Summary
Visceral leishmaniasis (VL) is a disseminated and lethal disease of reticulo‐endothelial system caused by protozoan parasites Leishmania donovani and L. infantum, which are known to induce host T cell suppression. To understand the impact of parasite load on T cell function, the present was focused on parasite load with T cell function in bone marrow of 26 VL patients. We observed significant enrichment of forkhead box protein 3 (FoxP3)+ (P = 0·0003) and interleukin (IL)‐10+ FoxP3+ regulatory T cells (Treg) (P = 0·004) in the bone marrow (BM) of patients with high parasite load (HPL) compared with low parasite load (LPL). Concordantly, T effector cells producing interferon (IFN)‐γ (P = 0·005) and IL‐17A (P = 0·002) were reduced in the BM of HPL. Blocking of Treg‐cell derived suppressive cytokines [(IL‐10 and transforming growth factor (TGF)‐β] rescued the effector T cells and their functions. However, it was observed that TGF‐β levels were dominant, favouring Treg cell differentiation. Furthermore, the low ratio of IL‐6/TGF‐β favours the suppressive milieu in HPL patients. Here we show the change in levels of various cytokines with the parasitic load during active VL, which could be helpful in devising newer immunotherapeutic strategies against this disease.
Keywords: bone marrow aspirate (BMA), Leishmania donovani, T regulatory cells (Treg), visceral leishmaniasis (VL)
Introduction
Visceral leishmaniasis (VL) is a chronic infectious disease, fatal if left untreated, caused by a group of protozoan parasites of the genus Leishmania and transmitted by phlebotomine sandflies 1. In the Indian subcontinent, visceral leishmaniasis is caused primarily by L. donovani, while L. infantum is the pathogen responsible for the disease in Latin America and the Mediterranean regions 2, 3. Demonstration of the amastigote form of Leishmania parasite in aspirates of lymph node, spleen or bone marrow is still the gold standard for diagnosis 4, 5, 6; parasitic grading is usually used as per the World Health Organization (WHO) guidelines (0–6+) of splenic aspirate 7. The WHO grading system has also been used for bone marrow (BM) aspirate 8, 9, even though the possibility of dilution by peripheral blood remains a concern. Severe parasitic infestation within the reticulo‐endothelial system (RES), including visceral organs such as the liver, spleen and in the BM, is the pathological hallmark of the disease 10. Dissemination of the disease is believed to be due to the suppressed state of immunity induced by the high parasite load (HPL) 2. However, the role of regulatory T cells (Treg) in such parasite‐induced immune suppression at the disease site, i.e. bone marrow, remains unexplored.
Clearance of leishmania parasites from the infected macrophages critically requires a strong T helper type 1 (Th1)‐like response with biased production of inflammatory cytokines. Such cytokines, namely interferon (IFN)‐γ and interleukin (IL)‐17, favour parasite clearance via macrophage activation leading to enhanced production of reactive oxygen and nitrogen species. A strong Th1‐like inflammatory response has been demonstrated to be protective in both the murine model as well as in VL patients 3, 11, 12. A state of immune suppression has been documented as characteristic of VL 13. Therefore, it had been proposed and demonstrated subsequently that the suppressed state of immune response at the pathological sites facilitates parasitic growth and dissemination, leading to their infiltration in the RES of the subjects. We have shown previously that, in spite of a higher frequency of IFN‐γ‐positive T cells, the parasite remains in the BM of the VL patients 14. We also demonstrated a higher frequency of Treg cells in the patients' BM 14. Higher levels of IL‐17 and IL‐22 have been proposed to be protective among endemic healthy contacts of VL patients 15. Thus, along with several other groups, we proposed that a suppressed state of T cell response at the pathological sites of disease is critical for parasitic growth, and this may be an immune evasion strategy of the parasite. We also showed that Mycobacterium tuberculosis induces Treg cell‐mediated suppression of the immune response 16, especially at the pathological sites of miliary tuberculosis and their frequency correlates with the bacillary load of the patients 17. We thus proposed that reciprocal levels of Treg versus inflammatory/effector T cells (IFN‐γ+, IL‐17+) dictate the fate of parasitic survival and pathogen growth within the macrophage. We demonstrated enrichment of Treg cells and IL‐10 secreted by them in the BM of VL patients 14. Here we investigated the status of Treg cells, their suppressive effect on the inflammatory cytokine production relating to the parasite load of the patients [high parasitic load (HPL) versus low parasitic load (LPL)]. We show a higher frequency of Treg cells in the BM of the HPL group as opposed to that of the LPL group. We also observed a higher frequency of Treg cells producing IL‐10 among HPL patients, suggesting that those enriched Treg cells are the major cellular source of IL‐10. These suppressive immune parameters in HPL patients correlate inversely with the lower frequencies of IFN‐γ+ and IL‐17A+ T cells and inflammatory cytokines. Furthermore, blocking of IL‐10 and transforming growth factor (TGF)‐β rescued inflammatory cytokine‐producing T cells in VL. Overall, our results show the direct relationship between Treg cells in the BM and the parasitic burden, suggesting that either Treg‐mediated suppression of the local immune response facilitates parasite load or a higher parasite burden induces Treg‐mediated suppression of immune response at the disease site.
We propose that Treg cells and their suppressive cytokines such as IL‐10, TGF‐β may be indicative of the patients' parasite burden, and blocking the Treg cell function may be critical for rescuing protective immunity and subsequent parasite clearance.
Material and methods
Study subject (VL patients)
BM aspirates from 26 parasitologically confirmed VL patients were included in this study after obtaining the patients' informed consent (mean age = 33·81 years, range = 10–60 years; 17 males and nine females) (Table 1). These patients were visited at the out‐patient department (OPD) of Balaji Utthan Sansthan Patna, Bihar for diagnosis and treatment of their illness. The patients presented with complaints of prolonged high fever, weight loss and swelling of abdomen. Further examination and laboratory findings revealed splenomegaly, hepatomegaly, pancytopenia and hypergammaglobulinaemia, etc. BM samples (1–1·5 ml) were collected in heparinized tubes at the time of diagnosis. Microscopic L‐D bodies were demonstrated as per the 2010 WHO guideline used for splenic aspirate 7. Utmost care was taken to avoid any significant dilution of BM aspirate by bleeding, and any such specimens were excluded. The parasitic load in the BM of these patients was evaluated before recruitment into the study and the start of therapy. The study subjects were divided into HPL and LPL groups based on the parasite density in BM of the study subjects (Supporting information, Fig. S1).
Table 1.
Demographic and haematological investigation table: showing the details of visceral leishmaniasis patient included in this study
| Number of patients | n = 26 | |
|---|---|---|
| Demographic characteristic | ||
| Age (mean ± s.d.) | 33·81 ± 11·35 | |
| Sex (M/F) | 17/09 | |
| Ethnicity | Indian | |
| Diagnosis | ||
| * Parasitic demonstration | No. of patients | Parasitic load |
| In bone marrow | 14 | +1 |
| 07 | +2 | |
| 05 | +3 | |
| Haematological investigations | ||
| † WBC count (mean ± s.d. × 103) | 3·72 ± 2·18 | |
| † Platelets count (mean ± s.d. × 103) | 100·9 ± 43·59 | |
| ‡ Hb (mean ± s.d.) | 8·98 ± 2·08 | |
*Parasitic load in bone marrow was calculated according to standard criteria of WHO (2010). †White blood cell count (WBC) and platelets count in thousands. ‡Haemoglobin (Hb) in mg/dl; s.d. = standard deviation.
Reagents
Isolated BM mononuclear cells (MNCs) were cultured in RPMI medium, supplemented with L‐glutamine (G‐5763; Sigma Chemicals Co., St Louis, MO, USA), antibiotics (Pen‐Strep‐Ampho Sol; Biological Industries, Kibbutz Beit Haemek, Israel) and 10% fetal calf serum (FCS) (Biological Industries); 0·5% bovine serum albumin (BSA) in phosphate‐buffered saline (PBS) was used as the staining buffer for surface staining, while 2% formaldehyde in PBS was used for cell fixation. Cell permeabilization for intracellular antigen detection was performed with 0·5% saponin (S‐7900; Sigma). Monoclonal antibodies were used for surface and intracellular antigen detection. Fluorescein isothiocyanate (FITC)‐conjugated IL‐17A, forkhead box protein 3 (FoxP3) (BD Pharmingen, San Diego, CA, USA), phycoerythrin (PE)‐conjugated CD25, IFN‐γ (BD Pharmingen), peridinin chlorophyll/cyanin (PerCP/Cy5) PE‐conjugated CD4 (BD Pharmingen) and FoxP3 staining kits were purchased from e‐Biosciences (Cat. no. 71–5776‐40; e‐Biosciences, San Diego, CA, USA).
Demonstration of parasite in BM aspirate
Clean labelled slides were used for thin BM smear preparation. After air‐drying, the slides were stained with Giemsa stain (GS500‐500ML; Sigma‐Aldrich), according to the manufacturer's protocol. Following identification of parasitic density on the slides using eyepiece (×10) and objective (×100), the parasite density was scored by means of a logarithmic scale ranging from 0 (no parasite per 1000 oil immersion fields) to +6 (> 100 parasites per field) based on the WHO diagnosis criteria (WHO, March 2010).
Preparation of whole cell lysate antigen from L. donovani
Parasites from late log phase growth were collected by centrifugation (6500 g) for 10 min at 4°C. The pellet of LD was washed twice with sterile PBS and finally resuspended in PBS and then sonicated. Protein concentration was assessed with an ND‐1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Ten µg of sonicated soluble protein (titrated dose) was used to stimulate 1–2 million cells in 1‐ml culture.
Isolation of MNCs from BM aspirate
Briefly, 1–1·5 ml of BM samples was collected in heparinized tubes at the time of diagnosis (microscopic parasitic demonstration). Collected BM samples were centrifuged at 845 g for 5 min in a micro‐centrifuge. The pellets containing cells were separated from the supernatant. Supernatant was collected and stored at −80°C and used for the detection of soluble cytokines in BM aspirate (IL‐17A, IL‐6 and TGF‐β). BM MNCs were isolated from pellets of BM aspirate through Ficoll Hypaque gradient centrifugation; isolated cells were washed thrice with incomplete RPMI (without FBS) and finally suspended in complete RPMI‐1640 supplemented with 10% FBS (Caisson Laboratories, Logan, UT, USA). The cell viability was estimated by trypan blue dye exclusion test. Samples with more than 95% viability were used for in‐vitro culture.
In‐vitro cell culture
Isolated BM MNCs were cultured (2 × 106 cells/ml) in 96‐well U‐bottomed microtitre plates (BD Falcon/BD Biosciences). Plates were coated with purified α‐CD28 and α‐CD49d antibody, 5 µg/ml in carbonate buffer, pH 9·6 (AbdSerotec, Oxford, UK; LE/AF, Cat. no. MCA70EL and MCA923EL, respectively); 0·5 million cells were seeded per well and stimulated with LD antigen (whole cell lysate, 10 µg/ml) for 48 h at 5% CO2 at 37°C. For blocking studies, purified monoclonal antibodies of IL‐10 (10 µg/ml, NA/LE; BD Pharmingen) and TGF‐β (10 µg/ml; BD Pharmingen) was mixed individually or in combination with BM MNCs (2 million/ml). Monensin (Golgi transport inhibitor; 1 mM; Sigma‐Aldrich) was added in the last 6 h of culture.
Surface and intracellular staining
Ex‐vivo and in‐vitro isolated and cultured cells were incubated directly with fluorescence‐labelled monoclonal antibodies in staining buffer (5% BSA in PBS) for 15 min on ice. After washing, fixation was performed in 2% formaldehyde in PBS for 15 min at room temperature. Cells were then permeabilized by using permeabilization buffer (0·5% saponin) for 15 min at room temperature. After washing, the cells were incubated at room temperature for 30 min with a titrated dose of intracellular monoclonal antibodies (IL‐10, IL‐17A and IFN‐γ, etc.) in permeabilization buffer. Finally, after washing twice with staining buffer, cells were transferred to a fluorescence activated cell sorter (FACS) tube (BD Falcon; BD Biosciences) for data acquisition by three/four‐colour flow cytometer (FACSCalibur; BD Biosciences).
Cells staining for FoxP3 antigen
Briefly, 1 × 106 MNCs were first surface‐stained for CD4 and CD25 and were further stained intracellularly for FoxP3 antigen using a FoxP3 staining (FITC/PE) kit (Cat. no. 00–5123‐43; eBiosciences). To ensure the specificity of CD4, CD25 and FoxP3 staining, we performed fluorescence minus one (FMO) and isotype staining of the same specimens. To enumerate the IL‐10‐producing FoxP3+ cells, antigen‐stimulated cells were stained for IL‐10 and FoxP3 using the same protocol. To confirm the staining specificity, we performed FMO for IL‐10. For enumeration of FoxP3+ Treg cells, 2 × 105 events were acquired to obtain the analysable number of FoxP3+ cells.
Estimation of IL‐6, TGF‐β, IL‐10 and IL‐17A in BM aspirates
To evaluate the soluble levels of various cytokines in the BM aspirate, sandwich enzyme‐linked immunosorbent assay (ELISA) was performed using kits specific for IL‐6 (eBioscience; cat no. 88–7066‐22), TGF‐β (eBioscience; cat no.88–8350‐88), IL‐17A (eBioscience; cat no.88–7176‐88) and IL‐10 (eBioscience; cat no.88–7106‐88), following the manufacturer's protocol.
Ethics declaration
The research project was approved by the Institutional Ethics Committee of AIIMS, New Delhi (Ref. no. IEC/NP‐331/2010; 28 December 2010). Written informed consent was obtained from all study subjects. For minor children and for patients who could not read and write, written informed consent was provided by their legal guardian.
Statistical analysis
GraphPad Prism‐5 was used for statistical analysis. Median and standard deviation (s.d.) were used for scatter analysis. The significance between two groups was calculated by unpaired two‐tailed and Mann–Whitney U‐tests. When more than two groups were compared simultaneously, significance was determined by analysis of variance (anova) using a Bonferroni correction for multiple comparisons. A P‐value < 0·05 was considered significant.
Results
Enrichment of Treg cells in BM of VL patients correlates with parasite load
To reveal the status of Treg cells and suppressive cytokines (IL‐10 and TGF‐β) in patients with HPL in BM, we enumerated the frequency of activated T (CD4+CD25+) cells and Treg (CD4+CD25br FoxP3+) cells in BM. We observed a significantly higher percentage frequency of activated T cells in HPL patients (n = 12) compared with LPL patients (n = 14) (Fig. 1b) (P = 0.0002; unpaired two‐tailed t‐test). Furthermore, we observed a significantly higher percentage frequency of FoxP3+ Treg cells (CD4+ CD25brFoxP3+) in patients with HPL compared with low LPL (Fig. 1c) (P = 0·0001; unpaired two‐tailed t‐test). Moreover, we observed a significantly lower percentage frequency of T effector cells (CD4+CD25br FoxP3–) in HPL patients (n = 12: 46·2 ± 4·06) compared with LPL patients (n = 14: 75·1 ± 2·71) (Fig. 1d) (P = 0·0001; unpaired two‐tailed t‐test). The percentage frequencies of activated T (CD4+CD25+) cells also correlated significantly with increased frequency of Treg (FoxP3+) cells (Fig. 1e) (P = 0·004; Pearson's correlation coefficient r 2 = 0·535). Moreover, a higher Treg frequency in BM was correlated positively with parasitic load in the BM of VL patients (Fig. 1f) (P < 0·0001; Pearson's correlation coefficient r 2 = 0·583). These results established a direct correlation between parasitic burden in BM with T cell activation (CD4+CD25+), increased Treg cells (FoxP3+) and decreased T effector cell (FoxP3–) frequency.
Figure 1.
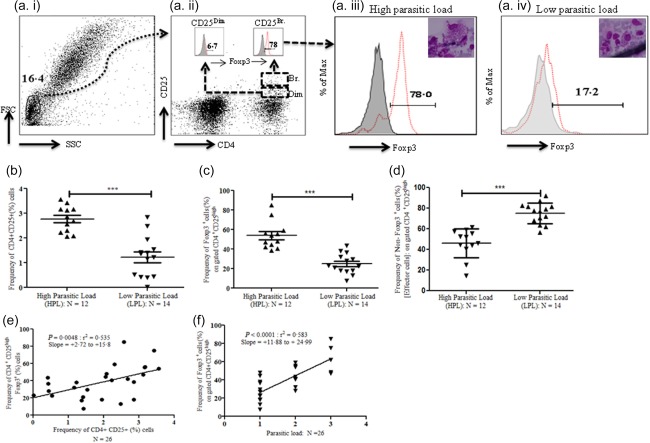
Enrichment of regulatory T cells (Treg) cells in bone marrow of visceral leishmaniasis (VL) patients correlates with parasite load. (a) Enumeration of Treg [forkhead box protein 3 (FoxP3+)] frequency: bone marrow mononuclear cells (BM MNCs) from VL patients were used for immunophenotyping of Treg cells. CD4+CD25+ population of T cells was gated on pregated lymphocytes (based on forward‐ versus side‐scatter); further, the dual population of CD4+CD25+ T cells was divided into CD4+CD25dim and CD4+CD25br (Fig. 1a, ii: inset). Intranuclear expression of FoxP3 was observed in these two populations. Histogram plot of FoxP3 expressing CD4+CD25dim or CD4+CD25br (thin red dotted line) was overlaid on FoxP3 fluorescence minus one (FMO) (solid dark field). CD4+CD25br were found to be the major population of activated T cells expressing FoxP3 (br = bright). (b) Increased percentage frequency of activated (CD4+CD25+) T cells in BM of VL patients with high parasitic load (HPL). The percentage frequency of activated T cells (CD4+CD25+) was analysed from BM‐derived MNCs of VL patients (n = 26). We observed a significantly higher percentage frequency of CD4+CD25+T cells in VL patients with HPL [(2+and 3+: n = 12), mean ± standard deviation (s.d.): 2·76 ± 0·15%] compared with low parasitic load [(1+: n = 14) mean ± s.d.: 1·23 ± 0·21%; P = 0·002, unpaired t‐test]. Horizontal lines in scatter‐plot depicted as the mean value (br = bright). (c) Increased Treg frequency (CD4+CD25+FoxP3+) in BM of VL patients with HPL: significantly increased percentage frequency of FoxP3+ T cells on pregated CD4+CD25+ were observed in BM MNCs of VL patients with HPL (n = 12: mean ± s.d.: 53·84 ± 4·06%) compared to the patients with low parasitic load (LPL) (n = 14: mean ± s.d.: 24·89 ± 2·70%) (P = 0·0001: unpaired t‐test). Horizontal lines in scatter‐plot depicted as mean value. (d) Significantly decreased frequency of T effector cells (CD4+CD25+FoxP3–) in bone marrow of VL patients with HPL: significantly decreased percentage frequency of T effector cells [(CD4+CD25+FoxP3–): i.e. 100%: % frequency of CD4+CD25+FoxP3+] in BM MNCs of VL patients with HPL (n = 12: mean ± s.d.: 46·16 ± 4·06%) compared to the patients with LPL (n = 14: mean ± s.d.: 75·12 ± 2·70%) (P = 0·0001: unpaired t‐test). Horizontal lines in scatter‐plot depicted as mean value. (e) Increased frequency of Treg (CD4+CD25+FoxP3+) cells in BM of VL patients was correlated positively with higher percentage frequency of activated T cells (CD4+CD25+): further, we observed a significant positive correlation between percentage frequency of Treg (CD4+CD25+FoxP3+) cells with percentage frequency of activated cells (CD4+CD25+) in VL patients (P = 0·004: Pearson's correlation coefficient r 2 = 0·535). (f) Increased frequency of Treg (CD4+CD25+FoxP3+) cells in BM of VL patients was correlated positively with parasitic load: we observed significant positive correlation between percentage of Treg frequency (CD4+CD25+FoxP3+) with parasitic load in VL patients (P < 0·0001: Pearson's correlation coefficient r 2 = 0·583). [Colour figure can be viewed at wileyonlinelibrary.com]
Enrichment of IL‐10‐producing Treg cells in VL patients with HPL
We further characterized the source of IL‐10 producing T cell, whether it is CD4+CD25br. Foxp3+ or CD4+CD25dim Foxp3+ T cells. We observed that CD4+CD25brFoxP3+ T cells (Fig. 2a, iii, iv) (red dotted line of overlay histogram) are the major producers of IL‐, rather than CD4+CD25dimFoxP3+ cells. Interestingly, a significantly higher proportion of Treg cells were IL‐10 producers in HPL patients (n =10) compared to that of LPL patients (n = 12) (Fig. 2b) (P = 0·004; unpaired two‐tailed t‐test). When we compared the ELISA‐based soluble level of IL‐10 among these groups, we observed that the level of IL‐10 was significantly higher in the HPL group (n = 11) (Fig. 2c) (P = 000·1: 208·4 ± 16·09) than that compared with the LPL group (n = 13) (106·3 ± 3·44). This indicates that the parasitic load may influence IL‐10 production by Treg cells in the BM of visceral leishmaniasis patients. Conversely, the higher frequency of IL‐10‐producing Treg cells may facilitate parasite growth by suppressing the host effector immune response.
Figure 2.
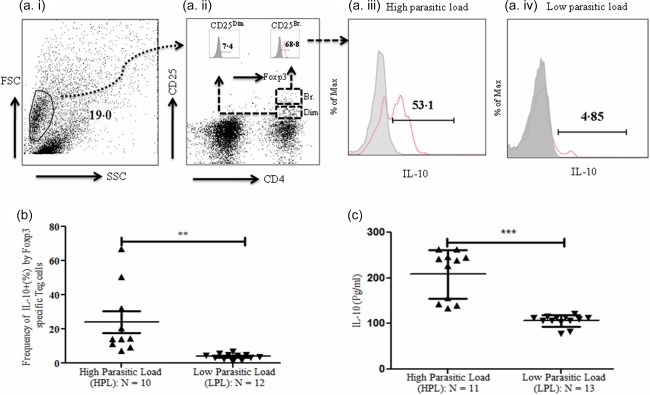
Enrichment of interleukin (IL)‐10 producing regulatory T cells (Treg) cells in visceral leishmaniasis (VL) patients at disease site. (a) Enumeration of IL‐10‐producing Treg (CD4+FoxP3+IL‐10+) cells in bone marrow mononuclear cells (BM MNCs): isolated MNCs from bone marrow of VL patients were cultured for the assessment of IL‐10 production by antigen‐specific Treg cells in response to Leishmania donovani antigen (whole cell lysate; 10 μg/ml). Cells were cultured for 72 h in animal cell culture conditions (incubation at 37°C with 5% CO2). Monensin (Golgi transport inhibitor; 1 mM) was added in the last 6 h of culture. On forward‐ versus side‐scatter gated lymphocytes, the dual population of CD4+CD25+ was defined and divided further into CD4+CD25dim and CD4+CD25br T cells. Histogram plot of FoxP3 expressing CD4+CD25dim or CD4+CD25br (thin red dotted line) was overlaid on FoxP3 fluorescence minus one (FMO) (Fig. 2a, ii: inset). IL‐10 expression was then assessed on CD4+CD25br FoxP3+ T cells. For this, IL‐10 expressing CD4+CD25br FoxP3+ T cells were overlaid on IL‐10 FMO (Fig. 2, iii and iv). (b) Increased frequency of IL‐10‐producing Treg cells at disease site in VL patients with high parasitic load (HPL): CD4+FoxP3+ pregated lymphocytes were used for illustration of the IL‐10‐producing Treg population (histogram). Treg‐specific IL‐10 population was increased significantly in VL patients with HPL in response to whole cell lysate of L. donovani (10 μg/ml) [n = 10: mean ± standard deviation (s.d.): 24·03 ± 6·31%] compared to the patients with low parasitic load (LPL) (n = 12: mean ± s.d.: 3·87 ± 0·49%) (P = 0·002: unpaired t‐test). Horizontal lines in scatter‐plot depicted as mean value. (c) Significantly increased soluble level of IL‐10 in VL patients with HPL: enzyme‐linked immunosorbent assay (ELISA)‐based soluble level of IL‐10 in BM aspirate of HPL (n = 11: mean ± s.d.: 208·5 ± 16·09 pg/ml) patients was significantly higher compared with LPL (n = 13: mean ± s.d.: 106·3 ± 3·44 pg/ml) patients (P = 0·0001: unpaired t‐test). Horizontal lines in scatter‐plot depicted as mean value. [Colour figure can be viewed at wileyonlinelibrary.com]
Decreased frequency of IFN‐γ‐ and IL‐17A‐producing cells in VL patients with HPL
To evaluate the status of effector T cells in cytokine production, we enumerated the frequency of IFN‐γ‐ and IL‐17A‐producing cells. The percentage frequency of both IFN‐γ‐ and IL‐17A‐producing CD4+ T cells (Fig. 3a, b) was significantly lower in HPL patients (n = 11) compared to that of LPL patients (n = 13) (P = 0·005 and 0·002, respectively; unpaired two‐ tailed t‐test). Furthermore, we correlated the percentage frequency of Treg cells with CD4‐specific IFN‐γ‐ and IL‐17A‐producing cells in the HPL group. We observed a negative correlation for both IFN‐γ and IL‐17A with Treg cells. Moreover, the correlation was highly significant for IFN‐γ than IL‐17A (n = 12) (Fig. 3c). These findings strongly suggest a parasite load role in dampening the effector T cells producing proinflammatory cytokines (IFN‐γ and IL‐17A), most probably by augmenting the suppressive Treg cells and their function.
Figure 3.
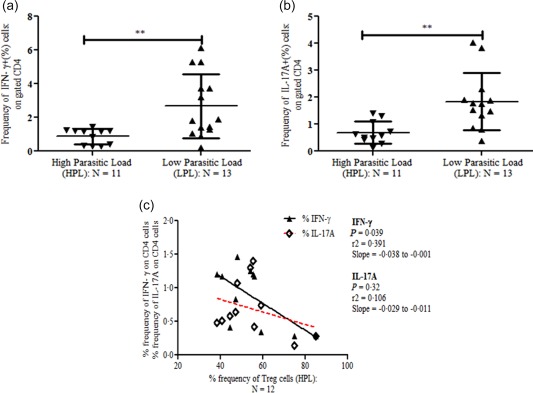
Decreased frequency of interferon (IFN)‐γ‐ and interleukin (IL)‐17A‐producing cells in visceral leishmaniasis (VL) patients with high parasitic load (HPL). Enumeration of IFN‐γ‐ and IL‐17A‐producing cells in bone marrow mononuclear cells (BM MNCs): isolated MNCs (n = 24) from bone marrow of VL patients were cultured for IFN‐γ and IL‐17A production: BM ‐MNCs were cultured for 24 h. At the 0 h time‐point, whole cell lysate of Leishmania donovani antigen (10 µg/ml) and at the 18 h time‐point, monensin was supplemented in culture. Finally, at the end of 24 h of culture, cells were harvested and used for intracellular staining of IFN‐γ‐ and IL‐17A‐producing cells using anti‐human IFN‐γ and IL‐17A monoclonal antibodies along with anti‐human CD4 antibodies. (a) Scatter‐plot of IFN‐γ‐producing cells on gated CD4+ T cells: significantly decreased percentage frequency of IFN‐γ‐producing cells in patients with HPL (n = 11: mean ± s.d.: 0·87 ± 0·13%) in comparison with patients with LPL (n = 13: mean ± s.d.: 2·72 ± 0·54%) (P = 0·005). (b) Scatter‐plot of IL‐17A‐producing cells on gated CD4+ T cells: as of IFN‐γ; the percentage frequency of IL‐17A also follows the same trend between HPL (n = 11: mean ± s.d.: 0·68 ± 0·12%) and low parasitic load (LPL) (n = 13: mean ± s.d.: 1·83 ± 0·29%) (P < 0·002). (c) Increased percentage frequency of regulatory T cells (Treg) (CD4+CD25+FoxP3+) cells in BM of HPL patients was correlated negatively with proinflammatory cytokine‐ (IFN‐γ and IL‐17A)‐producing cells. We observed a significant negative correlation between percentage frequency of Treg (CD4+CD25+FoxP3+) cells with a percentage frequency of IFN‐γ (P = 0·03: r 2 = 0·39 with slope = −0·038 to −0·001) and IL‐17A (P = 0·32: r 2 = 0·10 with slope = −0·029 to −0·011)‐producing cells on gated CD4+ T cells. [Colour figure can be viewed at wileyonlinelibrary.com]
Increased soluble level of IL‐6 and TGF‐β in VL patients with HPL
In vitro, naive T cells can be differentiated into Th17 cells in the presence of IL‐6 and TGF‐β 18, 19. Conversely, soluble TGF‐β promotes Treg cells by enhancing the expression of FoxP3 in CD4+ T cells. It has also been observed that IL‐6 inhibits TGF‐β‐induced Treg differentiation 18. To evaluate the status of soluble levels of IL‐6 and TGF‐β in the study group, we performed ELISA and observed that IL‐6 was significantly higher in the HPL (n = 12) (Fig. 4a) (P = 0·053: 187 ± 16·8) compared with the LPL (n = 11) (144·4 ± 12·5) groups. Similarly, the soluble level of TGF‐β was significantly higher in HPL patients (n = 11) (Fig. 4b) (1123 ± 155·4) compared with LPL patients (n = 13) (533·0 ± 101·4). Further, we observed that the soluble level of IL‐17A was relatively low in the HPL group (n = 12) (Fig. 4c) (57·99 ± 5·2) compared with the LPL group (n = 14) (72·21 ± 4·19). Why, in spite of high levels of both IL‐6 and TGF‐β, we observed lower numbers of Th17 cells and IL‐17A levels in VL HPL patients was surprising. To understand and explain this apparent discrepancy, we calculated the ratio of IL‐6 over TGF‐β, which shows the relative dominance of one cytokine over the other. We observed a significantly lower IL‐6/TGF‐β ratio among HPL patients (n = 11) (P = 0·011) relative to that of LPL patients (n =11) (Fig. 4d). This indicates TGF‐β dominance in the BM of HPL patients favouring Treg cell differentiation, resulting in the suppression of Th17 function or differentiation. Therefore, we conclude that HPL somehow triggers a dominant TGF‐β environment in BM, and probably that inhibits the protective Th17 cells.
Figure 4.
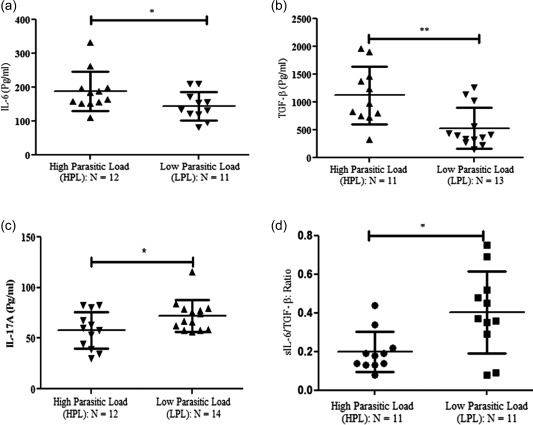
Soluble level of interleukin (IL)‐6, transforming growth factor (TGF)‐β and IL‐17 in visceral leishmaniasis (VL) patients with parasitic load: sandwich enzyme‐linked immunosorbent assay (ELISA)‐based assay was performed to measure the soluble levels of IL‐6, TGF‐β and IL‐17 in bone marrow (BM) aspirates of VL patients. Cytokine (IL‐6, TGF‐β and IL‐17) levels were evaluated based on company protocols. (a) Significantly increased level of IL‐6 in BM aspirate of high parasitic load (HPL) [n = 12: mean ± standard deviation (s.d.): 187·9 ± 16·84 pg/ml] patients compared with low parasitic load (LPL) (n = 11: mean ± s.d.: 144·4 ± 12·5 pg/ml) (P = 0·05: unpaired t‐test). Horizontal lines in scatter‐plot depicted as mean value. Similarly, for (b) TGF‐β in BMA of HPL (n = 11) compared with LPL (n = 13) (P = 0·003: Mann–Whitney U‐test) and (c) IL‐17A: significantly decreased soluble level of IL‐17A was observed in patients with HPL (n = 12: mean ± s.d.: 57·9 ± 5·27 pg/ml) compared with LPL (n = 14: mean ± s.d.: 72·21 ± 4·19 pg/ml) (P = 0·04: unpaired t‐test). (d) sIL‐6/TGF‐β: significantly decreased ratio of sIL‐6 versus TGF‐β was observed in HPL patients (n = 11) compared with LPL (n = 11) patients (P = 0·02: Mann–Whitney U‐test, unpaired t‐test).
Rescue of IFN‐γ‐ and IL‐17A‐producing cells upon blocking TGF‐β and IL‐10
To delineate the suppression of effector T cells by Treg cells in in‐vitro culture, BM MNCs (n = 8) VL patients were stimulated with leishmanial antigen in the presence or absence of neutralization/blocking monoclonal antibodies against IL‐10 and TGF‐β (10 μg/ml each). Using one‐way anova, we observed the significant rescue of both IFN‐γ‐ (P = 0·0001; Fig. 5a) and IL‐17A‐ (P = 0·0001; Fig. 5b) producing CD4+ T cells upon blocking or neutralization of these suppressive cytokines. When we analysed the data further for Bonferroni correction for multiple comparisons, significant rescue of IFN‐γ was observed among antigen stimulation and blocking of both suppressive cytokines (α‐IL‐10 and α‐TGF‐β). In the case of IL‐17A, a significant rescue was observed among antigen stimulation and blocking of IL‐10 (α‐IL‐10) only. Therefore, our findings indicate the suppression of IFN‐γ‐ and IL‐17A‐producing effector T cells in the BM of HPL by suppressive cytokines such IL‐10 and TGF‐β derived from Treg cells present in the BM. This is substantiated further by higher expression of IL‐10RA receptor (CDw210A) on both peripheral and BM‐derived T cells of HPL patients (data not shown).
Figure 5.
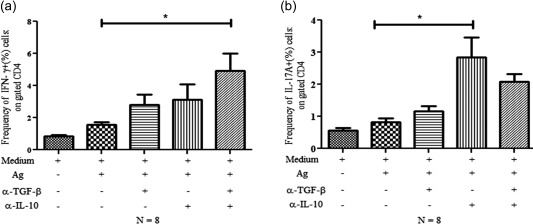
Rescue of both interferon (IFN)‐γ‐ and interleukin (IL)‐17A‐producing cells upon blocking of suppressive cytokines [transforming growth factor (TGF)‐β and IL‐10]. Mononuclear cells (MNCs) from bone marrow of visceral leishmaniasis (VL) patients were cultured for 48 h with/without Leishmania donovani antigen (10 µg/ml, (whole cell lysate) along with neutralizing antibodies for IL‐10 and TGF‐β (10 μg/ml) alone or in combination. Monensin was supplemented in the last 6 h of culture. Rescue of IFN‐γ and IL‐17A on CD4+ T cells was assessed by flow cytometry. (a) Column graph showing the one‐way analysis of variance (anova) significant rescue of IFN‐γ+ cells on CD4+ T cells. Data are average of eight independent experiments. Statistical Bonferroni correction for multiple alignment revealed significant rescue of IFN‐γ by blocking of both suppressive cytokines (TGF‐β and IL‐10) (Fig. 5a); (b) Similar to IFN‐γ; column graph of IL‐17A showing the one‐way anova significant rescue of IL‐17A+ cells on gated CD4+ T cells. Data are average of eight independent experiments. Statistical Bonferroni correction for multiple alignments revealed the significant rescue of IL‐17A upon blocking of IL‐10 alone (Fig. 5b).
Discussion
The gold standard of leishmaniasis diagnosis (VL) is the microscopic demonstration of the amastigote form of parasite (LD bodies) in splenic and BM aspirates 4, 20. The visceral organs are well‐known homing organs of parasites 3. Therefore, understanding the suppressed state of the host immune response against the parasites necessitates the study of immune cells or parameters of BM. Suppression of the T cell response in VL is well demonstrated, and contributes to the dissemination of parasites in various visceral organs of reticulo‐endothelial organs. This appears to be one of the major immune evasion strategies of L. donovani. However, the precise relation between parasite burden and T cell response in these affected organs remains inconclusive. Some critical and unresolved issues are: (i) which immune cells are involved in local suppression of T cells, (ii) how do they influence the parasite burden or (iii) how does the parasite load influence the host immune state at the disease site? To address some of these issues, we focused our attention on the T effector and Treg cells in the BM of VL patients with HPL as opposed to LPL patients. Few studies from this viewpoint have been carried out using peripheral blood 21. However, a correlation between immune parameters with parasitic load at the BM remains to be resolved.
A significant correlation was noted between the distinct function of certain T cell subsets and parasite load in the BM of the patients. Taking cues from our previous study, where we noted a significant enrichment of Treg cells in the BM of VL patients (mean 48·7%) compared to the BM of healthy subjects (mean 10·1%) 14, we enumerated Treg cells in the BM of VL patients and their correlation with the parasite load. We observed a higher frequency of Treg cells (CD4+CD25brFoxP3+) than that of T effector cells (CD4+CD25brFoxP3–) in the BM of HPL patients and the reverse was true for the LPL patients. This indicates a strong Treg‐mediated suppression of effector T cells in the BM of VL. The frequency of IL‐10‐producing Treg cells was also higher in the BM of HPL VL patients. Moreover, a higher frequency of suppressive (IL‐10+) Treg cells in the BM correlated negatively with the frequencies of IFN‐γ+ and IL‐17A+ T effector cells among HPL patients. We also observed a reverse pattern in LPL patients. Therefore, our results indicate that HPL correlates closely with the suppressive state of the T cell response in the BM. IL‐10 may also be produced by recently activated Tr1 cells, which are essentially FoxP3–. However, we did not observe any significant presence of FoxP3– IL‐10‐producing Tr1 cells in the BM of the VL patients that we studied. We believe that a causal link exists between the parasite load and functional status of T cell responses in the BM of HPL VL patients. However, which one is the cause or effect is not evident in our study between the immune suppression and parasite load. Further mechanistic study is needed to decipher the issue conclusively. Either HPL induces Treg cells and suppresses the protective T cell response or the dominance of Treg cells abrogates the immune containment of parasites, thus failing to control the parasite multiplication as well as the dissemination observed in VL patients. Delineating a precise causal link between parasite load and the Treg‐mediated suppression requires further detailed studies, including experiments in animal models.
As expected from our positive correlation between the parasite load and Treg cell frequency, we observed significantly higher levels of Treg cell‐associated cytokines IL‐10 and TGF‐β in HPL patients. However, we also noted a simultaneous and significant rise in IL‐6 levels in the BM of HPL patients compared to those of the LPL group. In our HPL group, therefore, there were higher levels of both IL‐6 and TGF‐β. However, the frequency of IL‐17A+ T cells (Th17) was significantly low. This appears paradoxical, as a combined elevation of these two cytokines is likely to facilitate Th17 cells, conferring an immune checkpoint against parasite multiplication 22. To address this paradox, we evaluated the ratio of TGF‐β to IL‐6 present in the BM of patients. We found a significantly lower IL‐6 : TGF‐β ratio among HPL and the reverse in LPL. This indicates a direct correlation between higher levels of TGF‐β and the increased parasitic burden of either one leading to the other, causing the dominance of Treg cell differentiation suppressing the differentiation of protective Th17 cells. Our results indicate the possibility of a parasite load‐dependent preferential rise in TGF‐β (along with IL‐6), thereby creating a cytokine milieu conducive for the generation of suppressive Treg cells compared to that of protective Th17 cells, albeit in the presence of IL‐6. This vicious cycle may result in unchecked parasite multiplication. Alternatively, HPL may also drive such a preferential production of TGF‐β. Therefore, our result does not rule out either of these two possibilities. A longitudinal immune profiling study will be required to resolve the causality relationship of our observational findings. The cellular source of parasite‐induced elevation of TGF‐β may be infected macrophages 22, 23. We envisage that preferential secretion of TGF‐β in the BM of VL patients may represent a critical immune evasion strategy of leishmanial parasites. Once such TGF‐β bias is achieved, Treg cells differentiate to increase TGF‐β further to suppress the effector T cells actively, resulting in uncontrolled parasite multiplication. This may then be apparent in a positive correlation between HPL and the suppressive immune parameters, as we observed that high Treg cells elevated suppressive cytokines TGF‐β/IL‐10 and low effector cells and cytokines IFN‐γ+ and IL‐17A+ T cells. Our findings of rescuing both IFN‐γ+ and IL‐17A+ T cells upon blocking of TGF‐β/IL‐10, either alone or in combination, substantiate that TGF‐β/IL‐10 present in HPL BM indeed suppress the effector T cells, leading to failure in the immune containment of parasites. This, in turn, manifests as a close correlation between the parasite load and suppressive immune parameters in the BM. However, this effect may be due to either a direct effect of these cytokines on the effector T cells or an indirect effect on the antigen‐presenting cells. Further study will be required for resolving this issue.
Overall, our findings reiterate certain critical issues of immune suppression observed in VL. In this study, we demonstrate (i) a close relationship between parasite burden and T cell suppression mediated by Treg cells in BM and (ii) a TGF‐β (and also IL‐10)‐dominant local milieu facilitates Treg cell differentiation, resulting in the suppression of protective T cells. Major cellular sources of TGF‐β may be infected macrophages 22 and Treg cells, as we have shown previously 16, 17. Whatever the initial source of this cytokine may be, once the infection sets the milieu suitable to drive Treg cell generation, a perpetual state of T cell suppression ensues in the BM. This leads eventually to uncontrolled parasite multiplication and dissemination. However, the contribution of a similar pathway may not be inferred for other pathological sites of visceral leishmaniasis due to differences in the architectural and immune milieu in spleen, liver and lymph nodes. Suppression of the host T cell response has also been well documented in L. infantum infection 2, 3, 24. Considering the similarity of the immune response, we envisage that the parasite burden may also suppress the T cell response during L. infantum infection. We propose that blocking suppressive elements such as Treg cell‐favouring cytokines may rescue the protective immune response and immune containment of the leishmania parasites. Conversely, evaluating these parameters may prove critical, as biomarkers for disease severity pave the ways to immune rescue therapy and vaccination.
Disclosure
The authors declare no financial or commercial conflicts of interest.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Microphotograph showing the intracellular Leishmania donovani bodies (LD bodies) in bone marrow aspirate of visceral leishmaniasis study subject. Thin microscopic smear slides were stained with Giemsa stain and examined microscopically under 10 × 100 magnifications. Presence of LD bodies was graded on a scale from 1+ to 6+. Amastigote (LD bodies) 1–10 in 10, 100 or 1000 fields were graded as 3+, 2+ and 1+. (a) Representative bone marrow LD bodies demonstration in the low parasitic load (LPL) (1+) study group. (b) Bone marrow LD bodies demonstration in the high parasitic load group (HPL) (2+) and (c) bone marrow LD bodies demonstration in the HPL group (HPL) (3+).
Acknowledgements
The authors thank all the patients who volunteered to participate in this study. We also thank the Department of Biotechnology, Government of India, for funding the work (Grant no. Ref: DBT/PR14092/MED/29/190/2010; dated 13/12/10).
References
- 1. Akhoundi M, Kuhls K, Cannet A et al A historical overview of the classification, evolution, and dispersion of leishmania parasites and sandflies. PLOS Negl Trop Dis 2016; 10:e0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Freitas EO, Leoratti FM, Freire‐de‐Lima CG, Morrot A, Feijo DF. The contribution of immune evasive mechanisms to parasite persistence in visceral leishmaniasis. Front Immunol 2016; 7:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodrigues V, Cordeiro‐da‐Silva A, Laforge M, Silvestre R, Estaquier J. Regulation of immunity during visceral Leishmania infection. Parasit Vectors 2016; 9:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Srivastava P, Dayama A, Mehrotra S, Sundar S. Diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg 2011; 105:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jetley S, Rana S, Khan S et al Bone marrow negative visceral leishmaniasis in an adolescent male. Iran J Parasitol 2013; 8:182–5. [PMC free article] [PubMed] [Google Scholar]
- 6. de Ruiter CM, van der Veer C, Leeflang MMG et al Molecular tools for diagnosis of visceral leishmaniasis: systematic review and meta‐analysis of diagnostic test accuracy. J Clin Microbiol 2014; 52:3147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization (WHO) . Control of the Leishmaniases: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March 2010. Tech Rep Ser 2010; 2010:01–202. [Google Scholar]
- 8. Sayyahfar S, Ansari S, Mohebali M, Behnam B. Visceral leishmaniasis without fever in an 11‐month‐old infant: a rare clinical feature of Kala‐azar. Korean J Parasitol 2014; 52:189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yegnasew Takele TA, Weldegebreal T, Hailu A et al Arginase activity in the blood of patients with visceral leishmaniasis and HIV infection. PLOS Negl Trop Dis 2012; 7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Philips CA‐OX, Kalal CR, Kumar KN, Bihari C, Sarin SK. Simultaneous occurrence of ocular, disseminated mucocutaneous, and multivisceral involvement of leishmaniasis. Case Rep Infect Dis 2014; 2014:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nylén S, Gautam S. Immunological perspectives of leishmaniasis. J Glob Infect Dis 2010; 2:135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Das A, Ali N. Vaccine development against leishmania donovani. Front Immunol 2012; 3:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khalil EA, Khidir SA, Musa AM et al Post‐Kala‐Azar dermal leishmaniasis: a paradigm of paradoxical immune reconstitution syndrome in non‐HIV/AIDS patients. J Trop Med 2013; 2013:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rai AK, Thakur CP, Singh A et al Regulatory T cells suppress T cell activation at the pathologic site of human visceral leishmaniasis. PLOS ONE 2012; 7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pitta MG, Romano A, Cabantous S et al IL‐17 and IL‐22 are associated with protection against human kala azar caused by Leishmania donovani . J Clin Invest 2009; 119:2379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh A, Dey AB, Mohan A, Sharma PK, Mitra DK, Unutmaz D. Foxp3+ regulatory T cells among tuberculosis patients: impact on prognosis and restoration of antigen specific IFN‐gamma producing T cells. PLOS ONE 2012; 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sharma PK, Saha PK, Singh A et al FoxP3+ regulatory T cells suppress effector T‐cell function at pathologic site in miliary tuberculosis. Am J Respir Crit Care Med 2009; 179:1061–70. [DOI] [PubMed] [Google Scholar]
- 18. Sanjabi S, Zenewicz L, Kamanaka M et al Anti‐inflammatory and pro‐inflammatory roles of TGF‐beta, IL‐10, and IL‐22 in immunity and autoimmunity. Curr Opin Pharmacol 2009; 9:447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kimura A, Kishimoto T. IL‐6: regulator of Treg/Th17 balance. Eur J Immunol 2010; 40:1830–5. [DOI] [PubMed] [Google Scholar]
- 20. Salam MA, Khan MG, Bhaskar KR, Afrad MH, Huda MM, Mondal D. Peripheral blood buffy coat smear: a promising tool for diagnosis of visceral leishmaniasis. J Clin Microbiol 2012; 50:837–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhattacharya P, Ghosh S, Ejazi SA et al Induction of IL‐10 and TGFbeta from CD4+CD25+FoxP3+ T cells correlates with parasite load in Indian Kala‐azar patients infected with Leishmania donovani. PLOS Negl Trop Dis 2016; 10:e0004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gantt KR, Schultz‐Cherry S, Rodriguez N et al Activation of TGF‐ by Leishmania chagasi: importance for parasite survival in macrophages. J Immunol 2003; 170:2613–20. [DOI] [PubMed] [Google Scholar]
- 23. Kumar R, Nylén S. Immunobiology of visceral leishmaniasis. Front Immunol 2012; 3:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Figueiredo WME, Viana SM, Alves DT et al Protection mediated by chemokine CXCL10 in BALB/c mice infected by Leishmania infantum. Mem Inst Oswaldo Cruz 2017; 112:561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Microphotograph showing the intracellular Leishmania donovani bodies (LD bodies) in bone marrow aspirate of visceral leishmaniasis study subject. Thin microscopic smear slides were stained with Giemsa stain and examined microscopically under 10 × 100 magnifications. Presence of LD bodies was graded on a scale from 1+ to 6+. Amastigote (LD bodies) 1–10 in 10, 100 or 1000 fields were graded as 3+, 2+ and 1+. (a) Representative bone marrow LD bodies demonstration in the low parasitic load (LPL) (1+) study group. (b) Bone marrow LD bodies demonstration in the high parasitic load group (HPL) (2+) and (c) bone marrow LD bodies demonstration in the HPL group (HPL) (3+).


