Summary
Preoperative glucocorticoid administration reduces the systemic inflammatory response. Pentraxin 3 (PTX3) is a novel inflammatory marker belonging to the humoral arm of innate immunity exerting a potentially protective host response. This study evaluated PTX3 and other complement marker changes after preoperative methylprednisolone (MP) early after total knee arthroplasty (TKA). Seventy patients were randomized (1 : 1) to preoperative intravenous (i.v.) MP 125 mg (group MP) or isotonic saline i.v. (group C). The outcomes included change in plasma PTX3, mannose‐binding lectin (MBL), ficolins (ficolin‐1, −2 and −3), complement components (C4 and C3), terminal complement complex (TCC) and C‐reactive protein (CRP) concentrations. Blood samples were analysed at baseline and 2, 6, 24 and 48 h after surgery with complete sampling from 63 patients for analyses. MP resulted in an increase in circulating PTX3 compared to saline from baseline to 24 h postoperatively (P < 0·001), while MP reduced the systemic inflammatory response (CRP) 24 and 48 h postoperatively (P < 0·001). However, the small postoperative changes in MBL, ficolin‐1, −2 and −3, C4, C3 and TCC concentrations did not differ between groups (P > 0·05). In conclusion, preoperative MP 125 mg increased circulating PTX3 and reduced the general inflammatory response (CRP) early after TKA, but did not affect other complement markers.
Keywords: acute‐phase proteins, arthroplasty, complement, glucocorticoids, inflammation
Introduction
The systemic immunological and inflammatory stress responses after major joint replacement are important for postoperative recovery and where patients with an exaggerated response may have delayed recovery 1. The tissue damage and subsequent inflammation initiates the rapid synthesis of acute phase molecules 2. The short pentraxin C‐reactive protein (CRP) is produced at a systemic level in the liver by hepatocytes and represents the main acute‐phase protein (inflammatory marker) in humans 3. Pentraxin 3 (PTX3), a new inflammatory marker, is produced by a wide range of different cell types, mainly by macrophages, dendritic cells (DCs) and endothelial cells, in response to inflammatory cytokines 4. As part of the innate immune system PTX3 functions as a regulatory molecule and is released early after experimental inflammatory stimuli in mice activating complement pathways and enhancing leucocyte recruitment 4, 5. Evidence suggests that PTX3 regulate inflammatory reactions in two parallel ways, locally and systemically 4, 6. PTX3 dampens localized excessive neutrophil recruitment, while systemic PTX3 responses may facilitate down‐regulation of proinflammatory reactions 4, 7. In this respect, increased levels of PTX3 could reflect a complex protective response of the host 8. As PTX3 is produced locally by various cells in response to tissue trauma, it is reasonable to consider PTX3 released in the blood stream also to be a marker of local inflammation 9.
The systemic anti‐inflammatory effects of glucocorticoids after surgery are well known 10, 11. In the hitherto only clinical study, glucocorticoid administration led to an increase in PTX3 in paediatric cardiac surgery, associated possibly with decreasing inflammatory parameters [CRP and interleukin (IL)‐1] 7. However, no other data from elective surgical trauma are available.
Thus, the aim of the present study was to compare the effect of a single preoperative intravenous dose of methylprednisolone (MP) versus placebo (saline) on changes in complement marker concentrations within 48 h after total knee arthroplasty (TKA) assessed by plasma PTX3, mannose‐binding lectin (MBL), ficolin‐1 (FCN‐1), ficolin‐2 (FCN‐2), ficolin‐3 (FCN‐3), complement components (C4 and C3) and the terminal complement complex (TCC), as well as CRP.
Materials and methods
Study design and setting
This single‐centre, randomized, placebo‐controlled trial was conducted from February 2015 to April 2016 at Copenhagen University Hospital, Bispebjerg and Frederiksberg. The study was registered on ClinicalTrials.gov under the US National Library of Medicine (NCT02332616, date 13/01/2015), embedded in a primary study on the effect of glucocorticoids on postoperative quadriceps dysfunction (NCT02319343). The principles of the Helsinki Declaration and the International Conference on Harmonization guidelines for Good Clinical Practice (GCP) were followed, and the trial was monitored by the GCP unit of Copenhagen University Hospital, Copenhagen, Denmark. Approval by the Ethics Committee for the Capital Region of Denmark (H‐6–2014‐101), the Danish Data Protection Agency and the Danish Health and Medicine Authority (EudraCT 2014–003395‐23) was obtained before patient enrolment. Oral and written informed consent was collected from all patients before participation and the manuscript was prepared according to the Consolidated Standards of Reporting Trials (CONSORT) recommendations for reporting randomized, controlled, clinical trials 12.
Participants
Patients undergoing elective, unilateral, primary TKA were assessed for eligibility, and were included if being able to speak and understand Danish and to provide informed oral and written consent. Patients were excluded if aged < 55 and > 80 years, general anaesthesia, allergy towards glucocorticoids, daily use of systemic glucocorticoids, cancer, insulin‐dependent diabetes mellitus, autoimmune disease including rheumatoid arthritis, local or systemic infection, treatment of peptic ulcer within 30 days from inclusion and fertile women. Patients were enrolled prior to surgery. Screening for eligibility, enrolment and allocation of patients was carried out by the principal investigator.
Randomization, trial intervention and blinding
After baseline assessment, 70 included patients were allocated randomly to two groups of 35. A random allocation sequence generated by computer and concealed in 70 consecutively numbered, opaque, sealed envelopes determining treatment was created by an independent research assistant not otherwise involved in the trial. The envelopes were opened consecutively on the morning of surgery, and two independent anaesthetist nurses prepared the trial drug consisting of either a single dose of MP, 125 mg (2 ml) intravenously (i.v.) (Solu‐Medrol®; Pfizer, Ballerup, Denmark) (group MP) or isotonic saline (2 ml) i.v. (group C), both of which being transparent; in addition, the syringes were masked. The trial solution was administered by the principal investigator just after induction of spinal anaesthesia. Investigators, care providers, data collectors and trial participants were blinded to the allocation. After study termination, the randomization list was dispatched enabling blinded analyses by the primary investigator. After carrying out all biochemical and statistical analyses, the list was un‐blinded with respect to intervention type.
Anaesthesia and surgery
Anaesthesia, surgery and analgesia followed standard procedures. One hour prior to surgery, patients received oral paracetamol 1 g and naproxen 500 mg. The surgical procedures were performed under lumbar spinal anaesthesia with 7·5–12·5 mg hyperbaric bupivacaine (5 mg ml−1, 0·5%). If required, additional sedation with propofol (1–5 mg kg−1 h−1) was administered. Intravenous cefuroxime 1·5 g for infectious prophylaxis and tranexamic acid 1 g for control of haemostasis were administered just after induction of spinal anaesthesia. Standardized intra‐operative fluid therapy was applied and consisted of 0·9% saline 12 ml kg−1 h−1 in the first hour of surgery and 6 ml kg−1 h−1 if surgery was prolonged beyond 1 h. Hypotension induced by spinal anaesthesia was treated with either i.v. 10 mg ephedrine or 0·1–0·2 mg phenylephrine at the discretion of the attending anaesthesiologist.
TKA was performed without the use of a femoral tourniquet using a standard medial para‐patellar approach. At the end of surgery, local infiltration analgesia was injected consisting of 150 ml ropivacaine 0·2%. Thromboembolic prophylaxis was administered 6–8 h after surgery and consisted of rivaroxaban 10 mg day−1 (Xarelto; Bayer Pharma, Berlin, Germany). Postoperatively, the patients received oral paracetamol 1 g four times daily and naproxen 500 mg twice daily, with rescue opioids on request. At night‐time, patients received zolpidem 10 mg and postoperative nausea and vomiting was treated with ondansetron 4 mg.
Study end‐points
The primary outcome was change in plasma PTX3 concentration (ng ml−1) from baseline to 48 h postoperatively. Secondary outcomes included changes in plasma MBL (ng ml−1), FCN‐1 (ng ml−1), FCN‐2 (μg ml−1), FCN‐3 (μg ml−1), C4 (g l−1), C3 (g l−1) and TCC (AU), as well as plasma CRP (mg l−1) concentrations. Outcomes were assessed preoperatively (baseline) and 2 (T2), 6 (T6), 24 (T24) and 48 (T48) h postoperatively, respectively.
Blood sampling and analyses
Peripheral venous blood was collected in ethylenediamine tetraacetic (EDTA) tubes and empty vials. The EDTA blood samples were ice cooled immediately and centrifuged at 3000 g for 10 min within 30 min of collection. Empty vial samples were set to coagulate for 30 min before being centrifuged for 10 min at 3000 g. Plasma was stored in a −80°C freezer awaiting analyses. The enzyme‐linked immunosorbent assay (ELISA) analyses for MBL, FCN‐1, ‐2 and ‐3, TCC and PTX‐3 were conducted by trained laboratory technicians, essentially described previously 13, 14, 15, 16, 17, while C4 and C3 were tested using the SPAPLUS machinery according to the manufacturer's recommendations (Binding Site, Birmingham, UK) at the Laboratory of Molecular Medicine, Department of Clinical Immunology, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark. Additionally, in‐vivo analyses on PTX3 levels were performed where MP was incubated with whole blood for up to 72 h and hirudin‐anti‐coagulated blood for up to 48 h at 37°C from two healthy volunteers.
Sample size calculation
The study was considered explorative and hypothesis‐generating, and the sample size calculation was based on the primary study on the effect of glucocorticoids on quadriceps muscle function (NCT02319343). The calculations resulted in a total sample size of 52 patients (an alpha of 0·05, a beta of 0·80) and allowing for 25% dropouts, a total of 70 patients were included in the trial.
Statistics
All data were validated by double entry, and evaluated for normal distribution by histograms and Q–Q plots before statistical analyses. PTX3 and CRP data did not follow normal distribution and underwent log transformation prior to statistical analysis. There were no missing data regarding patient characteristics. All continuous variables were compared between allocation groups using a univariate linear regression analysis incorporating baseline values as covariate investigating each sampling time‐point. Adjusted means are estimated marginal means, i.e. model‐predicted means when having the same baseline (T0) value (here set to the mean baseline value). These adjusted means may simplify evaluation of the intervention effect, as they adjust for differences in baseline values (baseline values are incorporated as covariates) 18. Continuous data are presented as mean [95% confidence interval (CI)] and categorical data as number (%). Statistical analyses were performed using spss version 22.0 (IBM Corporation, Troy, NY, USA).
A two‐sided P‐value < 0·05 was considered statistically significant.
Data availability statement
The data sets generated and analysed during the current study are available from the corresponding author on request.
Results
Participants
One hundred and sixty‐three patients were assessed for eligibility, leaving 70 patients for randomization, seven patients of whom were excluded following randomization (Fig. 1). Accordingly, a total of 33 (group MP) and 30 (group C) patients were available for analysis of the primary outcome using the non‐missing scores only. Baseline characteristics were comparable between allocation groups (Table 1). Similarly, we found no between‐group differences in intraoperative data (Table 1). Blood components were not administered during or after surgery.
Figure 1.
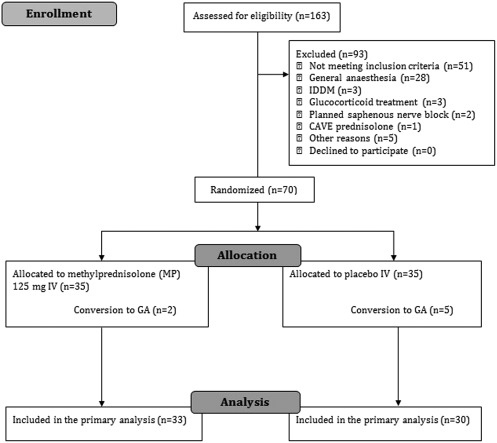
Consolidated Standards of Reporting Trials (CONSORT) flowchart for screening, inclusion and exclusion of trial participants. GA = general anaesthesia. IDDM = insulin‐dependent diabetes mellitus.
Table 1.
Baseline patient characteristics and intraoperative data illustrated by allocation group
| Variables | Group MP (n = 33) | Group C (n = 30) | P‐value |
|---|---|---|---|
| Age, years | 65·0 (62·5–67·5) | 67·7 (65·0–70·3) | 0·146 |
| Female sex | 20 (61%) | 15 (50%) | 0·453 |
| Body mass index, kg m−2 | 31·5 (29·6–33·5) | 30·1 (27·9–32·2) | 0·300 |
| ASA physical status classification | |||
| I | 10 (30%) | 4 (14%) | |
| II | 23 (70%) | 23 (76%) | |
| III | – | 3 (10%) | |
| Duration of surgery, min | 57 (52–63) | 60 (54–66) | 0·502 |
| Spinal bupivacaine dose, mg | 11·7 (11·3–12·1) | 11·3 (10·9–11·7) | 0·117 |
| Intraoperative propofol sedation, mg | 208 (129–286) | 217 (158–276) | 0·850 |
| Crystalloids, ml | 1016 (929–1103) | 1022 (910–1133) | 0·934 |
| HES | 0 | 0 | – |
| Packed erythrocytes | 0 | 0 | –– |
| Blood loss, ml | 117 (69–164) | 128 (91–164) | 0·719 |
Continuous variables are presented as mean (95% confidence interval), categorical variables as number (%). P‐values refer to between‐group analyses (group MP versus group C) using an independent‐sample t‐test. ASA = American Society of Anesthesiologists; HES = hydroxyethyl starch.
End‐points
Baseline and within‐group postoperative changes in complement markers are shown in Table 2. Baseline values were similar between groups regarding PTX3, the other complement markers and CRP. Over time, PTX3 concentrations increased compared to baseline values within both group MP and C (Table 2), and CRP also increased within both groups. FCN‐1, C4 and C3 concentrations decreased compared to baseline values in both groups and TCC increased in both groups (Table 2).
Table 2.
Unadjusted concentrations of the different biomarkers
| Group MP (n = 33) | Group C (n = 30) | P‐value | |
|---|---|---|---|
| PTX3, ng ml−1 | |||
| T0 | 3·9 (1·6–6·3) | 3·7 (2·1–5·4) | 0·590 |
| T2 | 5·0 (3·1–6·9) * | 4·5 (2·4–6·6) | |
| T6 | 14·6 (11·3–17·9) ** | 4·7 (2·9–6·6) * | |
| T24 | 16·8 (11·9–21·7) ** | 8·1 (6·2–10·0) ** | |
| T48 | 6·8 (4·3–9·2) ** | 8·9 (6·6–11·2) ** | |
| CRP, mg l−1 | |||
| T0 | 4·5 (2·2–6·7) | 6·9 (2·4–11·5) | 0·324 |
| T2 | 4·4 (2·3–6·6) | 6·4 (2·3–10·6) * | |
| T6 | 4·8 (2·7–7·0) * | 7·3 (2·9–11·7) | |
| T24 | 36·6 (29·3–43·8) ** | 74·4 (64·2–84·7) ** | |
| T48 | 95·6 (74·8–116·5) ** | 198·2 (172·4–224·0) ** | |
| FCN‐1, ng ml−1 | |||
| T0 | 486 (403–569) | 441 (375–507) | 0·280 |
| T24 | 394 (303–485) * | 369 (293–445) | |
| T48 | 371 (320–422) ** | 383 (331–435) * | |
| FCN‐2, μg ml−1 | |||
| T0 | 9·0 (7·6–10·5) | 8·1 (6·7–9–5) | 0·260 |
| T24 | 8·5 (7·3–9·7) | 7·4 (6·0–8·7) | |
| T48 | 10·1 (8·8–11·4) | 8·5 (7·1–9·9) | |
| FCN‐3, μg ml−1 | |||
| T0 | 41·1 (33·9–48·3) | 39·1 (32·9–45·3) | 0·372 |
| T24 | 36·4 (29·3–43·4) | 32·0 (24·5–39·5) * | |
| T48 | 34·4 (28·2–40·7) | 34·7 (24·7–44·6) | |
| C4, g l−1 | |||
| T0 | 0·25 (0·22–0·28) | 0·24 (0·21–0·26) | 0·519 |
| T24 | 0·22 (0·19–0·25) ** | 0·22 (0·19–0·24) * | |
| T48 | 0·22 (0·19–0·25) ** | 0·22 (0·20–0·24) * | |
| C3, g l−1 | |||
| T0 | 1·36 (1·30–1·42) | 1·35 (1·26–1·44) | 0·880 |
| T24 | 1·24 (1·17–1·31) ** | 1·24 (1·16–1·33) ** | |
| T48 | 1·24 (1·16–1·33) * | 1·29 (1·19–1·39) | |
| MBL, ng ml−1 | |||
| T0 | 944 (632–1256) | 981 (687–1274) | 0·814 |
| T24 | 949 (624–1274) | 912 (617–1207) | |
| T48 | 975 (662–1288) | 1049 (701–1397) | |
| TCC (AU) | |||
| T0 | 0·122 (0·095–0·150) | 0·153 (0·078–0·228) | 0·410 |
| T24 | 0·117 (0·096–0·137) | 0·138 (0·069–0·206) | |
| T48 | 0·175 (0·128–0·221) * | 0·205 (0·125–0·285) * | |
Baseline values are similar between groups using an independent‐sample t‐test. Values are presented as mean (95% confidence interval). Significant postoperative changes from baseline value within groups (paired t‐test) are marked * (P < 0·05) or ** (P < 0·001). PTX3 = pentraxin‐3; CRP = C‐reactive protein; FCN = ficolin; MBL = mannose‐binding lectin; TCC = terminal complement complex.
In the between‐group analysis adjusted for baseline values 18, PTX3 concentrations increased in group MP at T6 (P < 0·001) and T24 (P < 0·001) compared to group C (Fig. 2). At T48 PTX3 concentrations were lower and approaching baseline values in group MP compared to group C (P = 0·005). CRP concentrations were lower in group MP at T24 (P < 0·001) and T48 (P < 0·001) compared to group C. Regarding the other investigated complement markers, we found no difference in concentrations between groups for any given time‐points (P > 0·124), except for C4 concentrations at T48 being nearly significantly lower in group MP compared to group C (P = 0·052) (Table 2). The analysis of in‐vivo MP‐incubated whole blood and hirudin‐anti‐coagulated blood showed no increase in PTX3 levels (data not shown).
Figure 2.
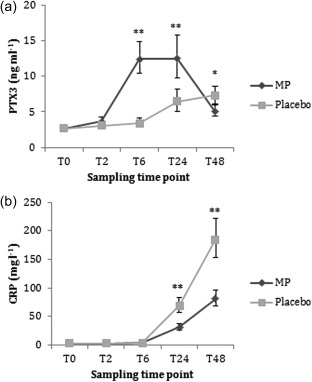
Comparison of the adjusted means for pentraxin‐3 (PTX3) and C‐reactive protein (CRP) concentrations. Adjusted means by univariate analysis incorporating baseline values as covariates 18 for (a) PTX3 and (b) CRP at different time‐points in patients undergoing total knee arthroplasty allocated to preoperative methylprednisolone (group MP) or saline (group C). Significant difference between groups is shown as *P < 0·05 or **P < 0·001. Whiskers represent 95% confidence interval.
There were no wound complications, deep infections or clinically apparent venous thromboembolism (VTE) in either group during the study period.
Discussion
This study demonstrates for the first time that MP prophylaxis in adult patients undergoing elective unilateral TKA is associated with a significantly early increase in plasma levels of PTX3, despite a marked reduced CRP response. Changes in complement markers in elective surgery have received little attention compared to acute illness, nor has the effect of added glucocorticoid in a standardized fast‐track set‐up. Thus, one study found a less pronounced increase in PTX3 levels compared to CRP after elective orthopaedic surgery 4 days after surgery, although not investigating the earlier response 19. In this context, the PTX3 response in acute myocardial infarction is characterized by a rapid increase reaching a peak in 6–8 h compared to 36–48 h for CRP 20, supporting the need for early assessment of PTX3. In children undergoing cardiopulmonary bypass (CPB), dexamethasone prophylaxis led to an increase in PTX3 response compared with untreated subjects 7, compatible with observations in our study. In agreement with this, it has been observed that patients with Cushing syndrome also have increased levels of circulating PTX3, whereas PTX3 levels were decreased in subjects with iatrogenic hypocortisolism 21.
An experimental study has shown that in myeloid DCs glucocorticoids inhibits PTX3 expression and synthesis 21. By contrast, glucocorticoids enhanced and extended the PTX3 expression in fibroblasts and endothelial cells under inflammatory conditions. This agrees with our observations, where we found no increase in PTX3 levels when MP was incubated with whole blood for up to 72 h as well as hirudin‐anti‐coagulated blood at 37°C for up to 48 h (data not shown).
In non‐haematopoietic cells, the glucocorticoid hormone receptor functions as a ligand‐dependent transcription factor (dimerization‐dependent) inducing PTX3 gene expression 21. In contrast, in haematopoietic cells, the glucocorticoid hormone receptor affects PTX3 gene transcription by interfering (dimerization‐independent) with the action of other signalling pathways 21. Thus, the divergent effects of glucocorticoid hormones on PTX3 expression is related to different functions, whether present on myeloid or non‐myeloid cells. In‐vivo observations in mice demonstrate that PTX3 can modulate neutrophil recruitment at inflamed sites through the interaction with P‐selectin, a molecule involved in the early steps of leucocyte recruitment 4. These data may provide some of the possible mechanisms underlining the regulatory role on inflammation exerted by PTX3. Recombinant PTX3 has been suggested as both an infectious and anti‐inflammatory treatment option 22, which could be interesting to consider based on the findings from the present study. Also, it may be that part of the downstream beneficial analgesic and anti‐fatigue effects of MP in relation to TKA 23, 24 is mediated partly by PTX3. In this context, the previous demonstration of PTX3 to correlate with severity of disease 25 in patients with cardiovascular disease 20, 26, cancer 27, acute respiratory distress syndrome 28 and sepsis 17, 29, 30 should be interpreted carefully, as it may not imply that PTX3 contributes to morbidity, but rather reflect the body's anti‐inflammatory response to disease. In this context, PTX3 may potentially be used for early clinical monitoring of glucocorticoid‐induced anti‐inflammatory processes.
As part of the same study, MP also reduced circulating biomarkers of endothelial damage 24 h postoperatively compared to saline, suggesting a protective function against vascular damage 31.
The strengths of the present trial include the high degree of standardized anaesthetic, surgical and multi‐modal analgesic regimen. All patients received spinal anaesthesia using bupivacaine only, the same surgical approach and local infiltration analgesia in all patients. In addition, the use of only one data collector may have minimized the risk of performance bias. Limitations include a short follow‐up period and lack of power and later follow‐up of clinical implications. Furthermore, the selection of biomarkers analysed in this study does not exclude the importance of other markers in the immune reaction to surgical interventions.
Conclusions
In this hypothesis‐generating clinical study, preoperative systemic administration of MP 125 mg increased circulating PTX3 concentrations but reduced the inflammatory response measured by CRP early after fast‐track TKA. No effect was seen on other complement marker changes. Further studies should evaluate the pathophysiological and clinical effects of high‐dose MP in major surgical trauma.
Disclosure
None declared. No benefits in any form have been received from a commercial party related directly or indirectly to the subject of this paper.
Author contributions
V. L. L., H. K., K. P., J. B., M. L. R. and P. G. conceived and designed the study protocol. V. L. L., H. K. and P. G. designed the protocol for the outcome assessment. V. L. L. performed data collection as a blinded assessor. V. L. L. did the blinded statistical analyses with assistance from K. P. and P. G. All authors were involved in the analysis and interpretation of the data. V. L. L. drafted the manuscript, and all authors revised the manuscript. All authors approved the final manuscript.
Acknowledgements
Laboratory technician Jesper Andresen is thanked for skilled technical assistance. We also wish to thank the nurses at the Department of Anaesthesiology as well as the surgeons and nurses at the Department of Orthopaedic Surgery, Copenhagen University Hospital, Bispebjerg and Frederiksberg, Denmark, for helpful assistance. This work was supported by The Lundbeck Foundation Centre for Fast‐Track THA and TKA. The Svend Andersen Research Foundation, Danish Council for Independent Research, the Danish Heart Foundation and the Novo Nordic Research Foundation.
References
- 1. Gaudilliere B, Fragiadakis GK, Bruggner RV et al Clinical recovery from surgery correlates with single‐cell immune signatures. Sci Transl Med 2014; 6:255ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manfredi AA, Rovere‐Querini P, Bottazzi B, Garlanda C, Mantovani A. Pentraxins, humoral innate immunity and tissue injury. Curr Opin Immunol 2008; 20:538–44. [DOI] [PubMed] [Google Scholar]
- 3. Deban L, Jaillon S, Garlanda C, Bottazzi B, Mantovani A. Pentraxins in innate immunity: lessons from PTX3. Cell Tissue Res 2011; 343:237–49. [DOI] [PubMed] [Google Scholar]
- 4. Deban L, Russo RC, Sironi M et al Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol 2010; 11:328–34. [DOI] [PubMed] [Google Scholar]
- 5. Cotena A, Maina V, Sironi M et al Complement dependent amplification of the innate response to a cognate microbial ligand by the long pentraxin PTX3. J Immunol 2007; 179:6311–7. [DOI] [PubMed] [Google Scholar]
- 6. Salio M, Chimenti S, De Angelis N et al Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation 2008; 117:1055–64. [DOI] [PubMed] [Google Scholar]
- 7. Lerzo F, Peri G, Doni A et al Dexamethasone prophylaxis in pediatric open heart surgery is associated with increased blood long pentraxin PTX3: potential clinical implications. Clin Dev Immunol 2011; 2011:730828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kunes P, Holubcova Z, Kolackova M, Krejsek J. Pentraxin 3(PTX 3): an endogenous modulator of the inflammatory response. Mediators Inflamm 2012; 2012:920517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jaillon S, Bonavita E, Gentile S et al The long pentraxin PTX3 as a key component of humoral innate immunity and a candidate diagnostic for inflammatory diseases. Int Arch Allergy Immunol 2014; 165:165–78. [DOI] [PubMed] [Google Scholar]
- 10. Kardash KJ, Sarrazin F, Tessler MJ, Velly AM. Single‐dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg 2008; 106:1253–7. [DOI] [PubMed] [Google Scholar]
- 11. de la Motte L, Kehlet H, Vogt K et al Preoperative methylprednisolone enhances recovery after endovascular aortic repair: a randomized, double‐blind, placebo‐controlled clinical trial. Ann Surg 2014; 260:540–8. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Hopewell S, Schulz KF et al CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garred P, Madsen HO, Kurtzhals JA et al Diallelic polymorphism may explain variations of the blood concentration of mannan‐binding protein in Eskimos, but not in black Africans. Eur J Immunogenet 1992; 19:403–12. [DOI] [PubMed] [Google Scholar]
- 14. Munthe‐Fog L, Hummelshoj T, Honore C et al Variation in FCN1 affects biosynthesis of ficolin‐1 and is associated with outcome of systemic inflammation. Genes Immun 2012; 13:515–22. [DOI] [PubMed] [Google Scholar]
- 15. Munthe‐Fog L, Hummelshoj T, Hansen BE et al The impact of FCN2 polymorphisms and haplotypes on the Ficolin‐2 serum levels. Scand J Immunol 2007; 65:383–92. [DOI] [PubMed] [Google Scholar]
- 16. Mollnes TE, Froland SS, Harboe M. Increased plasma levels of the terminal complement complex in patients with evidence of complement activation. Complement 1985; 2:175–84. [DOI] [PubMed] [Google Scholar]
- 17. Bastrup‐Birk S, Skjoedt M‐O, Munthe‐Fog L et al Pentraxin‐3 serum levels are associated with disease severity and mortality in patients with systemic inflammatory response syndrome. PLOS ONE 2013; 8:e73119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Altman DG. Practical statistics for medical research. London: Chapman & Hall/CRC, 1991;1–611. [Google Scholar]
- 19. Akerfeldt T, Larsson A. Pentraxin 3 increase is much less pronounced than C‐reactive protein increase after surgical procedures. Inflammation 2011; 34:367–70. [DOI] [PubMed] [Google Scholar]
- 20. Peri G, Introna M, Corradi D et al PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation 2000; 102:636–41. [DOI] [PubMed] [Google Scholar]
- 21. Doni A, Mantovani G, Porta C et al Cell‐specific regulation of PTX3 by glucocorticoid hormones in hematopoietic and nonhematopoietic cells. J Biol Chem 2008; 283:29983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cappuzzello C, Doni A, Dander E et al Mesenchymal stromal cell‐derived PTX3 promotes wound healing via fibrin remodeling. J Invest Dermatol 2016; 136:293–300. [DOI] [PubMed] [Google Scholar]
- 23. Lunn TH, Kristensen BB, Andersen LO et al Effect of high‐dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo‐controlled trial. Br J Anaesth 2011; 106:230–8. [DOI] [PubMed] [Google Scholar]
- 24. Toner AJ, Ganeshanathan V, Chan MT, Ho KM, Corcoran TB. Safety of perioperative glucocorticoids in elective noncardiac surgery: a systematic review and meta‐analysis. Anesthesiology 2017; 126:234–48. [DOI] [PubMed] [Google Scholar]
- 25. Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C‐reactive protein to the long pentraxin PTX3. J Clin Immunol 2008; 28:1–13. [DOI] [PubMed] [Google Scholar]
- 26. Latini R, Maggioni AP, Peri G et al Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation 2004; 110:2349–54. [DOI] [PubMed] [Google Scholar]
- 27. Infante M, Allavena P, Garlanda C et al Prognostic and diagnostic potential of local and circulating levels of pentraxin 3 in lung cancer patients. Int J Cancer 2016; 138:983–91. [DOI] [PubMed] [Google Scholar]
- 28. Mauri T, Coppadoro A, Bellani G et al Pentraxin 3 in acute respiratory distress syndrome: an early marker of severity. Crit Care Med 2008; 36:2302–8. [DOI] [PubMed] [Google Scholar]
- 29. Muller B, Peri G, Doni A et al Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med 2001; 29:1404–7. [DOI] [PubMed] [Google Scholar]
- 30. Mauri T, Bellani G, Patroniti N et al Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med 2010; 36:621–9. [DOI] [PubMed] [Google Scholar]
- 31. Lindberg‐Larsen V, Ostrowski SR, Lindberg‐Larsen M, Rovsing ML, Johansson PI, Kehlet H. The effect of pre‐operative methylprednisolone on early endothelial damage after total knee arthroplasty: a randomised, double‐blind, placebo‐controlled trial. Anaesthesia 2017; 72: 1217–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and analysed during the current study are available from the corresponding author on request.


