Abstract
Background:
Approximately 20%–25% of the world adult population and nearly 30% of Indians have metabolic syndrome disorder. Our objective was designed to find out the association between important nutrients and potential lifestyle risk factors such as diet, physical inactivity, and smoking and alcohol consumption with the number of metabolic syndrome components.
Methods:
This was a cross-sectional study. A total of 205 patients of metabolic syndrome were enrolled for this study. Diagnosis of metabolic syndrome was done on the basis of National Cholesterol Education Program Adult Treatment Panel III criteria (NCEP ATP III 2004). Dietary data were collected with the validated food frequency questionnaire and 24 h dietary recall method, and the nutrient intake was calculated with the specially designed software.
Results:
Unhealthy dietary habits were seen more among the participants who had more than 3 risk factors. Results showed the odds of taking >5 times junk foods was 3 times higher (odds ratio [OR]: 2.97; 95% confidence interval [CI]: 1.61–5.47), and sweet dishes was 2.3 times higher (OR: 2.33; 95% CI: 1.28–4.24) among the participants who had 4–5 risk factors. However, milk and dairy products > 4 servings/day (OR: 0.54; 95% CI: 0.175–1.67) and pulses and legumes more than 2 servings/day (OR: 0.57; 95% CI: 0.25–1.29) was protective against hypertension. Mean carbohydrate, saturated fat, and sodium intake was significantly higher in the participants who had 4–5 metabolic risk factors compared to 3 risk factors (P < 0.0001).
Conclusions:
It was concluded that low intake of fruits, vegetables, and higher intake of flesh food and inadequate physical activity significantly associated with the metabolic syndrome risk factors.
Keywords: Dietary pattern, hypertension, lifestyle risk factors, metabolic syndrome, physical activity
Introduction
Metabolic syndrome is a constellation of interrelated risk factors characterized by the co-occurrence of hyperglycemia, hypertension, high triglyceride levels, and low high-density lipoprotein (HDL) cholesterol levels and has become a major public health concern throughout the world.[1,2,3] It is well appreciated that the metabolic syndrome increased the risk of type 2 diabetes mellitus,[4] cardiovascular disease by 2-fold,[4,5] and 5-fold increase in the mortality over a 5–10 years period.[6] The third report of the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP-III) also suggested that metabolic syndrome increases the risk of Cardiovascular disease by 2-fold, and it will soon have a greater impact on premature coronary artery disease.[7]
It is estimated that 20%–25% of the world adult population suffer from metabolic syndrome disorders.[8] In India, the prevalence of metabolic syndrome is increasing exponentially as determined by 33.5% overall, 24.9% in males and 42.3% in females on the basis of diagnostic criteria given by (NCEP ATP III).[9] The specific etiology of metabolic syndrome is not clear until now, but it is a mixed outcome of genetic, metabolic, and some environmental factors.[10] Among adaptable environmental risk factors, a dietary habit seems to play a prime importance in the treatment and prevention of metabolic syndrome.[11] Some probable dietary risk factors, such as high intakes of saturated fatty acids and low intakes of omega-3 fatty acids also increase the risk of cardiovascular disease.[12] In addition, inadequate physical activity, smoking, and extreme alcohol consumption have been linked with increased risk of central obesity and other metabolic abnormalities.[13,14] On the basis of conventional approach in nutritional epidemiology, researchers identifying only one nutrients or food products, as a substitute of assessing the whole dietary patterns.[15] Although people consumes diets which included many types of foods with many nutrients. When we want to assess the pooled effects of different nutrients and various foods, we need to assess whole dietary pattern. Therefore, assessing the entire dietary pattern instead of assessing the individual nutrient can give the clear picture of relationship with the health outcomes. A randomized controlled trial which was conducted on metabolic syndrome patients observed that a dietary pattern, which is dietary approaches to stop hypertension diet plan can improve all the five metabolic syndrome component in men and women.[16] To the best of our knowledge, very few data are available concluding the association between potential lifestyle risk factors with the components of metabolic syndrome. Thus, the present study was designed to find out the association between important nutrients and potential lifestyle risk factors such as diet, physical inactivity, and smoking and alcohol consumption with the number of metabolic syndrome components.
Study population and design
Study population
This study was performed on Indian population at the Department of Cardiology, Sir Sunderlal Hospital, Banaras Hindu University, Varanasi, on an outdoor patient basis from February 2013 to June 2015. Participants were men and women aged 30–68 years who were visiting the outpatient department for the first time were considered for this study. The criteria for identifying the patients of metabolic syndrome are depending on the definition given by the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (NCEP ATP III) (modified 2004). The inclusion criteria were as follows: Waist circumference (males: ≥90 cm and for females: ≥80 cm), triglycerides ≥150 mg/dl, low HDL (males <40 mg/dl and for females <50 mg/dl), systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg, and fasting blood sugar ≥100 mg/dl. To be enrolled in the study, patients had to have ≥3 of the above-mentioned criteria to be classified as having metabolic syndrome. The exclusion criteria were as follows: participants were excluded if they were diagnosed with the following diseases such as thyroid disease or diabetes mellitus, cardiovascular diseases, chronic liver or kidney disease, advanced cancer, or any other chronic diseases or they were not willing to participate. Sample size was calculated on the basis of variability among the metabolic risk factors components which was assessed by the pilot study conducted on 30 patients of metabolic syndrome.
Study design
This was a hospital-based cross-sectional study. A metabolic screen was carried out for all the individuals (1020) during the study period for eligibility in the study. A total of 205 men and women who had ≥3 components of the metabolic syndrome and met the inclusion criteria were included in the present study. After screening details of dietary history and lifestyle risk factors were assessed for each study participants. The present study was approved by the Institutional Ethics Committee on Biomedical Research in Humans of Institute of Medical Sciences, Banaras Hindu University (BHU), Varanasi, Uttar Pradesh, India (EC Registration No. ECR/526/Inst/UP/2014 Dt. 31.1.14), and written informed consent was obtained from all potential participants at the screening visit.
Dietary assessment
Dietary assessment was performed with the food frequency questionnaire (FFQ) and 24 h dietary recall methods. FFQ is the standard method to know the dietary pattern in studies of chronic disease all over the world. All the respondents were asked to report every food items which they were taking to find out the frequency of consumption in the form of (never, seldom, once a month, once a week, two-three times a week, and daily). A 24-h dietary recall method was used to collect the information pertaining to dietary intake. The participants were asked about to report everything that they had eaten or drunk during previous day over the past 24-h period. To estimate portion size of food consumed, standardized set of cups and spoons suggested by National Institute of Nutrition, India, with varying capacities (volumes) was displayed to estimate the exact amount of cooked food eaten in each meal. From the size and volume of food consumption obtained by this method, the quantities consumed by the participants were converted in to exchanges and the equivalent weight of raw food in terms of grams or milliliters was calculated using a conversion table for Indian foods formulated at the National Institute of Nutrition ICMR 2010.[17]
Nutritional analysis
All food records were analyzed by a specially designed computerized program using the food database of Nutritive value of Indian foods (ICMR 2010).[17] Data obtained from the 24-h food records were processed and converted to the gram equivalents using the Indian system of food equivalents. Each food and beverage was then coded according to the software and entered into a computerized nutrition database which contains the nutritional values of all Indian foods for analysis.
Measurements
Waist circumference was measured with the help of unlengthened tape meter at the narrowest level over light clothing without any strain on the body surface. All the clinical and biochemical assessment was performed by the hospital staff using the standard procedure. Blood pressure was measured after 5 min of rest in the sitting position on the right arm using a standard mercury sphygmomanometer. Fasting blood samples were analyzed for plasma concentrations of glucose, triacylglycerol, total cholesterol, HDL cholesterol, and low-density lipoprotein cholesterol.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences software SPSS 16 (trail version). Continuous variables were expressed as mean ± standard error (SE), and categorical variables are expressed as frequencies and proportions (percent). Continuous variables were assessed using the Kolmogorov–Smirnov Z test to examine the distribution type; if the data did not follow a normal distribution; they were logarithmically transformed before analysis. Participants were divided into 3 groups according to the number of risk factors they had; 3 risk factors, 4 risk factors, and 5 risk factors. Chi-square and Independent t-test was used to assess the differences across the risk factors for categorical variables and independent t-test were used for continuous variables. Logistic regression analysis was used to calculate the odds ratios (ORs) and their 95% confidence intervals (CIs) to determine the associations of all categorical variables such as dietary pattern, smoking, physical activity across the number of risk factors and hypertension, and to determine the effect of demographic variables on the metabolic syndrome risk factors. One-way analysis of variance (ANOVA) was used to know differences in macro and micro intakes across the 3 risk factor groups. However, analysis of covariance (ANCOVA) was used to adjust the potential confounders such as age, gender, and physical activity. The various models which I have used to adjust the potential confounders are as follows: model 1, one-way ANOVA with no covariates, Model 2, ANCOVA with the adjustment for age and gender as covariates, and Model 3 ANCOVA adjusted for age, sex, and physical activity. Since the physical activity is considered a major risk factors to develop the metabolic syndrome that's why the model also included the physical activity (other than daily work) as a covariate. If the significant differences were found in ANOVA and ANCOVA across the risk factors, Tukey's post hoc test were used for further multiple comparison. All the statistical level of significance was set at 0.05 for all the continuous and categorical variables.
Results
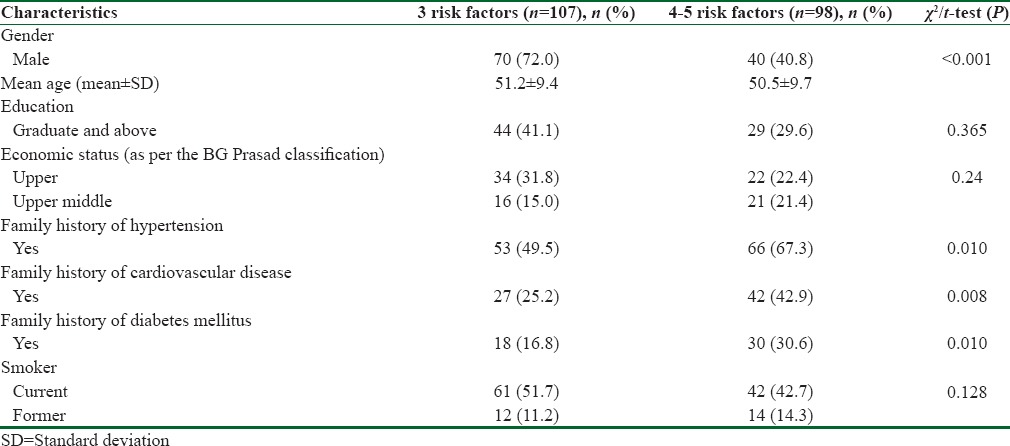
General characteristics of participants across the various risk factors are presented in Table 1. Mean age of the participants across the two risk factors were 51 ± 9.4 and 50.5 ± 9.7, respectively. There were significant differences were observed regarding gender, occupation and family history of hypertension, diabetes, and cardiovascular diseases among the two groups. P < 0.001.
Table 1.
General characteristics of the participants across the various risk factors
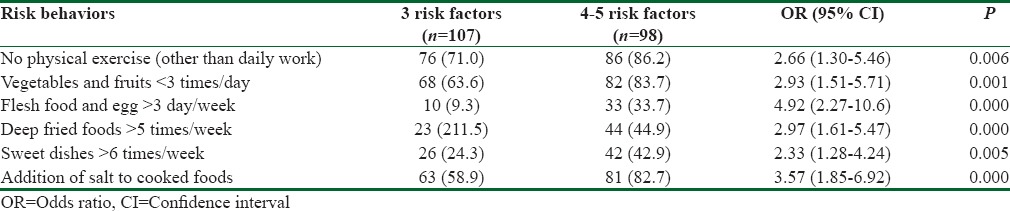
Results showed the differential intake frequencies for each food groups which is commonly known as the important risk factors to develop metabolic syndrome [Table 2]. Unhealthy eating practices were seen more among the participants who had more than 3 risk factors than in the participants who had 3 risk factors. Food pattern included fruits and vegetables intake <3 serving/day was significantly associated with the more number of risk factors (OR: 2.93; 95% CI: 1.51–5.71). Significantly higher proportion of participants (33.7%) were in the habit of taking flesh foods and egg > four times/week among the participants who had 4–5 risk factors in comparison to 9.3% among participants who had 3 risk factors. However, the odds of taking flesh foods and egg about five times higher in participants who had 4–5 number of risk factors (OR: 4.92; 95% CI: 2.2–10.6). Binary logistic regression showed the odds of taking >5 times junk foods/week was 3 times higher (OR: 2.97; 95% CI: 1.61–5.47) and sweet dishes was 2.3 times higher (OR: 2.33; 95% CI: 1.28–4.24) among the participants who had 4–5 risk factors when compared to the participants who had 3 risk factors.
Table 2.
Risk difference across the various numbers of risk factors among the participants
Logistic regression showed male gender carried a higher risk of developing hypertension (OR: 1.30; 95% CI: 0.71to2.41); however, increasing age was not associated with hypertension (OR: 1.00; 95% CI: 0.731to1.36).
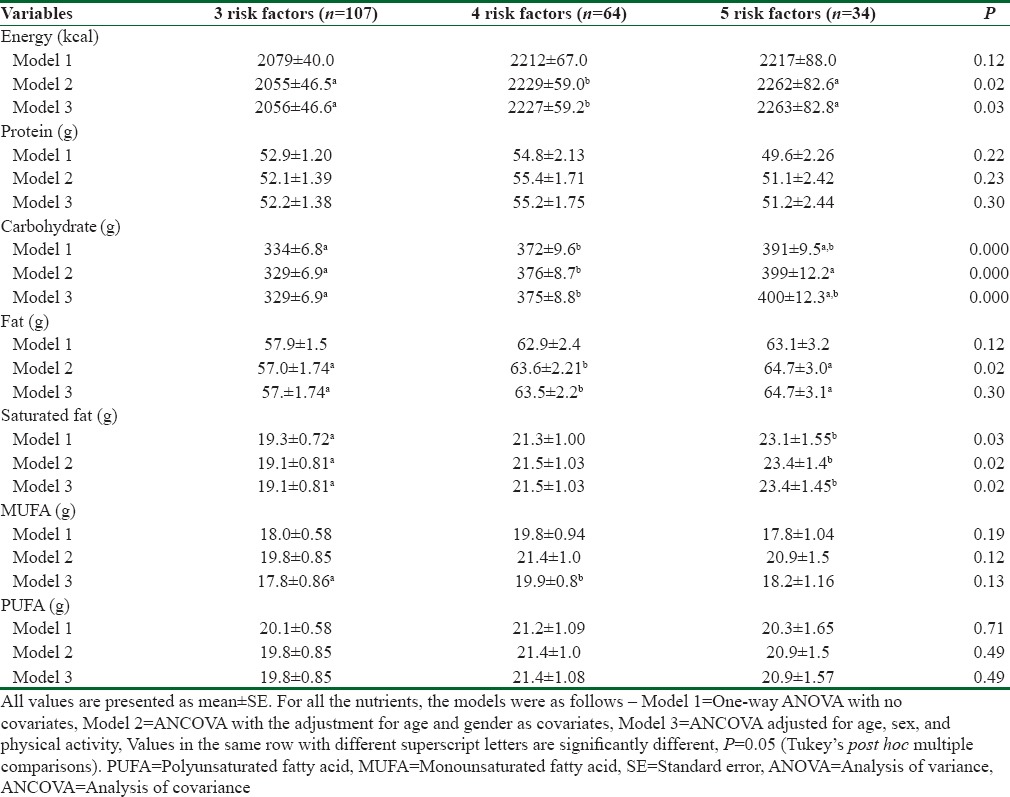
The means and SEs for various macronutrients intakes across the 3 metabolic syndrome risk factors are presented in Table 3. The first model (Model 1) compared only dietary intakes among the three risk groups with no covariates and the second model (Model 2) was adjusted for age and sex and (Model 3) further adjusted for age, sex, and physical activity. There was no significant differences were found in the mean total energy and protein intake across the various risk factors group. However, Model 2 and 3 showed significant differences in terms of energy intake, but no significant differences were found in protein intake even after the adjustment for age and sex and physical activity. Model 1 showed no significant differences in the total fat intakes across the various risk factors, but it turn out to be significant in Model 2. Whereas, mean carbohydrate and saturated fat was significantly higher in the participants who had 4–5 risk factors compared to 3 risk factors even after the adjustment with all covariates P < 0.05.
Table 3.
Daily intakes of major nutrients according to number of metabolic syndrome risk factors among participants
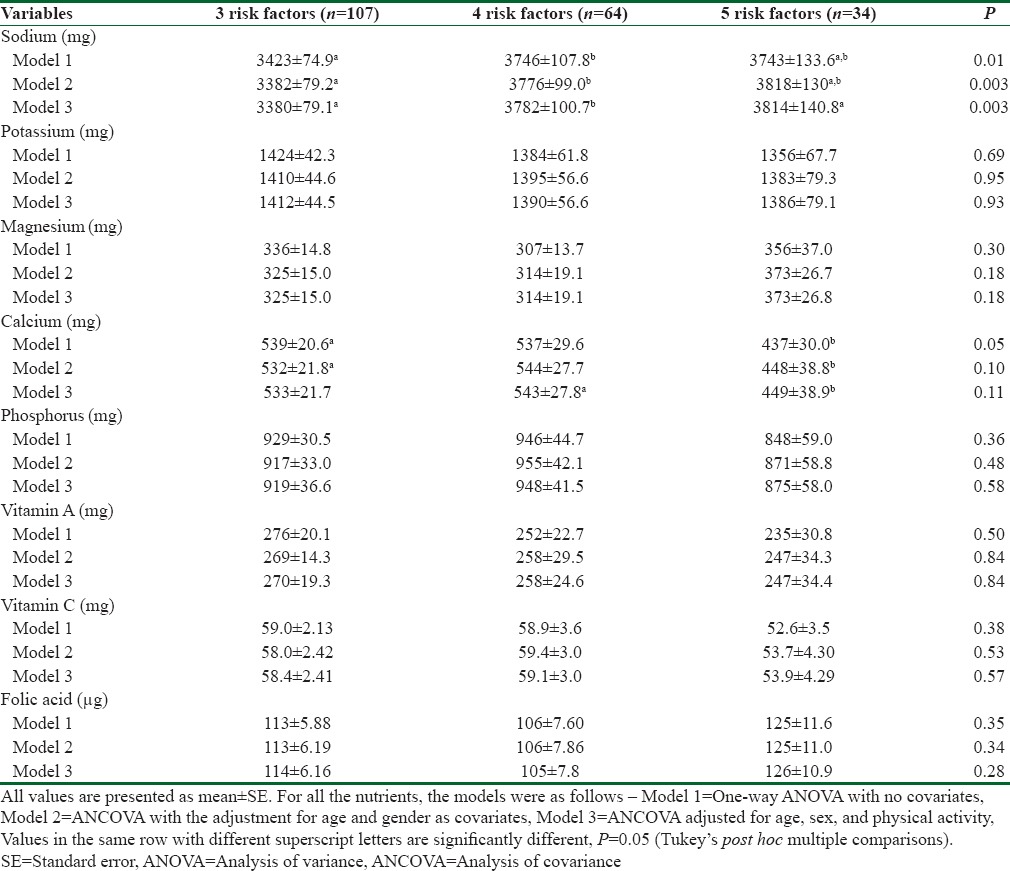
Regarding micronutrient, evidence suggest that high intake of sodium is a major risk factors for cardiovascular diseases. Results showed that mean intake of sodium is significantly higher on the participants who had 4–5 risk factors (P < 0.001) [Table 4]. These differences remained significant after the adjustment for age and sex and physical activity. Potassium, Vitamin A, and phosphorus intake was lower among participants who had 4–5 risk factors, whereas magnesium intake was higher among those who had 4–5 risk factors, but these differences were not reached to the significant level in any of the model. ANOVA showed mean daily intake of zinc, iron, manganese, and folic acid remained insignificant across the 3 risk factors group in the entire model. However, phosphorus and Vitamin C intake was higher in participants who had 3 risk factors, but these differences were not reached to the significant level P > 0.05.
Table 4.
Daily intakes of micronutrient according to number of metabolic syndrome risk factors among participants
Discussion
Metabolic syndrome is a complex interaction of different risk factors including genetic, environmental, and metabolic factors.[18] The difference in the prevalence of this syndrome across gender observed in this study could be attributed to the higher prevalence of waist circumferences in women [Table 1] which also has been reported in other recent epidemiological studies.[19] Another study reported that higher prevalence of metabolic syndrome among females is because of gender-specific factors.[20] This study was carried out on a sample of 205 men and women to find out the association of major food groups with the number of risk factors they had. Among these some, but not all, of these food groups were positively associated with multiple risk factors of metabolic syndrome. Unhealthy eating practices were seen more among the participants who had more than 3 risk factors. Participants consumed more fruits and vegetables who had 3 risk factors than the participants who had more than 3 risk factors (OR: 2.93; 95% CI: 1.51–5.71) and had a healthy lifestyle in general. This finding also supported by the series of other studies, It has been suggested that inclusion of more fruits and vegetables in a diet has been associated with lower blood pressure and may be associated with an improved fasting lipid profile.[21] The findings from Bogalusa Heart Study revealed that young adults who had no risk factors consumed high fruits and vegetables and less sweetened beverages than those who had 1 risk factor.[22] A cross-sectional study which was conducted on 486 Tehranian adult females in the highest quintile of fruit and vegetable consumption showed a 10% to 40% and 14% to 38% lower likelihood of having metabolic syndrome compared to those in the lowest quintile of the fruit and vegetables intake, respectively.[23] Although in the present study, there was no significant association was found for fruits intake. Association of other food groups with the risk of metabolic syndrome has been also reported by several other studies. It has been suggested that the daily consumption of Western diet including meat, fried foods, and more salt was adversely associated with the prevalence of metabolic syndrome and a protective effect was found for the intake of dairy foods.[24] Similar association was also found in the present study, it showed that the odds of taking flesh foods and egg about five times higher and junk foods about three times higher among the participants who had 4–5 risk factors compared to those who had 3 risk factors. In contrast with this study, one study reported no association between intakes of meat with metabolic syndrome.[25] Few studies has been investigated the association between sweetened beverages and metabolic syndrome.[26] Similar to the findings of this study, researchers suggest that high consumption of sweets and beverages positively associated with overweight, particularly in children and adolescents.[27] Another recent epidemiological study also reported a positive association between sweetened beverage and metabolic syndrome risk.[28] Inadequate physical activity and smoking habits were seen more among the participants who had 4–5 risk factors. Similarly, in a study, the odds of having the metabolic syndrome who reported light to moderate physical activity was 0.75, whereas for those who have done regular exercise, the OR was 0.57.[29] The data on macronutrient and micronutrient intake and its association with the prevalence of metabolic syndrome are controversial. From the data observed from 24-h recall, It was found that mean intakes of carbohydrate, saturated fat, and total fat and sodium intake were significantly higher on the participants who had 4–5 risk factors. However, soluble fiber, calcium, magnesium, and Vitamin C were lower among the participants who had 4–5 risk factors. Similarly, many reports suggest positive association of saturated fats with glucose intolerance and insulin resistance.[30] On the other hand, omega-3 fatty acids increases insulin sensitivity, and decreases the risk of type 2 diabetes mellitus, and also hinder the production of inflammatory cytokines, thus improving the risk factors of metabolic syndrome.[31] Low intake of micronutrients such as magnesium and calcium has been linked to hyperglycemia and insulin dysfunction and the importance of folic acid in the prevalence of cardiovascular risk factors has been documented.[32,33] This finding is also supported the present study. Another study suggested that low intake of fruits, vegetables, n-3 fatty acids MUFA, polyunsaturated fatty acids, whole grain foods, and low protein is a major contributing factor to the development of diabetes, hypertension, and heart disease.[34]
Limitations
A few limitations have been noted in this study.First, we have used FFQ and 24-h recall for the dietary information. Due to the nature of the self-reporting technique, recall bias and inaccuracy in the measurement of portion sizes, it was difficult to find out the exact amount of foods they had consumed. Measurement error as a result of self-reporting for dietary intake and other lifestyle risk behaviors may introduce nondifferential misclassification leading to the association toward to the null. However, there are many evidence suggesting that information on the basis of self-reported techniques is applicable in epidemiologic studies for chronic diseases.[35] Second, the participants who were aware of their metabolic disturbances might have changed their food habits before.
Conclusions
Despite these limitations, the present study concludes that high consumption of flash foods, physical inactivity, and low consumption of fruits and vegetables is independently linked with the multiple metabolic risk factors. The finding from this study concluded that lifestyle interventions such as diet and physical activity would be the first line approach for improving the metabolic risk factors related to cardiovascular disease. Participants should be recommended to consume less junk foods, high amount of fruits, and vegetables and also encouraged to do moderate level of physical activity (e.g., jogging, brisk walking, swimming, yoga, and bicycling) for 40–45 min at least 5 times in a week. More prospective studies are needed to confirm these findings in a novel ways of intervening in the diet of Indians, and to explore the association between different food groups and the metabolic syndrome.
Ethical approval
This study was approved by the Human Ethics Committee of Institute of Medical Sciences, Banaras Hindu University, Varanasi. Written informed consents were also obtained from each participant after an oral explanation of the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.George A, Zimmet P, Shaw J. The IDF consensus worldwide definition of the metabolic syndrome. International Diabetes Federation. 2006. [Last accessed on 2012 Nov 29]. Available from: http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf .
- 2.National Institute of Nutrition. Dietary Guidelines for Indians – A Manual. 2nd. Hyderabad: National Institute of Nutrition; 2012. [Google Scholar]
- 3.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 4.Sattar N, Gaw A, Scherbakova O, Ford I, O’Reilly DS, Haffner SM, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the west of Scotland coronary prevention study. Circulation. 2003;108:414–9. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 5.McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–90. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 6.Greenstone CL. The metabolic syndrome: A lifestyle medicine foe worthy of a seek and destroy mission. Am J Lifestyle Med. 2008;2:109–12. [Google Scholar]
- 7.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 8.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):s9–30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 9.Prasad DS, Kabir Z, Dash AK, Das BC. Prevalence and risk factors for metabolic syndrome in Asian Indians: A community study from urban Eastern India Kalinga Institute of Medical Sciences, Bhubaneswar, Orissa. India J Cardiovasc Dis Res. 2008;3:204–11. doi: 10.4103/0975-3583.98895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldeisen SE, Tucker KL. Nutritional strategies in the prevention and treatment of metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:46–60. doi: 10.1139/h06-101. [DOI] [PubMed] [Google Scholar]
- 11.Hegsted DM, Kritchevsky D. Diet and serum lipid concentrations: Where are we? Am J Clin Nutr. 1997;65:1893–6. doi: 10.1093/ajcn/65.6.1893. [DOI] [PubMed] [Google Scholar]
- 12.Poehlman ET, Toth MJ, Bunyard LB, Gardner AW, Donaldson KE, Colman E, et al. Physiological predictors of increasing total and central adiposity in aging men and women. Arch Intern Med. 1995;155:2443–8. [PubMed] [Google Scholar]
- 13.Wirfält E, Hedblad B, Gullberg B, Mattisson I, Andrén C, Rosander U, et al. Food patterns and components of the metabolic syndrome in men and women: A cross-sectional study within the malmö diet and cancer cohort. Am J Epidemiol. 2001;154:1150–9. doi: 10.1093/aje/154.12.1150. [DOI] [PubMed] [Google Scholar]
- 14.Salmerón J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545–50. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- 15.Hu FB. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi T, Azizi F. Beneficial effects of a dietary approaches to stop hypertension eating plan on features of the metabolic syndrome. Diabetes Care. 2005;28:2823–31. doi: 10.2337/diacare.28.12.2823. [DOI] [PubMed] [Google Scholar]
- 17.Gopalan C, Sastri R, Balasubramaniyam SC. Nutritive Value of Indian Foods. Hyderabad: National Institute of Nutrition, ICMR. 2004:2–161. [Google Scholar]
- 18.Cho YA, Kim J, Cho ER, Shin A. Dietary patterns and the prevalence of metabolic syndrome in Korean women. Nutr Metab Cardiovasc Dis. 2011;21:893–900. doi: 10.1016/j.numecd.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Sonnenberg L, Pencina M, Kimokoti R, Quatromoni P, Nam BH, D’Agostino R, et al. Dietary patterns and the metabolic syndrome in obese and non-obese Framingham women. Obes Res. 2005;13:153–62. doi: 10.1038/oby.2005.20. [DOI] [PubMed] [Google Scholar]
- 20.Ren J, Kelley RO. Cardiac health in women with metabolic syndrome: Clinical aspects and pathophysiology. Obesity (Silver Spring) 2009;17:1114–23. doi: 10.1038/oby.2009.8. [DOI] [PubMed] [Google Scholar]
- 21.American Heart Association Nutrition Committee. Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 22.Yoo S, Nicklas T, Baranowski T, Zakeri IF, Yang SJ, Srinivasan SR. Comparison of dietary intakes associated with metabolic syndrome risk factors in young adults: The Bogalusa Heart Study. Am J ClinNutr. 2004;80:841–8. doi: 10.1093/ajcn/80.4.841. [DOI] [PubMed] [Google Scholar]
- 23.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC, et al. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr. 2006;84:1489–97. doi: 10.1093/ajcn/84.6.1489. [DOI] [PubMed] [Google Scholar]
- 24.Panagiotakos DB, Pitsavos C, Skoumas Y, Stefanadis C. The association between food patterns and the metabolic syndrome using principal components analysis: The ATTICA study. J Am Diet Assoc. 2007;107:979–87. doi: 10.1016/j.jada.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Panagiotakos DB, Pitsavos C, Skoumas Y, Stefanadis C. The association between food patterns and the metabolic syndrome using principal components analysis: The ATTICA study. J Am Diet Assoc. 2007;107:979–87. doi: 10.1016/j.jada.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: A prospective study. Arch Intern Med. 2005;165:997–1003. doi: 10.1001/archinte.165.9.997. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: A prospective, observational analysis. Lancet. 2001;357:505–8. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 28.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116:480–8. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 29.Panagiotakos DB, Pitsavos C, Chrysohoou C, Skoumas J, Tousoulis D, Toutouza M, et al. Impact of lifestyle habits on the prevalence of the metabolic syndrome among Greek adults from the ATTICA study. Am Heart J. 2004;147:106–12. doi: 10.1016/s0002-8703(03)00442-3. [DOI] [PubMed] [Google Scholar]
- 30.Narasimhan S, Nagarajan L, Vaidya R, Gunasekaran G, Rajagopal G, Parthasarathy V, et al. Dietary fat intake and its association with risk of selected components of the metabolic syndrome among rural South Indians. Indian J Endocrinol Metab. 2016;20:47–54. doi: 10.4103/2230-8210.172248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpentier YA, Portois L, Malaisse WJ. N-3 fatty acids and the metabolic syndrome. Am J Clin Nutr. 2006;83:1499S–504S. doi: 10.1093/ajcn/83.6.1499S. [DOI] [PubMed] [Google Scholar]
- 32.Humphries S, Kushner H, Falkner B. Low dietary magnesium is associated with insulin resistance in a sample of young, nondiabetic black Americans. Am J Hypertens. 1999;12:747–56. doi: 10.1016/s0895-7061(99)00041-2. [DOI] [PubMed] [Google Scholar]
- 33.Abby S, Harris I, Harris K. Homocysteine and cardiovascular disease. J Am Board Fam Pract. 2000;11:391–8. doi: 10.3122/15572625-11-5-391. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Diet, Nutrition and the Prevention of Non-Communicable Diseases: Report of a Joint WHO/FAO Expert Consultation. 2003. [Last accessed on 2014 Aug 20]. p. 916. Available from: http://www.who.int/dietphysicalactivity/publications/trs916/download/en/
- 35.McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW, Jacques PF, et al. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham offspring cohort. Diabetes Care. 2004;27:538–46. doi: 10.2337/diacare.27.2.538. [DOI] [PubMed] [Google Scholar]


