Summary
In coeliac disease (CD), anti‐tissue transglutaminase 2 immunoglobulin (Ig)A antibodies (anti‐TG2) are produced and deposited in the intestine. PreventCD (www.preventcd.com) is a European multi‐centre study, which investigates the influence of infant nutrition and that of genetic, immunological and other environmental factors on the risk of developing CD. The aim of the current study was to evaluate the appearance of intestinal anti‐TG2 deposits in very early intestinal biopsies from at‐risk infants and their predictive value for villous atrophy. Sixty‐five small bowel biopsies, performed in 62 children, were investigated for the presence of intestinal anti‐TG2 extracellular IgA deposits by using double immunofluorescence. The biopsies were performed in the presence of elevated serum levels of CD‐associated antibodies and/or symptoms suggesting disease. Deposits of anti‐TG2 IgA were present in 53 of 53 CD patients and three of three potential CD patients. In potential CD patients, mucosal deposits showed a patchy distribution characterized by some areas completely negative, whereas active CD patients had uniformly present and evident mucosal deposits. Only one of six patients without CD (negative for serum anti‐TG2 and with normal mucosa) had intestinal deposits with a patchy distribution and a weak staining. Two of the 53 CD patients received a definitive diagnosis of CD after a second or third biopsy; mucosal deposits of anti‐TG2 IgA were evaluated in all samples. Before developing villous atrophy, both patients had anti‐TG2 deposits in normal mucosal architecture, antibodies in one patient being absent in serum. We demonstrated that in CD the intestinal deposits of anti‐TG2 are a constant presence and appear very early in the natural history of disease.
Keywords: coeliac disease, intestinal anti‐TG2 antibodies, intestinal deposits, PreventCD
Introduction
Coeliac disease (CD) is an immune‐mediated systemic disorder elicited by wheat gliadin and related prolamines in genetically susceptible individuals and is characterized by the presence of variable combination of gluten‐dependent clinical manifestations, CD‐specific antibodies, human leucocyte antigen (HLA) DQ2 and DQ8 haplotypes and enteropathy 1.
In 1997 Dieteric et al. 2 discovered that the antigen against which endomysial antibodies reacted was the enzyme tissue transglutaminase (TG2), an enzyme catalyzing Ca2+‐dependent cross‐linking between glutamine residues in peptides and some polyamines and primary amino groups. Since then, TG2‐specific serum antibodies have proved to be extremely sensitive and specific markers of CD in both adults and children, becoming increasingly important in the diagnostic work‐up of disease 3.
Evidence shows that the production of anti‐TG2 autoantibodies occurs in the small intestinal mucosa 4, and the serum autoantibodies are thought to be the result of ‘spillover’ from the intestine. In adult and paediatric populations, anti‐TG2 immunoglobulin (Ig)A deposited on the surface of locally present extracellular TG2 are in the small bowel of coeliac patients with overt disease, and are seen most often below the epithelial layer and around blood vessels 5, 6, 7. Interestingly, patients with early‐stage CD with normal small bowel mucosa villous morphology may show these mucosal TG2‐targeted autoantibody deposits 8, 9, and in most cases these deposits predict a later evolution to villous atrophy 8, 10, 11.
PreventCD (www.preventcd.com) is a European multi‐centre study which investigates the influence of infant nutrition and that of genetic, immunological and environmental factors on the risk of developing CD 12. It is a prospective, randomized, placebo‐controlled intervention study based on early gluten introduction in young children who have at least one first‐degree relative with CD and who are positive for HLA‐DQ2 or DQ8. According to the research protocol, infants from at‐risk families are followed‐up strictly with serological tests, and those with consistently elevated CD‐associated antibodies, or with symptoms suggesting CD, are offered small bowel biopsies to confirm the diagnosis. One main advantage of this study has been to have the opportunity to obtain duodenal biopsies in the early and often asymptomatic phases of the disease which may provide important data on the natural history of this condition.
In this paper we took advantage of the availability of these very early biopsies from at‐risk infants to investigate the appearance of intestinal anti‐TG2 deposits and their predictive value for villous atrophy.
Materials and methods
Patients
During the study period 2007–14, 94 children underwent diagnostic biopsies, as described in a previous paper 12. Frozen intestinal tissue fragments suitable for the investigation of anti‐TG2 deposits were available from 62 of these children, who comprised the study group of the present evaluation. The median age was 2 years and 8 months (range 1 year–5 years and 5 months); 61% were female. They were from five different countries: 21 from Italy, 17 from Hungary, one from Poland, 16 from Spain and seven from Israel. According to the study protocol, they fulfilled the criteria for diagnostic small bowel biopsies; namely, the presence of elevated serum levels of coeliac disease‐associated antibodies and/or symptoms suggesting coeliac disease 12. Twenty‐six children presented positive serum anti‐TG2 antibodies and symptoms suggesting disease (12 diarrhoea, three abdominal pain, four failure to thrive, four anorexia, two iron deficiency and one constipation); four children presented only symptoms (two failure to thrive, one diarrhoea, one abdominal pain) and 32 only elevated serum levels of antibodies (27 anti‐TG2 and 5 anti‐gliadin antibodies).
Methods
Anti‐TG2 and anti‐gliadin antibodies
Measurements of serum anti‐TG2 and anti‐gliadin antibodies were performed centrally (Thermo Fisher Scientific, Freiburg, Germany) at least seven times during the first 3 years of age and then annually thereafter. Serum antibodies were determined using Varelisa gliadin IgA and Celikey, as reported previously 12.
Histology assessment
According to the study protocol, the biopsy specimens were assessed histologically at the study sites and were also reviewed centrally by a pathologist who made a blinded evaluation of each slide without any clinical, serological or additional genetic information. As detailed in the PreventCD protocol, the diagnosis of CD was made according to the 1990 criteria of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) 13 in the presence of at least Marsh grade 3 villous atrophy, while crypt hyperplasia alone was considered insufficient for the diagnosis. The pathology reports were sent to the coordinator centre at the Leiden University Medical Center, the Netherlands for inclusion into the common database of the PreventCD project, and the cases were allocated to CD or non‐CD by a diagnostic committee created within the project.
Intestinal deposits of anti‐TG2
One of the biopsy fragments was embedded immediately in optimal cutting temperature (OCT) compound (BioOptica, Milan, Italy), frozen and stored in liquid nitrogen. It was evaluated for the presence of IgA anti‐TG2 extracellular deposits. In brief, 5‐µm frozen sections from each patient were fixed in acetone and examined by double immunofluorescence. After a 15‐min preincubation with normal rabbit serum the sections were covered with a monoclonal mouse antibody against TG2 (1 : 200; CUB7402; NeoMarkers, Portsmouth, NH, USA) for 1 h at room temperature in a humid chamber. The sections were washed in phosphate‐buffered saline (PBS) and incubated for 30 min in the dark with a mixture of fluorescein isothiocyanate‐labelled rabbit antibody against human IgA (1 : 100; Dako, Glostrup, Denmark) to reveal (in green) tissue‐bound IgA produced by the patient, and R‐phycoerythrin‐labelled (or rhodamine‐labelled) rabbit anti‐mouse antibody (1 : 40; Dako) to reveal (in red) TG2. Finally, the sections were rinsed several times in PBS and mounted by glycerol/PBS. The co‐localization of IgA and TG2 that resulted in yellow bands, under surface epithelium, around crypts and blood vessels representing mucosal deposits, were analysed using fluorescence microscopy. IgA deposits of anti‐TG2 were graded semiquantitatively as 0 = staining absent, 2 = weak positive staining with a patchy distribution or 3 = positive staining with uniform distribution, according to their intensity and diffusion in the tissue samples. The stainings were performed in four laboratories (Naples, Italy; Budapest, Hungary; Petah Tikva, Israel; Valencia, Spain). In all laboratories sections from all patients were analysed by two investigators blindly without knowledge of clinical history. Sections from 14 patients were stained and analysed for the detection of mucosal deposits in three different laboratories (Naples, Budapest and Petach Tikva); the concordance rate was 86%.
Ethics
The study was approved by the medical ethics committee at each participating center and complied with Good Clinical Practice guidelines.
Statistics
Statistical analysis was performed using GraphPad Prism 4 for Windows, version 4·03 (GraphPad Software, San Diego, CA, USA). The Mann–Whitney U‐test was used to compare titres of anti‐TG2 antibodies in serum between the patients with a patchy distribution of mucosal deposits and the patients with a uniform distribution. Pearson's correlation test was used to compare titres of anti‐TG2 IgA in serum and score of the staining of mucosal deposits in all patients. A P‐value of < 0·05 was considered significant. The rate of concordance between serum titres of anti‐TG2 antibodies and mucosal deposits was calculated by standard statistical formulae.
Results
Histology
A total of 65 small bowel biopsies were performed in 62 children, and they were sent to the referral pathology centre for histopathology assessment. Two patients received biopsies at different time‐points; in particular, one patient underwent biopsies twice and the other three times. After the evaluation of the diagnostic committee of the PreventCD project, the subjects enrolled into this study were divided into three groups on the basis of serum and histological findings.
The first group included 53 children who received the definitive diagnosis of CD on the basis of an intestinal mucosa type Marsh 3a–c 14; all but one had serum levels of anti‐TG2 higher than the cut‐off (> 6 U/ml). In the second group there were three patients with positive serology for CD but architecturally normal intestinal mucosa (2 Marsh 0, 1 Marsh 1); they received a diagnosis of potential CD. Finally, the third group included six patients without CD, all negative for serum anti‐TG2 and with normal mucosa (5 Marsh 0 and 1 Marsh 1) (Table 1).
Table 1.
Clinical and laboratory features of the 60 PreventCD patients that performed one biopsy
| Active CD | Potential CD | Non‐CD | |||||
|---|---|---|---|---|---|---|---|
| Patients | 51 | 3 | 6 | ||||
| Age (mean) | 2 years and 8 months | 2 years and 3 months | 2 years and 4 months | ||||
| HLA | DQ2 and/or DQ8‐positive | DQ2 and/or DQ8‐positive | DQ2 and/or DQ8‐positive | ||||
| Sex | 33 female, 20 male | 3 female | 4 female, 2 male | ||||
| Symptoms and anti‐TG2+ | No symptoms, only anti TG2+ | Only symptoms, anti‐TG2+ and AGA negative | Symptoms and anti‐TG2+ | No symptoms only anti‐TG2+ | Only symptoms, anti‐TG2‐ and AGA‐negative | No symptoms, anti‐TG2– and AGA+ | |
| Criteria for diagnostic small‐bowel biopsies | n = 25 | n = 24 | n = 2 | n = 1 | n = 2 | n = 2 | n = 4 |
| Histology | All Marsh 3 | All Marsh 3 | All Marsh 3 | Marsh 0 |
Marsh 1 Marsh 0 |
n = 1, Marsh 0 n = 1, Marsh 1 |
n = 4, Marsh 0 |
| Mucosal deposits staining |
n = 18, strong uniform n = 7, weak patchy |
n = 18, strong uniform n = 6, weak patchy |
n = 1, strong uniform n = 1, weak patchy |
Weak patchy | Weak patchy | n = 2, absent |
n = 1, weak patchy n = 3, absent |
CD = coeliac disease; anti‐TG2 = serum anti‐tissue transglutaminase2 antibodies; AGA = serum‐anti gliadin antibodies; mucosal deposits = intestinal anti‐TG2 deposits; HLA = human leucocyte antigen.
Two of the 53 children who received a definitive diagnosis of CD had more than one biopsy procedure (Table 2). At time of the first biopsy one patient presented normal architecture of duodenal mucosa, positive serum IgA anti‐gliadin (cut‐off = 16 U/ml), but after approximately 3 years he received a diagnosis of CD on the basis of villous atrophy (Marsh 3c) and elevated serum anti‐TG2 antibodies. At two different times, the other patient had serum‐positive anti‐TG2 antibodies with normal mucosal architecture of duodenal biopsies; after approximately 2 years he received a third biopsy because of the persistence of high serum antibodies and the appearance of symptoms suggestive of CD; on this occasion he presented a mucosa type Marsh 3b and received a definitive diagnosis of CD (Table 2).
Table 2.
Clinical and laboratory features of the two PreventCD patients that performed more than one biopsy
| Patient 1 | Patient 2 | ||||
|---|---|---|---|---|---|
| First biopsy | Second biopsy | Third biopsy | First biopsy | Second biopsy | |
| Age | 3 years 3 months | 3 years 10 months | 4 years 3 months | 1 year 6 months | 4 years |
| Symptoms | No | No | Delayed growth | No | No |
| HLA | DQ2+DQ8+ | DQ2+ | |||
|
Serum anti‐TG2 Cut‐off = 6 U/ml |
12·9 | 8·4 | 24·8 | 0·1 | 100 |
|
Serum AGA Cut‐off = 16 U/ml |
11·2 | 18 | 31·2 | 40·2 | 100 |
| Histology | Marsh 0 | Marsh 1 | Marsh 3b | Marsh 1 | Marsh 3b |
| Anti‐TG2 mucosal deposits | Patchy | Patchy | Uniform | Weak patchy | Uniform |
Serum anti‐TG2 = serum anti‐tissue transglutaminase 2 antibodies; serum AGA = serum anti‐gliadin antibodies; HLA = human leucocyte antigen.
Intestinal IgA anti‐TG2 deposits
All 53 patients with definitive diagnoses of CD were positive for intestinal deposits of anti‐TG2 IgA. The one CD patient with negative serum CD‐associated antibodies also presented mucosal deposits of anti‐TG2 IgA. The pattern of positivity was characterized by a thick yellow band with a clear localization below the basement membrane, along the villous and crypt as well as around mucosal vessels (Figure 1a–c). The three potential CD patients presented intestinal deposits of anti‐TG2 IgA with a patchy distribution characterized by some areas being completely negative, whereas patients with overt CD presented uniformly distributed mucosal deposits of anti‐TG2 IgA (Fig. 1d–f). In the group of six patients without CD (negative for serum anti‐TG2 and with normal mucosa), only one patient showed intestinal deposits with a patchy distribution and with weak staining. This patient received biopsy for positive serum anti‐gliadin antibodies only (Table 1).
Figure 1.
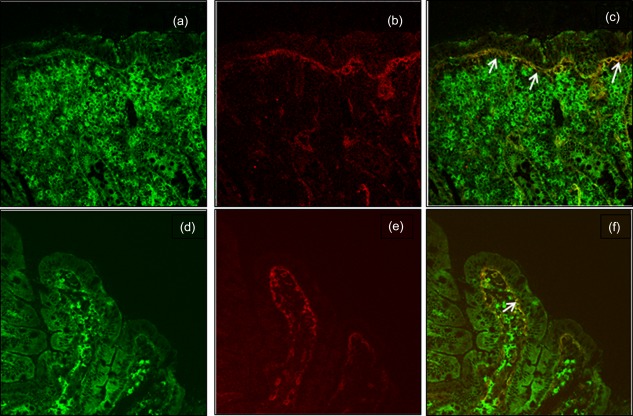
Duodenal mucosa sections from an active coeliac disease (CD) patient (a–c) and from a potential CD patient (d–f) presenting deposits of immunoglobulin (Ig)A anti‐transglutaminase 2 (TG2). IgA secreted by plasma cells is detected in green (a,d), TG2 with a subepithelial localization is shown in red (b,e). Mucosal deposits of IgA anti‐TG2 are visible in yellow (c,f; white arrows).
In two of the 53 CD patients who received more than one biopsy, as described above, mucosal deposits of anti‐TG2 IgA were evaluated in biopsies taken at different times (Table 2). In both patients, before showing villous atrophy, anti‐TG2 intestinal deposits were already present; in one patient the serum anti‐TG2 antibodies were absent despite the positivity of intestinal deposits, thus mucosal anti‐TG2 IgA deposition preceded the seropositivity observed later.
Based on these results, in at‐risk infants for CD detection of mucosal deposits of anti‐TG2 IgA showed a positive predictive value of 88·3% and a rate of concordance with serum anti‐TG2 antibodies of 90·7%. Interestingly, we observed a direct correlation between titres of serum anti‐TG2 and staining score (Pearson's r = 0·7, P < 0·0001); in particular, the patients with a uniform distribution of mucosal deposits had titres of serum antibodies significantly higher (median 100, range = 3·5–128 U/ml) than patients with patchy distribution (median = 38·35, range = 0·1–100; P < 0·001).
Follow‐up
All 53 patients with definitive diagnoses of CD began a gluten‐free diet with good compliance. As expected, they became negative for serum anti‐TG2. The three potential CD patients continued a gluten‐containing diet; two became negative for clinical and serum CD‐related antibodies after approximately 1 year of follow‐up. The patient without CD, but with weak intestinal deposits, is still on a gluten‐containing diet showing negative CD serology.
Discussion
In this paper we studied a paediatric population at high risk of CD, being positive for HLA‐DQ2 or HLA‐DQ8 and having at least one first‐degree relative with CD. Biopsies were taken very early in the course of disease; intestinal biopsies were taken as soon as these infants showed symptoms or serum associated‐CD antibodies. In all cases in whom a diagnosis of CD was made, we observed the presence of deposits at these very early stages. Their intensity was strong, confirming previous findings 6. Interestingly, mucosal anti‐TG2 deposits were also detected in the only CD patient without significant serum levels of these autoantibodies. This confirms the finding in previous studies showing that the presence of these autoantibodies at the gut level reinforces the diagnosis in borderline cases, mainly in seronegative CD 7, 15.
Furthermore, we observed that all patients with positive serology for CD, but architecturally normal intestinal mucosa, defined as potential CD patients, had intestinal deposits of anti‐TG2 IgA. In agreement with previous studies 7, 16, these cases with potential CD showed more often a patchy distribution characterized by some areas being completely negative, whereas others had evident mucosal deposits of anti‐TG2 IgA. We think that patchy staining of mucosal deposits of anti‐TG2 antibodies, noted in potential CD, could be due to the low affinity displayed by these antibodies to their antigen and/or to their lower concentration. On the other hand, we found a direct correlation between the score of staining and serum titres of anti‐TG2 antibodies.
These patients need close follow‐up, as previous studies have suggested that intestinal anti‐TG2 antibody deposits in the mucosa are more frequent in cases that progress to mucosal damage than in those remaining potential CD 17. In this specific setting, we had six samples with normal architecture and anti‐TG2 IgA deposits belonging to five subjects, but the limited number of cases does not allow firm conclusions to be drawn. We observed two subjects who have evolved to villous atrophy (see below) and three others who have become seronegative in the subsequent follow‐up. However, our experience in other cases not belonging to this cohort suggests that, despite becoming seronegative, most such subjects continue to have intestinal deposits of such autoantibodies and may develop manifest CD at a later time. In both patients in whom we observed evolution to villous atrophy there were intestinal anti‐TG2 deposits in the early biopsies, emphasizing once again that TG2‐specific IgA deposition precedes the formation of the gluten‐induced flat jejunal lesion; interestingly, in one of them the intestinal deposits were already present even when the serum anti‐TG2 antibodies were still negative. This is in agreement with the hypothesis that coeliac autoantibodies are deposited in the morphologically normal small intestinal mucosa before they can be detected in the circulation 5, 10.
Finally, in a group of patients without CD, with negative serum anti‐TG2 and normal mucosa, we observed a patient who showed intestinal deposits with patchy distribution and with very weak staining. It is known that intestinal anti‐TG2 IgA deposits are detected in a minority of non‐coeliac subjects, with a prevalence ranging from 5 to 20% 6, 7, 16. This occurs often in patients with other autoimmune diseases, but this was not the case in our patient. In fact, in this population at high risk of CD the presence of intestinal deposits may have a different meaning and identify patients with abnormal immune response to gluten; it remains to be seen whether these patients will develop gluten‐related disease in future.
Our study has several strengths, as it is a randomized, double‐blind, placebo‐controlled prospective study in which we studied an at‐risk population by obtaining biopsies early in the course of disease. Further, the analysis of intestinal deposits was performed in different laboratories, and the same technique applied in more centres showed highly reproducible results. At the same time, it presents some limitations. The number of enrolled patients is relatively small. Furthermore, in these infants the biopsies were performed only if they had symptoms or positive serum antibodies.
We can conclude that in a paediatric population at high risk of CD there is a rapid progression to villous atrophy accompanied by the early presence of both intestinal and serum anti‐TG2 antibodies. Our experience confirms the strict concordance between the presence of anti‐TG2 deposits in the gut and their increased levels in the serum, both in the presence and absence of villous atrophy. The few discrepant cases support previous suggestions of the earlier appearance in the intestinal mucosa in comparison to serum, while the ability of intestinal deposits of these autoantibodies to predict the evolution to villous atrophy needs to be confirmed in large groups of patients.
Disclosure
All authors have nothing to disclose.
Author contributions
M. B.: analysis and interpretation of data and drafting of the manuscript. M. M. collected laboratory and histological data, analysis and interpretation of data and drafting of the manuscript. I. R. K.‐S.: acquisition, analysis and interpretation of data, drafting of the manuscript. M. L. M.: study concept and design, critical revision of the manuscript. C. M.: collected clinical, laboratory and histological data, analysis of data, critical revision of the manuscript. H. N.‐D.: collected clinical, laboratory and histological data, analysis of data, critical revision of the manuscript. C. R.‐K.: collected clinical, laboratory and histological data, analysis of data, critical revision of the manuscript. M. R.: collected clinical, laboratory and histological data, analysis of data, critical revision of the manuscript. R. S.: study concept and design, critical revision of the manuscript. R. T.: study concept and design, critical revision of the manuscript. R. A.: collected clinical, laboratory and histological data, analysis of data and critical revision of the manuscript.
Acknowledgements
This study was supported by the European Commission FP6–2005‐FOOD‐4B‐36383 and Hungarian National Research Funds OTKA 101788 and NKFI 120392, GINOP 2.3.2–15‐2016‐00015 projects (co‐financed by the European Union and the European Regional Development Fund).
References
- 1. Husby S, Koletzko S, Korponay‐Szabó IR et al European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012; 54:136–60. [DOI] [PubMed] [Google Scholar]
- 2. Dieterich W, Ehnis T, Bauer M et al Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 1997; 3:797–801. [DOI] [PubMed] [Google Scholar]
- 3. Giersiepen K, Lelgemann M, Stuhldreher N et al Accuracy of diagnostic antibody tests for coeliac disease in children: summary of an evidence report. J Pediatr Gastroenterol Nutr 2012; 54:229–41. [DOI] [PubMed] [Google Scholar]
- 4. Marzari R, Sblattero D, Florian F et al Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J Immunol 2001; 166:4170–6. [DOI] [PubMed] [Google Scholar]
- 5. Korponay‐Szabo IR, Halttunen T, Szalai Z et al In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut 2004; 53:641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koskinen O, Collin P, Lindfors K, Laurila K, Mäki M, Kaukinen K. Usefulness of small‐bowel mucosal transglutaminase‐2 specific autoantibody deposits in the diagnosis and follow‐up of celiac disease. J Clin Gastroenterol 2010; 44:483–8. [DOI] [PubMed] [Google Scholar]
- 7. Maglio M, Tosco A, Auricchio R et al Intestinal deposits of anti‐tissue transglutaminase IgA in childhood celiac disease. Dig Liver Dis 2011; 43:604–8. [DOI] [PubMed] [Google Scholar]
- 8. Kaukinen K, Peräaho M, Collin P et al Small‐bowel mucosal transglutaminase 2‐specific IgA deposits in coeliac disease without villous atrophy: a prospective and randomized clinical study. Scand J Gastroenterol 2005; 40:564–72. [DOI] [PubMed] [Google Scholar]
- 9. Tosco A, Aitoro R, Auricchio A et al Intestinal anti‐tissue transglutaminase antibodies in potential coeliac disease. Clin Exp Immunol 2013; 171:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salmi TT, Collin P, Jarvinen O et al Immunoglobulin A autoantibodies against transglutaminase 2 in the small intestinal mucosa predict forthcoming celiac disease. Aliment Pharmacol Ther 2006; 24:541–52. [DOI] [PubMed] [Google Scholar]
- 11. Tosco A, Salvati MV, Auricchio R et al Natural history of potential celiac disease in children. Clin Gastroenterol Hepatol 2011; 9:320–5. [DOI] [PubMed] [Google Scholar]
- 12. Vriezinga SL, Auricchio R, Bravi E et al Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med 2014; 371:1304–15. [DOI] [PubMed] [Google Scholar]
- 13. Walker SJA, Guandalini S, Schmitz J et al Revised criteria for the diagnosis of celiac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child 1990; 65:909–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999; 11:1185–94. [DOI] [PubMed] [Google Scholar]
- 15. Salmi TT, Collin P, Korponay‐Szabó IR, Laurila K et al Endomysial antibody‐negative coeliac disease: clinical characteristics and intestinal autoantibody deposits. Gut 2006; 55:1746–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tosco A, Maglio M, Paparo F et al Immunoglobulin A anti‐tissue transglutaminase antibody deposits in the small intestinal mucosa of children with no villous atrophy. J Pediatr Gastroenterol Nutr 2008; 47:293–8. [DOI] [PubMed] [Google Scholar]
- 17. Auricchio R, Tosco A, Piccolo E et al Potential celiac children: 9‐year follow‐up on a gluten‐containing diet. Am J Gastroenterol 2014; 109:913–21. [DOI] [PubMed] [Google Scholar]


