C.C. Neonatal transection of the corpus callosum affects rotational side preference in adult Swiss mice. Neurosci Lett. 2007;415:159–163. doi: 10.1016/j.neulet.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Schalomon P.M., Wahlsten D. Wheel running behavior is impaired by both surgical section and genetic absence of the mouse corpus callosum. Brain Res Bull. 2002;57:27–33. doi: 10.1016/s0361-9230(01)00633-5. [DOI] [PubMed] [Google Scholar]
- 48.Flynn K.M., Michaud M., Madri J.A. CD44 deficiency contributes to enhanced experimental autoimmune encephalomyelitis: a role in immune cells and vascular cells of the blood-brain barrier. Am J Pathol. 2013;182:1322–1336. doi: 10.1016/j.ajpath.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flynn K.M., Michaud M., Canosa S., Madri J.A. CD44 regulates vascular endothelial barrier integrity via a PECAM-1 dependent mechanism. Angiogenesis. 2013;16:689–705. doi: 10.1007/s10456-013-9346-9. [DOI] [PubMed] [Google Scholar]
- 50.McCrea H.J., Ment L.R. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol. 2008;35:777–792. doi: 10.1016/j.clp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stenson B.J. Oxygen targets for preterm infants. Neonatology. 2013;103:341–345. doi: 10.1159/000349936. [DOI] [PubMed] [Google Scholar]
- 52.Vose L.R., Vinukonda G., Jo S., Miry O., Diamond D., Korumilli R., Arshad A., Zia M.T., Hu F., Kayton R.J., La Gamma E.F., Bansal R., Bianco A.C., Ballabh P. Treatment with thyroxine restores myelination and clinical recovery after intraventricular hemorrhage. J Neurosci. 2013;33:17232–17246. doi: 10.1523/JNEUROSCI.2713-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scafidi J., Hammond T.R., Scafidi S., Ritter J., Jablonska B., Roncal M., Szigeti-Buck K., Coman D., Huang Y., McCarter R.J., Jr., Hyder F., Horvath T.L., Gallo V. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature. 2014;506:230–234. doi: 10.1038/nature12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orellana S.A., Sweeney W.E., Neff C.D., Avner E.D. Epidermal growth factor receptor expression is abnormal in murine polycystic kidney. Kidney Int. 1995;47:490–499. doi: 10.1038/ki.1995.62. [DOI] [PubMed] [Google Scholar]
- 55.Tang J., Liu N., Zhuang S. The role of epidermal growth factor receptor in acute and chronic injury. Kidney Int. 2013;83:804–810. doi: 10.1038/ki.2012.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wegner M. SOX after SOX: SOXession regulates neurogenesis. Genes Dev. 2011;25:2423–2428. doi: 10.1101/gad.181487.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weidemann A., Johnson R.R. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 58.Chen A., Xiong L.J., Tong Y., Mao M. The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed Rep. 2013;1:167–176. doi: 10.3892/br.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jantzie L.L., Corbett C.J., Firl D.J., Robinson S. Postnatal erythropoietin mitigates impaired cerebral cortical development following subplate loss from prenatal hypoxia-ischemia. Cereb Cortex. 2014, Apr 9 doi: 10.1093/cercor/bhu066. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmitz T., Endesfelder S., Chew L.J., Zaak I., Bührer C. Minocycline protects oligodendroglial precursor cells against injury caused by oxygen-glucose deprivation. J Neurosci Res. 2012;90:933–944. doi: 10.1002/jnr.22824. [DOI] [PubMed] [Google Scholar]
- 61.Stolt C.C., Wegner M. SoxE function in vertebrate nervous system development. Int J Biochem Cell Biol. 2011;42:437–440. doi: 10.1016/j.biocel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 62.Tsuneki M., Madri J.A. CD44 regulation of endothelial cell proliferation and apoptosis via modulation of CD31 and VE-cadherin expression. J Biol Chem. 2014;289:5357–5370. doi: 10.1074/jbc.M113.529313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsuneki M., Madri J.A. Adhesion molecule-mediated hippo pathway modulates hemangioendothelioma cell behavior. Mol Cell Biol. 2014;34:4485–4499. doi: 10.1128/MCB.00671-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Y., Xiao M., He W., Cai Z. Minocycline upregulates cyclic AMP response element binding protein and brain-derived neurotrophic factor in the hippocampus of cerebral ischemia rats and improves behavioral deficits. Neuropsychiatr Dis Treat. 2015;11:507–516. doi: 10.2147/NDT.S73836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scheinost D., Lacadie C., Vohr B.R., Schneider K.C., Papademetris X., Constable R.T., Ment L.R. Cerebral lateralization is protective in the very prematurely born. Cereb Cortex. 2015;25:1858–1866. doi: 10.1093/cercor/bht430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin Z., Liang J., Wang J., Kolattukudy P.E. MCP-induced protein 1 mediates the minocycline-induced neuroprotection against cerebral ischemia/reperfusion injury in vitro and in vivo. J Neuroinflammation. 2015;12:264–276. doi: 10.1186/s12974-015-0264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Y., Salayandia V.M., Thompson J.F., Yang L.Y., Estrada E.Y., Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflammation. 2015;12:245–260. doi: 10.1186/s12974-015-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pytel P., Karrison T., Can Gong, Tonsgard J.H., Krausz T., Montag A.G. Neoplasms with schwannian differentiation express transcription factors known to regulate normal schwann cell development. Int J Surg Pathol. 2010;18:449–457. doi: 10.1177/1066896909351698. [DOI] [PubMed] [Google Scholar]
- 69.Wang L., He S., Yuan J., Mao X., Cao Y., Zong J., Tu Y., Zhang Y. Oncogenic role of SOX9 expression in human malignant glioma. Med Oncol. 2012;29:3484–3490. doi: 10.1007/s12032-012-0267-z. [DOI] [PubMed] [Google Scholar]
- 70.Shakhova O., Zingg D., Schaefer S.M., Hari L., Civenni G., Blunschi J., Claudinot S., Okoniewski M., Beermann F., Mihic-Probst D., Moch H., Wegner M., Dummer R., Barrandon Y., Cinelli P., Sommer L. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat Cell Biol. 2012;14:882–890. doi: 10.1038/ncb2535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative autoradiograph images of C57BL/6 and CD1 neural stem cell (NSC) lysates blotted with anti-cleaved caspase 3, anti-Sox10, anti–HIF-1α, anti–cleaved notch1, anti-PCNA, and anti–β-actin, and C57/BL6 and CD1 postnatal day 20 (P20) and P40 SVZ tissue lysates blotted with anti-Sox10, anti–HIF-1α, anti-EPO, anti-BDNF, anti–phospo-Akt, anti-Akt, anti–cleaved notch1, anti-YAP, anti-survivin, anti-GPR124, anti-CD31, anti-PCNA, anti-cleaved caspase 3, anti-MAG, anti-PLP, anti-MOBP, anti-synapsin, and anti–β-actin. BDNF, brain-derived neurotrophic factor; CT, control; EPO, erythropoietin; GPR, G-protein coupled receptor; HIF, hypoxia-inducible factor; HX, hypoxic; MAG, myelin associated glycoprotein; MOBP, myelin-associated oligodendrocyte basic protein; NX, normoxic; PCNA, proliferating cell nuclear antigen; PLP, proteolipid protein; SVZ, subventricular zone; YAP, yes-associated protein 1.
Ratios of control/control [CT/CT] and [minocycline]/control ([MC]/CT) for normoxic- and reduced oxygen-cultured C57BL/6 and CD1 neural stem cells (NSC). Using the data accrued in our Western analysis of normoxic- and reduced oxygen–cultured C57BL/6 and CD1 NSC illustrated in Figure 1, we illustrated the effects of minocycline using ratios of the expression of each protein following minocycline treatment divided by the expression of each protein without minocycline treatment. The ratios of minocycline-treated divided by untreated expression levels increases from 1.0 only in the reduced oxygen, minocycline-treated C57BL6/B NSC. Additionally, dramatic decreases in ratios are noted at 50 mmol/L minocycline in all four protein determinations. (A, Sox10; B, HIF-1α; C, cleaved notch1; D, PCNA). HIF, hypoxia-inducible factor; HX, hypoxic; NX, normoxic; PCNA, proliferating cell nuclear antigen.
Western blot analysis of postnatal day 40 (P40) isolated subventricular zone (SVZ) tissues harvested from normoxia-reared nursing pups treated with either plain water, 5% sucrose (vehicle), or minocycline, and reduced oxygen–reared nursing pups treated with either plain water, 5% sucrose (vehicle), or minocycline. Similar to our results at the P20 time point, minocycline treatment of hypoxic-reared nursing C57BL/6 pups elicits statistically significant increases in Sox10 (A), HIF-1α (B), EPO (C), BDNF (D), phospho-AKT (E), cleaved notch1 (F), YAP (G), survivin (H), GPR124 (I), CD31 (J), PCNA (K), MAG (M), PLP (N), MOBP (O), and synapsin (P) and decreases in CCASP3 (L) compared to vehicle-only and normoxia-reared pups. Specifically, SVZ tissues of minocycline-treated, hypoxic-reared C57BL/6 pups exhibited increases in expression of Sox10, HIF-1α, HIF-2α, EPO, BDNF, phospho-AKT, PCNA, cleaved notch1 (proliferation markers); GPR124 and CD31 (microvascular endothelial cell markers); MAG, MOBP, and PLP (components of myelin); and synapsin (a component of synapses), while exhibiting a decrease in CCASP3 (an apoptosis marker). Data are expressed as means ± SD. n = 3. ∗P < 0.05, ∗∗P < 0.005, and ∗∗∗P < 0.0005. BDNF, brain-derived neurotrophic factor; CCASP3, cleaved caspase 3. EPO, erythropoietin; GPR, G-protein coupled receptor; HIF, hypoxia-inducible factor; HX, hypoxic; MAG, myelin associated glycoprotein; MC, minocycline-treated; MOBP, myelin-associated oligodendrocyte basic protein; NX, normoxic; PCNA, proliferating cell nuclear antigen; PLP, proteolipid protein; SC, sucrose (vehicle); SVZ, subventricular zone; YAP, yes-associated protein 1.
Immunofluorescence analyses of postnatal day 70 (P70) vehicle-only and minocycline-treated C57 pup SVZ and cerebral tissues reveals increased SVZ proliferation and decreased SVZ apoptosis of neural progenitors; increased PLP expression in CC and myelinated fiber tracts and increased expression of synapsin in the hippocampus of minocycline-treated pups. A and B: Frozen sections of the SVZ zone of vehicle-only and minocycline-treated pups revealed increased proliferation of neural progenitors, labeled bright pink due to colocalization of nestin and Ki-67, in the minocycline-treated pups. C and D: By contrast, apoptosis (denoted by colocalization of nestin and cleaved caspase 3) is significantly decreased in minocycline-treated pups. E and F: Frozen sections of the SVZ zone of vehicle-only and minocycline-treated pups reveal increased proliferation of SVZ microvascular endothelial cells, labeled bright yellow due to colocalization of CD31 and PCNA, in the minocycline-treated pups. Nestin is indicated by green fluorescence, Ki-67 and cleaved caspase 3 by red fluorescence, CD13 by red fluorescence, PCNA by green fluorescence, and DAPI by blue fluorescence. Frozen sections of the corpus callosum and cortex of vehicle-only and minocycline-treated pups reveal increased expression of PLP in the corpus callosum (G–J) and myelinated fiber tracts in the cortex (K and L) in the minocycline-treated pups compared to the vehicle-only treated pups. PLP is indicated by red fluorescence, DAPI by blue fluorescence. Frozen sections of the hippocampus of vehicle-only and minocycline-treated pups reveal increased punctate expression of synapsin in the hilus region (H) of the dentate gyrus bounded by the GL in the minocycline-treated pups compared to the vehicle-only treated pups (M–R). Synapsin is indicated by red fluorescence, DAPI by blue fluorescence. CC, corpus callosum; GL, granular layer; LV, lateral ventricle; PCNA, proliferating cell nuclear antigen; PLP, proteolipid protein; SVZ, subventricular zone.
Working model of a minocycline-induced signaling cascade comprising the induction of Sox10 and HIF-1α (and their downstream signaling pathways and myelination-associated genes); the up-regulation of YAP and survivin, both of which positively influence each other's expression, increasing proliferation and decreasing apoptosis; hypoxia-induced SIAH2, an E3 ligase, destabilizing PHDs and LATS2, reducing Hippo pathway activation, increasing YAP signaling, affecting proliferation and apoptosis; and YAP complexing with HIF-1α in the nucleus, stabilizing HIF-mediated signaling, affecting survival, proliferation, and apoptosis and ultimately leading to improved behaviors. Dashed lines denote data derived from Ma et al.34 The overall scheme was derived from the current data and previous studies.14, 18, 19, 23, 34, 35, 36, 42, 62, 63, 64 HIF, hypoxia-inducible factor; PHD, prolyl hydroxylase domain; YAP, yes-associated protein 1.