Summary
Natural killer (NK) cells participate in the regulation of the immune response. However, the immunomodulatory function of NK cells in systemic lupus erythematosus (SLE) is not well understood. The aim of this study was to evaluate the regulatory function of NK cells in SLE patients and to identify the NK cells involved in the pathogenesis of this complex disease. We analysed the expression of NK receptors and co‐stimulatory molecules in peripheral NK cells (CD3–CD56+) from SLE patients, as well as the numbers of human leucocyte antigen D‐related (HLA‐DR)/CD11c+ NK cells. In addition, NK cell regulatory function was assessed by the detection of NK cell‐mediated dendritic cell (DC) lysis. We found that SLE patients showed increased numbers of immunoglobulin‐like transcript 2 (ILT2)+, CD86+ and CD134+ NK cells. Furthermore, NK cells from SLE patients induced higher levels of DC lysis. We were able to identify a new subset of NK cells co‐expressing CD11c and HLA‐DR. These atypical NK cells were increased in SLE patients when compared with controls. We have identified an expanded new subset of NK cells in SLE patients. This is the first study, to our knowledge, which demonstrates that NK cells in SLE patients have an altered phenotype with a high expression of receptors characteristic of dendritic cells. Our results suggest that the impairment in the regulatory function of NK cells, together with the increased number of DC‐like NK cells, could play an important role in the development of SLE and highlight the importance of NK cells as a future therapeutic target.
Keywords: atypical NK cell, dendritic cells, SLE
Introduction
Natural killer (NK) cells are large granular cells that belong to the innate immunity system and to the family of innate lymphoid cells (ILC) 1. Their principal function is to kill virus‐infected and tumoral cells 2. The activation and function of NK cells are regulated by several membrane receptors, including killer immunoglobulin‐like receptors (KIR), CD94/NKG2 lectin‐like receptors and the immunoglobulin‐like transcript receptors (ILT) 3. These molecules are designated commonly as NK cell receptors (NKR), and they can have activating or inhibitory functions. The CD94/NKGA heterodimer is an inhibitory receptor, while CD94/NKG2C and NKG2D homodimer are activating receptors 4. Natural cytotoxicity receptors (NCR) NKp46 (CD335), NKp30 (CD337) and NKp44 (CD336) also regulate the activation of NK cells 5. Immunoglobulin‐like transcript 2 (ILT2), CD85j or leucocyte immunoglobulin‐like receptor B1 (LILRB1), is detected in monocytes, B cells, NK and T cell subsets, and functions as an inhibitory receptor 6.
Although the major function of NK cells is to kill infected and tumoral cells, it is now accepted that they display characteristics of adaptive immune response, such as antigen‐specific memory 7. Also, NK cells are able to express major histocompatibility complex class II (MHC‐II) proteins 8. Interleukin (IL)‐2‐activated NK cells express OX40L (CD252) and CD86 upon NKG2D cross‐linking, and can induce T cell proliferation 9.
Dendritic cells (DCs) are professional antigen‐presenting cells, whose main functions are to engulf and process antigens and to present them to the T cells in order to induce an immune response. NK cells can influence the adaptive immune system through the interaction with DCs 10. It has been shown that NK cells induce the maturation of DCs in vitro. Subsequently, DCs are capable of stimulating proliferation of naive CD4+ T cells and enhancing the cytotoxic activity of NK cells 11. Furthermore, it has been reported that NK cells can induce the production of interferon (IFN)‐α by plasmacytoid DCs stimulated with RNA‐containing immune complexes 12.
Conversely, NK cells are able to negatively regulate the adaptive immune system. It has been reported that autologous immature dendritic cells (iDCs) are susceptible to NK cell‐mediated lysis through the activating NKR, NKp30 13. However, the exact mechanism involved in the NKp30‐mediated lysis of DC is not well understood. It has been reported that NKp30 recognizes the molecule B7‐H6, which is expressed by tumoral cells and by monocytes and neutrophils after ligation of Toll‐like receptor (TLR)‐4 14. The killing of immature DCs guarantees that only iDCs responding to the infectious stimuli can participate in processing antigens and undergo maturation 15. Mature DCs (mDCs) are resistant to NK‐mediated‐lysis, probably by the up‐regulation of MHC‐I molecules 16. The lysis of dendritic cells might act as quality control by eliminating non‐immunogenic iDCs, hence allowing the total activation of the adaptive immune system. However, NK cells not only inhibit adaptive immune response through killing iDCs; it has also been reported that NK cells can prevent T cell function 17. NK cells can kill autologous activated CD4+ T cells efficiently, but not resting CD4+ T cells 18. The lysis of activated T cells represents another way in which NK cells regulate the immune response by killing autoreactive CD4+ T cells and avoiding an autoimmune response.
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with a wide clinical spectrum, which includes arthritis, cutaneous, neurological and renal manifestations. It affects mainly women between puberty and menopause. In SLE, numerous alterations in immune system has been well described, such as autoantibodies produced by B cells, formation of immune complexes, increase in the production of IFN‐α and self‐reactive T cells 19, 20, but little it is known about the role of NK cells in this autoimmune disease. It has been reported that NK cells are significantly diminished in SLE patients compared with healthy volunteers, and that these cells show an enhanced capacity to secrete IFN‐γ while displaying reduced cytotoxicity 21. In SLE patients with recent diagnosis, an increased proportion of NKp46+ NK cells and a diminished percentage of NKG2C+, KIR2DL3+ and KIR2DL1+ NK cells has been reported 22. In another study carried out by Hervier et al., the authors reported an increase in the expression of the inhibitory receptor NKG2A+ in the CD56dim NK cell subset, along with an activated phenotype 23.
Moreover, a subpopulation of NK cells that expresses MHC‐II and CD11c, expressed normally in DCs, was found in a TLR‐7 transgenic murine model of lupus (TLR‐7tg, which contains approximately 10 copies of the endogenous Tlr7 gene). This NK cell population could present antigen efficiently, and could induce autoimmune disease 24, 25.
Little is known about the immunoregulatory function of NK cells in SLE. Therefore, the aim of this study was to assess the capacity of NK cells to modulate DC function in SLE patients and healthy volunteers, as well as to assess the expression of NK receptors and other molecules reported in lupus‐like murine models.
Materials and methods
Subjects
Sixty patients with a diagnosis of SLE were recruited from the department of Rheumatology of the Hospital Dr Ignacio Morones Prieto and from the department of Immunology and Rheumatology of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. SLE diagnosis was made according to the criteria of the American College of Rheumatology 26. The mean age of patients was 35·81 ± 13·23 years. The disease activity score was evaluated according to the SLE disease activity index (SLEDAI) 27. Fifty‐five healthy volunteers with similar age and same sex as the SLE patients were included as controls (mean age = 34·65 ± 12·85 years). All clinical characteristics are summarized in Tables 1 and 2. In all cases, an informed written consent was obtained, and the local Ethics Committees (Ethics and Research Committee of the Hospital Central Dr Ignacio Morones Prieto and Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán) approved this study. This work was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for human experimentation.
Table 1.
Characteristics clinic and demographics of systemic lupus erythematosus (SLE) patients
| SLE | Controls | |
|---|---|---|
| n | 60 | 55 |
| Sex (F/M) | 57/3 | 52/3 |
| Age (mean ± s.d.) | 35·81 ± 13·23 | 34·65 ± 12·85 |
| SLEDAI (mean ± s.d.) | 5·51 ± 4·44 | |
| ≤ 4 | 29 | |
| > 4 | 16 | |
| Remission | 15 | |
| Treatment | 44 | |
| No treatment | 16 |
Data are shown as the mean and standard deviation for sex, age and SLE Disease Activity Index (SLEDAI). The patients were divided into three groups according to SLEDAI (≤ 4, > 4 and remission patients); s.d. = standard deviation.
Table 2.
Combination therapy schemes used in systemic lupus erythematosus (SLE) patients
| Drug combination | Number of patients |
|---|---|
| Methotrexate + anti‐malarial | 3 |
| Methotrexate + prednisone + cyclosporin | 1 |
| Methotrexate + prednisone | 11 |
| Prednisone + azathioprine | 1 |
| Methotrexate + prednisone + azulfidine | 1 |
| Methotrexate + prednisone + anti‐malarial | 6 |
| Methotrexate + prednisone + anti‐malarial + cyclophosphamide | 1 |
| Prednisone + anti‐malarial | 1 |
| Anti‐malarial | 5 |
| Prednisone + anti‐malarial + azathioprine | 1 |
| Anti‐malarial + mycophenolate mofetil | 1 |
| Prednisone + anti‐malarial + cyclophosphamide + mycophenolate mofetil | 1 |
| Methotrexate + prednisone + azathioprine | 1 |
| Mycophenolate mofetil | 2 |
| methotrexate + prednisone + anti‐malarial + mycophenolate mofetil | 1 |
| Prednisone | 2 |
| Mycophenolate mofetil + tacrolimus | 1 |
| Methotrexate | 4 |
Antibodies
The following monoclonal anti‐human antibodies were used: anti‐human CD3 labelled with peridinin chlorophyll (PerCP), anti‐human NKp46‐phycoerythrin (PE), anti‐human NKG2D‐PE, anti‐human CD107a [lysosomal‐associated membrane protein 1 (LAMP‐1)] coupled to PE/cyanin 7 (Cy7), anti‐human CD11c‐PerCP/Cy5 and anti‐human human leucocyte antigen D‐related (HLA‐DR) coupled to allophycocyanin (APC)/Cy7 (BioLegend, San Diego, CA), anti‐human CD56 labelled with APC, anti‐human ILT2‐PE, anti‐human CD80‐fluorescein isothiocyanate (FITC), anti‐human CD86 coupled to PE and anti‐human CD134 (OX40)‐PE, anti‐HLA‐DR‐PE (eBioscience, San Diego, CA, USA), anti‐human CD161‐PE (BD Biosciences, San José, CA, USA), anti‐human CD159a/NKG2A labelled with phycoerythrin [magnetic affinity cell sorting (MACS); Miltenyi Biotec, Bergisch Gladbach, Germany] and anti‐human NKG2C PE‐conjugated anti‐human NKp30‐PE (R&D Systems, Minneapolis, MN, USA). For functional assays the following antibodies were employed: low endotoxin, azide‐free (LEAF) purified anti‐human NKp30, clone P30‐15 and anti‐human NKG2D, clone 1D11 (BioLegend).
Flow cytometry analysis
Peripheral blood mononuclear cells (PBMC) were incubated with Fc‐blocking antibody (Human TruStain FcX; BioLegend) and stained with specific conjugated monoclonal antibodies to CD3, CD56, CD161, ILT2, NKG2A, NKG2C, NKG2D, NKp46, NKp30, CD86, HLA‐DR, CD134 and CD80. To identify a specific subset of NK cells, PBMC were stained with anti‐CD3‐PerCP, CD56‐APC, CD11‐PerCP‐Cy5.5, HLA‐DR‐APC‐Cy7 and NKp46‐PE. Cells were then washed, resuspended in phosphate‐buffered saline (PBS) and analysed by multi‐parametric flow cytometry. The cells were acquired using the fluorescence activated cell sorter (FACS) Diva Software (BD Biosciences) in a FACSAria II cytometer (BD Biosciences). In order to set gates we used the fluorescence minus one (FMO) strategy. All data were analysed with FlowJo version 10.1 software (TreeStar, Inc., Ashland, OR, USA).
Cell isolation
Heparinized peripheral venous blood was obtained through peripheral venipuncture. PBMCs were isolated by a gradient of Ficoll‐PaqueTM PLUS (GE Healthcare Bio‐Sciencies AB, Upsala, Sweden) centrifugation. A fraction of the total PBMC obtained was designated for the analysis NK cell receptors by flow cytometry, while the other fraction was used to purify NK cells and monocytes. For NK cell isolation, PBMC was incubated with the EasySep™ human NK cell enrichment kit, followed by negative selection through the ‘Big Easy’ EasySepTM magnet (Stemcell Technologies, Vancouver, Canada). NK cell purity was assessed by flow cytometry analysis and was always > 90%. Isolated NK cells were frozen in 10% of fetal bovine serum (FBS) and dimethylsulphoxide (DMSO) and stored at −80°C until used. Monocytes were isolated from PBMC by incubation with anti‐CD14 monoclonal antibody (mAb)‐coated microbeads followed by positive selection using MACS Miltenyi Biotec MS columns.
In‐vitro DC generation
For the in‐vitro generation of monocyte derivated DCs (moDC), purified monocytes were plated at a final concentration of 1 × 106 monocytes/ml in RPMI‐1640 (×1) culture medium (Gibco, Grand Island, NY, USA) supplemented with 15% of FBS (Gibco), 2 mM glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin (Gibco), non‐essential amino acids and sodium pyruvate (Sigma Chemical Co., St Louis, MO, USA). Monocytes were cultured in the presence of recombinant human recombinant human granulocyte–macrophage colony‐stimulating hormone (rhGM‐CSF) (50 ng/ml) and recombinant human IL‐4 (15 ng/ml) for 6 and 8 days. At day 6 a fraction of immature DCs (iDCs) was harvested for co‐culture with NK cells. Another fraction of iDCs was cultured for 24 additional hours with lipopolysaccharide (LPS) (100 ng/ml) in order to induce maturation. Cells were harvested at day 8, washed, labelled and analysed for expression of the indicated maturation markers. Culture medium and cytokines were replaced every 2 days.
Carboxyfluorescein diacetate succinimidyl ester (CFSE)‐based cytotoxicity assay
Autologous iDCs or mDCs were harvested and washed with PBS. Cells were loaded with 5 mM of CFSE (Molecular Probes, Eugene, OR, USA) and incubated for 10 min at 37°C. NK cells were thawed before use and cultured overnight in RPMI supplemented with 10% FBS, penicillin (100 U/ml), streptomycin (100 mg/ml) and glutamine (2 mM). Human recombinant IL‐2 (R&D Systems, Inc., Minneapolis, MN, USA) was added for NK cell activation at a final concentration of 50 ng/ml. CFSE‐labelled autologous DCs were used as target cells. Co‐culture of NK : iDCs or NK : mDCs was performed at different ratios [effector : target (0 : 1, 1 : 5, 1 : 1 and 5 : 1)] for 8 and 24 h. We maintained the number of DCs in each condition and the number of NK cells was adjusted accordingly. After 8 and 24 h, cells were harvested and analysed using the FACSCalibur flow cytometer (BD Biosciences). To assess CFSE‐labelled cell lysis, harvested cells were stained with 7‐aminoactinomycin D (7‐AAD) solution, according to the manufacturer's instructions, in order to detect and exclude dead cells. Samples were then acquired in the flow cytometer for 1 min per sample. In some experiments, an anti‐CD107a (LAMP‐1) antibody labelled with PerCP‐Cy7 was added at the beginning of the co‐culture. Cells were harvested at 8 and 24 h and labelled with anti‐CD3‐PerCP and anti‐CD56‐APC antibodies. The percentage of NK cell degranulation (CD107a expression) was assessed by flow cytometry. For positive control, NK cells were stimulated with phorbol myristate acetate (PMA)/ionomycin.
Analysis of NKR function
In order to evaluate the role of NKG2D and NKp30 receptors in NK cell cytotoxic function against DCs, NK cells were incubated for 30 min on ice with the following functional degree antibodies, anti‐NKG2D (5 μg/ml) and/or anti‐NKp30 (3 μg/ml), in three different conditions: anti‐NKG2D, anti‐NKp30 or anti‐NKG2D plus anti‐NKp30. After incubation, NK cells were washed with PBS and co‐culture with previously CFSE‐loaded iDCs. In brief, NK/iDCs were co‐cultured for 24 h at different ratios [NK : DC (1 : 5, 1 : 1, 5 : 1)] in the presence of anti‐CD107a‐PE‐Cy7 antibody. Cells were then harvested and analysed by flow cytometry.
Cytokine production assay
For supernatants from NK cells, DC co‐cultures were collected and stored at −80°C. Cytokine levels were quantified using the cytokine bead array (CBA) human T helper type 1 (Th1)/Th2 Cytokine Kit II (BD Biosciences), according to the manufacturer's instructions, and then analysed in a FACS Canto II (BD Biosciences).
Statistical analysis
Data were analysed with the GraphPad Prism version 5.01 software. Flow cytometry data were evaluated by using the Mann–Whitney U‐test. When indicated, the Kruskall–Wallis test was also performed. Post‐hoc analysis was made using Dunnet's post‐test. The analysis of correlations between variables was based on Spearman's rank test. P < 0·05 was considered statistically significant.
Results
Phenotype of circulating NK cells from SLE patients
First, we assessed the levels of circulating NK cells and NKR from SLE patients and controls. We evaluated 29 patients and controls. Four patients were without treatment at the time of the study. Twenty‐five patients were receiving immunosuppressive therapy and median of SLEDAI activity was 5·7. The gating tree is shown in Fig. 1a. NK cells were defined as CD3–CD56+ cells. As shown in Fig. 1b, SLE patients showed lower percentages of CD3–CD56+ cells compared with healthy controls (median = 5·68 versus 9·14%, respectively, P = 0·04). We evaluated the expression of inhibitory and activating receptors in NK cells and observed that although the levels of NK cells positive for the activating receptors NKG2C, NKG2D, NKp30 (Fig. 1c) and NKp46 (data not shown) were similar in SLE patients and controls, the percentages of NK cells positive for the inhibitory receptor ILT2 were significantly higher in SLE patients compared to the control group (median = 38·28 versus 22·70% respectively, P = 0·002) (Fig. 1d). However, we did not observe differences in the levels of NK cells expressing NKG2A; furthermore, the surface expression of all the receptors studied, measured as mean fluorescence intensity (MFI), showed similar results in both groups (data not shown).
Figure 1.
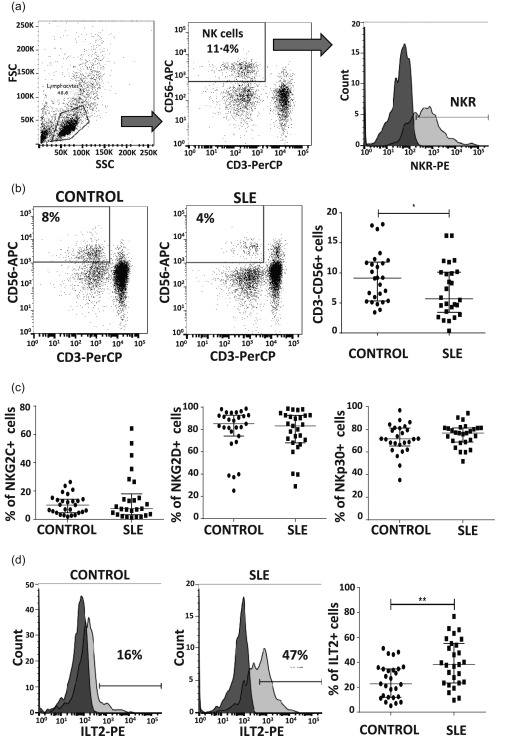
Expression of natural killer receptors (NKR) in peripheral NK cells from systemic lupus erythematosus (SLE) patients and healthy controls. Peripheral blood mononuclear cells (PBMCs) from SLE patients (n = 29) and healthy controls (n = 29) were immunostained for CD3, CD56 and the NK receptors (NKR): NKG2A, NKG2C, NKG2D and immunoglobulin‐like transcript 2 (ILT2), and analysed by flow cytometry. (a) Flow cytometry gating strategy for the analysis of NKR expression in the CD3–CD56+ cell subset is shown. The data in (b), (c) and (d) were generated based on similar gating strategies. Cut‐off for background fluorescence was defined based on isotype negative controls and fluorescence minus one (FMO) strategy. (b) Left and middle panel: dot‐plot from an SLE patient and a healthy control is shown. Numbers represent percentages of NK cells. Right panel: analysis of the NK cell percentages from SLE patients and controls. (c) Percentages of positive cells for activating receptors NKG2C, NKG2D and NKp30 were analysed in CD3–CD56+ cells from SLE and healthy controls. (d) Left and middle panels: dot‐plot from an SLE patient and a healthy control is shown. Numbers represent percentages of ILT2‐positive NK cells. Right panel: analysis of the ILT2 expression in the CD3–CD56+ subset. Data are represented as the median and the interquartile range. *P < 0·05; **P < 0·005; ***P < 0·0001.
NK cells expressing CD86, CD134 and HLA‐DR are increased in SLE patients
It has been reported that NK cells express co‐stimulatory molecules CD80 and CD86, as well as MHC‐II molecules 28. We decided to evaluate the expression of these important activating molecules, as well as the expression of CD134, as this molecule has an important role in the interaction of NK : DC. The levels of CD80+ NK cells were similar in SLE patients and controls (Fig. 2a). Interestingly, the percentages of NK cells positive for CD86 and CD134 (P = 0·02) were significantly higher in SLE patients compared to controls (P < 0·05 in both cases) (Fig. 2b,c). Importantly, the percentage of NK cells positive for HLA‐DR was significantly higher in SLE patients. Notably, we found SLE patients with more than 90% of NK cells positive for this MHC class II molecule (Fig. 2d). When we analysed MFI (data not shown) we found no difference between both groups studied, suggesting that it is the number of positive cells, but not the density of molecules on one cell surface, that differs in SLE patients. We assessed the possible impact of immunosuppressive therapy in the expression of these molecules and we found no significant difference between patients receiving treatment and those who were not (P > 0·05).
Figure 2.
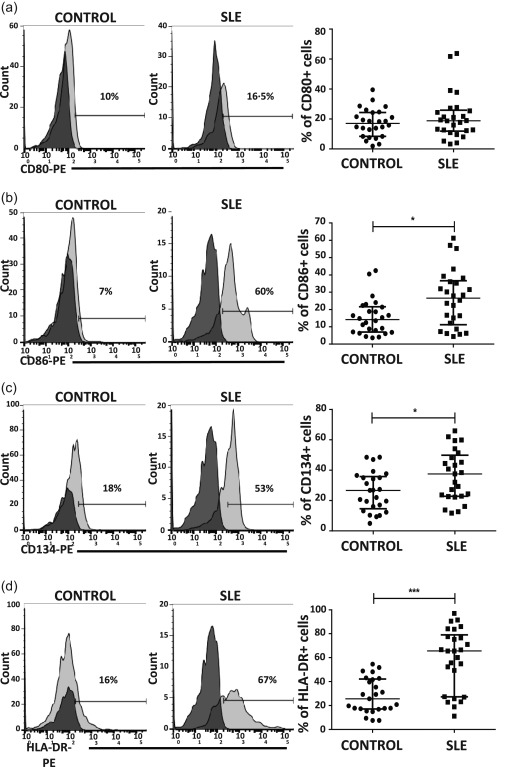
Expression of co‐stimulatory and major histocompatibility complex (MHC) class II molecules in natural killer (NK) cells from healthy volunteers and systemic lupus erythematosus (SLE) patients. Peripheral blood mononuclear cells (PBMCs) from SLE patients (n = 29) and healthy controls (n = 29) were immunostained for CD3, CD56, CD80, CD86, CD134 and human leucocyte antigen D‐related (HLA‐DR). Co‐stimulatory molecules and HLA‐DR were analysed from the CD3–CD56+ subset. Left and middle panel in (a–d). Dot‐plots from one healthy control and one SLE patient are shown. Numbers represent the percentage of CD80, CD86, CD134 or HLA‐DR‐positive cells from the CD3–CD56+ population. The cut‐offs for background fluorescence were based on isotype‐matched immunoglobulin (Ig)‐negative controls and fluorescence minus one (FMO) strategy. Right panel in (a–d). Analysis of the expression of CD80, CD86, CD134 or HLA‐DR in the CD3–CD56+ subset from SLE patients and healthy controls. Data are represented as the median and the interquartile range. *P < 0·05; **P < 0·005; ***P < 0·0001.
NK cells regulate adaptive immune responses by lysing DCs and this function is altered in SLE
In order to evaluate the cytotoxic capacity of NK cells against autologous DCs in SLE, we performed co‐cultures of NK cells with immature or mature DCs as targets. DCs were labelled previously with CFSE and the co‐cultures performed at different ratios [NK (effector) : DC (target cells): 0 : 1, 1 : 5, 1 : 1 and 5 : 1]. DCs were harvested at 8 or 24h, acquired in a flow cytometer, and analysed using the following approach: CFSE‐positive DCs are living cells, and we refer to them as percentage of DC survival (Fig. 3a). We assumed that the percentage of DC survival correlated inversely with NK cell‐mediated cytotoxicity. We also assessed the degranulation of NK cells by measuring CD107a expression (Fig. 3b). When we analysed immature DC survival after 24 h of culture we found that the percentage of DC survival was lower in SLE samples at the three different ratios of NK : DC (Fig. 3c, left panel). As expected, NK‐mediated lysis of DCs was observed at a higher NK cell proportion only in healthy controls (NK : DC, 5 : 1) (Fig. 3c).
Figure 3.
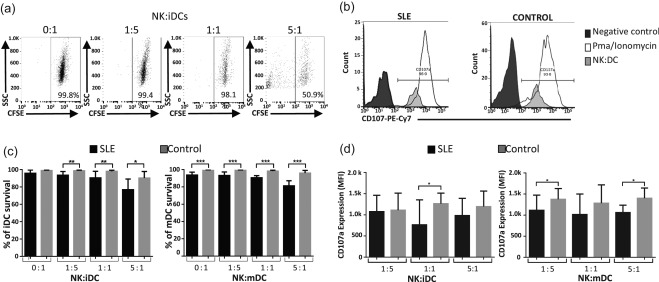
Regulatory function of natural killer (NK) cells is impaired in systemic lupus erythematosus (SLE) patients. Co‐cultures of NK cells and carboxyfluorescein diacetate succinimidyl ester (CFSE)‐labelled immature and mature dendritic cells were performed. Cells were harvested at 8 or 24 h and analysed by flow cytometry. Percentage of dendritic cell (DC) survival corresponds to % of CFSE‐positive DCs. (a) Representative dot‐plots of CFSE assays from an SLE patient are shown. Flow cytometry acquisition was performed during 1 min per sample. Numbers represent percentage of CFSE‐positive DCs. (b) Percentage of NK cell degranulation (CD107a expression) was assessed by flow cytometry. Representative histograms of CD107a expression from one SLE patient and one healthy control are shown. Numbers represent the percentage of NK cells positive for CD107a. (c) Right panel: percentage of immature DC survival from SLE patients and healthy controls at the following effector : target ratios 0 : 1, 1 : 5, 1 : 1 and 5 : 1. Left panel: percentage of mature DC survival from SLE patients and healthy controls at the following effector : target ratios 0 : 1, 1 : 5, 1 : 1 and 5 : 1. (d) Surface expression of CD107a in NK cells from SLE patients and healthy controls, harvested at 8 h after co‐culture with (c) immature and (d) mature DCs is displayed. Data are shown as the median and interquartile range. Controls are displayed in grey bars and SLE patients in black bars. *P < 0·05; **P < 0·005; ***P < 0·0001.
We observed that the percentage of mature DC survival is diminished significantly in SLE patients compared with controls at 8 (data not shown) and 24 h in all ratios, including in the absence of NK cells, indicating that mature DCs in SLE have less survival ability independently of NK cell function (Fig. 3c, right panel). In this regard, when surface expression of CD107a was evaluated at 8 h of co‐culture, we found that NK cells from SLE patients showed diminished CD107a expression compared to controls. This was observed in co‐cultures with iDCs at a ratio of 1 : 1 effector : target cells (Fig. 3d, right panel) and in co‐cultures with mDC at 1 : 5 and 5 : 1 ratios (Fig. 3d, left panel). However, at 24 h of co‐culture the surface expression of CD107a was similar in SLE patients compared with controls in all ratios (data not shown). Our results show that NK cells from SLE patients display an impaired cytotoxic function, whereas their DCs have less survival capacity.
To analyse the possible involvement of the inflammatory milieu related to SLE in this phenomenon, we decided to analyse a cohort of patients in remission of SLE and compare them with a group of patients with a SLEDAI activity index > 4 (Fig. 4). As described previously, NK cells from healthy controls induce immature DC lysis at a higher NK cell ratio. Immature and mature DCs from healthy controls were resistant to lysis at 8 h (data not shown), but at 24 h we observed a slight but significant increase in DC lysis at higher NK cell proportions (5 : 1) (Fig. 4a). Interestingly, NK : DC co‐cultures from patients in remission had a similar behaviour to those from healthy controls (Fig. 4a,c). It is important to point out that in healthy controls and in most of the remission patients we did not observe spontaneous lysis of DCs. Conversely, DCs from patients with active SLE showed reduced survival in the absence of NK cells; this was true for immature and mature DC (Fig.4b,d). Furthermore, the reduction in immature and mature DC survival was promoted by co‐culture with NK cells, suggesting that in this case NK cells mediated DC lysis (Fig. 4a,c).
Figure 4.
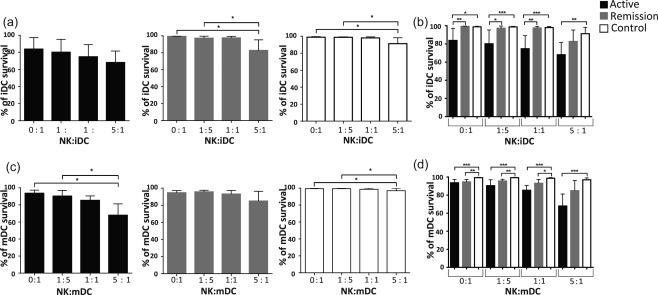
Regulatory function of natural killer (NK) cells is impaired in systemic lupus erythematosus (SLE) patients and depends upon disease activity. Co‐culture of NK cells and carboxyfluorescein diacetate succinimidyl ester (CFSE)‐labelled immature or mature dendritic cells (DCs) were performed for 24 h at different ratios; the percentage of dendritic cell survival corresponds to CFSE+ cells. (a) Percentage of immature DC survival following co‐culture with NK cells from active, remission and control subjects for 24 h at different ratios. (b) Comparison of the percentage of DC survival between the three groups. (c) Results of the percentage of mature DC survival at 24 h from active, remission and control subjects are displayed. (d) Comparisons between the three groups. Data are shown as the median and interquartile range. Controls are displayed in white bars, remission patients in grey bars and patients with SLEDAI > 4 in black bars. *P < 0·05; **P < 0·005; ***P < 0·0001.
In all cases, the possible association of our results with the immunosuppressive therapy was evaluated. Patients were divided according to their therapy: (i) prednisone plus anti‐malarial, (ii) methotrexate plus prednisone, (iii) methotrexate, prednisone and a combination of azathioprine or mycophenolate mofetil (MMF), and (iv) no treatment. We found no difference between groups in any case (P > 0·05, Kruskal–Wallis test and Dunn's multiple comparison test).
Role of NKp30 and NKG2D in the immunoregulatory function of NK cells
To evaluate the role of NKp30 and NKG2D in NK cell function, we performed NK/DC co‐cultures at different ratios, as mentioned previously, and in the presence or absence of the following antibodies – anti‐NKp30, anti‐NKG2D or anti‐NKp30 plus anti‐NKG2D – and evaluated CD107a expression in NK cells (Fig. 5). In healthy controls, we could not observe any effect by blocking these activating receptors. Interestingly, we found that the preincubation of NK cells with anti‐NKp30 promotes a slight increase in degranulation of NK cells in SLE patients when compared with the non‐blocking condition (Fig. 5a–c); this effect could also be observed when NK cells were incubated in the presence of anti‐NKp30 plus anti‐NKG2D at a 1 : 5 NK : DC ratio (Fig. 5a). With regard to the NKG2D receptor, its inhibition showed no effect on NK cell degranulation (Fig. 5a–c).
Figure 5.
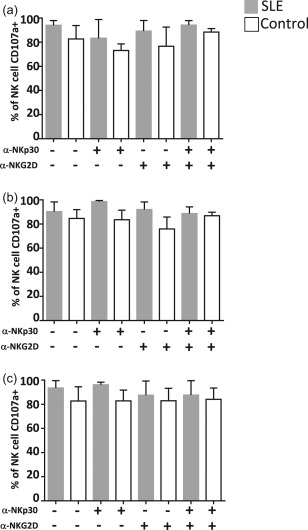
Dendritic cell (DC) lysis is not dependent on natural killer (NK)p30 and NKG2D function. NK cells were treated previously with α‐NKp30, α‐NKG2D or both α‐NKp30 plus α‐NKG2D blocking antibodies then co‐cultured with immature DC at different ratios – NK : DC 1 : 5 (a), 1 : 1 (b) and 5 : 1 (c), and harvested after 24 h. CD107a‐phycoerythrin/cyanin 7 (PE/Cy7) antibody was added at the beginning of the co‐culture and the percentage of CD107a‐positive cells was analysed by flow cytometry. Data are shown as the median and the interquartile range.
Cytokine levels on co‐cultures of NK : DCs
We observed that DCs from SLE patients presented increased levels of lysis in co‐cultures with NK cells, even when NK degranulation was diminished. Therefore, we decided to evaluate cytokine levels in the supernatants of these co‐cultures. We found similar levels of inflammatory cytokines in all the conditions analysed. IFN‐γ and tumour necrosis factor (TNF)‐α concentrations were similar in those co‐cultures from SLE patients and healthy controls (Fig. 6a,b). The same was found for IL‐10 (Fig. 6c). Interestingly, we detected significantly lower levels of IL‐6 (P = 0·02) in supernatants from SLE cultures at a ratio of NK : DC 1 : 1 (Fig. 6d).
Figure 6.
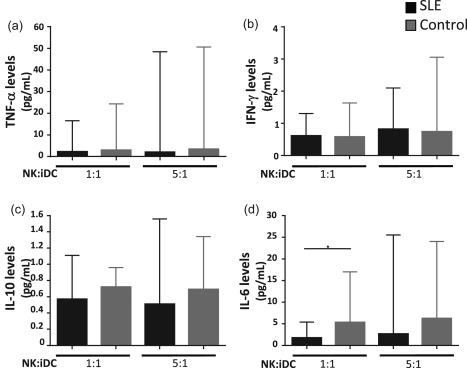
Interleukin (IL)‐6 levels are reduced in systemic lupus erythematosus (SLE) co‐cultures. Co‐cultures of natural killer (NK) and immature dendritic cells (DCs) were performed at different ratios. After 24 h cells were harvested and the supernatants were collected. Levels of interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α, IL‐6, and IL‐10 were determined by flow cytometry. SLE patients are shown in black bars and healthy controls in grey bars. Data are represented as the median and the interquartile range. *P < 0·05.
Identification of a new subset of CD11c+ HLA‐DR+ NK cells increased in SLE patients
New subsets of NK cells, referred to as NK : DC or atypical NK cells 24, 25, 29, have been described. These NK cell subsets are characterized by the co‐expression of NK cell markers, including NK1.1 and some DCs markers, including CD11c, MHC‐II and co‐stimulatory molecules. Nevertheless, these new NK cell subsets are not well characterized in humans. We decided to assess whether this population of atypical NK cells could also be identified in SLE patients. We evaluated 10 SLE patients. All patients were under immunosuppressive therapy. The mean SLEDAI activity index was 3·3. We chose the lymphocyte gate based on size and granularity characteristics, and then identified CD3–CD56+ cells. We analysed NKp46 expression to guarantee NK cell lineage and the percentages of CD11c+HLA‐DR+ cells from the CD3–CD56+NKp46+ subpopulation (Fig. 7a). Surprisingly, approximately 10% of NK cells display the atypical phenotype in healthy controls, and this was significantly higher in SLE patients (mean = 25%, P = 0·007) (Fig. 7b,c). Interestingly, in the SLE group the percentage of atypical NK cells correlated negatively with the SLEDAI activity index (Fig. 7d).
Figure 7.
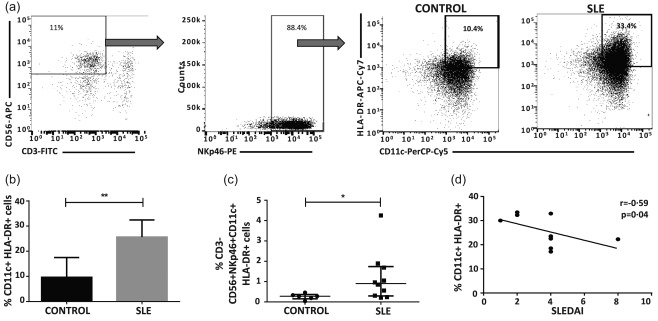
Systemic lupus erythematosus (SLE) patients display higher numbers of atypical natural killer (NK) cells. Peripheral blood mononuclear cells (PBMC) from SLE patients (n = 10) and healthy controls (n = 6) were immunostained for CD3, CD56, the NK receptor, NKp46, CD11c and human leucocyte antigen D‐related (HLA‐DR) and analysed by flow cytometry. (a) Flow cytometry gating strategy for the analysis of CD11c and HLA‐DR expression in the CD3–CD56+NKp46+ cell subset (NK cells) from one healthy control is shown. Right panel: histogram from one SLE patient. Numbers in histograms represent HLA‐DR/CD11c+ cells analysed from the CD3–CD56+NKp46+ population. The data in (b), (c) and (d) were generated based on the same gating strategy. Cut‐off for background fluorescence was defined based on isotype negative controls and fluorescence minus one (FMO) strategy. (b) Percentage of HLA‐DR+CD11c+ NK cells from SLE patients and healthy controls, analysed as described in (a). (c) Frequency of CD3–CD56+NKp46+CD11c+ HLA‐DR+ cells from SLE patients and healthy controls, analysed from lymphocyte gate in the forward‐ and side‐scatter dot‐plots. (d) Correlation between CD11c+HLA‐DR+ NK cells and SLE Disease Activity Index (SLEDAI) index. Data are shown as the median and the interquartile range. *P < 0·05; **P < 0·001.
Discussion
NK cells have been described as playing a pivotal role in the innate immune responses. Currently, it is widely accepted that they have regulatory functions, which is clear from studies in animal models of autoimmune diseases. An impairment of NK cell‐mediated regulatory function has been described in multiple sclerosis 30. In this study, we analysed for the first time, to our knowledge, the regulatory functions of NK cells in SLE. We found reduced levels of NK cells in peripheral blood from SLE patients compared to healthy controls, as it has been described previously 31, 32. This reduction in the numbers of circulating NK cells may be explained by the recruitment of these cells into sites of inflammation, as it has been reported for other inflammatory diseases such as rheumatoid arthritis, where an increase of CD56bright NK cells has been observed in synovial fluid of inflamed joints. However, we cannot exclude the possibility of recruitment to lymph nodes or to increased levels of NK cell apoptosis 33, 34. Circulating NK cells expressing the inhibitory receptor ILT2 were increased in SLE patients. ILT2 is an inhibitory receptor that inhibits NK cell cytolytic function 35. We have reported previously an impaired function of ILT2 in peripheral blood mononuclear cells from SLE patients 36. However, the function of this receptor in NK cells from SLE donors has not been yet explored. Although the expression of NKR assessed in this study was similar in both our groups, it was extremely interesting that SLE patients displayed a high variation of percentages of NK cells expressing NKG2C. This may reflect the complex scenario of analysing the NK cell phenotype. It has been reported that human cytomegalovirus (HCMV) infection is associated significantly with an increased expression of some cell membrane receptors expressed by NK lymphocytes. In particular, HCMV‐seropositive apparently healthy adult individuals exhibit a significantly enhanced proportion of NK and T lymphocytes that express the CD94/NKG2C NK receptor, which is accompanied by increased levels of ILT2 expression 37. It has been reported that the risk of acquiring HCMV infection through life is high, and the presence of anti‐HCMV antibodies in serum from apparently healthy individuals can reach 100% in developing countries 38. The possible impact of HCMV infection in our results was not fully evaluated. However, we were not able to detect differences in the expression of NKG2C in NK cells from SLE patients compared to controls, suggesting that HCMV infection may not have a relevant influence in our results.
HLA‐DR is a MHC‐II molecule, which expression is restricted to antigen‐presenting cells. However, it has been described that in human NK cells the expression of MHC‐II can be induced by stimulation with IL‐2 39. Remarkably, we found that SLE patients have significantly increased levels of NK cells expressing HLA‐DR molecule, which suggests that NK cells in lupus displaying an activating phenotype may be due to the inflammatory milieu found on this autoimmune disease 40. In support of this, we also found that the co‐stimulatory molecules CD86 and CD134 are also increased in NK cells from SLE individuals. It has been reported previously that the expression of CD86 in NK cells confers them with an activating phenotype and enhances cytotoxicity against tumours 41, 42. In this context, the higher proportions of CD86+ NK cells we detected in SLE patients could be implicated importantly in the deregulation of the immune response observed in this pathology.
CD134 (OX40) is a molecule expressed in T cells, interacts with OX40L (CD252), expressed on DCs and regulates T cell survival 43. Moreover, it is known that this molecule is expressed on NK cells after activation 9, but its function in NK cells remains unknown. We decided to evaluate the expression of CD134, as this molecule could intervene in the interaction between NK and DC. We found an increased frequency of CD134+ NK cells in SLE patients. An increased percentage of circulating OX40L+ myeloid DCs has been reported in blood from active SLE patients, and the OX40–OX40L axis may contribute to the pathogenesis of SLE 44. Our results provide new evidence in this regard; the expression of CD134 in NK cells may allow them to interact with OX40L+ myeloid DCs, prompting adaptive immune responses. Together, NK cells in SLE may participate in the initiation of adaptive immune responses, in this manner promoting the production of autoantibodies.
We observed that NK cells from SLE patients kill immature DCs more efficiently than controls. We hypothesized that in SLE the over‐killing of immature dendritic cells could be a two‐edged sword. On one hand, NK cells may kill immature DCs that have captured and processed self‐antigens, thus limiting the number of iDCs that may undergo maturation and present autoantigens to autoreactive T cells. On the other hand, however, the killing of immature dendritic cells may contribute to the loss of peripheral tolerance, which is a clue to the pathogenesis of SLE. We also found that mature DCs from SLE patients are more susceptible to lysis mediated by NK cells. In this regard, it has been reported that IL‐10 reverses the susceptibility of mature DCs from NK cell‐mediated deletion. Although we did not evaluate IL‐10 plasma levels in SLE patients, it is well known that this cytokine plays a role in SLE. The higher levels of IL‐10 observed in SLE patients may explain the susceptibility of mature DCs from NK cell‐mediated lysis in this group 45. It is possible that the killing of mature DCs represents a mechanism that counteracts the activation of autoreactive T cells and may be involved in achieving remission of the disease 46.
It is well known that activating NKR regulate NK cell activity. Surprisingly, blocking NKp30 and NKG2D receptors did not affect the lysis mediated by NK cells in both SLE patients and controls. This observation, together with the fact that we could not detect differences in the expression of CD107a in our different experimental conditions, indicates that another contact‐dependent or soluble factor may be involved in the lysis of immature DCs. In this regard, it is feasible that the Fas–FasL or TNF‐related apoptosis‐inducing ligand (TRAIL)–TRAIL‐R interaction are involved in this phenomenon 47, 48.
In mice, the expression of MHC‐II molecules by NK cells identifies a specific population of NK cells with a DC phenotype 29. This population of atypical NK cells is increased in an experimental TLR‐7 transgenic murine model of lupus. Atypical NK cells have been shown to participate as antigen‐presenting cells with lytic functions 24, 25. In humans, a similar NK population has been described in multiple sclerosis, where the levels of CD11c+/HLA‐DR+ NK cells correlate with disease activity 30. This is the first report identifying an atypical population of NK cells in healthy subjects and in patients with SLE, characterized by the phenotype CD3–/CD56+/NKp46+/HLA‐DR+/CD11c+. Furthermore, we observed that SLE patients showed significantly higher numbers of these cells and the numbers correlate inversely with disease activity, suggesting that atypical NK cells could be expanded in SLE, but their number decreases as the inflammatory conditions augment, suggesting that these cells may have a protective role in the pathogenesis of this complex disease.
In summary, we have identified a new subset of atypical NK cells HLA‐DR+CD11c+ expanded in SLE patients. Furthermore, NK cells from SLE patients display an aberrant phenotype together with an impaired regulatory function, which supports its important role in the pathogenesis of this disease, and indicates them as a possible target for future therapies.
Disclosure
The authors have declared no conflicts of interest.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Expression of human leucocyte antigen D‐related (HLA‐DR) in peripheral natural killer (NK) cells from systemic lupus erythematosus (SLE) patients and healthy controls. Peripheral blood mononuclear cells (PBMC) from SLE patients (n = 29) and healthy controls (n = 29) were immunostained for HLA‐DR. HLA‐DR was analysed from the CD3–CD56+ subset. The cut‐offs for background fluorescence were based on isotype‐matched immunoglobulin (Ig)‐negative controls and fluorescence minus one (FMO) strategy. Mean fluorescence intensity of HLA‐DR expression from SLE patients and controls is shown.
Acknowledgements
We fully appreciate the collaboration of Dr A. Villasenor from Max Planck Institute for Heart and Lung Research, Bad Nauheim, Germany, for her invaluable help in reviewing this manuscript. This work was supported by the grant CB‐2012‐ 01 CONACYT no.180094, México (to A.M.‐U.).
References
- 1. Caligiuri MA. Human natural killer cells. Blood 2008; 112:461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer‐cell subsets. Trends Immunol 2001; 22:633–40. [DOI] [PubMed] [Google Scholar]
- 3. Middleton D, Curran M, Maxwell L. Natural killer cells and their receptors. Transpl Immunol 2002; 10:147–64. [DOI] [PubMed] [Google Scholar]
- 4. Borrego F, Masilamani M, Marusina AI, Tang X, Coligan JE. The CD94/NKG2 family of receptors. From molecules and cells to clinical relevance. Immunol Res 2006; 35:263–77. [DOI] [PubMed] [Google Scholar]
- 5. Kruse PH, Matta J, Ugolini S, Vivier E. Natural cytotoxicity receptors and their ligands. Immunol Cell Biol 2014; 92:221–9. [DOI] [PubMed] [Google Scholar]
- 6. Brown D, Trowsdale J, Allen R. The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens 2004; 64:215–25. [DOI] [PubMed] [Google Scholar]
- 7. Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature 2009; 457:557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakayama M, Takeda K, Kawano M, Takai T, Ishii N, Ogasawara K. Natural killer (NK)‐dendritic cell interactions generate MHC class II‐dressed NK cells that regulate CD4+ T cells. Proc Natl Acad Sci USA 2011; 108:18360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross‐talk between activated human NK cells and CD4+ T cells via OX40–OX40 ligand interactions. J Immunol 2004; 173:3716–24. [DOI] [PubMed] [Google Scholar]
- 10. Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol 2004; 25:47–52. [DOI] [PubMed] [Google Scholar]
- 11. Gerosa F, Baldani‐Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med 2002; 195:327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hagberg N, Berggren O, Leonard D et al IFN‐alpha production by plasmacytoid dendritic cells stimulated with RNA‐containing immune complexes is promoted by NK cells via MIP‐1beta and LFA‐1. J Immunol 2011; 186:5085–94. [DOI] [PubMed] [Google Scholar]
- 13. Wilson JL, Heffler LC, Charo J, Scheynius A, Bejarano MT, Ljunggren HG. Targeting of human dendritic cells by autologous NK cells. J Immunol 1999; 163:6365–70. [PubMed] [Google Scholar]
- 14. Matta J, Baratin M, Chiche L et al Induction of B7‐H6, a ligand for the natural killer cell‐activating receptor NKp30, in inflammatory conditions. Blood 2013; 122:394–404. [DOI] [PubMed] [Google Scholar]
- 15. Ferlazzo G, Morandi B, D'Agostino A et al The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur J Immunol 2003; 33:306–13. [DOI] [PubMed] [Google Scholar]
- 16. Ferlazzo G, Semino C, Melioli G. HLA class I molecule expression is up‐regulated during maturation of dendritic cells, protecting them from natural killer cell‐mediated lysis. Immunol Lett 2001; 76:37–41. [DOI] [PubMed] [Google Scholar]
- 17. Trivedi PP, Roberts PC, Wolf NA, Swanborg RH. NK cells inhibit T cell proliferation via p21‐mediated cell cycle arrest. J Immunol 2005; 174:4590–7. [DOI] [PubMed] [Google Scholar]
- 18. Nielsen N, Ødum N, Ursø B, Lanier LL, Spee P, Sandberg JK. Cytotoxicity of CD56(bright) NK cells towards autologous activated CD4+ T cells is mediated through NKG2D, LFA‐1 and TRAIL and dampened via CD94/NKG2A. PLOS ONE 2012; 7:31959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaul A, Gordon C, Crow MK et al Systemic lupus erythematosus. Nat Rev Dis Primers 2016; 2:16039. [DOI] [PubMed] [Google Scholar]
- 20. Tsokos GC. Systemic lupus erythematosus. N Engl J Med 2011; 365:2110–21. [DOI] [PubMed] [Google Scholar]
- 21. Spada R, Rojas JM, Barber DF. Recent findings on the role of natural killer cells in the pathogenesis of systemic lupus erythematosus. J Leukoc Biol 2015; 98:479–87. [DOI] [PubMed] [Google Scholar]
- 22. Ye Z, Ma N, Zhao L, Jiang ZY, Jiang YF. Differential expression of natural killer activating and inhibitory receptors in patients with newly diagnosed systemic lupus erythematosus. Int J Rheum Dis 2016; 19:613–21. [DOI] [PubMed] [Google Scholar]
- 23. Hervier B, Beziat V, Haroche J et al Phenotype and function of natural killer cells in systemic lupus erythematosus: excess interferon‐gamma production in patients with active disease. Arthritis Rheum 2011; 63:1698–706. [DOI] [PubMed] [Google Scholar]
- 24. Voynova EN, Skinner J, Bolland S. Expansion of an atypical NK cell subset in mouse models of systemic lupus erythematosus. J Immunol 2015; 194:1503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Voynova E, Qi CF, Scott B, Bolland S. Cutting edge: induction of inflammatory disease by adoptive transfer of an atypical NK cell subset. J Immunol 2015; 195:806–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan EM, Cohen AS, Fries JF et al The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25:1271–7. [DOI] [PubMed] [Google Scholar]
- 27. Bombardier C, Gladman DD, Urowitz MB et al Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992; 35:630–40. [DOI] [PubMed] [Google Scholar]
- 28. Luque I, Reyburn H, Strominger JL. Expression of the CD80 and CD86 molecules enhances cytotoxicity by human natural killer cells. Hum Immunol 2000; 61:721–8. [DOI] [PubMed] [Google Scholar]
- 29. Chan CW, Crafton E, Fan HN et al Interferon‐producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med 2006; 12:207–13. [DOI] [PubMed] [Google Scholar]
- 30. Aranami T, Miyake S, Yamamura T. Differential expression of CD11c by peripheral blood NK cells reflects temporal activity of multiple sclerosis. J Immunol 2006; 177:5659–67. [DOI] [PubMed] [Google Scholar]
- 31. Henriques A, Teixeira L, Ines L et al NK cells dysfunction in systemic lupus erythematosus: relation to disease activity. Clin Rheumatol 2013; 32:805–13. [DOI] [PubMed] [Google Scholar]
- 32. Park YW, Kee SJ, Cho YN et al Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum 2009; 60:1753–63. [DOI] [PubMed] [Google Scholar]
- 33. Dalbeth N, Callan MF. A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum 2002; 46:1763–72. [DOI] [PubMed] [Google Scholar]
- 34. Shibatomi K, Ida H, Yamasaki S et al A novel role for interleukin‐18 in human natural killer cell death: high serum levels and low natural killer cell numbers in patients with systemic autoimmune diseases. Arthritis Rheum 2001; 44:884–92. [DOI] [PubMed] [Google Scholar]
- 35. Favier B, Lemaoult J, Lesport E, Carosella ED. ILT2/HLA‐G interaction impairs NK‐cell functions through the inhibition of the late but not the early events of the NK‐cell activating synapse. FASEB J 2010; 24:689–99. [DOI] [PubMed] [Google Scholar]
- 36. Monsivais‐Urenda A, Nino‐Moreno P, Abud‐Mendoza C et al Analysis of expression and function of the inhibitory receptor ILT2 (CD85j/LILRB1/LIR‐1) in peripheral blood mononuclear cells from patients with systemic lupus erythematosus (SLE). J Autoimmun 2007; 29:97–105. [DOI] [PubMed] [Google Scholar]
- 37. Echaniz‐Aviles G, Tamayo‐Legorreta E, Cruz‐Valdez A et al Prevalence of antibodies against cytomegalovirus in women of reproductive age. Salud Publica Mex 1993; 35:20–6. [PubMed] [Google Scholar]
- 38. Guma M, Budt M, Saez A et al Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus‐infected fibroblasts. Blood 2006; 107:3624–31. [DOI] [PubMed] [Google Scholar]
- 39. Evans JH, Horowitz A, Mehrabi M et al A distinct subset of human NK cells expressing HLA‐DR expands in response to IL‐2 and can aid immune responses to BCG. Eur J Immunol 2011; 41:1924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yano N, Endoh M, Nomoto Y, Sakai H, Rifai A. Increase of HLA‐DR‐positive natural killer cells in peripheral blood from patients with IgA nephropathy. Hum Immunol 1996; 49:64–70. [DOI] [PubMed] [Google Scholar]
- 41. Chen L, Flies DB. Molecular mechanisms of T cell co‐stimulation and co‐inhibition. Nat Rev Immunol 2013; 13:227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peng Y, Luo G, Zhou J et al CD86 is an activation receptor for NK cell cytotoxicity against tumor cells. PLOS ONE 2013; 8:e83913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Croft M. Control of immunity by the TNFR‐related molecule OX40 (CD134). Annu Rev Immunol 2010; 28:57–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jacquemin C, Schmitt N, Contin‐Bordes C et al OX40 ligand contributes to human lupus pathogenesis by promoting T follicular helper response. Immunity 2015; 42:1159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alter G, Kavanagh D, Rihn S et al IL‐10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. J Clin Invest 2010; 120:1905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chiesa MD, Vitale M, Carlomagno S, Ferlazzo G, Moretta L, Moretta A. The natural killer cell‐mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig‐like receptors. Eur J Immunol 2003; 33:1657–66. [DOI] [PubMed] [Google Scholar]
- 47. Hayakawa Y, Screpanti V, Yagita H et al NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J Immunol 2004; 172:123–9. [DOI] [PubMed] [Google Scholar]
- 48. El‐Karaksy SM, Kholoussi NM, Shahin RM, El‐Ghar MM, Gheith Rel S. TRAIL mRNA expression in peripheral blood mononuclear cells of Egyptian SLE patients. Gene 2013; 527:211–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Expression of human leucocyte antigen D‐related (HLA‐DR) in peripheral natural killer (NK) cells from systemic lupus erythematosus (SLE) patients and healthy controls. Peripheral blood mononuclear cells (PBMC) from SLE patients (n = 29) and healthy controls (n = 29) were immunostained for HLA‐DR. HLA‐DR was analysed from the CD3–CD56+ subset. The cut‐offs for background fluorescence were based on isotype‐matched immunoglobulin (Ig)‐negative controls and fluorescence minus one (FMO) strategy. Mean fluorescence intensity of HLA‐DR expression from SLE patients and controls is shown.


