Abstract
Background:
Morphine is a pain medication. It is mostly processed in liver and reasons disturbing effects. It can increase the production of free radicals. Thymoquinone is a phytochemical compound found in the plant Nigella sativa. It has diverse pharmacological properties such as antioxidant and anticancer. This study was intended to assess the effects of thymoquinone against morphine damages on the liver of mice.
Methods:
In this study, various doses of thymoquinone (4.5, 9, and 18 mg/kg) and thymoquinone plus morphine was administered (once a day) intraperitoneally to 48 male mice for 20 consequent days. These mice were randomly assigned to eight groups (n = 6). Aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, serum nitric oxide (NO) levels, liver weight, and histology have been studied.
Results:
The results indicated that morphine administration significantly increased the mean diameter of central hepatic vein and hepatocyte, blood serum NO level, liver enzymes level, and decreased liver weight compared to saline group (P < 0.05). However, thymoquinone and thymoquinone plus morphine administration significantly enhanced liver weight and reduced the mean diameter of hepatocyte, central hepatic vein, liver enzymes, and NO levels in all groups compared to morphine group (P < 0.05).
Conclusions:
It seems that antioxidant effect of thymoquinone could protect damage of liver parameters against morphine toxicity.
Keywords: Liver, mice, morphine, thymoquinone
Introduction
At present, the use of medicinal plants, owing to their protective properties against diseases such as cancer, cardiovascular and liver diseases, is increasing steadily. Some plant extracts used in conventional medicine are full of compounds with preventive and protective properties, especially on liver diseases.[1] Black cumin (Nigella sativa L.) is a member of ranunculus family with white or light blue flowers that turn black when exposed to air.[2] The main compounds of the aqueous extract of this plant are thymoquinone, dithymoquinone, thymohydroquinone, and thymol.[3] Black cumin has antioxidant, anti-inflammatory, and antihistamine properties and strengthens the immune system. It exert numerous pharmacological effects including, reduction of blood sugar, lipid and high blood pressure, excretion of bile and uric acid, protection of kidney, heart, and liver tissues as well as antimicrobial and antiparasitic effects.[4] Farag et al. has shown that thymoquinone advances the liver and kidney changes persuaded by chronic cyclosporine.[5] A major part of the drugs imported into the body is metabolized by the hepatocytes, and it seems that the liver tissue is damaged during the metabolism of these materials.[6] Li et al. showed a significant correlation between morphine consumption and hepatitis C infection.[7] Oxidative stress plays a pivotal role in toxic liver damage.[8] The food and supplement enriched with antioxidant can protect the liver against various oxidative damages of reactive oxygen species (ROS).[9] Daba and Abdel-Rahman indicated that the black cumin extract can produce antioxidant effects against the liver toxicity induced by carbon tetrachloride.[10] Increased ROS causes DNA failure, inactivates some proteins, and impairs biologic membranes through induction of oxidative stress.[11] Crystal morphine is white or light brown and is extracted from opium and is frequently used in medicine as an analgesic drug.[12] Moreover, morphine induces oxidative stress and apoptosis of neurons, and hepatocyte, glial, and immune system cells.[13] Bekheet showed that administration of morphine can reduce the size of liver cells, thereby leading to their death.[14] The cell damage and dysfunction of endocrine and metabolic activities of liver can be recognized, to some extent, by measuring some of the liver enzymes.[15] Chen reported that morphine consumption increases liver toxicity and damage through increased induction of lipid peroxidation.[16] Furthermore, Abdel-Wahhab and Aly showed that black cumin can inhibit aflatoxin administration effect on blood and biochemical pathways in the liver of rats.[17] Given the toxicity effect of morphine, especially inducing oxidative stress and its application as an analgesic drug in medicine as well as thymoquinone properties, especially antioxidative stress, and that no study has evaluated the antioxidative effects of thymoquinone on the morphine-induced liver disorders, the present study was conducted to assess the effects of thymoquinone on the morphine-induced liver disorders in mice.
Methods
Laboratory animals
In this study, a total of 48 male BALB/c mice, with a weight range of 27–30 g, were purchased from Razi Institute. The mice were kept at the animal house for 1 week before the commencement of the study under laboratory conditions at 20°C ± 2°C, 12:12 day/night cycle, and free access to food and water. The animals were kept in the standard cages of the animal house of school of medicine; six mice in each cage.[18]
Experimental protocol
The mice were divided into eight groups: experimental Group 1: 0.9% normal saline, experimental Group 2: 20 mg/kg morphine, experimental Group 3: 4.5 mg/kg thymoquinone + 20 mg/kg morphine, experimental group 4: 9 mg/kg thymoquinone + 20 mg/kg morphine, experimental group 5: 18 mg/kg thymoquinone + 20 mg/kg morphine, experimental group 6: 4.5 mg/kg thymoquinone, experimental group 7: 9 mg/kg thymoquinone, and experimental group 8: 18 mg/kg thymoquinone. Morphine administered by intraperitoneally injecting as follows: 20 mg/kg once daily within thefirst 5 days and twice per day within the next 5 days. On days 11–20, a dose of up to 30 mg/kg twice per day.[19,20] Mice with thymoquinone as follows: on days 1–20, thymoquinone once daily, intraperitoneally injecting.[21] Mice with morphine plus thymoquinone as follows: on days 1–20, thymoquinone once daily plus morphine, intraperitoneally injecting.[19,21] The same volume of saline was administered.
Chemicals
Morphine (C16H19NO3) and thymoquinone (2-isopropyl-5-methylbenzo-1,4-quinone; C10H12O2) were obtained from Sigma Chemical Company (St. Louis, USA) and were dissolved in saline (0.9%) for administration.
Serum blood collection and liver weight measurement
The mice were anesthetized with chloroform and blood samples were taken from the heart. The blood samples were incubated at 37°C to coagulate. The coagulated blood samples were then centrifuged for 15 min at 3000 rpm until the serum was separated. The separated serum was kept at -20°C until the measurement of liver enzymes and nitric oxide (NO).[20] Animals were killed and sacrificed. Livers were removed and weighed on a microbalance sensitive to 0.001 mg (Precisa 125A, Switzerland) and recorded.
Measurement of liver enzymes
The liver was fragmented and turned into a uniform solution. To separate the enzymes, the obtained solution was centrifuged for 20 min twice at 10,000 rpm. The upper part of the solution was separated to measure the enzymes. Alkaline phosphatase (ALP) actions were determined rendering to the procedure described in laboratory applied manual.[22] Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were analyzed by the method of Reitman and Frankel.[23]
Nitric oxide measurement
NO measurement was carried out indirectly by measuring its stable metabolite, nitrate, using the microplate method. A total of 6 mg zinc sulfate powder was mixed with 400 μl serum sample. The samples were centrifuged for 10 min at 10000 rpm, and supernatant was used for nitrate measurement. Nitrate was converted into nitrite by vanadium chloride recovery (III) method and the nitrite level (μmol/L) of serums was measured by Griess assay.[6,24]
Tissue preparation
Tissue preparation was performed by an automatic tissue processor machine. Briefly, after fixation (10% formalin at room temperature for 72 h), the tissues were dehydrated with ascending alcohol, they were passed through xylene to clear and finally through paraffin to impregnate and fill the spaces created in the tissues. Fine slices (5 μm) by microtome (Leica RM2125, Germany) and stained with H and E. The preparation was observed under Olympus BX51T32E01 microscope linked to a DP12 Camera with 3.34 million pixel determination and Olysia Bio software (Olympus Optical, Japan).[25]
Measurement of hepatocytes and central hepatic vein
As a minimum fifty hepatocytes (total 100) from each region were measured in each liver. The entire cellular area was measured for each hepatocyte. Hepatocyte outline was measured after taking a picture with a ×40 objective. To estimate the mean diameter (mean axis), the shortest and longest axis were measured in the drawing of each hepatocyte. Central hepatic vein measurement was performed, using the same methodology.[1,6,20]
Statistical analysis
All the quantitative data were presented as mean ± standard deviation. One-way analysis of variance followed by least significant difference posthoc test was performed to determine the statistical significance between different groups using SPSS software (Statistical Package for the Social Sciences, version 16.0, SPSS Inc., Chicago, Illinois, USA). The P < 0.05 was considered statistical significant.
Results
Liver weight
The effective doses of morphine (20 mg/kg (and thymoquinone (4.5, 9, and 18 mg/kg) plus morphine caused a significant decrease in liver weight compared to the saline group (P < 0.05).
Thymoquinone and thymoquinone plus morphine improved liver weight in treated animals of all doses (4.5, 9, and 18 mg/kg) compared with the morphine group (P < 0.05) [Figure 1].
Figure 1.
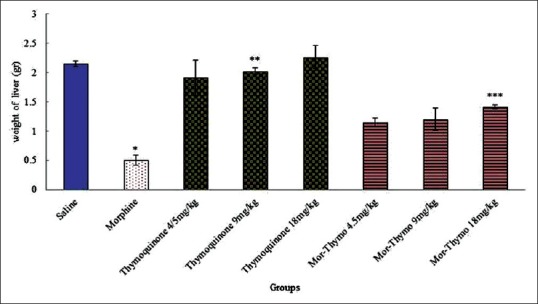
expression experimental groups were equally divided into eight groups. *Significant decrease of liver weight in morphine group compared to saline group (P < 0.05). **Significant increase in all groups of thymoquinone compared to morphine group (P < 0.05). ***Significant increase in all groups of thymoquinone plus morphine compared to morphine group (P < 0.05)
Measurement of liver enzymes
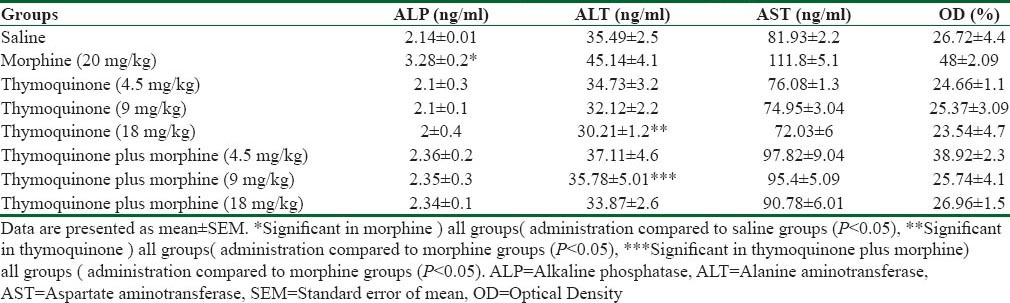
Morphine caused a significant increase in the mean of ALT, AST, and ALP enzymes compared to saline group (P < 0.05). In addition, the mean of ALT, AST, and ALP enzymes decreased significantly in thymoquinone and thymoquinone plus morphine (4.5, 9, and 18 mg/kg) administration compared to morphine group (P < 0.05) [Table 1].
Table 1.
Effect of morphine, thymoquinone, and thymoquinone plus morphine administration on liver enzymes and the mean nitric oxide levels of expression experimental groups
Nitric oxide measurement
The mean level of NO in blood serum increased significantly in the morphine (20 mg/kg) and thymoquinone plus morphine (4.5, 9, and 18 mg/kg) groups compared to the saline group (P < 0.05). Furthermore, the mean of NO in blood serum decreased significantly in thymoquinone (4.5, 9, and 18 mg/kg) and thymoquinone (4.5, 9, and 18 mg/kg) plus morphine compared to morphine group (P < 0.05) [Table 1].
Measurement of hepatocytes and central hepatic vein
The mean diameter of hepatocytes and central hepatic vein were significantly increased in morphine group in comparison with saline group (P < 0.05).
Furthermore, thymoquinone (4.5, 9, and 18 mg/kg) and thymoquinone (4.5, 9, and 18 mg/kg) plus morphine caused a significant decrease in the mean diameter of hepatocytes and central hepatic vein comparison with morphine group administration (P < 0.05) [Figure 2].
Figure 2.
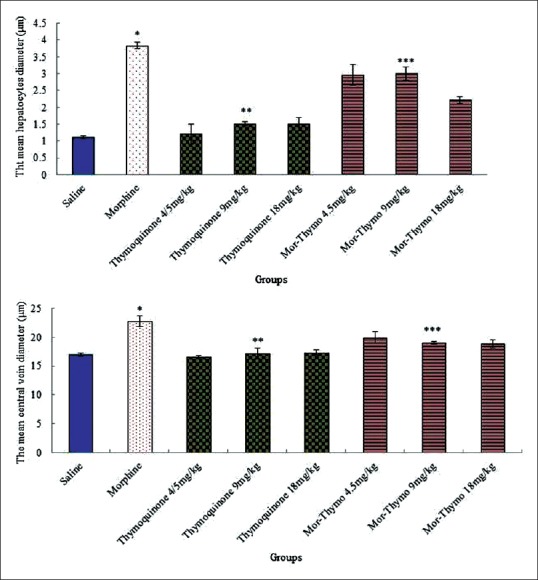
Effect of morphine, thymoquinone, and thymoquinone plus morphine administration on the mean of hepatocytes and central hepatic vein diameter of expression experimental groups. *Significant increase of the mean hepatocytes and central hepatic vein diameters in morphine groups compared to saline groups (P < 0.05). **Significant decrease in all groups of thymoquinone administration compared to morphine groups (P < 0.05). ***Significant decrease in all groups of thymoquinone plus morphine administration compared to morphine groups (P < 0.05)
Histopathological observations
At 20 days of treatment with morphine (examination of H and E sections), the liver section seemed with variable variations and noticeable injury. These modifications were showed by more distributed Kupffer cells around central vein and enlargement of central hepatic vein compared to saline stage. Treatment with morphine plus thymoquinone (18 mg/kg) showed thymoquinone reduced liver injury due to morphine toxicity and largely suppressed lymphocytic infiltration [Figure 3].
Figure 3.
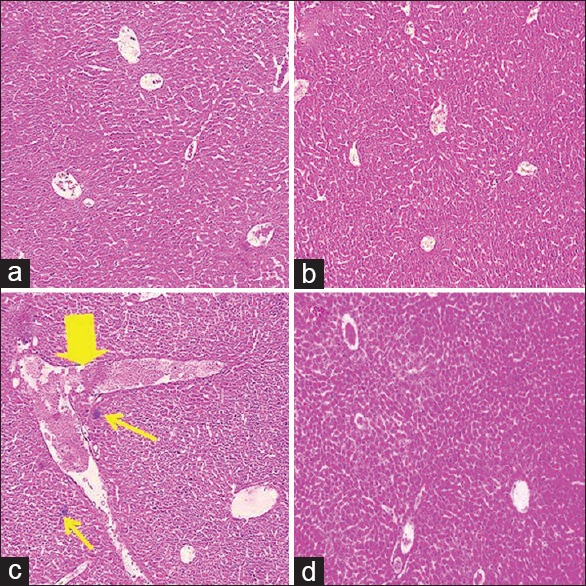
Histological changes of liver H and E staining (magnification, ×100). (a) micrograph of liver section of mice in saline group showing a normal liver structure, (b) micrograph of liver section treated with thymoquinone (18 mg/kg) showing a normal liver structure, (c) micrograph of liver section treated with morphine showing more distributed Kupffer cells around central vein (thin arrows) and enlargement of central hepatic vein (thick arrow), and (d) micrograph of liver section treated with morphine plus thymoquinone (18 mg/kg) showing a normal liver structure
Discussion
Liver is one of the vital body organs that play a major role in detoxification of the toxic elements, environmental pollutants, and chemical drugs. However, oxidative stress is a factor that greatly cause liver failure caused by drugs and toxins.[22] The increased activity of liver enzymes performance indices, AST, ALT, and ALP, in serum is indicative of liver damage, which leads to reduced performance of liver cells.[6] Therefore, in the present study, the increased serum level of these enzymes in the groups receiving morphine can be indicative of the incidence of damage to the liver cells. Morphine can be metabolized into free radicals. Furthermore, lipid peroxidation is observed in the people using heroin.[15] In the current research, treatment with thymoquinone significantly inhibited the elevation of serum liver enzymes. The compounds in black cumin such as thymoquinone have a scavenger effect against free radicals.[26] On the other hand, thymoquinone is an inhibitor of lipid peroxidation in vitro. Thymoquinone, as an active ingredient of N. sativa, protects the liver against the toxicity induced by tert-butyl hydroperoxide by inhibiting glutathione reduction and decreasing the serum level of enzymes such as ALT and AST in the hepatocytes of mice.[27] The study of Nili-Ahmadabadi et al. showed that thymoquinone reduces the serum level of liver enzymes such as ALT, AST, and ALP, which is in line with the results of the present study.[21] In the current study, changes were observed in the liver tissue in the groups receiving morphine, as hyperemia of the sinusoids, accumulation of macrophages around the central veins, and infiltration of lymphoid cells in the port space. The increased activity of opioid system can induce liver damage.[28] It seems that macrophages are activated in response to tissue damage and release toxic mediators such as tumor necrosis factor-alpha, interleukin-1, and nitric acid. The liver macrophages are the same kupffer cells that exist in liver sinusoids. Studies have shown that accumulation of these cells and secretion of toxic mediators in the nonnecrotic areas are involved in induction of liver toxicity and cause liver necrosis.[29] Due to simultaneous administration of thymoquinone and morphine, only poor degenerative changes were observed and no trace of necrosis was found, which can show the protective effects of thymoquinone extract against morphine toxicity. It seems that these beneficial effects of N. sativa are associated with the antioxidant properties and reducing oxidative stress of the compounds existing in thymoquinone.[30] The results of the study of Salahshoor et al. indicated that administration of kerosene, as an antioxidant, reduces the inflammatory effects of morphine on the liver, which is in agreement with the findings of the current study.[20] The results of the analysis of liver weight among the studied groups demonstrated a significant reduction in the liver weight between the saline group and morphine groups, indicating that the effects of morphine on the reduction of liver weight were eliminated to a large extent after treatment with thymoquinone. It seems that morphine administration reduces liver weight through impairing the liver cells and disturbing the real metabolism of the mice.[6] The study carried out by Sumathi et al. showed that subcutaneous injection of morphine reduced the body weight, which is parallel with the results of the present research.[31] Increased liver weight can be indicative of the improved nutrition of the animals under treatment with thymoquinone. This weight increase can be due to the effects of thymoquinone on the enhanced diet of the studied animals.[32] The results of this study also showed a significant increase in the mean diameter of hepatocytes and central hepatic vein between saline and morphine groups, and thymoquinone administration reduced the mean diameter of hepatocytes and central hepatic vein in all groups compared to the morphine group. It seems that morphine has hepatotoxic properties, due to increased glutathione, and causes the loss of liver cytoplasmic cells.[33] Changing in the size of liver's central vein and hepatic cells could be the consequence of the elevation in the metabolic activity of cells to excrete toxin from the body during detoxification process.[34] Morphine metabolism induced free radicals then it caused lipid peroxidation, reaction with DNA and membrane proteins, and consequently cell damage through various mechanisms.[35] Thymoquinone as antioxidant compound may exert inhibitory effect on cytochrome P450, prevent further morphine metabolism and reduce the production of free radicals, consequently.[36] The findings of the present study are similar to the study carried out by Salahshoor et al., in which they showed curcumin administration resulted in the increasing diameter of hepatocytes.[6] Moreover, the findings indicated that morphine could significantly increase NO level in blood serum; however, NO was significantly reduced in thymoquinone groups. These results may confirm the antioxidant and anti-inflammatory effects of thymoquinone. NO is a free radical that is produced in the mammalian cells, it is involved in the regulation of physiologic processes and its increased production is followed by induction of various diseases.[20,37] Morphine can increase the production of NO through intracellular regulation of calcium and activation of calcium/calmodulin-dependent NO synthesis. Morphine can also increase NO production directly through naloxone-sensitive receptors.[38] On the other hand, the hydroxyl radicals generated by NO and superoxide anion interfere with the pathogenesis process and liver toxicity.[39] Owing to the ability of antioxidants in removal of free radicals, these substances can destroy and impair NO system (protein enzymes, substrates, and cofactors) and consequently reduce NO production.[40] Thymoquinone through induction of oxidative stress, inhibit the increased production of NO.[41] Owing to the ability of antioxidants in removal of free radicals, these substances can destroy and impair nitric oxide system (protein enzymes, substrates and cofactors) and consequently reduce nitric oxide production.[42] Thymoquinone through induction of oxidative stress, inhibit the increased production of nitric oxide.[43] The results of the current study confirm the findings of Salahshoor et al. indicating that nicotine could increase serum NO and curcumin, as an antioxidant, was able to prevent the elevation of blood serum NO in the laboratory animals.[6]
Conclusions
The findings of this study showed that administration of thymoquinone, as a potent antioxidant, could have protective properties against the destructive effects of morphine in the liver of mice. In addition, the effects of thymoquinone on the liver enzymes and NO level were observed, which indicated thymoquinone could have protective effects against the free radicals produced by morphine and could consequently improve the performance and structure of liver.
Financial support and sponsorship
The authors gratefully acknowledge the Research Council of Kermanshah University of Medical Sciences (Grant Number: 951123) for the financial support of this project.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors gratefully acknowledge the Research Council of Kermanshah University of Medical Sciences (Grant Number: 951123) for the financial support of this project. This work was performed in partial fulfillment of the requirements for MD degree of Arman Vahabi at faculty of medicine in Kermanshah University of Medical Sciences, Kermanshah, Iran.
References
- 1.Jalili C, Tabatabaei H, Kakaberiei S, Roshankhah S, Salahshoor MR. Protective role of crocin against nicotine-induced damages on male mice liver. Int J Prev Med. 2015;6:92. doi: 10.4103/2008-7802.165203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salem ML, Hossain MS. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int J Immunopharmacol. 2000;22:729–40. doi: 10.1016/s0192-0561(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 3.Razavi BM, Hosseinzadeh H. A review of the effects of Nigella sativa L. and its constituent, thymoquinone, in metabolic syndrome. J Endocrinol Invest. 2014;37:1031–40. doi: 10.1007/s40618-014-0150-1. [DOI] [PubMed] [Google Scholar]
- 4.Geng D, Zhang S, Lan J. Analysis on chemical components of volatile oil and determination of thymoquinone from seed of Nigella glandulifera. Zhongguo Zhong Yao Za Zhi. 2009;34:2887–90. [PubMed] [Google Scholar]
- 5.Farag MM, Ahmed GO, Shehata RR, Kazem AH. Thymoquinone improves the kidney and liver changes induced by chronic cyclosporine a treatment and acute renal ischaemia/reperfusion in rats. J Pharm Pharmacol. 2015;67:731–9. doi: 10.1111/jphp.12363. [DOI] [PubMed] [Google Scholar]
- 6.Salahshoor M, Mohamadian S, Kakabaraei S, Roshankhah S, Jalili C. Curcumin improves liver damage in male mice exposed to nicotine. J Tradit Complement Med. 2015;6:176–83. doi: 10.1016/j.jtcme.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Zhang T, Douglas SD, Lai JP, Xiao WD, Pleasure DE, et al. Morphine enhances hepatitis C virus (HCV) replicon expression. Am J Pathol. 2003;163:1167–75. doi: 10.1016/S0002-9440(10)63476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murer SB, Aeberli I, Braegger CP, Gittermann M, Hersberger M, Leonard SW, et al. Antioxidant supplements reduced oxidative stress and stabilized liver function tests but did not reduce inflammation in a randomized controlled trial in obese children and adolescents. J Nutr. 2014;144:193–201. doi: 10.3945/jn.113.185561. [DOI] [PubMed] [Google Scholar]
- 9.El-Gendy KS, Aly NM, Mahmoud FH, Kenawy A, El-Sebae AK. The role of Vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem Toxicol. 2010;48:215–21. doi: 10.1016/j.fct.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Daba MH, Abdel-Rahman MS. Hepatoprotective activity of thymoquinone in isolated rat hepatocytes. Toxicol Lett. 1998;95:23–9. doi: 10.1016/s0378-4274(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 11.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–22. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 12.Brokjær A, Kreilgaard M, Olesen AE, Simonsson US, Christrup LL, Dahan A, et al. Population pharmacokinetics of morphine and morphine-6-glucuronide following rectal administration – A dose escalation study. Eur J Pharm Sci. 2015;68:78–86. doi: 10.1016/j.ejps.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Dehghan M, Mahmoudian A. The effect of morphine administration on structure and ultrastructure of uterus in pregnant mice. Iran J Reprod Med. 2010;8:111–8. [Google Scholar]
- 14.Bekheet SH. Morphine sulphate induced histopathological and histochemical changes in the rat liver. Tissue Cell. 2010;42:266–72. doi: 10.1016/j.tice.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Samarghandian S, Afshari R, Farkhondeh T. Effect of long-term treatment of morphine on enzymes, oxidative stress indices and antioxidant status in male rat liver. Int J Clin Exp Med. 2014;7:1449–53. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn Mag. 2010;6:135–41. doi: 10.4103/0973-1296.62900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdel-Wahhab MA, Aly SE. Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J Appl Toxicol. 2005;25:218–23. doi: 10.1002/jat.1057. [DOI] [PubMed] [Google Scholar]
- 18.Jalili C, Salahshoor MR, YousefiI D, KhazaeiI M, Darehdori AS. Mokhtari T. Morphometric and hormonal study of the effect of Urtica diocia extract on mammary glands in rats. Int J Morphol. 2015;33:983–7. [Google Scholar]
- 19.Zhang YT, Zheng QS, Pan J, Zheng RL. Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic Clin Pharmacol Toxicol. 2004;95:53–8. doi: 10.1111/j.1742-7843.2004.950202.x. [DOI] [PubMed] [Google Scholar]
- 20.Salahshoor MR, Khashiadeh M, Roshankhah S, Kakabaraei S, Jalili C. Protective effect of crocin on liver toxicity induced by morphine. Res Pharm Sci. 2016;11:120–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Nili-Ahmadabadi A, Tavakoli F, Hasanzadeh G, Rahimi H, Sabzevari O. Protective effect of pretreatment with thymoquinone against Aflatoxin B(1) induced liver toxicity in mice. Daru. 2011;19:282–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Sadashivam S, Manickam A. Biochemical Methods. 2nd. New Delhi, India: New Age International; 1996. pp. 121–4. [Google Scholar]
- 23.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Jalili C, Salahshoor MR, Naderi T. The effect of hydroalcoholic extract of P. crispum on sperm parameters, testis tissue and serum nitric oxide levels in mice. Adv Biomed Res. 2015;4:40. doi: 10.4103/2277-9175.151249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jalili C, Salahshoor MR, Naseri A. Protective effect of Urtica dioica L against nicotine-induced damage on sperm parameters, testosterone and testis tissue in mice. Iran J Reprod Med. 2014;12:401–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Jalili C, Salahshoor MR, Hoseini M, Roshankhah S, M Sohrabi. A Shabanizadeh.Protective effect of thymoquinone against morphine injuries to kidneys of mice. Iran J Kidney Dis. 2017;11:142–50. [PubMed] [Google Scholar]
- 27.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–8. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 28.Aboutabl EA, El-Azzouny AA, Hammerschmidt FJ. DeGruyter: Berlin; 1986. seeds; pp. 49–55. [Google Scholar]
- 29.Jalili C, Salahshoor MR, Moradi MT, Ahookhash M, Taghadosi M, Sohrabi M. Expression Changes of Apoptotic Genes in Tissues from Mice Exposed to Nicotine. Asian Pac J Cancer Prev. 2017;18:239–44. doi: 10.22034/APJCP.2017.18.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumathi T, Niranjali Devaraj S. Effect of Bacopa monniera on liver and kidney toxicity in chronic use of opioids. Phytomedicine. 2009;16:897–903. doi: 10.1016/j.phymed.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Amouoghli Tabrizi B. Protective effect of edible turmeric powder on early hepatic injury in diabetic rats. J Kashan Univ Med Sci. 2010;14:190–9. [Google Scholar]
- 32.Al-Khafaji N. Protective effect of crude oil of Nigella Sativa on liver in male albino mice treated with low toxic dose of paracetamol. Med J Babylon. 2013;10:930–6. [Google Scholar]
- 33.Sumathi T, Nathiya VC, Sakthikumar M. Protective effect of Bacoside-A against morphine-induced oxidative stress in rats. Indian J Pharm Sci. 2011;73:409–15. doi: 10.4103/0250-474X.95624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tubesha Z, Imam MU, Mahmud R, Ismail M. Study on the potential toxicity of a thymoquinone-rich fraction nanoemulsion in Sprague Dawley rats. Molecules. 2013;18:7460–72. doi: 10.3390/molecules18077460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becq ME, Brechot GE. Liver function tests. J Hepatol. 1996;23:1030. doi: 10.1002/hep.510230514. [DOI] [PubMed] [Google Scholar]
- 36.Sakr SA. Ameliorative effect of ginger (Zingiber officinale) on Mancozeb Fungicide induced liver injury in albino rats. Aust J Basic Appl Sci. 2007;1:650–6. [Google Scholar]
- 37.Cordova CA, Siqueira IR, Netto CA, Yunes RA, Volpato AM, Cechinel Filho V, et al. Protective properties of butanolic extract of the Calendula officinalis L. (marigold) against lipid peroxidation of rat liver microsomes and action as free radical scavenger. Redox Rep. 2002;7:95–102. doi: 10.1179/135100002125000325. [DOI] [PubMed] [Google Scholar]
- 38.Heaton MB, Paiva M, Siler-Marsiglio K. Ethanol influences on Bax translocation, mitochondrial membrane potential, and reactive oxygen species generation are modulated by Vitamin E and brain-derived neurotrophic factor. Alcohol Clin Exp Res. 2011;35:1122–33. doi: 10.1111/j.1530-0277.2011.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qureshi M. In-vitro antioxidant and in-vivo hepatoprotective activity of leucas ciliata leaves. Rec Nat Prod. 2010;2:124–30. [Google Scholar]
- 40.Zarrindast MR, Javadi-Paydar M, Delphi L, Vousooghi N. Morphine-induced nitric oxide production in PC12 cells. Arch Iran Med. 2012;15:404–8. [PubMed] [Google Scholar]
- 41.Hon WM, Lee KH, Khoo HE. Nitric oxide in liver diseases: Friend, foe, or just passerby? Ann N Y Acad Sci. 2002;962:275–95. doi: 10.1111/j.1749-6632.2002.tb04074.x. [DOI] [PubMed] [Google Scholar]
- 42.Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–9. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 43.Nagi MN, Almakki HA, Sayed-Ahmed MM, Al-Bekairi AM. Thymoquinone supplementation reverses acetaminophen-induced oxidative stress, nitric oxide production and energy decline in mice liver. Food Chem Toxicol. 2010;48:2361–5. doi: 10.1016/j.fct.2010.05.072. [DOI] [PubMed] [Google Scholar]


