Abstract
Published systematic reviews concluded that there is moderate to strong evidence to infer a potential role of lead and cadmium, widespread metal exposures, as cardiovascular risk factors. For other non-essential metals, the evidence has not been appraised systematically. Our objective was to systematically review epidemiologic studies on the association between cardiovascular disease in adults and the environmental metals antimony, barium, chromium, nickel, tungsten, uranium, and vanadium. We identified a total of 4 articles on antimony, 1 on barium, 5 on chromium, 1 on nickel, 4 on tungsten, 1 on uranium and 0 on vanadium. We concluded that the current evidence is not sufficient to inform on the cardiovascular role of these metals because the small number of studies. Few experimental studies have also evaluated the role of these metals in cardiovascular outcomes. Additional epidemiologic and experimental studies, including prospective cohort studies, are needed to understand the role of metals, including exposure to metal mixtures, in cardiovascular disease development.
Keywords: cardiovascular, atherosclerosis, metals, systematic review, epidemiologic studies
Introduction
Substantial epidemiologic and experimental evidence supports the role of lead and cadmium, widespread environmental metals, in the development of cardiovascular disease of atherosclerotic origin. The epidemiologic evidence for those two metals has been summarized in recent systematic reviews [1, 2]. In animal studies, lead and cadmium induced aortic atherosclerosis [3, 4]. The potential cardiovascular effect of these divalent cations, moreover, has been reinforced with the finding that repeated edetate disodium chelation can prevent cardiovascular disease outcomes compared to placebo [2, 5••], although other essential divalent cations may also be involved. Multiple metals can induce oxidative stress, a main proposed mechanism for their potential atherogenic effects [6, 7]. Specifically, metals can produce reactive radicals, deplete glutathione and other proteins with sulfhydryl groups and bind enzymes involved in redox balance [1, 6]. Several metals can also disrupt endocrine and endothelial vascular functions [8–11]. Recent epidemiologic and experimental evidence points to the possibility that environmental metals can interfere with enzymes involved in the one-carbon and citric acid metabolism and in histone modification pathways, resulting in anomalous DNA-methylation status throughout the genome and changes in gene expression [12–15]. The potential atherogenicity of metals, thus, is likely not restricted to lead and cadmium.
While in humans, the accumulated evidence strongly suggests a potential role of metals such as cadmium and lead in cardiovascular risk [1, 2], for other non-essential metals, the evidence has not been systematically appraised. Most occupational studies, unfortunately, have been limited in their ability to inform the role of metals in cardiovascular disease due to the use of indirect measures of exposure instead of individual exposure assessment measures, such as biomarkers, potential residual confounding by not adjusting for cardiovascular risk factors and the healthy worker survivor effect bias, and potential co-exposures.
Our objective was to conduct a systematic review and synthesis of results from epidemiologic studies evaluating the association of biomarkers of exposure to environmental non-essential metals beyond lead and cadmium with cardiovascular disease of atherosclerotic origin. We did not include inorganic arsenic, a metalloid, nor mercury, a metal with a complex body of evidence on its relationship with cardiovascular disease, due to major confounding by seafood exposure [16–18]. For chromium, although there is some evidence that chromium (III) could be an essential element, this is not proven and chromium (VI) on the other hand is an established toxic metal. Metals reviewed in this systematic review included antimony, barium, chromium, nickel, tungsten, uranium, and vanadium. In addition to the systematic review, for each metal we also provide a background on exposure sources, biomarker interpretation, and main health effects. We organized the presentation of the results by environmental metal. We also provide a summary table with the characteristics and the interpretation of most commonly used metal biomarker (Table 1).
Table 1.
Characteristics of biomarkers of metal exposure
| Metal | Biomarker | Timing of exposure reflected |
Characteristics of biomarker |
|---|---|---|---|
| Antimony | Urine | Recent exposure | Rapidly excreted in urine. Intravenous exposure: 90% excreted in 24 hours [158]; Inhalation exposure: half-life of 94 hrs (SbIII) and 24 hrs (SbV) [69,159]. |
| Tungsten | Urine | Recent exposure | Rapidly excreted in urine[95]; half-life in kidneys of 5 days (70%) and 100 days (30%) [109] |
| Chromium | Serum | Recent exposure | Reflects Cr(III) but not Cr(VI) exposure, as Cr(VI) is taken up by erythrocytes [96]. |
| Urine | Recent exposure | Reflects dietary Cr(III) intake (1–2 days), considered inadequate for Cr(VI) exposure [95,96]. | |
| Whole blood | Species dependent | Reflects more recent exposure of Cr(III) (intravenous half-life of 10–40 hrs), but less recent Cr(VI) exposure (intravenous half-life of 25–35 days). Cr(VI) is taken up by erythrocytes, while Cr(III) is not [96]. | |
| Toenail | Long term exposure | Considered less reliable than blood or urine [96]. Moderate reproducibility across 5–6 years [100]. | |
| Barium | Urine | Recent exposure | No well-established biomarkers of Ba exposure. Intravenous exposure: 75% cleared in 3 days [160]. |
| Nickel | Whole blood | Recent exposure | Rapidly excreted in urine; half-life of 20–34 hrs in plasma [133] and 3.6–3.8 hrs in rats following intravenous exposure [161]. |
| Uranium | Urine | Recent exposure | Primary biomarker of U exposure [139]. Rapidly excreted in urine and feces; half-life in kidneys of 1–6 days (99%). Kidney excretion reflects inhalation or dermal exposure [138]. |
Methods for the Systematic Review
Search strategy, study selection and data abstraction
We searched PubMed for relevant studies published through April 1, 2016 using the search strategy described in Supplemental File 1. The search strategy retrieved a total of 3,445 citations (including duplicates). We included all articles assessing environmental metal exposure using biomarkers. The search had no language restrictions. Two investigators (A.E. N. and A.R.H.) independently reviewed each of all the abstracts and selected 57 references applying the following study exclusion criteria (Figure 1): a) No original research (i.e. reviews, editorials, non-research letters); b) No human study; c) No atherosclerosis outcomes; d) No environmental metal exposure levels measured in biological tissues (e.g. environmental measures such as water or air, or distance from a source, including radiation for uranium), e) Case report or case series. For antimony, we identified two additional studies by manual search [19••, 20]. In this systematic review the focus was on the role of environmental metals exposure in atherosclerotic cardiovascular disease in adults. Age, sex and smoking are major determinants of metal levels in the human body and major risk factors for cardiovascular disease. We thus excluded, as a second layer of exclusion, 41 studies not adjusting for age, sex or smoking [21–56]. Any discrepancies were resolved by consensus and if necessary a third reviewer was involved. A native speaker reviewed the full text of any non-English article that could not be included or excluded based on the initial abstract review. We included in the final review 10 papers, some of them measuring multiple environmental metals evaluated in unique study populations [20, 57–59] (Figure 1). Our review identified no publications investigating the association between vanadium and atherosclerotic disease. After retrieval of articles from the search, the reference lists of selected articles were checked for other potentially relevant articles, identifying no additional studies.
Figure 1. Flow diagram of the study selection process.
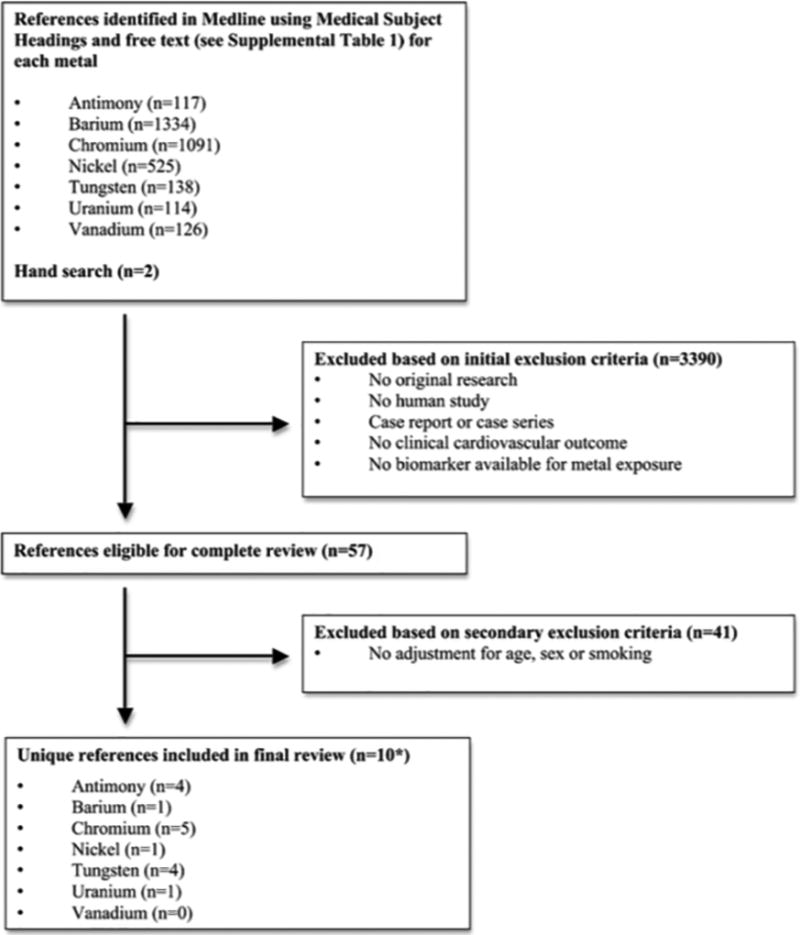
Summary of inclusion and exclusion criteria used in this systematic review of studies investigating the association between environmental metals and atherosclerotic cardiovascular disease, 1 April 2016. * 10 references include the following studies with multiple environmental metals evaluated in unique study populations: Agarwal et al. (2011)[57] examined in NHANES 1999–2006 population urine antimony and tungsten. Navas-Acien et al. (2005)[58] examined in NHANES 1999–2000 urine antimony, barium and tungsten. Mendy et al. (2012)[20] examined in the NHANES 2007–2008 populations urine antimony, tungsten and uranium. Lind et al. (2012)[59] examined in the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) population whole blood chromium and nickel.
To assess study quality, we adapted the criteria used by Longnecker et al. [60] for observational studies (Supplemental File 2). We followed the criteria proposed by the 2004 US Surgeon General Report on the health consequences of smoking [61], which include the evaluation of consistency, temporality, strength, dose-response relationship, and biological plausibility including confounding. As a result, the evidence for each environmental metal and atherosclerosis endpoint was classified in four groups as modified from the Surgeon General Report: sufficient evidence, suggestive but not sufficient evidence, insufficient evidence to infer a relationship, and suggestive of no relationship.
Statistical analysis
We collected the following data for each study: first author, year of publication, study design, size and population characteristics, exposure assessment and categories for comparison, endpoint ascertainment and endpoint definition, measures of association for a change in metal levels and 95% confidence interval or p-values and adjustment factors. For some studies that reported only the association for metal categories, we reported the estimated relative risk (RR) comparing the highest to the lowest categories. For chromium only, a pooled RR estimate for CVD was calculated across five studies using an inverse-variance weighted random effects model in the meta package in Stata version 13.1 [62]. The estimated pooled RR for chromium studies is provided for descriptive purposes only and should be interpreted with caution, as there is substantial heterogeneity in the biomarker type, comparison unit, and CVD outcome definition across studies. Thus, the pooled RRs and confidence intervals must be taken with caution. Pooled RR estimates for CVD were also calculated for each study that assessed multiple CVD outcomes. While, most studies reported cross-sectional odds ratios (ORs), we interpret the pooled estimates as RR estimates. However, several studies reported high prevalence of the outcome such that the OR may over or underestimate the RR [63].
Two study populations had information on both prevalent and incident CVD endpoints [19••, 64]. For these studies, we used incident outcomes only when pooling. We evaluated heterogeneity between studies using the I2 statistic, which describes the total variability across all studies due to heterogeneity [65]. Additionally, we tested for influential studies by omitting each study sequentially and assessed publication bias using funnel plots.
Current perspectives and result
Antimony
Antimony is naturally occurring in the Earth’s crust and can also be released from anthropogenic sources, particularly coal and refuse combustion, and nonferrous metal mining, smelting, and refining [66]. The general population is exposed to antimony from food and water [67], ambient air [66], and through antimonial medicines [68]. Antimony is mainly excreted through urine and feces, with a half-life that varies by species, ranging from 24 hours for Sb(V) to 94 hours for Sb(III) [69]. In epidemiologic studies, antimony concentrations in urine reflect recent exposure [69].
Antimony at high exposure levels has been related to respiratory illness [70], gastrointestinal effects [71], dermatitis [72], and cardiovascular effects such as altered electrocardiography readings and elevated blood pressure [68, 73]. Relatively little is known, however, about antimony toxicity and atherosclerosis. In an occupational study of Hispanic men employed at an antimony smelter in Texas, antimony exposure was not associated to cardiovascular mortality [74]. In the general population, antimony exposure has been associated with elevated blood pressure [75] and diabetes [76]. Fatal arrhythmias, QT prolongation after correcting for heart rate, and other electrocardiogram abnormalities are known, but uncommon, side effects of long-term antimonial medicine use [68, 77, 78]. In vitro evidence suggests that antimony exposure is associated with oxidative stress [69] and intracellular calcium dysregulation [79] in cardiac myocytes. In vivo evidence shows that antimony exposure is associated with elongated cardiac action potentials (53) and altered ECG readings and cardiomyopathy [80]. Antimony can also modify arsenic toxicity by altering arsenic metabolism, and co-exposure is likely as both metals occur together in the environment [69].
In the systematic review, we identified four publications investigating the association between antimony and atherosclerotic disease that met the inclusion criteria (Table 2) [19••, 20, 57, 58]. These studies were all conducted in the general US population using the National Health and Nutrition Examination Survey [19••, 20, 57, 58]. Antimony exposure was measured in urine only. Cardiovascular disease endpoints were based on examination (peripheral arterial disease) [58], linkage to the National Death Index (heart disease mortality) [19••] and self-report (prevalence of combined cardiovascular disease [57] and specific endpoints such as coronary heart failure, coronary heart disease, heart attack, and stroke [20]). Two studies reported dose-response associations of urinary antimony with prevalent endpoints using flexible splines [19••, 58]. Three studies took into account urine dilution by adjusting the regression models by urine creatinine. One study both adjusted models for creatinine and divided urinary antimony levels by creatinine, with sensitivity analyses showing an attenuation of effect estimates for heart disease mortality when only adjusting the regression model for creatinine [19••]. Effect estimates for prevalent self-reported atherosclerotic disease remained unchanged.
Table 2.
Studies of antimony exposure biomarkers and atherosclerosis outcomes (4 studies available)
| Study, year |
Population | Men (%) |
Age Range (years) |
Biomarker | Exposed vs. Reference |
Endpoint Ascertain- ment |
Outcome (s) | No. of cases / non-cases |
Relative Risk estimate (95% CI) |
Adjustment Factors |
|---|---|---|---|---|---|---|---|---|---|---|
| Cross-sectional studies | ||||||||||
| Agarwal et al. 2011[57] | General US population, NHANES 1999–2006 N=5037 | 48.3 | 46.5 (mean) |
|
Per log µg/mg | Self-report |
|
|
|
Age, sex, race, education, hypertension, diabetes, hypercholesterolemia, chronic kidney disease, body mass index, C-reactive protein, smoking status, serum cotinine. Sb levels divided by creatinine. |
| Guo et al. 2016 [19••] | General US population, NHANES, 1999–2010 N=1857 | 49.7 | ≥20 |
|
Quartiles 1 (≤0.048) vs 2 to 4 (>0.048–0.075, >0.075–0.121, >0.121 µg/g) | Self-report |
|
|
|
Age, gender, race, smoking, drinking, marital status, education, family poverty-income ratio, BMI, hypertension, diabetes, eGFR, and ln-transformed urinary creatinine. Sb levels also divided by urinary creatinine. |
| Navas-Acien et al. 2005 [58] | General US population, NHANES 1999–2000 N=790 | NR | ≥40 |
|
75th (0.17 µg/L) vs. 25th (0.07 µg/L) percentile | Measured ankle-brachial index<0.9 |
|
|
|
Age, sex, race, education, smoking, urinary creatinine |
| Mendy et al. 2012 [20] | General US population, NHANES 2007–2008 (N=1857) | 49.6 | 20–80 |
|
Above vs. below the GM (0.06 µg/g) | Self-report |
|
|
|
Age, sex, race/ethnicity, education level, ratio family income to poverty, alcohol consumption, cigarette smoking, urinary barium, cadmium, cobalt, cesium, molybdenum, lead, thallium, tungsten, and uranium. Sb levels divided by urinary creatinine |
| Prospective studies | ||||||||||
| Guo et al. 2016 [19••] | General US population, NHANES, 1999–2010N=1857 | 49.7 | ≥20 |
|
Quartiles 1 (≤0.048) vs 2 to 4 (>0.048–0.075, >0.075–0.121, >0.121 µg/g) | NCHS linkage to National Death Index |
|
|
|
Age, gender, race, smoking, drinking, marital status, education, family poverty-income ratio, BMI, hypertension, eGFR, and ln-transformed urinary creatinine. Sb levels also divided by urinary creatinine. |
BMI= body mass index; NR: not reported; eGFR: estimated glomerular filtration rate; NCHS: National Center for Health Statistics. GM: geometric mean.
In general, studies mostly showed a trend toward an increased risk of atherosclerotic disease with increased antimony exposure, although only the associations with the prevalence of combined CVD [57], peripheral arterial disease [58], and self-reported congestive heart failure and heart attack [19••] were statistically significant. Confounding by other cardiovascular risk factors, including smoking, was generally addressed. Two studies reported adjustments for other heavy metals such as lead and cadmium [19••, 20].
Chromium
Chromium is found in nature primarily as chromite ore. Chromium +3 (Cr(III)) and +6 (Cr(VI)) oxidation states are the most common in chromium compounds [81]. Cr(III) is the dominating species in the environment, but in some areas, the ground water can have an elevated content of Cr(VI) [82]. Chromium and its salts are used in metallurgical, refractory and chemical industries. The essentiality of Cr(III) and the use of it as supplement for glycemic control in type 2 diabetes is controversial [83]. Several clinical trials have evaluated the effect of chromium supplementation on glycemic control, with inconsistent findings [84–87]. Some studies have reported a potential role of Cr(III) in the maintenance of normal glucose tolerance through increasing the activity of insulin-stimulated tyrosine kinase [88]. Alternatively, exposure to Cr(VI) compounds have shown associations with increased risk of dermatitis, ulcerative upper airway disease, kidney disease and respiratory cancer [89–92].
Stainless steel welders are exposed to Cr(VI) through air [93, 94] in co-exposure with oxides of nickel and other metals (Mn, Fe, Al) [94]. Because Cr(VI) is rapidly reduced to Cr(III) in the lung and intestinal tract lining or upon cell entrance, Cr in biological material is most likely always trivalent, except shortly after exposure to Cr(VI) and in erythrocytes [95, 96]. The general population is mainly exposed to trivalent chromium Cr(III) through food and dietary supplements [95]. Other known sources of chromium are water, ambient air and tobacco smoke [97]. Interestingly, tobacco smoke contains Cr(VI), thus co-exposure with other metals such as cadmium, lead, and nickel is possible [98].
Chromium is mainly excreted through urine with a half-life of 15–41 hours [99] while the half-life of whole Cr in blood is 13.9 days. Chromium both in urine and blood reflects relatively short term exposure. Biomonitoring of Cr(VI) exposure is complicated by the high dietary intake of Cr(III) in the general population. The relative contribution of Cr(III) versus Cr(VI) to biomarkers of exposure is biospecimen-specific, with urine and serum Cr levels reflecting mostly Cr(III) exposure and whole blood Cr levels reflecting Cr(VI) exposure (Table 1). Urinary Cr(VI) levels may be low after exposure to Cr(VI), as Cr(VI) is reduced to Cr(III) in vivo. Because Cr(VI), but not Cr(III), is taken up by erythrocytes, whole blood best reflects Cr(VI) exposure, and Cr(VI) has a half-life in blood of 25–35 days (Table 1) [96]. Since toenails have a slow growth rate, it has been estimated that toenail measurements represent exposures over last 3–12 months [100]. Overall, total biomarker levels are likely mostly reflecting Cr(III) in all the evaluated studies, especially studies using urine as biological matrix. Future epidemiologic studies, especially those based on whole blood, require chromium speciation to assess potentially adverse cardiovascular effects of Cr(VI).
Given the rapid reduction of Cr(VI) to Cr(III) and the inability of Cr(III) to enter cells, it is unlikely that the endothelium will be exposed to Cr(VI). Thus, there is no in vivo evidence linking exposure to chromium with atherosclerosis or endothelial function. Nonetheless, in experimental studies Cr(VI) induced DNA damage due to reactive oxygen species caused by the reduction to Cr(III) [101–103]. Alternatively, experimental studies in rabbits describe an improvement of serum lipids using Cr(III) compounds [104, 105].
In the systematic review, we identified five publications investigating the association between chromium and atherosclerotic disease that met the inclusion criteria (Table 3). These studies were conducted in the US [64], Kingdom of Saudi Arabia [106], Sweden [59], Finland [107] and Europe [108]. Three studies restricted to men only [64, 106, 108], and a fourth study population included 92% men [107]. Chromium exposure was measured in whole blood [59], serum [106], urine [106, 107] and toenail [64, 108]. Cardiovascular disease endpoints were based on review of clinical and mortality records (coronary heart disease incidence [106, 108] and mortality [107], and combined CVD endpoint including myocardial infarction, coronary artery bypass graft, percutaneous transluminal coronary angioplasty or stroke [64]) and examination (presence of plaque in carotids [59]). Chromium speciation was not conducted in any of these studies. Three studies [59, 64, 108] reported dose-response associations of urinary chromium with prevalent endpoints using quartile [64] and quintile [59, 108] categories. While the two studies [106, 107] using urine biomarkers divided urine chromium by urine creatinine, none of them conducted sensitivity analyses by showing effect estimates when no adjustment or only adjusting the regression model for creatinine.
Table 3.
Studies of chromium biomarkers and clinical cardiovascular disease outcomes (5 studies available)
| Study, year |
Population | Men (%) |
Age Range (yrs) |
Biomarker | Exposed vs. Reference |
Endpoint Ascertainment |
Outcome (s) | No. of cases / non-cases |
Relative Risk estimate (95% CI) |
Adjustment Factors |
|---|---|---|---|---|---|---|---|---|---|---|
| Cross-sectional study | ||||||||||
| Lind et al. 2012[59] | Subjects of Uppsala, (Sweden) PIVUS Study (N=1016) | 49.8 | = 70 |
|
|
Local thickening of the IMT more than 50% thicker than the surrounding IMT, measured by external B-mode ultrasound imaging | Plaque presence prevalence |
|
|
Gender, waist circumference, BMI, fasting blood glucose, systolic and diastolic blood pressure, high and low-density lipoprotein cholesterol, serum triglycerides, smoking, antihypertensive treatment and statin use. |
| Rajpathak et al. 2004 [64] | The Health Professionals Follow-up Study N=886 | 100 | 40–75 |
|
|
Self-report and medical record review | Prevalent CVD: Myocardial infarction, coronary artery bypass graft (CABG), percutaneous transluminal coronary angioplasty (PTCA), or stroke |
|
|
Age, BMI, alcohol intake, smoking status, family history of myocardical infarction, physical activity, high cholesterol, hypertension, dietary score, toenail levels of selenium and mercury. |
| Case-control study | ||||||||||
| Alissa et al. 2009 [106] | Inpatients of coronary care unit of King Fahd Military Hospital and King Abdulaziz University Hospital (Kingdom of Saudi Arabia) N=260 | 100 | ≥43,4 |
|
|
Review of hospital records | Myocardial infarction |
|
|
Height, smoking status, oral hypoglycemic drugs, serum triglycerides. Urinary Cr levels divided by urinary creatinine. |
| Guallar et al. 2005[108] | Incident case-control Inpatients of coronary care unit of EURAMIC Study (N=1408) | 100 | ≤70 |
|
|
Review of hospital records | Incident acute coronary heart disease |
|
|
Age, study center, smoking, alcohol drinking, BMI, high density lipoprotein cholesterol, diabetes, history of hypertension, family history of coronary heart disease, toenail selenium adipose tissue levels of α-tocopherol, β-carotene, and lycopene, and major fatty acid peaks. |
| Niskanen et al. 1986 [107]a | Finland Social Insurance Survey participants from Jamsankoski and Jamsa,1966–1972 N=96 | 92 | Mean=49.7 |
|
|
Review of hospital records | Coronary heart disease mortality |
|
|
Matching by age, sex, place of residence, and smoking status |
| Prospective study | ||||||||||
| Rajpathak et al. 2004 [64] | Nested case-control (incidence density sampling) from The Health Professionals Follow-up Study (N=563) | 100 | 40–75 |
|
|
Self-report and medical record review | Incident CVD: Myocardial infarction, coronary artery bypass graft (CABG), percutaneous transluminal coronary angioplasty (PTCA), or stroke |
|
|
Age, BMI, alcohol intake, smoking status, family history of myocardical infarction, physical activity, high cholesterol, hypertension, dietary score, toenail levels of selenium and mercury. Cr levels divided by urinary creatinine. |
BMI: body mass index; IQR: interquartile range; NR: not reported
Only mean Cr levels for cases and controls were originally reported; we derived the IQR, RR, and 95% CI via the linear discrimination method [157].
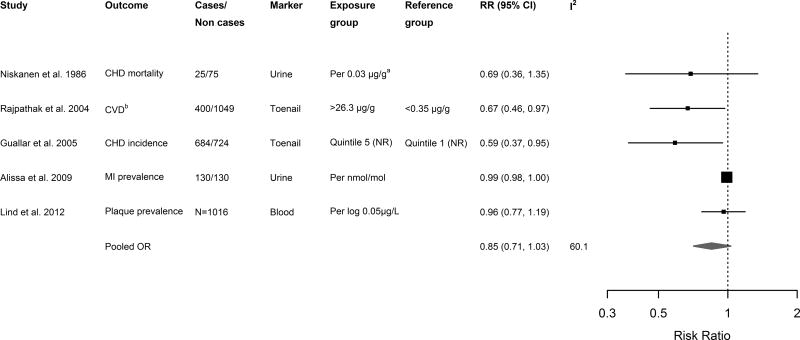
All the studies included in the systematic review reported a trend toward inverse associations of chromium exposure and atherosclerotic disease (Table 3), although it was statistically significant only in two studies [107, 108]. For descriptive purposes only, we performed a meta-analysis and estimated the combined relative risks from the 5 retrieved studies, obtaining a pooled relative risk that was marginally significant [0.89 (0.75, 1.05)] (Figure 2). Sequentially excluding each study did not change the pooled effect estimates (data not shown). Although analyses of bias are limited by the small number of studies, funnel plots and Egger’s test indicated the potential for publication bias in studies evaluating Cr exposure (p= 0.104; p<0.1 often considered significant in meta-analyses [62]). We observed some heterogeneity between studies (I2 =48.0%). One [64] and two [64, 108] studies reported adjustments for mercury and selenium, respectively. No study adjusted for lead and cadmium.
Figure 2. Relative risks (RRs) for cardiovascular disease endpoints for a given change in chromium level.
Squares and diamonds represent effect estimates and are proportional to the inverse of the variance of the log odds ratios, and lines represent 95% CIs. a Niskanen et al. only reported mean levels of urinary Cr among cases and controls; we derived RR and 95% CI via the linear discrimination method [157]. Abbreviations: NR, not reported; CHD, coronary heart disease; MI, myocardial infarction. Total N was reported where number of cases/non cases was not available. Pooled estimates within and across studies were pooled via inverse-variance weighted random effects.
Tungsten
Tungsten is naturally occurring in rock and soil, and enters the environment from industrial output or naturally occurring contamination [109]. While the main source of tungsten exposure is occupational via inhalation of hard metal dust, the general population may be exposed through drinking water, food, or industrial releases into the environment [110, 111]. The elimination time of tungsten in most tissues, except bone, is 5 days (70% of the dose) and 100 days (30% of the dose). Consequently, urinary tungsten reflects recent exposure [109, 112]. Occupational inhalation of hard metal dust containing tungsten and cobalt causes asthma and fibrosis called hard metal disease [113, 114]. Although toxicological evidence regarding cardiovascular disease is sparse, in vivo studies suggest tungsten causes histological lesions in the heart [115] and can inactivate molybdenum-enzymes by replacing molybdenum binding sites [116]. Tungsten likely causes oxidative stress [7, 11, 112, 117] and can modify cobalt toxicity [118]. There is little epidemiological evidence of tungsten exposure and cardiovascular disease in the general population, although tungsten exposure has been associated with elevated blood pressure [119], excretion of reactive oxygen species [117], and DNA methylation and hydroxymethylation [120].
In the systematic review, we identified four publications investigating the association between tungsten and cardiovascular disease (Table 4). All studies were cross-sectional and conducted in the general US population in NHANES. Tungsten exposure was measured in urine only. Among the four retrieved studies, one cardiovascular endpoint was based on examination (peripheral arterial disease) [58], while other endpoints were based on self-report of composite cardiovascular disease [57, 121•], coronary heart failure, coronary heart disease, heart attack and stroke [57, 121•]. The association of elevated tungsten levels and composite cardiovascular disease [57, 121•], peripheral arterial disease [58], and stroke [121•] were statistically significant. The associations of tungsten with heart failure, coronary heart disease, heart attack, and stroke in one study [20] were in the positive direction but non-significant. One study reported a dose-response associations using flexible splines [58]. All three studies took into account urine dilution by adjusting the regression models by urine creatinine. Two studies adjusted for molybdenum and cobalt [20, 121•]. Only one study also adjusted for cadmium and lead [20]
Table 4.
Studies of tungsten exposure biomarkers and atherosclerosis outcomes (4 studies available)
| Study, year |
Population | Men (%) |
Age Range (yrs) |
Biomarker | Exposed vs. Reference |
Endpoint Ascertainmen t |
Outcome (s) | No. of cases / non-cases |
Relative Risk estimate (95% CI) |
Adjustment Factors |
|---|---|---|---|---|---|---|---|---|---|---|
| Cross-sectional studies | ||||||||||
| Agarwal et al. 2011 [57] | General US population, NHANES 1999–2006 N=5037 | 48.3 | 46.5 (mean) |
|
Per log µg/mg | Self-report |
|
|
|
Age, sex, race, education, hypertension, diabetes, hypercholesterolemia, chronic kidney disease, body mass index, C-reactive protein, smoking status, serum cotinine. W levels divided by urine creatinine. |
| Navas-Acien et al. 2005 [58] | General US population, NHANES 1999–2000 N=790 | NR | ≥40 |
|
75th (0.13 µg/L) vs. 25th (0.03 µg/L) percentile | Measured ankle-brachial index<0.9 |
|
|
|
Age, sex, race, education, smoking, urinary creatinine |
| Mendy et al. 2012 [20] | General US population, NHANES 2007–2008 (N=1857) | 49.6 | 20–80 |
|
Above vs. below the GM (0.09 µg/g) | Self-report |
|
|
|
Age, sex, race/ethnicity, education level, ratio family income to poverty, alcohol consumption, cigarette smoking, urinary barium, cadmium, cobalt, cesium, molybdenum, lead, thallium, antimony, and uranium. W levels divided by urinary creatinine |
| Tyrrell et al. 2013 [121•] | General US population, NHANES 1999–2010 N=8614 | 49.0 | 18–74 |
|
Per log µg/L | Self-report |
|
|
|
Age, sex, ethnicity, SES, smoking, occupation, BMI, hypertension, hypercholesterolemia, urinary molybdenum and cobalt. W levels divided by urinary creatinine |
BMI: body mass index; GM: geometric mean; SES: socioeconomic status; NR: not reported;
Other metals
Other non-essential metals are also of potential cardiovascular concern. Barium sulfide is produced from mineral barite and it is used mainly in oil and gas drilling industry and in the manufacture of alloys, glass, cement, ceramics, electronics, radiopaque contrast, sugar refining and as pigment. The general population is exposed to barium from gasoline [122], soil, air, water [123] and food, especially nuts [124]. Chronic effects of barium on the cardiovascular system are unclear. An occupational study on barium workers, who were exposed to other chemicals, found higher incidence of elevated blood pressure in the barium exposed group [125]. Studies in populations exposed through drinking water, however, found no significant differences in blood pressure, heart disease, stroke, kidney disease or lung disease [126, 127]. Different studies have measured barium concentration in hair, urine and blood samples. Barium is mainly excreted through feces (91%), sweat (6%) and urine (3%) [128]. The pattern of total excretion fits a three-component exponential function with biological half-times of 3.6, 34.2, and 1033 days, respectively [129].
Nickel is a natural element present in sulphide or oxide ores and it is used in steel and alloy industries, batteries and chemical catalysis. Nickel is widely used in coins, jewelry, watches, buttons, orthodontic and orthopaedic uses and stents. The general population is exposed to nickel from combustion of fossil fuel and pollution through air, soil and water [130], food (cacao, nuts [131]), and tobacco smoking [132]. Nickel is an established carcinogen in occupational settings (respiratory cancers), especially insoluble nickel sulphide and nickel oxide [133–135]. Other chronic health effects associated to nickel include rhinitis, sinusitis, nasal septum perforations, asthma, skin allergies and reproductive effects [133]. Although biomarkers of nickel exposure are not well validated, nickel has been measured in whole blood, serum, plasma, and urine [133]. It is well established that nickel is rapidly excreted through urine with a half-life of 20 to 27 hours [136], with salivary and sweat excretion being secondary [137]. .
Uranium is used in energy production, glass tinting agents, ceramic glazes, gyroscope wheels, chemical catalysts, shields for high-intensity radioactive sources, X-ray tube targets, and military munitions [138]. Uranium is ubiquitous in the environment, for that reason, the general population is exposed to uranium from soil, air, water and food [139]. Although uranium is both a chemical and a radioactive material, it has been determined that its adverse health effects are primarily a result of its chemical rather than radiological toxicity [139]. Chemical exposure in humans has been related with hepatitis, lung toxicity and renal disease caused by oxidative stress [140, 141] and these effects have not been demonstrated in radiological studies [142]. Among uranium miners, the causal relationship between exposure to radon progeny and lung cancer is well-established, although the carcinogenicity of uranium itself remains unknown [139]. Uranium in body fluids generally exists as a uranyl ion UO2 complex. It accumulates in tissues (especially bone) or it is excreted quickly by urine, with a two-phase model in kidneys from 1–6 days (99%) to 1500 days (100%)[138].
Experimental evidence indicating a potential role in atherogenesis of these metals is scarce. The barium ion is a physiological antagonist of potassium and it is related to acute effects in radiopaque barium sulfate intoxications with smooth, skeletomuscular and cardiac symptoms like areflexia and heart fibrillation [143]. One study in rat has assessed the effect induced by chronic ingestion of uranium, in reducing the activity of cholesterol 7 alpha-hydroxylase (CYP7A1) [144], which is involved in lipid metabolism. In vivo evidence on the potential role of these metals in atherosclerosis is needed.
Our systematic review identified very few articles that met the inclusion criteria on the association of these other metals with cardiovascular atherosclerotic disease (Table 5, Supplemental File 3). For each of these metals, we only identified one publication investigating the association with atherosclerotic cardiovascular disease: barium and peripheral arterial disease [58], nickel and carotid atherosclerosis [59], and uranium and the prevalence of cardiovascular endpoints including coronary heart disease, stroke and heart failure (Table 5). These studies were conducted in the USA [20, 58] and Sweden [59]. Exposure was measured in urine only [20, 58] or whole blood only [59]. Cardiovascular endpoints were assessed by physical examination in 2 studies (one measuring peripheral arterial disease with ankle-brachial index [58] and one measuring carotid atherosclerosis by intima-media thickness and plaque presence with ultrasounds [59]) and by self-report in 1 studies [20]. For barium, there was a trend toward inverse association with peripheral arterial disease although it was not significant [58]. For nickel and uranium, the evaluated studies mostly showed a trend towards increased cardiovascular risk with increasing levels of exposure [20, 59], which was statistically significant for heart failure and heart attack. Two studies adjusted for age, sex and smoking, but failed to adjust for traditional cardiovascular risk factors.
Table 5.
Studies of barium, nickel and uranium biomarkers and clinical cardiovascular disease outcomes (3 studies available)
| Study, year |
Population | Men (%) |
Age Range (yrs) |
Biomarker | Exposed vs. Reference |
Endpoint Ascertainment |
Outcome (s) | No. of cases / non-cases |
Relative Risk estimate (95% CI) |
Adjustment Factors |
|---|---|---|---|---|---|---|---|---|---|---|
| Cross-sectional studies | ||||||||||
| Barium | ||||||||||
| Navas-Acien et al. 2005 [58] | General US population NHANES 1999–2000 N=790 | NR | ≥40 |
|
|
Measured ankle-brachial index<0.9 |
|
45/659 |
|
Age, sex, race,education, smoking status, and urinary creatinine |
| Nickel | ||||||||||
| Lind et al. 2012 [59] | Subjects of Uppsala, (Sweden) PIVUS Study (N=1016) | 49.8 | = 70 |
|
|
Local thickening of the IMT more than 50% thicker than the surrounding IMT, measured by external B-mode ultrasound imaging |
|
NR |
|
P-value adjusted for gender, waist circumference, body mass index, fasting blood glucose, systolic and diastolic blood pressure, high and low-density lipoprotein cholesterol, serum triglycerides, smoking, antihypertensive treatment and statin use |
| Uranium | ||||||||||
| Mendy et al. 2012 [20] | General US population NHANES 2007–2008 (N=1857) | 50.4 | ≥ 20 |
|
|
Self-report |
|
|
|
Age, sex, race/ethnicity, education level, ratio family income to poverty, alcohol consumption, cigarette smoking, urinary barium, cadmium, cobalt, cesium, molybdenum, lead, thallium, antimony, and uranium. U levels divided by urinary creatinine |
BMI: body mass index. GM: geometric mean; NR: not reported
General discussion and needs for future epidemiologic research
Few studies have evaluated the association between other metals beyond lead and cadmium, or the metalloid arsenic, with cardiovascular disease development including information on age, sex and smoking status. The metals for which we found at least 2 or more studies included antimony, tungsten, and chromium. For the association of antimony and tungsten exposures with different atherosclerotic endpoints, all the studies were conducted in NHANES, a representative sample of the general US population. Although these studies found an increased risk of CVD related outcomes with increased antimony and tungsten concentrations, more studies in other population are needed to evaluate the consistency of the findings. For chromium, epidemiologic studies in distinct populations consistently found an inverse association between chromium biomarkers including serum, urine, whole blood and toenail, and incident and prevalent atherosclerotic disease. A graphical display analysis, however, indicates there is possibility of risk of publication bias. This finding highlights the importance of publishing all studies, including null studies, for chromium, but also for other metals. Additional research is thus needed to confirm the relationship between chromium and cardiovascular disease, including the shape of the dose-response, and most importantly, to distinguish if the association is different by chromium species. For other environmental metals, the small number of studies did not allow us to recognize any type of patterns in their associations with atherosclerotic disease, although the association for uranium was suggestive of increased risk. These epidemiologic associations of a potential increased risk of CVD for antimony, tungsten, and uranium are also supported by a few experimental studies specifically conducted for those metals. Additional experimental research is needed to better understand the potential mechanisms, the dose-response, and the impact of different routes of administration. These experimental studies are critical to facilitate the interpretation and the conduction of human research. While the small number of studies limits the conclusion of this review, the evidence accrued so far supports the importance of environmental metals as cardiovascular risk factors, with different directions of the association for chromium vs. the other metals.
Table 1 summarizes the characteristics and interpretation of the metal biomarkers relevant for this systematic review. Spot urine biomarkers were the most commonly used biospecimen among the reviewed studies. Limitations of urine biomarkers, include within-individual variability in urinary metal excretion and the need to adjust or correct for urine dilution [58]. Variation associated with the laboratory technique for metals determination (typically inductively coupled plasma-mass spectrometry), including relatively high limits of detection that results in a large proportion of the study population with unobserved metal concentrations, also introduces measurement error. Only 4 studies [58, 106–108] reported the intra and inter-assay coefficient of variations of the laboratory method, which typically should fall below the 10% threshold. Only 5 studies [19••, 20, 58, 108, 121•] reported the percent of undetectable values, the limit of detection for the specific metals or the methods to handle undetectable values. While traditional approaches to handle left-censored data, such as replacing concentrations below the limit of detection by the limit of detection divided by two or the square root of two, may induce bias when more than 10–20% or the study population display undetectable values [145], recently developed imputation approaches based on Markov Chain Monte Carlo predictive models have been recently applied with the objective to flexibly incorporate measurement error and left truncation, improve the estimation of dose-responses and increase sample size when values are missing completely at random [146, 147]. Typically, non-differential measurement error related to physiological urinary metal and creatinine excretion and artifactual variation could introduce conservative bias toward the null. Additionally, given the relatively short half lives of most urinary metals, the biomarkers may not reflect long-term exposures (Table 1), although short half-life biomarkers can reflect chronic exposure if exposure is constant over time.
A limitation of the retrieved studies was the substantial heterogeneity in the adjustment for traditional cardiovascular risk factors among the retrieved studies. We included adjustment for sex, age, and smoking status as required inclusion criteria because they are major confounders of the association between metal exposure and cardiovascular disease, and smoking is a major source of metal exposure (152, 153). Only one [59] study reported results stratified by age subgroups, two studies [59, 121•] reported results stratified by sex, and no studies reported results stratified by smoking status. Conducting stratified analysis can be difficult in studies with small sample sizes, as was common in the studies included in this review (median sample size 1247). Most of the studies adjusted for diabetes, hypertension, and dyslipidemia. An important issue is the adjustment for renal function, as some of the reported metals such as chromium [148–150], antimony [151], and uranium [152] are nephrotoxic and could, thus, be mediators of the association of metals and cardiovascular disease. Although there is discussion on the adequacy of urine creatinine to correct for urine dilution for metal biomarkers, adjustment for specific gravity cannot be interpreted in the presence of albuminuria, which also limits the value of specific gravity to account for urine dilution in the presence of kidney damage [153].
Only one prospective study, a nested case-control study conducted for chromium [64], allowed the assessment of temporality. The study found a prospective inverse association of baseline chromium with coronary heart disease incidence collected through 10 years of follow-up. This prospective association was directionally consistent with cross-sectional [59, 64] and case-control [106, 108] studies. Regarding the dose-response, some studies used flexible approaches (i.e. quantile categories or non-parametric splines) mostly showing approximately monotonic relationships of chromium [59, 64, 108], antimony [19••, 58] and tungsten [58] with cardiovascular disease.
Future prospective studies with sufficient repeated measurements over time, which can enable the evaluation of cardiovascular risk by changes in environmental metals, are needed. Another interesting area of future research is the role of joint exposures in atherosclerosis. It is important to evaluate mixtures of metals as metals co-occur together [154, 155]. While several of the retrieved studies adjusted the regression models for other metals, statistical methods to comprehensively tackle mixtures of compounds are needed.
In addition to primary prevention interventions to reduce exposure to atherogenic metals in general populations, future mechanistic and human experimental research is needed to clarify the effect of removing potentially atherogenic metal stores from the body via edetate disodium chelation treatment in the prevention of recurrent cardiovascular disease [5••]. For chromium, further experimental evidence is needed to clarify the potential mechanism for improved glycemic control, and further epidemiological studies using more precise biomarkers of Cr(III) and Cr(VI) exposure are needed to clarify the species-specific association with cardiovascular disease.
Conclusion
The accumulated evidence supports the role of environmental metals in atherosclerotic disease. For all the environmental metals evaluated, including chromium, we concluded that the current evidence is “insufficient” to support causality given the small number of studies, the heterogeneity in potential residual confounding of the associations by traditional cardiovascular risk factors and metal co-exposures, and the few number of prospective studies. For chromium, despite consistent inverse associations among published studies, the potential mechanisms are unclear, and we cannot discard possible publication bias. Important questions include the need for larger and prospective studies, the relevance of issues related with adjustment for urine dilution when using urinary biomarkers and the systematic evaluation of the dose-response relationships. Cardiovascular disease will remain the main cause of burden of disease world-wide in the next decades [156]. Given the potential associations between metal exposure and cardiovascular disease as well as the paucity of experimental literature on metal-induced cardiotoxicity, more experimental research is needed to determine the potential mechanism of metal-induced atherosclerosis.
Supplementary Material
Acknowledgments
Maria Tellez-Plaza was supported by the Strategic Action for Research in Health sciences (CP12/03080 and PI15/00071), which is an initiative from Carlos III Health Institute Madrid and the Spanish Ministry of Economy and Competitiveness and co-funded with European Funds for Regional Development (FEDER). Ana Navas-Acien and Anne E. Nigra were supported by grants R01ES021367 and R01ES025216 from the National Institute of Environmental Health Sciences.
Abbreviations
- AAS
atomic absorption spectrometry
- CC
case-control
- CI
confidence interval
- CO
cohort
- CS
cross-sectional
- IQR
interquartile range
Footnotes
Authors’ contributions
All authors conceptualized the review. A.E.N., A.R.H., M.T.P. and A.N.A. developed the search strategy. A.E.N., A.R.H. reviewed all the retrieved abstracts. A.N.A. and M.T.P. acted as third reviewers in case of inconsistent articles selection by A.E.N. and A.R.H. A.E.N., A.R.H., A.N.A. and M.T.P. drafted the data extraction tables. A.E.N. and A.R.H. assisted in editing data extraction tables. All the authors interpreted the data extraction tables. A.E.N., A.R.H., A.N.A. and M.T.P. wrote the initial draft of the manuscript. J.R. assisted in writing the manuscript. All authors read and approved the final manuscript.
Compliance with Ethics Guidelines
Conflict of Interest
Anne E. Nigra, Adrian Ruiz-Hernandez, Josep Redon, Ana Navas-Acien, and Maria Tellez-Plaza declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently (from 2013 to present), have been highlighted as:
•Of importance
••Of outstanding importance
- 1.Solenkova NV, Newman JD, Berger JS, Thurston G, Hochman JS, Lamas GA. Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am. Heart J. 2014;168:812–22. doi: 10.1016/j.ahj.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamas GA, Navas-Acien A, Mark DB, Lee KL. Heavy metals, cardiovascular disease, and the unexpected benefits of edetate chelation therapy. J Am Coll. Cardiol. 2016;67:2411–8. doi: 10.1016/j.jacc.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Revis NW, Zinsmeister AR, Bull R. Atherosclerosis and hypertension induction by lead and cadmium ions: an effect prevented by calcium ion. Proc. Natl. Acad. Sci. 1981;78:6494–8. doi: 10.1073/pnas.78.10.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messner B, Bernhard D. Cadmium and cardiovascular diseases: cell biology, pathophysiology, and epidemiological relevance. Biometals. 2010;23:811–22. doi: 10.1007/s10534-010-9314-4. [DOI] [PubMed] [Google Scholar]
- 5 ••.Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309:1241–50. doi: 10.1001/jama.2013.2107. This large, randomized, placebo-controlled trial found that edetate disodium chelation therapy significantly reduced cardiac events in stable post-myocardial infarction (MI) patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005;12:1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 8.Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J. Toxicol. Environ. Health. B. Crit. Rev. 2009;12:206–23. doi: 10.1080/10937400902902062. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y-CT, Ghio AJ. Vascular effects of ambient pollutant particles and metals. Curr. Vasc. Pharmacol. United Arab Emirates. 2006;4:199–203. doi: 10.2174/157016106777698351. [DOI] [PubMed] [Google Scholar]
- 10.Omanwar S, Fahim M. Mercury Exposure and Endothelial Dysfunction: An Interplay Between Nitric Oxide and Oxidative Stress. Int. J. Toxicol. 2010;34:300–7. doi: 10.1177/1091581815589766. [DOI] [PubMed] [Google Scholar]
- 11.Prozialeck WC, Edwards JR, Nebert DW, Woods JM, Barchowsky A, Atchison WD. The vascular system as a target of metal toxicity. Toxicol. Sci. 2008;102:207–18. doi: 10.1093/toxsci/kfm263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz-Hernandez A, Kuo C-C, Rentero-Garrido P, Tang W-Y, Redon J, Ordovas JM, et al. Environmental chemicals and DNA methylation in adults: a systematic review of the epidemiologic evidence. Clin. Epigenetics. 2015;7:55. doi: 10.1186/s13148-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee D-H, Jacobs DR, Porta M. Hypothesis: a unifying mechanism for nutrition and chemicals as lifelong modulators of DNA hypomethylation. Environ. Health Perspect. 2009;117:1799–802. doi: 10.1289/ehp.0900741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chia N, Wang L, Lu X, Senut M-C, Brenner C, Ruden DM. Hypothesis: environmental regulation of 5-hydroxymethylcytosine by oxidative stress. Epigenetics. 2011;6:853–6. doi: 10.4161/epi.6.7.16461. [DOI] [PubMed] [Google Scholar]
- 15.Dai H, Wang Z. Histone Modification Patterns and Their Responses to Environment. Curr. Environ. Heal. Reports. 2014;1:11–21. [Google Scholar]
- 16.Roman HA, Walsh TL, Coull BA, Dewailly É, Guallar E, Hattis D, et al. Evaluation of the cardiovascular effects of methylmercury exposures: current evidence supports development of a dose-response function for regulatory benefits analysis. Environ. Health Perspect. 2011;119:607–14. doi: 10.1289/ehp.1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guallar E, Sanz-Gallardo MI, Veer P van’t, Bode P, Aro A, Gómez-Aracena J, et al. Mercury, Fish Oils, and the Risk of Myocardial Infarction. N Engl J Med. 2002;347(22):1747–54. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D. Fish, mercury, selenium and cardiovascular risk: current evidence and unanswered questions. Int. J. Environ. Res. Public Health. 2009;6:1894–916. doi: 10.3390/ijerph6061894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19 ••.Guo J, Su L, Zhao X, Xu Z, Chen G. Relationships between urinary antimony levels and both mortalities and prevalence of cancers and heart diseases in general US population, NHANES 1999–2010. Sci. Total Environ. 2016 doi: 10.1016/j.scitotenv.2016.07.011. [Epub ahead of print] This large nationally representative prospective study from the US evaluated the association of antimony with self-reported heart disease prevalence and heart disease mortality after 11-year follow-up. [DOI] [PubMed] [Google Scholar]
- 20.Mendy A, Gasana J, Vieira ER. Urinary heavy metals and associated medical conditions in the US adult population. Int. J. Environ. Health Res. England. 2012;22:105–18. doi: 10.1080/09603123.2011.605877. [DOI] [PubMed] [Google Scholar]
- 21.Frustaci A, Magnavita N, Chimenti C, Caldarulo M, Sabbioni E, Pietra R, et al. Marked elevation of myocardial trace elements in idiopathic dilated cardiomyopathy compared with secondary cardiac dysfunction. J. Am. Coll. Cardiol. 1999;33:1578–83. doi: 10.1016/s0735-1097(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 22.Yoshinaga J, Suzuki T, Morita M, Hayakawa M. Trace elements in ribs of elderly people and elemental variation in the presence of chronic diseases. Sci. Total Environ. 1995;162:239–52. doi: 10.1016/0048-9697(95)04470-l. [DOI] [PubMed] [Google Scholar]
- 23.Abraham AS, Sonnenblick M, Eni M, Shemesh O, Batt AP. Serum chromium in patients with recent and old myocardial infarction. Am. Heart J. 1980;99:604–6. doi: 10.1016/0002-8703(80)90734-6. [DOI] [PubMed] [Google Scholar]
- 24.Afridi HI, Kazi TG, Kazi GH, Jamali MK, Shar GQ. Essential trace and toxic element distribution in the scalp hair of Pakistani myocardial infarction patients and controls. Biol. Trace Elem. Res. 2006;113:19–34. doi: 10.1385/BTER:113:3. [DOI] [PubMed] [Google Scholar]
- 25.Afridi HI, Kazi TG, Kazi N, Sirajuddin, Kandhro GA, Baig JA, et al. Chromium and manganese levels in biological samples of Pakistani myocardial infarction patients at different stages as related to controls. Biol. Trace Elem. Res. 2011;142:259–73. doi: 10.1007/s12011-010-8773-3. [DOI] [PubMed] [Google Scholar]
- 26.Anderson TW, Neri LC, Schreiber GB, Talbot FD, Zdrojewski A. Letter: Ischemic heart disease, water hardness and myocardial magnesium. Can. Med. Assoc. J. 1975;113:199–203. [PMC free article] [PubMed] [Google Scholar]
- 27.el-Yazigi A, Hannan N, Raines DA. Urinary excretion of chromium, copper, and manganese in diabetes mellitus and associated disorders. Diabetes Res. 1991;18:129–34. [PubMed] [Google Scholar]
- 28.Fujimoto S. [Studies on the relationships between blood trace metal concentrations and the clinical status of patients with cerebrovascular disease, gastric cancer and diabetes mellitus] Hokkaido Igaku Zasshi. 1987;62:913–32. [PubMed] [Google Scholar]
- 29.Huang BX, Lin SQ, Chen SY, Zhou G, Yin F, Lou ZP, et al. Hair chromium levels in patients with vascular diseases. Biol. Trace Elem. Res. 1991;29:133–7. doi: 10.1007/BF03032690. [DOI] [PubMed] [Google Scholar]
- 30.Li YQ, Zhao YH, Cao YH, Shao MZ. [Urine-Cr assay with diphenylcarbazide] Hua Xi Yi Ke Da Xue Xue Bao. 1989;20:85–7. [PubMed] [Google Scholar]
- 31.Pan TC, Chen YL, Wu WJ. Serum trace metals in Blackfoot disease patients. Kaohsiung J. Med. Sci. 1996;12:555–60. [PubMed] [Google Scholar]
- 32.Punsar S, Wolf W, Mertz W, Karvonen MJ. Urinary chromium excretion and atherosclerotic manifestations in two Finnish male populations. Ann. Clin. Res. 1977;9:79–83. [PubMed] [Google Scholar]
- 33.Tan C, Chen H, Xia C. The prediction of cardiovascular disease based on trace element contents in hair and a classifier of boosting decision stumps. Biol. Trace Elem. Res. 2009;129:9–19. doi: 10.1007/s12011-008-8279-4. [DOI] [PubMed] [Google Scholar]
- 34.Tang Y-R, Zhang S-Q, Xiong Y, Zhao Y, Fu H, Zhang H-P, et al. Studies of five microelement contents in human serum, hair, and fingernails correlated with aged hypertension and coronary heart disease. Biol. Trace Elem. Res. 2003;92:97–104. doi: 10.1385/BTER:92:2:97. [DOI] [PubMed] [Google Scholar]
- 35.Vlad M, Caseanu E, Uza G, Petrescu M. Concentration of copper, zinc, chromium, iron and nickel in the abdominal aorta of patients deceased with coronary heart disease. J. Trace Elem. Electrolytes Health Dis. 1994;8:111–4. [PubMed] [Google Scholar]
- 36.Zerbino DD, Solomenchuk TN. [Myocardial infarction at the age under 50: influence of occupational xenobiotics (analysis of chemical elements in hair of patients)] Med. Tr. Prom. Ekol. 2007:17–21. [PubMed] [Google Scholar]
- 37.Avtandilov GG. [Age dynamics of the trace element content of normal and atherosclerosis-modified portions of the human aorta] Arkh. Patol. 1967;29:40–2. [PubMed] [Google Scholar]
- 38.Bierenbaum ML, Fleischman AI, Dunn J, Arnold J. Possible toxic water factor in coronary heart-disease. Lancet (London, England) 1975;1:1008–10. doi: 10.1016/s0140-6736(75)91949-2. [DOI] [PubMed] [Google Scholar]
- 39.Conri C, Simonoff M, Besse P, Llabador Y, Fleury B, Simonoff GN. [Fall of plasma chromium in coronary diseases] Presse Med. 1986:1931. [PubMed] [Google Scholar]
- 40.Kuang P, Wu W, Lang S. Trace elements and ischemic cerebral vascular disease. Ann. N. Y. Acad. Sci. 1993;676:340–1. doi: 10.1111/j.1749-6632.1993.tb38749.x. [DOI] [PubMed] [Google Scholar]
- 41.Mozhaitseva AG. [Blood level dynamics of copper, titanium and chromium in patients with ischemic heart disease] Kardiologiia. 1970;10:147–8. [PubMed] [Google Scholar]
- 42.Polikarpov BM. [Disorder of chromium metabolism in myocardial infarct] Kardiologiia. 1975;15:130–2. [PubMed] [Google Scholar]
- 43.Schroeder HA. The role of trace elements in cardiovascular diseases. Med. Clin. North Am. 1974;58:381–96. doi: 10.1016/s0025-7125(16)32164-2. [DOI] [PubMed] [Google Scholar]
- 44.She LM, Hu CH, Luo ZM, Zhang DZ, Wang NM, Chen SQ, et al. [Chromium content of the hair of patients with acute cerebrovascular diseases] Hua Xi Yi Ke Da Xue Xue Bao. 1987;18:160–2. [PubMed] [Google Scholar]
- 45.Takemoto K, Kawai H, Kuwahara T, Nishina M, Adachi S. Metal concentrations in human lung tissue, with special reference to age, sex, cause of death, emphysema and contamination of lung tissue. Int. Arch. Occup. Environ. Health. 1991;62:579–86. doi: 10.1007/BF00381111. [DOI] [PubMed] [Google Scholar]
- 46.Wang XF, Ma TG, Xi J. [Relation of trace elements and lipids in erythrocytes and coronary artery stenosis] Zhonghua Xin Xue Guan Bing Za Zhi. 1988;16:347–9. 383. [PubMed] [Google Scholar]
- 47.Afridi HI, Kazi TG, Kazi N, Kandhro GA, Baig JA, Jamali MK, et al. Association of environmental toxic elements in biological samples of myocardial infarction patients at different stages. Biol. Trace Elem. Res. 2011;141:26–40. doi: 10.1007/s12011-010-8713-2. [DOI] [PubMed] [Google Scholar]
- 48.Afridi HI, Kazi TG, Kazi N, Kandhro GA, Baig JA, Shah AQ, et al. Evaluation of toxic elements in scalp hair samples of myocardial infarction patients at different stages as related to controls. Biol. Trace Elem. Res. 2010;134:1–12. doi: 10.1007/s12011-009-8450-6. [DOI] [PubMed] [Google Scholar]
- 49.Khan SN, Rahman MA, Samad A. Trace elements in serum from Pakistani patients with acute and chronic ischemic heart disease and hypertension. Clin. Chem. 1984;30:644–8. [PubMed] [Google Scholar]
- 50.Leach CNJ, Linden JV, Hopfer SM, Crisostomo MC, Sunderman FWJ. Nickel concentrations in serum of patients with acute myocardial infarction or unstable angina pectoris. Clin. Chem. 1985;31:556–60. [PubMed] [Google Scholar]
- 51.Sukumar A, Subramanian R. Relative element levels in the paired samples of scalp hair and fingernails of patients from New Delhi. Sci. Total Environ. 2007;372:474–9. doi: 10.1016/j.scitotenv.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 52.Chen H, Tan C, Lin Z, Wu T, Diao Y. A feasibility study of diagnosing cardiovascular diseases based on blood/urine element analysis and consensus models. Comput. Biol. Med. 2013;43:865–9. doi: 10.1016/j.compbiomed.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Howard JM. Serum nickel in myocardial infarction. Clin. Chem. 1980:1515. [PubMed] [Google Scholar]
- 54.McNeely MD, Sunderman FWJ, Nechay MW, Levine H. Abnormal concentrations of nickel in serum in cases of myocardial infarction, stroke, burns, hepatic cirrhosis, and uremia. Clin. Chem. 1971;17:1123–8. [PubMed] [Google Scholar]
- 55.Sakharchuk VM, Iatsula GS, Rakitskaia NV. [Clinico-experimental study of levels of several microelements in arteriosclerosis] Kardiologiia. 1972;12:131–3. [PubMed] [Google Scholar]
- 56.Babadzhanov SN. [Content of zinc, vanadium, and iron in the whole blood and their balance in arteriosclerosis and hypertensive disease] Kardiologiia. 1975;15:133–4. [PubMed] [Google Scholar]
- 57.Agarwal S, Zaman T, Tuzcu EM, Kapadia SR. Heavy metals and cardiovascular disease: results from the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Angiology. 2011;62:422–9. doi: 10.1177/0003319710395562. [DOI] [PubMed] [Google Scholar]
- 58.Navas-Acien A, Silbergeld EK, Sharrett R, Calderon-Aranda E, Selvin E, Guallar E. Metals in urine and peripheral arterial disease. Environ. Health Perspect. 2005;113:164–9. doi: 10.1289/ehp.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lind PM, Olsen L, Lind L. Circulating levels of metals are related to carotid atherosclerosis in elderly. Sci. Total Environ. 2012;416:80–8. doi: 10.1016/j.scitotenv.2011.11.064. [DOI] [PubMed] [Google Scholar]
- 60.Longnecker MP, Berlin JA, Orza MJ, Chalmers TC. A meta-analysis of alcohol consumption in relation to risk of breast cancer. JAMA. 1988;260:652–6. [PubMed] [Google Scholar]
- 61.US National Center for Chronic Disease Prevention and Health Promotion. Office on Smoking and Health. Centers for Diseases Control and Prevention. [Accessed August 2016];How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. 2010 Available from: http://www.ncbi.nlm.nih.gov/pubmed/21452462. [PubMed]
- 62.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 64.Rajpathak S, Rimm EB, Li T, Morris JS, Stampfer MJ, Willett WC, et al. Lower toenail chromium in men with diabetes and cardiovascular disease compared with healthy men. Diabetes Care. 2004;27:2211–6. doi: 10.2337/diacare.27.9.2211. [DOI] [PubMed] [Google Scholar]
- 65.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 66.Agency for Toxic Substances and Disease Registry. US Public Health Service. Toxicological profile for antimony and compounds. Atlanta, GA: 1992. [Accessed August 2016]. Available from: http://www.atsdr.cdc.gov/toxprofiles/tp23.pdf. [PubMed] [Google Scholar]
- 67.Iyengar GV, Tanner JT, Wolf WR, Zeisler R. Preparation of a mixed human diet material for the determination of nutrient elements, selected toxic elements and organic nutrients: a preliminary report. Sci. Total Environ. 1987;61:235–52. doi: 10.1016/0048-9697(87)90371-8. [DOI] [PubMed] [Google Scholar]
- 68.Sundar S, Chakravarty J. Antimony toxicity. Int. J. Environ. Res. Public Health. 2010;7:4267–77. doi: 10.3390/ijerph7124267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gebel T. Arsenic and antimony: comparative approach on mechanistic toxicology. Chem Biol Interact. 1997;107:131–44. doi: 10.1016/s0009-2797(97)00087-2. [DOI] [PubMed] [Google Scholar]
- 70.Potkonjak V, Pavlovich M. Antimoniosis: a particular form of pneumoconiosis. I. Etiology, clinical and X-ray findings. Int. Arch. Occup. Environ. Health. 1983;51:199–207. doi: 10.1007/BF00377752. [DOI] [PubMed] [Google Scholar]
- 71.Taylor PJ. Acute intoxication from antimony trichloride. Br. J. Ind. Med. 1966;23:318–21. doi: 10.1136/oem.23.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White GP, Mathias CG, Davin JS. Dermatitis in workers exposed to antimony in a melting process. J. Occup. Med. 1993;35:392–5. [PubMed] [Google Scholar]
- 73.Brieger H, Semisch CW, Stasney J, Piatnek DA. Industrial antimony poisoning. Ind. Med. Surg. 1954;23:521–3. [PubMed] [Google Scholar]
- 74.Schnorr TM, Steenland K, Thun MJ, Rinsky RA. Mortality in a cohort of antimony smelter workers. Am. J. Ind. Med. 1995;27:759–70. doi: 10.1002/ajim.4700270510. [DOI] [PubMed] [Google Scholar]
- 75.Shiue I. Higher urinary heavy metal, arsenic, and phthalate concentrations in people with high blood pressure: US NHANES, 2009–2010. Blood Press. 2014;23:363–9. doi: 10.3109/08037051.2014.925228. [DOI] [PubMed] [Google Scholar]
- 76.Menke A, Guallar E, Cowie CC. Metals in Urine and Diabetes in U.S. Adults. Diabetes. 2016;65:164–71. doi: 10.2337/db15-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Desjeux P, et al. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2002:494–501. doi: 10.1016/s1473-3099(02)00347-x. [DOI] [PubMed] [Google Scholar]
- 78.Chulay JD, Spencer HC, Mugambi M. Electrocardiographic changes during treatment of leishmaniasis with pentavalent antimony (sodium stibogluconate) Am. J. Trop. Med. Hyg. 1985;34:702–9. doi: 10.4269/ajtmh.1985.34.702. [DOI] [PubMed] [Google Scholar]
- 79.Wey HE, Richards D, Tirmenstein MA, Mathias PI, Toraason M. Toxicol. Appl. Pharmacol. Vol. 145. Academic Press; 1997. The Role of Intracellular Calcium in Antimony-Induced Toxicity in Cultured Cardiac Myocytes; pp. 202–10. [DOI] [PubMed] [Google Scholar]
- 80.Alvarez M, Malécot CO, Gannier F, Lignon JM. Antimony-induced cardiomyopathy in guinea-pig and protection by L-carnitine. Br. J. Pharmacol. 2005;144:17–27. doi: 10.1038/sj.bjp.0706030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greenwood NN, Earnshaw A. Chemistry of the Elements. 2. Butterworth-Heinemann; 1997. [Google Scholar]
- 82.Gonzalez AR, Ndung’u K, Flegal AR. Natural occurrence of hexavalent chromium in the Aromas Red Sands Aquifer, California. Environ. Sci. Technol. 2005;39:5505–11. doi: 10.1021/es048835n. [DOI] [PubMed] [Google Scholar]
- 83.Costello RB, Dwyer JT, Bailey RL. Chromium supplements for glycemic control in type 2 diabetes: limited evidence of effectiveness. Nutr. Rev. 2016;74:455–68. doi: 10.1093/nutrit/nuw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Althuis MD, Jordan NE, Ludington EA, Wittes JT. Glucose and insulin responses to dietary chromium supplements: a meta-analysis. Am. J. Clin. Nutr. 2002;76:148–55. doi: 10.1093/ajcn/76.1.148. [DOI] [PubMed] [Google Scholar]
- 85.Suksomboon N, Poolsup N, Yuwanakorn A. Systematic review and meta-analysis of the efficacy and safety of chromium supplementation in diabetes. J. Clin. Pharm. Ther. 2014;39:292–306. doi: 10.1111/jcpt.12147. [DOI] [PubMed] [Google Scholar]
- 86.Guimarães MM, Carvalho ACMS, Silva MS. Effect of chromium supplementation on the glucose homeostasis and anthropometry of type 2 diabetic patients: Double blind, randomized clinical trial: Chromium, glucose homeostasis and anthropometry. J. Trace Elem. Med. Biol. 2016;36:65–72. doi: 10.1016/j.jtemb.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 87.Paiva AN, Lima JG de, Medeiros ACQ de, Figueiredo HAO, Andrade RL de, Ururahy MAG, et al. Beneficial effects of oral chromium picolinate supplementation on glycemic control in patients with type 2 diabetes: A randomized clinical study. J. Trace Elem. Med. Biol. 2015;32:66–72. doi: 10.1016/j.jtemb.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 88.Davis CM, Vincent JB. Chromium oligopeptide activates insulin receptor tyrosine kinase activity. Biochemistry. 1997;36:4382–5. doi: 10.1021/bi963154t. [DOI] [PubMed] [Google Scholar]
- 89.Samitz MH, Katz SA, Scheiner DM, Gross PR. Chromium-protein interactions. Acta Derm. Venereol. 1969;49:142–6. [PubMed] [Google Scholar]
- 90.Kleinfeld M, Rosso A. Ulcerations of the nasal septum due to inhalation of chromic acid mist. Ind. Med. Surg. 1965;34:242–3. [PubMed] [Google Scholar]
- 91.Fristedt B, Lindqvist B, Schuetz A, Ovrum P. Survival in a case of acute oral chromic acid poisoning with acute renal failure treated by haemodialysis. Acta Med. Scand. 1965;177:153–9. doi: 10.1111/j.0954-6820.1965.tb01817.x. [DOI] [PubMed] [Google Scholar]
- 92.Costa M. Toxicity and carcinogenicity of CRVI) in animal models and humans. Crit. Rev. Toxicol. 1997;27:431–42. doi: 10.3109/10408449709078442. [DOI] [PubMed] [Google Scholar]
- 93.Gylseth B, Gundersen N, Langård S. Evaluation of chromium exposure based on a simplified method for urinary chromium determination. Scand. J. Work. Environ. Health. 1977;3:28–31. doi: 10.5271/sjweh.2794. [DOI] [PubMed] [Google Scholar]
- 94.Stern RM. Process-dependent risk of delayed health effects for welders. Environ. Health Perspect. 1981;41:235–53. doi: 10.1289/ehp.8141235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Langárd S, Costa M. Chapter 24 – Chromium. Handbook on the Toxicology of Metals. 2007:487–510. [Google Scholar]
- 96.Agency for Toxic Substances and Disease Registry. [Accessed August 2016];US Public Health Service. Toxicological Profile for Chromium. 2012 Available from: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=62&tid=17. [PubMed]
- 97.Schroeder HA, Balassa JJ, Tipton IH. Abnormal trace metals in man--chromium. J. Chronic Dis. 1962;15:941–64. doi: 10.1016/0021-9681(62)90114-5. [DOI] [PubMed] [Google Scholar]
- 98.Caruso RV, O’Connor RJ, Stephens WE, Cummings KM, Fong GT. Toxic metal concentrations in cigarettes obtained from U.S. smokers in 2009: results from the International Tobacco Control (ITC) United States survey cohort. Int. J. Environ. Res. Public Health. 2014;11:202–17. doi: 10.3390/ijerph110100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tossavainen A, Nurminen M, Mutanen P, Tola S. Application of mathematical modelling for assessing the biological half-times of chromium and nickel in field studies. Br. J. Ind. Med. 1980;37:285–91. doi: 10.1136/oem.37.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garland M, Morris JS, Rosner BA, Stampfer MJ, Spate VL, Baskett CJ, et al. Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiol. Biomarkers Prev. 1993;2:493–7. [PubMed] [Google Scholar]
- 101.Cheng L, Sonntag DM, de Boer J, Dixon K. Chromium(VI)-induced mutagenesis in the lungs of big blue transgenic mice. J. Environ. Pathol. Toxicol. Oncol. 2000;19:239–49. [PubMed] [Google Scholar]
- 102.Costa M, Zhitkovich A, Taioli E, Toniolo P. Preliminary report on a simple new assay for DNA-protein cross-links as a biomarker of exposures experienced by welders. J. Toxicol. Environ. Health. 1993;40:217–22. doi: 10.1080/15287399309531789. [DOI] [PubMed] [Google Scholar]
- 103.Wise SS, Holmes AL, Wise JP. Hexavalent chromium-induced DNA damage and repair mechanisms. Rev. Environ. Health. 2008;23:39–57. doi: 10.1515/reveh.2008.23.1.39. [DOI] [PubMed] [Google Scholar]
- 104.Abraham AS, Brooks BA, Eylath U. Chromium and cholesterol-induced atherosclerosis in rabbits. Ann. Nutr. Metab. 1991;35:203–7. doi: 10.1159/000177646. [DOI] [PubMed] [Google Scholar]
- 105.Abraham AS, Sonnenblick M, Eini M. The action of chromium on serum lipids and on atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 1982;42:185–95. doi: 10.1016/0021-9150(82)90149-6. [DOI] [PubMed] [Google Scholar]
- 106.Alissa EM, Bahjri SM, Ahmed WH, Al-Ama N, Ferns GAA. Chromium status and glucose tolerance in Saudi men with and without coronary artery disease. Biol. Trace Elem. Res. 2009;131:215–28. doi: 10.1007/s12011-009-8365-2. [DOI] [PubMed] [Google Scholar]
- 107.Niskanen J, Marniemi J, Piironen O, Maatela J, Maki J, Vuori I, et al. Trace element levels in serum and urine of subjects died of coronary heart disease. Acta Pharmacol. Toxicol. 1986;59(Suppl 7):340–3. doi: 10.1111/j.1600-0773.1986.tb02775.x. [DOI] [PubMed] [Google Scholar]
- 108.Guallar E, Jiménez FJ, van ’t Veer P, Bode P, Riemersma RA, Gómez-Aracena J, et al. Low toenail chromium concentration and increased risk of nonfatal myocardial infarction. Am. J. Epidemiol. 2005;162:157–64. doi: 10.1093/aje/kwi180. [DOI] [PubMed] [Google Scholar]
- 109.Agency for Toxic Substances and Disease Registry. [Accessed August 2016];US Public Health Service. Toxicological Profile for Tungsten. 2005 Available from: http://www.atsdr.cdc.gov/toxprofiles/TP.asp?id=806&tid=157.
- 110.Witten ML, Sheppard PR, Witten BL. Tungsten toxicity. Chem. Biol. Interact. 2012;196:87–8. doi: 10.1016/j.cbi.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 111.Grimes DJ, Ficklin WH, Meier AL, McHugh JB. Anomalous gold, antimony, arsenic, and tungsten in ground water and alluvium around disseminated gold deposits along the Getchell Trend, Humboldt County, Nevada. J. Geochemical Explor. 1995;52:351–71. [Google Scholar]
- 112.Lemus R, Venezia CF. An update to the toxicological profile for water-soluble and sparingly soluble tungsten substances. Crit. Rev. Toxicol. 2015;45:388–411. doi: 10.3109/10408444.2014.1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lison D. Human toxicity of cobalt-containing dust and experimental studies on the mechanism of interstitial lung disease (hard metal disease) Crit. Rev. Toxicol. 1996;26:585–616. doi: 10.3109/10408449609037478. [DOI] [PubMed] [Google Scholar]
- 114.Moulin JJ, Wild P, Romazini S, Lasfargues G, Peltier A, Bozec C, et al. Lung cancer risk in hard-metal workers. Am. J. Epidemiol. 1998;148:241–8. doi: 10.1093/oxfordjournals.aje.a009631. [DOI] [PubMed] [Google Scholar]
- 115.McInturf SM, Bekkedal MYV, Wilfong E, Arfsten D, Chapman G, Gunasekar PG. The potential reproductive, neurobehavioral and systemic effects of soluble sodium tungstate exposure in Sprague–Dawley rats. Toxicol. Appl. Pharmacol. 2011;254:133–7. doi: 10.1016/j.taap.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 116.Brondino CD, Romão MJ, Moura I, Moura JJ. Molybdenum and tungsten enzymes: the xanthine oxidase family. Curr. Opin. Chem. Biol. 2006;10:109–14. doi: 10.1016/j.cbpa.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 117.Graczyk H, Lewinski N, Zhao J, Sauvain J-J, Suarez G, Wild P, et al. Increase in oxidative stress levels following welding fume inhalation: a controlled human exposure study. Part. Fibre Toxicol. 2016;13:31. doi: 10.1186/s12989-016-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lasfargues G, Lison D, Maldague P, Lauwerys R. Comparative study of the acute lung toxicity of pure cobalt powder and cobalt-tungsten carbide mixture in rat. Toxicol. Appl. Pharmacol. 1992;112:41–50. doi: 10.1016/0041-008x(92)90277-y. [DOI] [PubMed] [Google Scholar]
- 119.Shiue I, Hristova K. Higher urinary heavy metal, phthalate and arsenic concentrations accounted for 3–19% of the population attributable risk for high blood pressure: US NHANES, 2009–2012. Hypertens. Res. 2014;37:1075–81. doi: 10.1038/hr.2014.121. [DOI] [PubMed] [Google Scholar]
- 120.Tellez-Plaza M, Tang W-Y, Shang Y, Umans JG, Francesconi KA, Goessler W, et al. Association of global DNA methylation and global DNA hydroxymethylation with metals and other exposures in human blood DNA samples. Environ. Health Perspect. 2014;122:946–54. doi: 10.1289/ehp.1306674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121 •.Tyrrell J, Galloway TS, Abo-Zaid G, Melzer D, Depledge MH, Osborne NJ. High urinary tungsten concentration is associated with stroke in the National Health and Nutrition Examination Survey 1999–2010. PLoS One. 2013;8:e77546. doi: 10.1371/journal.pone.0077546. This large nationally representative cross-sectional study from the US evaluated the association of tungsten with self-reported prevalence of stroke and cardiovascular disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bargagli R, Nimis PL, Monaci F. Lichen Biomonitoring of Trace Element Deposition in Urban, Industrial and Reference Areas of Italy. J. Trace Elem. Med. Biol. 1997;11:173–5. doi: 10.1016/S0946-672X(97)80049-1. [DOI] [PubMed] [Google Scholar]
- 123.Agency for Toxic Substances and Disease Registry. [Accessed August 2016];US Public Health Service. Toxicological Profile for Barium. 2007 Available from: http://www.atsdr.cdc.gov/toxprofiles/TP.asp?id=327&tid=57. [PubMed]
- 124.World Health Organization. Environmental Health Criteria, No. 107 - Barium. WHO. World Health Organization; 1990. [Accessed August 2016]. Available from: http://www.inchem.org/documents/ehc/ehc/ehc107.htm. [Google Scholar]
- 125.Choudhury H, Cary R, United Nations Environment Programme., International Labour Organisation., World Health Organization., Inter-Organization Programme for the Sound Management of Chemicals et al. [Accessed August 2016];Barium and barium compounds : first draft. 2001 Available from: http://www.who.int/ipcs/publications/cicad/en/cicad33.pdf.
- 126.Brenniman GR, Kojola WH, Levy PS, Carnow BW, Namekata T. High barium levels in public drinking water and its association with elevated blood pressure. Arch. Environ. Health. 1981;36:28–32. doi: 10.1080/00039896.1981.10667602. [DOI] [PubMed] [Google Scholar]
- 127.Wones RG, Stadler BL, Frohman LA. Lack of effect of drinking water barium on cardiovascular risk factors. Environ. Health Perspect. 1990;85:355–9. doi: 10.1289/ehp.85-1568324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schroeder HA, Tipton IH, Nason AP. Trace metals in man: strontium and barium. J. Chronic Dis. 1972;25:491–517. doi: 10.1016/0021-9681(72)90150-6. [DOI] [PubMed] [Google Scholar]
- 129.Rundo J. The retention of barium-133 in man. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1967;13:301–2. doi: 10.1080/09553006814550261. [DOI] [PubMed] [Google Scholar]
- 130.Nickel . (Environmental Health Criteria 108). 383 Seiten, 36 Tab. Vol. 36. World Health Organization; Geneva: 1991. pp. 102–102. [Google Scholar]
- 131.Rojas E, Herrera LA, Poirier LA, Ostrosky-Wegman P. Are metals dietary carcinogens? Mutat. Res. Toxicol. Environ. Mutagen. 1999;443:157–81. doi: 10.1016/s1383-5742(99)00018-6. [DOI] [PubMed] [Google Scholar]
- 132.Smith CJ, Livingston SD, Doolittle DJ. An international literature survey of "IARC Group I carcinogens" reported in mainstream cigarette smoke. Food Chem. Toxicol. 1997;35:1107–30. doi: 10.1016/s0278-6915(97)00063-x. [DOI] [PubMed] [Google Scholar]
- 133.Klein C, Costa M. Chapter 35 – Nickel. Handbook on the Toxicology of Metals. 2007:743–58. [Google Scholar]
- 134.Lee YW, Klein CB, Kargacin B, Salnikow K, Kitahara J, Dowjat K, et al. Carcinogenic nickel silences gene expression by chromatin condensation and DNA methylation: a new model for epigenetic carcinogens. Mol. Cell. Biol. 1995;15:2547–57. doi: 10.1128/mcb.15.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee Y-W, Broday L, Costa M. Effects of nickel on DNA methyltransferase activity and genomic DNA methylation levels. Mutat. Res. Toxicol. Environ. Mutagen. 1998;415:213–8. doi: 10.1016/s1383-5718(98)00078-3. [DOI] [PubMed] [Google Scholar]
- 136.Nielsen GD, Søderberg U, Jørgensen PJ, Templeton DM, Rasmussen SN, Andersen KE, et al. Toxicol. Appl. Pharmacol. Vol. 154. Academic Press; 1999. Absorption and Retention of Nickel from Drinking Water in Relation to Food Intake and Nickel Sensitivity; pp. 67–75. [DOI] [PubMed] [Google Scholar]
- 137.Onkelinx C, Becker J, Sunderman FW. Compartmental analysis of the metabolism of 63Ni(II) in rats and rabbits. Res. Commun. Chem. Pathol. Pharmacol. 1973;6:663–76. [PubMed] [Google Scholar]
- 138.Keith LS, Faroon OM, Fowler BA. Chapter 45 – Uranium. Handbook on the Toxicology of Metals. 2007:881–903. [Google Scholar]
- 139.Agency for Toxic Substances and Disease Registry. [Accessed August 2016];US Public Health Service. Toxicological Profile for Uranium. 2013 Available from: http://www.atsdr.cdc.gov/toxprofiles/TP.asp?id=440&tid=77. [PubMed]
- 140.Guéguen Y, Souidi M, Baudelin C, Dudoignon N, Grison S, Dublineau I, et al. Short-term hepatic effects of depleted uranium on xenobiotic and bile acid metabolizing cytochrome P450 enzymes in the rat. Arch. Toxicol. 2006;80:187–95. doi: 10.1007/s00204-005-0027-3. [DOI] [PubMed] [Google Scholar]
- 141.Monleau M, De Méo M, Frelon S, Paquet F, Donnadieu-Claraz M, Duménil G, et al. Distribution and genotoxic effects after successive exposure to different uranium oxide particles inhaled by rats. Inhal. Toxicol. 2006;18:885–94. doi: 10.1080/08958370600822524. [DOI] [PubMed] [Google Scholar]
- 142.Miller AC, Stewart M, Brooks K, Shi L, Page N. Depleted uranium-catalyzed oxidative DNA damage: absence of significant alpha particle decay. J. Inorg. Biochem. 2002;91:246–52. doi: 10.1016/s0162-0134(02)00391-4. [DOI] [PubMed] [Google Scholar]
- 143.Dorian P, Penkoske PA, Witkowski FX. Order in disorder: effect of barium on ventricular fibrillation. Can. J. Cardiol. 1996;12:399–406. [PubMed] [Google Scholar]
- 144.Racine R, Grandcolas L, Grison S, Stefani J, Delissen O, Gourmelon P, et al. Cholesterol 7α-hydroxylase (CYP7A1) activity is modified after chronic ingestion of depleted uranium in the rat. J. Steroid Biochem. Mol. Biol. 2010;120:60–6. doi: 10.1016/j.jsbmb.2010.03.066. [DOI] [PubMed] [Google Scholar]
- 145.Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Env. Hyg. 1990;5:46–51. [Google Scholar]
- 146.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Sharrett AR, Guallar E. Cadmium and peripheral arterial disease: gender differences in the 1999–2004 US National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2010;172:671–81. doi: 10.1093/aje/kwq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, Guallar E. Cadmium Exposure and All Cause and Cardiovascular Mortality in the US General Population. Environ. Health Perspect. 2012;20(7):1017–22. doi: 10.1289/ehp.1104352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Franchini I, Mutti A, Cavatorta A, Corradi A, Cosi A, Olivetti G, et al. Nephrotoxicity of chromium. Remarks on an experimental and epidemiological investigation. Contrib. Nephrol. 1978;10:98–110. [PubMed] [Google Scholar]
- 149.Wang X, Qin Q, Xu X, Xu J, Wang J, Zhou J, et al. Chromium-induced early changes in renal function among ferrochromium-producing workers. Toxicology. 1994;90:93–101. doi: 10.1016/0300-483x(94)90208-9. [DOI] [PubMed] [Google Scholar]
- 150.Petersen R, Mikkelsen S, Thomsen OF. Chronic interstitial nephropathy after plasma cutting in stainless steel. Occup. Environ. Med. 1994;51:259–61. doi: 10.1136/oem.51.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jolliffe DS. Nephrotoxicity of pentavalent antimonials. Lancet. 1985;1:584. doi: 10.1016/s0140-6736(85)91245-0. [DOI] [PubMed] [Google Scholar]
- 152.Vicente-Vicente L, Quiros Y, Pérez-Barriocanal F, López-Novoa JM, López-Hernández FJ, Morales AI. Nephrotoxicity of uranium: pathophysiological, diagnostic and therapeutic perspectives. Toxicol. Sci. 2010;118:324–47. doi: 10.1093/toxsci/kfq178. [DOI] [PubMed] [Google Scholar]
- 153.Voinescu GC, Shoemaker M, Moore H, Khanna R, Nolph KD. The relationship between urine osmolality and specific gravity. Am. J. Med. Sci. 2002;323:39–42. doi: 10.1097/00000441-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 154.Pang Y, Jones MR, Tellez-Plaza M, Guallar E, Vaidya D, Post WS, et al. Association of Geography and Ambient Air Pollution with Urine Metal Concentrations in Six US Cities: the Multi-Ethnic Study of Atherosclerosis. Int J Environ Res Public Health. 2016 Mar 15;13(3) doi: 10.3390/ijerph13030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pang Y, Peng RD, Jones MR, Francesconi KA, Goessler W, Howard BV, et al. Metal mixtures in urban and rural populations in the US: The Multi-Ethnic Study of Atherosclerosis and the Strong Heart Study. Environ. Res. 2016;147:356–64. doi: 10.1016/j.envres.2016.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol. Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 158.Tylenda CA, Fowler BA. Chapter 18 – Antimony. Handbook on the Toxicology of Metals. 2007:353–65. [Google Scholar]
- 159.Kentner M, Leinemann M, Schaller KH, Weltle D, Lehnert G. External and internal antimony exposure in starter battery production. Int. Arch. Occup. Environ. Health. 1995;67:119–23. doi: 10.1007/BF00572235. [DOI] [PubMed] [Google Scholar]
- 160.Oskarsson A. Reeves al. Chapter 20 – Barium. Handbook on the Toxicology of Metals. 2007:407–14. [Google Scholar]
- 161.Agency for Toxic Substances and Disease Registry. [Accessed August 2016];US Public Health Service. Toxicological Profile for Nickel. 2005 Available from: http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=245&tid=44.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.