Abstract
Background
Diagnostic re-evaluation is important for all patients with congenital hypothyroidism (CH) for determining the etiology and identifying transient CH cases. Our study is a first thyroxine therapy withdrawal study conducted in Macedonian CH patients for a diagnostic re-evaluation. We aimed to evaluate the etiology of CH, the prevalence of transient CH and identify predictive factors for distinguishing between permanent (PCH) and transient CH (TCH).
Materials and methods
Patients with CH aged >3 years underwent a trial of treatment withdrawal for 4 weeks period. Thyroid function testing (TFT), ultrasound and Technetium-99m pertechnetate thyroid scan were performed thereafter. TCH was defined when TFT remained within normal limits for at least 6-month follow-up. PCH was diagnosed when TFT was abnormal and classified according the imaging findings.
Results
42 (55%) patients had PCH and 34 (45.0%) patients had TCH. Thyroid agenesia was the most prevalent form in the PCH group. Patients with TCH had lower initial thyroid-stimulating hormone (TSH) values (P < 0.0001); higher serum thyroxine levels (P = 0.0023) and lower mean doses of levothyroxine during treatment period (P < 0.0001) than patients with PCH. Initial TSH level <30.5 IU/mL and levothyroxine dose at 3 years of age <2.6 mg/kg/day were a significant predictive factors for TCH; sensitivity 92% and 100%, specificity 75.6% and 76%, respectively.
Conclusion
TCH presents a significant portion of patients with CH. Initial TSH value and levothyroxine dose during treatment period has a predictive role in differentiating TCH from PCH. Earlier re-evaluation, between 2 and 3 years age might be considered in some patients requiring low doses of levothyroxine.
Keywords: congenital hypothyroidism, etiology, levothyroxine, transient
Introduction
Thyroid hormones play important role in the processes of neuronal migration and differentiation, myelination and synaptogenesis and are essential for proper neurodevelopment (1). Congenital hypothyroidism (CH) is generally classified into two main groups: permanent CH and transient CH depending on the lifelong therapy requirements. The vast majority of CH children will have permanent hypothyroidism: thyroid dysgenesis (TD) due to abnormal thyroid development or thyroid dyshormonogenesis due to defects of thyroid hormone biosynthesis. The etiologic evaluation of CH is possible through several examinations, such as ultrasonography, scintigraphy, thyroglobulin measurement and perchlorate discharge test. There are also cases where the results can be inconclusive even after performing several diagnostic tests. The etiology of CH is important for determining the severity of the disease and its prognosis, clinical management and the need for genetic counseling. A number of recent studies worldwide have reported a change in the epidemiology of CH with a doubling incidence of 1 in 1500 live newborns, mostly caused by the increasing number of the mild and potentially transient CH cases with eutopic thyroid gland (2, 3).
Long-term follow-up of CH cases is of particular interest in the last decade. Although unfavorable outcome of untreated CH is well established, recent evidence suggests that many CH children are no longer treated after the age of 3 years (4, 5, 6).
To distinguish between transient and permanent forms, the guidelines recommend re-evaluation after 3 years of age in all children with unexplained CH through a trial of treatment withdrawal. This is important since some cases of transient CH caused by identifiable or non-identifiable factors may require only a short-term therapy (7, 8).
Here, we report the results of the first thyroxine therapy withdrawal study conducted in Macedonia through a diagnostic re-evaluation following a standardized protocol. We aimed to evaluate the etiology of CH and determine the prevalence of transient hypothyroidism among CH children diagnosed in the newborn period after the neonatal screening.
Neonatal screening for CH in the Republic of Macedonia was introduced in 2002 as a pilot project and since 2007, it is mandatory in the entire country.
Materials and methods
Patients
Patients detected through the neonatal screening in the period April 2002–December 2015 are included. A total of 251,008 newborns were screened with a mean coverage of the 96.7%. The overall incidence of primary CH was 1/1967 and female-to-male ratio was 1.35:1 (9, 10). Children with CH detected by neonatal screening program were followed at a single center of the University Children’s Hospital. The neonatal screening was carried out by determination of whole-blood thyroid-stimulating hormone (TSH) on dried blood spot specimen obtained from newborns 48 to 72 h after birth by fluoroimmunometric DELFIA assay. The TSH cut-off level was 15 IU/L in the period 2002–2010 and 10 IU/L thereafter. Preterm or sick newborns were screened between the first and second week of life. Birth weight, gestational age and time of sampling were recorded on the blood spot card for adequate interpretation. Results between 10 and 15 IU/L were considered borderline and repeat analysis (new blood spot card) was requested usually 7 days after the previous test. Whenever the repeated blood sampling TSH concentration was higher than 10 IU/L, patients were recalled for biochemical and clinical evaluation. The diagnosis of CH was based on the abnormal thyroid function tests (TSH >10 IU/L and low or normal T4 or FT4) on confirmatory serum measurements.
Study design
All children diagnosed with CH were immediately initiated on levothyroxine (LT4) treatment. The patients underwent regular thyroid function tests (TFT), as well as assessment of growth and development, mainly at 3-month intervals. Children aged ≥3 years underwent trial off therapy for period of 4 weeks and were scheduled for re-evaluation thereafter. Parents were advised to monitor for signs and symptoms of hypothyroidism. After four weeks off therapy clinical assessment, TFT and imaging studies were performed.
Children with abnormal TFT were classified as having permanent hypothyroidism (PCH), thus, LT4 therapy was restarted at previous dose and titrated thereafter. Further classification of PCH was based on the ultrasound and scintigraphy findings (athyreosis, thyroid ectopia, hypoplasia or probable dyshormonogeneis). Probable dyshormonogenesis was defined when a large thyroid gland in the eutopic position with increased uptake was found on imaging studies. Children in whom TFT, ultrasound and scintigraphy were normal were followed with serial TFT tests every month for at least 6-month period. If the TFT remained normal, they were classified as transient hypothyroidism (TCH). Patients in whom the therapy was stopped between 2 and 3 years of age during the regular follow-up because of continuously normal TFT or low thyroxine dose underwent thyroid re-testing and ultrasonography. If the results were within normal limits, they were diagnosed as TCH, and no further follow-up was recommended.
Laboratory and imaging methods
TSH and T4/FT4 were measured using IMMULITE 2000 chemiluminescent enzyme immunoassay system (Siemens Healthcare Diagnostics Inc.). Values of thyroid hormones ranging from the 3rd to the 97th percentile standardized for age were considered normal (11). Reference values: T4: 4.5–10.9 µg/dL, FT4: 0.9–1.8 ng/dL, TSH 0.4–5.0 IU/L.
Thyroid ultrasound (SonoScape SSI-5000 Color Doppler Ultrasound System, SonoScape Medical Corp., Shenzhen, China) was performed to detect the presence of the thyroid gland and assess the size and echostructure. The size of thyroid gland was determined by measurement of the volume of the thyroid lobes and their comparison with the reference values from the literature (12). Thyroid scan was performed by double-head Mediso gamma camera after an intravenous injection of 99m-technetium pertechnetate (2 MBq/kg) and obtaining standard anterior and lateral images in supine position. The presence, size and location of areas of 99mTcO4 uptake were recorded.
Data analysis
Statistical analysis was performed using the SPSS for Windows statistical package (version 17.0). The following variables were analyzed: gestational age, sex, birthweight, initial values of TSH and T4, age at treatment initiation, initial LT4 dose, LT4 dose at 1, 2 and 3 years of age and at re-evaluation. Comparison between the two groups for numerical variables was performed with the Student’s t-test and the Mann–Whitney test. Pearson’s chi-square test was used in the comparison of proportions. One-way ANOVA analysis followed by post hoc Tukey HSD (honestly significant difference) test was used for comparison between the etiological subgroups of patients with permanent hypothyroidism.
Logistic regression analysis was used to investigate predictors for transient CH. Several parameters that showed significant difference between TCH and PCH groups were applied in the ROC (receiver-operating characteristic) analysis. The optimal cut-off point of each predictor was determined, and sensitivity and specificity were calculated for this cut-off value. For comparison of ROC curves, MedCalc for Windows (version 17.8, MedCalc Software, Ostend, Belgium) was used. P values lower than 0.05 were considered statistically significant.
Ethical approval
All procedures performed in study were in accordance with the ethical standards of the Helsinki Medical Declaration and its later amendments. The study protocol was approved by Medical Faculty’s Ethical Committee, and informed consent was obtained from the parents of the children included in the study.
Results
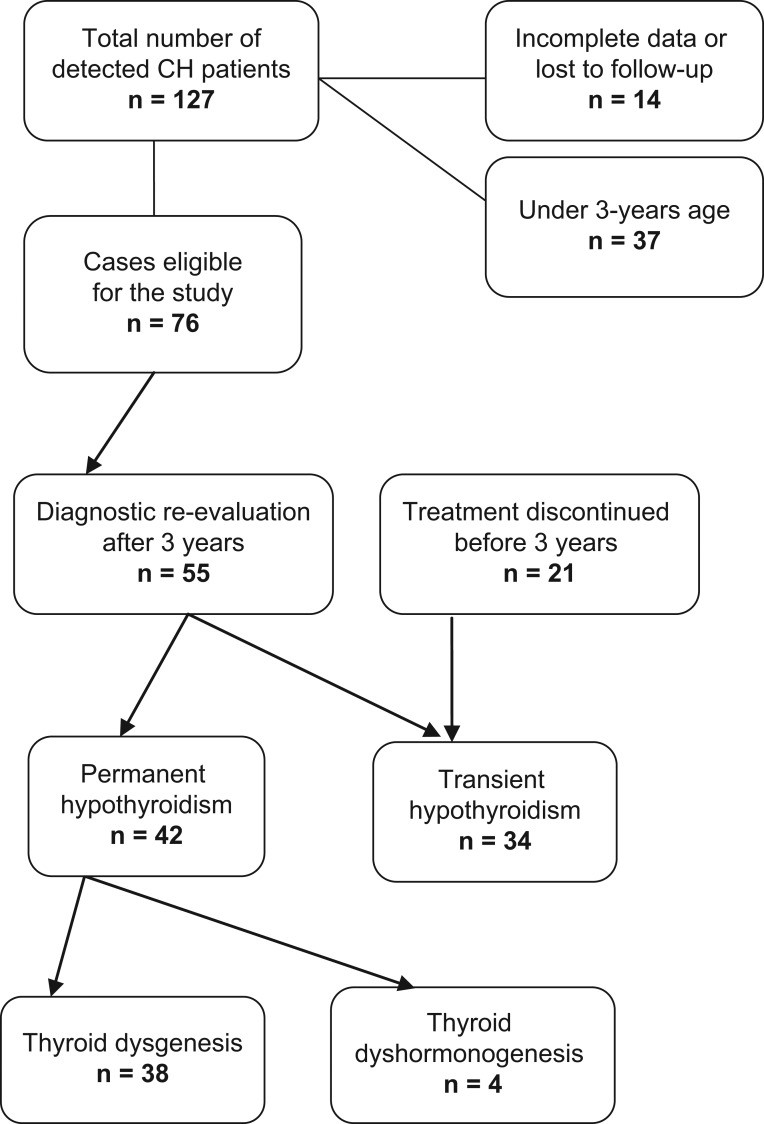
A total of 127 neonates detected by national neonatal thyroid screening were confirmed to have primary CH, in the period 2002–2015, with an overall incidence of 1:1967 live births. Thirty-seven children were excluded from the present study because of age less than 3 years and 14 children because of incomplete medical records, lost from follow-up, parents’ refusal or Down syndrome. Therapy was interrupted in 55 CH children for mean period of 30 days (range 28–40 days). All patients were clinically euthyroid on levothyroxine therapy at the time of enrollment. The mean age was 6.5 ± 2.8 years (range: 3–13 years); 35 were girls and 20 were boys. In 21 CH children treatment was discontinued during the regular follow-up at mean age of 25.4 ± 4.7 months (range: 18–33 months). Forty-two patients (55%) were classified in the PCH group and 34 (45%) in TCH group based on the defined criteria. The prevalence of PCH was 1/3586 and the prevalence of TCH was 1/4404. Figure 1 shows the flow diagram of the study. The ratio of sex was not significantly different between TCH and PCH groups (P = 0.091). Treatment for CH was initiated at a mean age of 12.1 (± 4.1) days, and it was not significantly different between the groups (Table 1). Patients in the TCH group exhibited significantly lower TSH levels compared to subjects in the PCH group (Table 1). The initial levothyroxine doses, as well as LT4 dose at 1, 2 and 3 years of age were significantly lower in TCH subjects (Table 1).
Figure 1.
Flow diagram of the follow-up study.
Table 1.
Comparison of the clinical and laboratory characteristics of patients with congenital hypothyroidism.
| Total (n = 76) | Permanent CH (n = 42) | Transient CH (n = 34) | P value |
|---|---|---|---|
| Sex (N) | |||
| Female | 29 | 17 | 0.091a |
| Male | 13 | 17 | |
| Birth weight (kg) | 3.4 ± 0.5 | 3.1 ± 0.6 | 0.303 |
| Gestational age (week) | 39.3 ± 1.2 | 38.9 ± 1.6 | 0.121 |
| Age of treatment initiation (day) | 11.3 ± 4.2 | 13.8 ± 3.4 | 0.076 |
| Thyroid tests at diagnosis | |||
| TSH (µIU/L) | 81.9 ± 56.8 | 22.7 ± 10.9 | <0.0001 |
| T4 (µg/dL) | 4.8 ± 3.9 | 6.7 ± 2.4 | 0.0023 |
| Levothyroxine dose (µg/kg) | |||
| Initial | 11.8 ± 2.1 | 9.2 ± 1.5 | 0.002 |
| 1 year | 3.7 ± 0.8 | 2.4 ± 0.7 | <0.0001 |
| 2 years | 3.3 ± 0.7 | 1.9 ± 0.6 (N = 30) | <0.0001 |
| 3 years | 3.2 ± 0.7 | 1.7 ± 0.6 (N = 14) | <0.0001 |
| At re-evaluation | 2.6 ± 0.6 | 1.4 ± 0.5 (N = 13) | |
aThe sex ratio between groups was calculated using the chi-square test.
Thyroid agenesia was the most prevalent cause of permanent hypothyroidism present in one half of the patients (n = 21), followed by thyroid ectopy (n = 13), hypoplasia (n = 4) and probable thyroid dyshormonogenesis in the other 4 cases (Table 2). Among the patients with ectopic thyroid gland (10 females and 3 males), two and five patients had submental and lingual thyroid gland, respectively, and the remaining six patients had sublingual uptake on scintigraphy.
Table 2.
Characteristics of patients with permanent congenital hypothyroidism.
| Permanent CH (n = 42) | |||||
|---|---|---|---|---|---|
| Etiology | Thyroid dysgenesis (n = 38) | Thyroid dyshormonogenesis (n = 4) | |||
| Athyreosis (n = 21) | Ectopy (n = 13) | Hypoplasia (n = 4) | P | ||
| Thyroid function tests | |||||
| At time of diagnosis | |||||
| TSH (µIU/L) | 122.5 ± 51.2 | 67.1 ± 40.7 | 31.3 ± 17.3 | 30.9 ± 18.7 | <0.05* |
| T4 (µg/dL) | 2.9 ± 2.7 | 6.4 ± 4.2 | 7.3 ± 4.6 | 6.8 ± 3.1 | <0.05* |
| After treatment discontinuation | |||||
| TSH (µIU/L) | 72.2 ± 10.6 | 58.8 ± 27.1 | 35.9 ± 21.8 | 27.3 ± 10.6 | <0.05* |
| T4 (µg/dL) | 1.1 ± 0.1 | 3.6 ± 2.8 | 6.8 ± 3.5 | 3.2 ± 3.0 | <0.05* |
| l-Thyroxine dose | |||||
| At time of diagnosis | 12.2 ± 2.2 | 11.5 ± 1.1 | 10.9 ± 2.8 | 10.3 ± 2.0 | 0.256 |
| At time of treatment discontinuation | 2.6 ± 0.5 | 2.9 ± 0.8 | 2.3 ± 0.9 | 2.37 ± 0.4 | 0.237 |
*The P value corresponding to the F statistic of one-way ANOVA for the initial TSH values and TSH at re-evaluation is lower than 0.05 suggesting for significant difference between the CH etiology groups.
A significant difference in the TSH and T4 values at diagnosis and after treatment discontinuation was also observed in children with PCH subdivided in different etiological groups: athyreosis, ectopy, hypoplasia and dyshormonogenesis, P < 0.05 (Table 2). Patients with thyroid agenesia had significantly higher TSH values at diagnosis compared to patients with ectopy, hypoplasia and putative dyshormonogenesis. After 4 weeks off therapy, TSH values did not significantly differ between athyreosis and ectopies (P = 0.427). The results of the Tukey HDS post hoc test indicating differences within PCH subgroups are presented in Table 3.
Table 3.
Tukey HDS (‘Honestly Significant Difference’) post hoc test results indicating which groups significantly differ from others providing 95% confidence interval.
| At diagnosis | After treatment discontinuation | |||
|---|---|---|---|---|
| Tukey HSD Q statistic | P value | Tukey HSD Q statistic | P value | |
| TSH value | ||||
| Athyreosis vs ectopy | 4.9351 | 0.0066** | 2.1721 | 0.4272 |
| Athyreosis vs hypoplasia | 5.7550 | 0.0013** | 6.8050 | 0.0010** |
| Athyreosis vs dyshormonogenesis | 5.9677 | 0.0010** | 8.4170 | 0.0010** |
| Ectopy vs hypoplasia | 2.0516 | 0.4923 | 4.7093 | 0.0094** |
| Ectopy vs dyshormonogenesis | 2.2164 | 0.4106 | 6.1942 | 0.0010** |
| Hypoplasia vs dyshormonogenesis | 0.1690 | 0.8999 | 1.2859 | 0.7772 |
| T4 value | ||||
| Athyreosis vs ectopy | 3.995 | 0.035** | 4.5641 | 0.0126** |
| Athyreosis vs hypoplasia | 4.378 | 0.018** | 8.0208 | 0.0010** |
| Athyreosis vs dyshormonogenesis | 3.047 | 0.153 | 2.9966 | 0.1643 |
| Ectopy vs hypoplasia | 1.058 | 0.866 | 4.1226 | 0.0282** |
| Ectopy vs dyshormonogenesis | 0.035 | 0.899 | 0.5289 | 0.8999 |
| Hypoplasia vs dyshormonogenesis | 0.923 | 0.899 | 4.0283 | 0.0332** |
**P< 0.05.
Predictive factors suggesting transient congenital hypothyroidism
According to the ROC curve analysis, initial TSH value <30.5 IU/mL was associated with TCH, showing 92% sensitivity and 75.6% specificity, with an area under the ROC curve (AUC) 0.850 (P < 0.001). Also initial serum T4 >3.6 µg/dL was associated with TCH with 92% sensitivity and 63.4% specificity, AUC 0.778 (P < 0.001).
The optimal cut-off points for the LT4 dose during treatment as a predictor for distinguishing PCH and TCH were as follows: initial LT4 dose 11.0 µg/kg/day, with 96% sensitivity and 70.7% specificity, AUC 0.857; LT4 dose at 1 year of age 3.0 µg/kg/day, 86.4% sensitivity and 76.5% specificity, AUC 0.880; LT4 dose at 2 years of age 2.8 µg/kg/day, 95.2% sensitivity and 82.6% specificity, AUC 0.904; LT4 dose at 3 years of age 2.6 µg/kg/day, 100% sensitivity and 76% specificity, AUC 0.921. In a logistic regression analysis with an initial TSH and T4 levels, and the levothyroxine dose during the treatment period as independent variables, the initial TSH levels and the levothyroxine dose at 3 years of age were significant predictors of a TCH diagnosis (Fig. 2 and Table 4).
Figure 2.
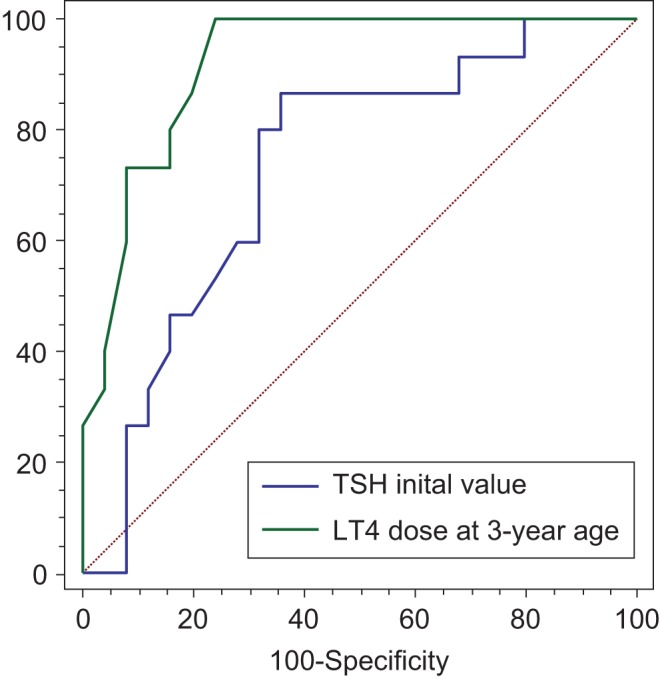
ROC curves shows both the initial TSH values and the levothyroxine dose at 3 years of age were predictive factors for discrimination between transient and permanent congenital hypothyroidism.
Table 4.
Logistic regression analysis of factors associated with transitory congenital hypothyroidism.
| Predictors | Coefficient | Sth. error | rpartial | t | P value |
|---|---|---|---|---|---|
| 2.3069 | |||||
| Initial TSH value | −0.004274 | 0.001965 | −0.3691 | −2.175 | 0.0376* |
| Initial T4 value | −0.003676 | 0.01800 | −0.03725 | −0.204 | 0.8396 |
| Initial LT4 dose | −0.04351 | 0.02828 | −0.2705 | −1.539 | 0.1343 |
| LT4 dose at 1-year age | −0.08257 | 0.06334 | −0.2315 | −1.304 | 0.2023 |
| LT4 dose at 2-year age | −0.1185 | 0.06672 | −0.3085 | −1.776 | 0.0858 |
| LT4 dose at 3-year age | −0.2374 | 0.07304 | −0.5104 | −3.251 | 0.0028* |
*P < 0.05.
Discussion
Early detection and attainment of euthyroid status as quickly as possible are essential for all children with primary CH for achieving an optimal neurodevelopment. Another significant point in addition to early treatment is specification of underlying cause of CH, thus identifying transient cases and preventing overtreatment. The results of our study showed that almost 45% of patients diagnosed with CH through neonatal screening had transient CH and do not require lifelong thyroid hormone supplementation. Although the prevalence of transient CH varies in different studies an increasing trend has been observed worldwide in the recent years (5, 6, 13, 14, 15, 16). One possible explanation for this increased incidence is the change in screening strategies, such as lowering the TSH cut-off values that allows more sensitive detection and early intervention. The TSH cut-off was lowered in our national thyroid screening program from 15 to 10 IU/L after 2010. The overall incidence of CH significantly increased from 1/2489 up to 2010 to 1/1585 thereafter, with increasing the prevalence of transient CH cases (10). However, the optimal cut-off in this study was 30.5 IU/L. Kang and coworkers reported a similar TSH cut-off point of 31 IU/L for distinguishing TCH and PCH (17). Other studies suggested initial TSH cut-off values of 28.4 IU/L and 34 IU/L (14, 18). Generally, it is safe to refer to the current guidelines, which suggest an immediate treatment if TSH >20 IU/L and clinicians individual approach for cases with TSH values between 6 IU/L and 20 IU/L (7). Prematurity is often reported to be associated with TCH (19, 20). In our study, there was no significant difference in the birth weight and the duration of gestation between the TCH and PCH group. Other factors that might have contributed to TCH are ethnic modifications in the population, variations in iodine supply, endocrine-disrupting chemicals exposure etc. (3). A recently published study accessing the iodine status through TSH measurements on newborn screening reported iodine sufficiency in Macedonia (21). Considering these facts the etiology of the most of our TCH cases remains unknown. Patients with TCH exhibited significantly lower TSH and higher T4 levels at the time of diagnosis compared to those with PCH. Some previous studies in the literature reported that the initial T4 did not differentiate between TCH and PCH cases (13, 17, 22).
Forty-two children had permanent hypothyroidism after re-evaluation, and thyroid agenesia was the most prevalent etiology. Thyroid dyshormonogenesis was suspected in 4 patients with PCH based on the thyroid volume ultrasound and abnormal scintigraphy uptake. However, definitive diagnosis of dyshormonogenesis requires a perchlorate discharge test or a molecular genetic analysis and unfortunately neither of them was available in our center. The initial TSH and T4 values were significantly different between the PCH subgroups, which corresponds to the reports from other studies in the literature (23, 24).
Another interesting finding in our study was the difference in the initial TSH values between patients with athyreosis and thyroid ectopy, which was not observed after trial off therapy. This might be due to the titration of the levothyroxine dose during the follow-up in patients with thyroid ectopy. Thus, the similar clinical course and therapy requirement of both athyreosis and ectopies is obvious. The levothyroxine dose was not significantly different between the PCH subgroups at initiation and at re-evaluation period.
However, the initial LT4 dose was significantly different between patients with transitory and permanent hypothyroidism. The current guidelines recommend starting dose of 10–15 µg/kg/day, but considering the heterogeneity of CH, some children may require smaller doses because of some endogenous thyroid hormone production. Since imaging studies were not performed in all of our patients at diagnosis, the initial dose was mainly based on the results of TFT. However, targeted LT4 dosing based on the laboratory and thyroid anatomy might be reasonable in some prospective study in the future.
During the treatment period, significant dose differences were observed between the patients with PCH and TCH (3.7 µg/kg/day vs 2.4 µg/kg/day at 12 months and 3.3 µg/kg/day vs 1.9 µg/kg/day at 24 months). Moreover, the levothyroxine dose at 3-year age was a positive predictor of TCH diagnosis. Many authors emphasize the LT4 dose as a discriminate factor between TCH and PCH. Messina and coworkers reported that LT4 requirements >4.9 µg/kg/day at 12 months age or >4.27 µg/kg/day at 24 months were highly suggestive of PCH, irrespective of gland ultrasonography (22).
Cho and coworkers reported that children requiring LT4 dose lower than 3.25 µg/kg/day at 12 and 24 months were likely to have TCH, suggesting that earlier re-evaluation is possible in these patients (between 12 and 24 months rather than after 3 years) (18). A significant proportion of patients with TCH in our study had discontinued treatment within 36 months and confirmed to have a transient hypothyroidism thereafter. Thus, the re-evaluation through one-month trial off therapy might be considered at 2 years of age in patients requiring low doses of LT4 during follow-up. In our study, a levothyroxine dose of 2.6 μg/kg at 3 years of age might be used to predict the diagnosis of TCH.
Our study presents a first diagnostic re-evaluation of Macedonian children with CH following a standardized protocol. However, it has several limitations. The small number of cases and unavailability of genetic analysis for the diagnosis of dyshormonogenesis are some of them. Extension of the study in the future with the newly diagnosed CH children, as well as the longer follow-up period of cases with transient hypothyroidism is warranted.
In conclusion, 45% of cases diagnosed with CH had of a transient form of hypothyroidism. Patients with TCH had lower initial TSH levels and higher initial T4 values, as well as lower levothyroxine dose requirements during the follow-up than PCH patients. A levothyroxine dose lower than 2.6 μg/kg/day at 3 years of age might predict TCH. Although inconsistent to the current guidelines, earlier re-evaluation of children younger than 3 years might be safe in patients requiring low doses of LT4, thus preventing unnecessary or excessive treatment of TCH.
Declaration of interest
All authors declare no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Horn S, Heuer H. Thyroid hormone action during brain development: more questions than answers. Molecular and Cellular Endocrinology 2010. 315 19–26. ( 10.1016/j.mce.2009.09.008) [DOI] [PubMed] [Google Scholar]
- 2.Harris KB, Pass KA. Increase in congenital hypothyroidism in New York State and in the United States. Molecular Genetics and Metabolism 2007. 91 268–277. ( 10.1016/j.ymgme.2007.03.012) [DOI] [PubMed] [Google Scholar]
- 3.Persani L. Congenital hypothyroidism with gland in situ is more frequent than previously thought. Frontiers in Endocrinology 2012. 3 18 ( 10.3389/fendo.2012.00018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruters A, Jenner A, Krude H. Long-term consequences of congenital hypothyroidism in the era of screening programmes. Best Practice and Research: Clinical Endocrinology and Metabolism 2002. 16 369–382. ( 10.1053/beem.2002.0202) [DOI] [PubMed] [Google Scholar]
- 5.Kemper AR, Ouyang LJ, Grosse SD. Discontinuation of thyroid hormone treatment among children in the United States with congenital hypothyroidism: findings from health insurance claims data. BMC Pediatrics 2010. 10 9 ( 10.1186/1471-2431-10-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eugster EA, LeMay D, Zerin JM, Pescovitz OH. Definitive diagnosis in children with congenital hypothyroidism. Journal of Pediatrics 2004. 144 643–647. ( 10.1016/j.jpeds.2004.02.020) [DOI] [PubMed] [Google Scholar]
- 7.Leger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, Polak M, Butler G, ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE, Congenital Hypothyroidism Consensus Conference Group et al European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Journal of Clinical Endocrinology and Metabolism 2014. 99 363–384. ( 10.1210/jc.2013-1891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics, Rose SR, Brown RS, Foley T, Kaplowitz PB, Kaye CI, et al Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 2006. 117 2290–2303. ( 10.1542/peds.2006-0915) [DOI] [PubMed] [Google Scholar]
- 9.Kocova M, Anastasovska V, Sukarova-Angelovska E, Tanaskoska M, Taseva E. Clinical practice: experience with newborn screening for congenital hypothyroidism in the Republic of Macedonia – a multiethnic country. European Journal of Pediatrics 2015. 174 443–448. ( 10.1007/s00431-014-2413-4) [DOI] [PubMed] [Google Scholar]
- 10.Anastasovska A, Kocova M. Impact of lower screening TSH cutoff level on the increasing prevalence of congenital hypothyroidism. International Journal of Neonatal Screening 2017. 3 007 ( 10.3390/ijns3020007) [DOI] [PubMed] [Google Scholar]
- 11.Kapelari K, Kirchlechner C, Hogler W, Schweitzer K, Virgolini I, Moncayo R. Pediatric reference intervals for thyroid hormone levels from birth to adulthood: a retrospective study. BMC Endocrine Disorders 2008. 8 15 ( 10.1186/1472-6823-8-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chanoine JP, Toppe V, Lagasse R, Spehl M, Delange F. Determination of thyroid volume by ultrasound from the neonatal period to late adolescence. European Journal of Pediatrics 1991. 150 395–399. ( 10.1007/BF02093716) [DOI] [PubMed] [Google Scholar]
- 13.Rabbiosi S, Vigone MC, Cortinovis F, Zamproni I, Fugazzola L, Persani L, Corbetta C, Chiumello G, Weber G. Congenital hypothyroidism with eutopic thyroid gland: analysis of clinical and biochemical features at diagnosis and after re-evaluation. Journal of Clinical Endocrinology and Metabolism 2013. 98 1395–1402. ( 10.1210/jc.2012-3174) [DOI] [PubMed] [Google Scholar]
- 14.Lim HK, Kim KH, Kim SH, No HY, Kim CJ, Woo YJ, Hwang TJ. Predictors of transient hypothyroidism in neonatal screening test. Journal of Korean Society of Pediatric Endocrinology 2006. 11 50–56. [Google Scholar]
- 15.Unuvar T, Demir K, Abacı A, Buyukgebiz A, Bober E. The role of initial clinical and laboratory findings in infants with hyperthyrotropinemia to predict transient or permanent hypothyroidism. Journal of Clinical Research in Pediatric Endocrinology 2013. 5 170–173. ( 10.4274/Jcrpe.931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korzeniewski SJ, Grigorescu V, Kleyn M, Young WI, Birbeck G, Todem D, Romero R, Paneth N. Transient hypothyroidism at 3-year follow-up among cases of congenital hypothyroidism detected by newborn screening. Journal of Pediatrics 2013. 162 177–182. ( 10.1016/j.jpeds.2012.06.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang MJ, Chung HR, Oh YJ, Shim YS, Hwang IT. Three-year follow-up of children with abnormal newborn screening results for congenital hypothyroidism. Pediatrics and Neonatology 2017. 58 442–448. ( 10.1016/j.pedneo.2017.01.002) [DOI] [PubMed] [Google Scholar]
- 18.Cho MS, Cho GS, Park SH, Jung MH, Suh BK, Koh DG. Earlier reevaluation may be possible in pediatric patients with eutopic congenital hypothyroidism requiring lower L-thyroxine doses. Annals of Pediatric Endocrinology and Metabolism 2014. 19 141–145. ( 10.6065/apem.2014.19.3.141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbetta C, Weber G, Cortinovis F, Calebiro D, Passoni A, Vigone MC, Beck-Peccoz P, Chiumello G, Persani L. A 7-year experience with low blood TSH cutoff levels for neonatal screening reveals an unsuspected frequency of congenital hypothyroidism (CH). Clinical Endocrinology 2009. 71 739–745. ( 10.1111/j.1365-2265.2009.03568.x) [DOI] [PubMed] [Google Scholar]
- 20.Radetti G, Fanolla A, Pappalardo L, Gottardi E. Prematurity may be a risk factor for thyroid dysfunction in childhood. Journal of Clinical Endocrinology and Metabolism 2007. 92 155–159. ( 10.1210/jc.2006-1219) [DOI] [PubMed] [Google Scholar]
- 21.Anastasovska V, Kocova M. Newborn screening for thyroid-stimulating hormone as an indicator for assessment of iodine status in the Republic of Macedonia. Journal of Medical Biochemistry 2016. 35 385–389. ( 10.1515/jomb-2016-0023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messina MF, Aversa T, Salzano G, Zirilli G, Sferlazzas C, De Luca F, Lombardo F. Early discrimination between transient and permanent congenital hypothyroidism in children with eutopic gland. Hormone Research in Paediatrics 2015. 84 159–164. ( 10.1159/000435811) [DOI] [PubMed] [Google Scholar]
- 23.Dias VM, Campos AP, Chagas AJ, Silva RM. Congenital hypothyroidism: etiology. Journal of Pediatric Endocrinology and Metabolism 2010. 23 815–826. ( 10.1515/jpem.2010.131) [DOI] [PubMed] [Google Scholar]
- 24.Perry RJ, Maroo S, Maclennan AC, Jones JH, Donaldson MDC. Combined ultrasound and isotope scanning is more informative in the diagnosis of congenital hypothyroidism than single scanning. Archives of Disease in Childhood 2006. 91 972–976. ( 10.1136/adc.2006.096776) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a