Abstract
Context:
Dyspnea is a subjective, multidimensional experience of breathing discomfort, commonly seen in patients with advanced cancer. To find the impact of dyspnea on the quality of life in this population, it is important to understand the prevalence and factors influencing dyspnea.
Aims:
This study aimed to determine the prevalence, intensity, and factors influencing dyspnea in advanced cancer and determine its impact on overall quality of life.
Settings and Design:
This was a prospective cross-sectional study. The prevalence of dyspnea and its impact on quality of life was determined in 500 patients registered with palliative medicine outpatient department.
Subjects and Methods:
The patients were asked to fill a set of questionnaires, which included the Cancer Dyspnea Scale (translated and validated Hindi and Marathi versions), visual analog scale for dyspnea and EORTC QLQ C 15 PAL. Details of demographics, symptomatology, and medical data were collected from the case record sheets of the patients.
Statistical Analysis Used:
Descriptive statistics, univariate, and multiple regression analysis were used to calculate the results.
Results:
About 44.37% of the patients experienced dyspnea. Dyspnea increased with worsening anxiety, depression, fatigue, appetite, well-being, pain, lung involvement by primary or metastatic cancer, performance status, and deteriorating overall quality of life and emotional wellbeing.
Conclusions:
The prevalence of dyspnea in advanced cancer patients is as high as 44.37% and has a negative impact on their overall quality of life.
Keywords: Advanced cancer, dyspnea, prevalence, quality of life
INTRODUCTION
Dyspnea is a term used to characterize a subjective experience of breathing discomfort that is comprised of qualitatively distinct sensations that vary in intensity. The experience derives from interactions among multiple physiological, psychological, social, and environmental factors that may induce secondary physiological and behavioral responses.[1] Dyspnea, or breathlessness, encompasses multiple somatic perceptions that are variously described as "air hunger," "increased effort of breathing," "chest tightness," "rapid breathing," "incomplete exhalation," or "a feeling of suffocation." Dyspnea is a multidimensional symptom, consisting of affective as well as physical aspects. Studies on Caucasian population report the prevalence of dyspnea in cancer in a range from 46%–59%,[2,3] and patients with primary lung cancer, it ranges from 75%–87%.[4,5] The intensity of dyspnea in cancer is moderate to severe,[2,3] and higher in primary and metastatic lung cancer.[4,5] These figures are highest at the end of life.[6,7]
Dyspnea in advanced cancer patients can be chronic or intermittent with episodic exacerbations.[8] The perception of dyspnea is an individualized experience and affected by demographic and sociocultural variables and other individual differences. Many studies describe that dyspnea correlates significantly with survival time in cancer.[9,10] Thus, dyspnea is also an important component of prognostication in cancer patients.
The World Health Organization has defined Quality of Life (QoL) as "an individual's perception of their position in life in the context of the culture and value systems in which they live, and in relation to their goals, expectations, standards, and concerns." Western literature has reported lower QoL in patients with advanced cancer suffering from dyspnea.[4,11]
It is well established that dyspnea significantly impacts all domains of QoL, but it is often overlooked.[12] This study considers the prevalence of dyspnea over a large sample of patients coming for palliative care consultations in a tertiary cancer center in India. It also strives to measure the impact of dyspnea on their QoL. To the best of our knowledge, this is the first prospective study done on such a unique set of patients in India.
SUBJECTS AND METHODS
Study subjects
This was a prospective observational study done over a period of 6 months from April to September 2014 at the Department of Palliative Medicine, Tata Memorial Centre (Mumbai). Posters were used to solicit the participation of prospective research subjects in the study. Due diligence was taken to ensure that the procedure for recruiting subjects was not coercive or stated or implied a certainty of favorable outcome or other benefits beyond what is outlined in the consent document and the protocol. All patients presenting to the outpatient clinic of the palliative care service were screened and accrued as per the inclusion criteria. The inclusion criteria were all literate adult patients (age ≥18) with advanced cancer with normal cognitive status (as determined by the physician) with the ability to understand the nature of the study and provide consent for the process. The exclusion criteria were patients admitted to Intensive Care Units, on noninvasive ventilation, or on disease-modifying therapy. All patients who participated in the study completed a written informed consent form at the time of their initial enrolments. Compensation in any form was not provided for taking part in this study. However, necessary facilities, emergency treatment, and professional services were made available to the patients, just as the usual procedures of the hospital. Due diligence was taken to protect the patients’ confidentiality. The Institutional Review Board of the hospital approved the study, and it was registered with clinical trials registry of India (CTRI REF/2014/05/006948).
Study procedures
All study-related procedures including data collection were done by the author and coauthors, all physicians trained in Palliative Medicine for 1–3 years. The patients consulted the palliative care team on the day of referral from oncology clinics. We did a one-time assessment of the participants at their first visit. It involved medical consultation, recording of socio-demographic information and symptom scores using Edmonton Symptom Assessment Scale (ESAS) (to be completed by study individuals), performance score using Eastern Cooperative Oncology Group (ECOG) scale, dyspnea by Cancer Dyspnea Scale (CDS), and QoL of the patients using European Organization for Research and Treatment of Cancer QoL Core 15 Palliative (EORTC QLQ-C15-pal) questionnaire.
Outcome measures
Outcome measures included socio-demographics and disease-related information; prevalence and severity of dyspnea in CDS/ESAS; factors correlating with the severity of dyspnea; and the impact of dyspnea on the QoL domain.
Statistical analyses
This was an observational prospective study. Based on the annual number of patients seen in the department, the projected sample of patients over a period of 6 months was estimated as 1500. To maintain heterogeneity in sampling, every third patient (i.e., 3rd, 6th, 9th, 12th…) was systematically screened and enrolled in the study. We conducted statistical analyses using SPSS version 20 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp).[13] Missing data were excluded from analyses and P = 0.05 or less were deemed to be statistically significant. Distribution patterns of data were noted and nonparametric equivalents of the parametric tests were used for analysis of skewed data. Statistical methods used were:
Descriptive statistics – to summarize details regarding age, gender, religion, address, educational status, marital status, family income (monthly), cancer diagnosis, stage, sites of metastasis, treatment, comorbidities (if any), ECOG score, ESAS symptom scores, intensity of overall distress related to dyspnea (effort, anxiety, and discomfort) using CDS, QoL using the EORTC-QLQ PAL15
Kruskal–Wallis/ANOVA– to determine whether the severity and perception of dyspnea is same across gender, age, cancer type, etc
Correlation coefficient – to determine if there is any association between dyspnea and demographic domains
Univariate analysis – to analyze the association of other physical symptoms on ESAS scale with dyspnea
Pearson correlation coefficient – to find out the relation between dyspnea score as given by the CDS and QOL score
Multiple regression analysis was used to test the secondary objective of the study that is to determine the correlation of clinical and demographic details with the degree of dyspnea.
RESULTS
A sample of 500 patients was recruited for the study. A total of 498 patients were included in the analysis, as 2 patients did not complete the questionnaires.
Demographic and clinical information
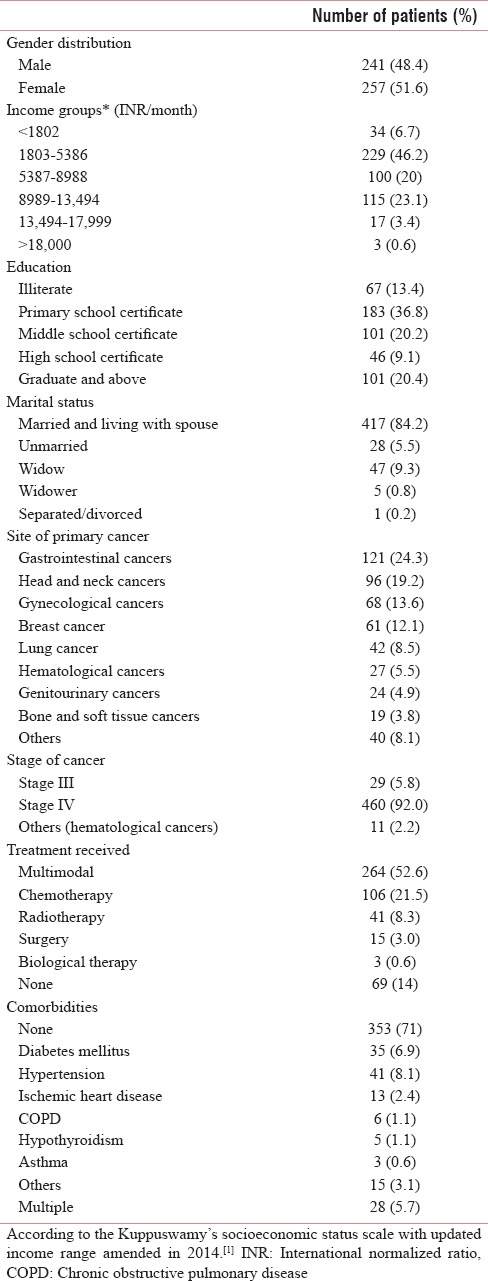
At baseline assessment, 51.6% of the individuals were women and had a median age of 52 years (range: 18–86 years). 46.2% of them were in the second category in Kuppuswamy's socioeconomic status scale with updated income range.[14]
(INR 1803-5386) per month and only about a third of them had a primary education (36.8%). About 84.2% were married. The most common primary cancer type was gastrointestinal cancer (24.3%). About 92% of the patients had stage IV cancer and 52.6% had received multimodal therapy for treatment of cancer. About 71% did not have any comorbidities [Table 1].
Table 1.
Data of sociodemographic details, treatment history, stage of disease (n=498)
Symptomatology
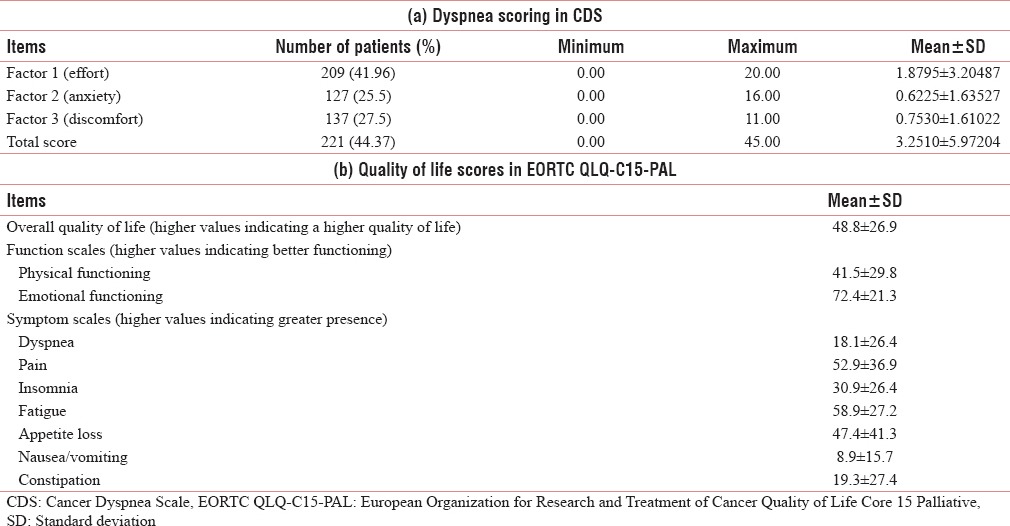
About 36.55% of patients were ECOG 3. At the initial visit 287 (57.4%) reported no dyspnea, whereas 134 (26.9%) had mild symptoms on ESAS scale [Table 2]. The prevalence of dyspnea as determined by the CDS scores was 44.37%. About 41.96% patients perceived sense of effort as a major component associated with dyspnea with a mean value of 1.88 (standard deviation 3.2) as compared to other two components of CDS (i.e., sense of anxiety and sense of discomfort). Higher values in Overall QoL indicated a higher QoL. The patients at presentation had good emotional functioning but comparatively poor physical functioning. Major symptoms were pain, fatigue, and loss of appetite whereas comparatively less number of patients presented dyspnea affecting QoL [Table 3].
Table 2.
Baseline clinical characteristics (n=498)
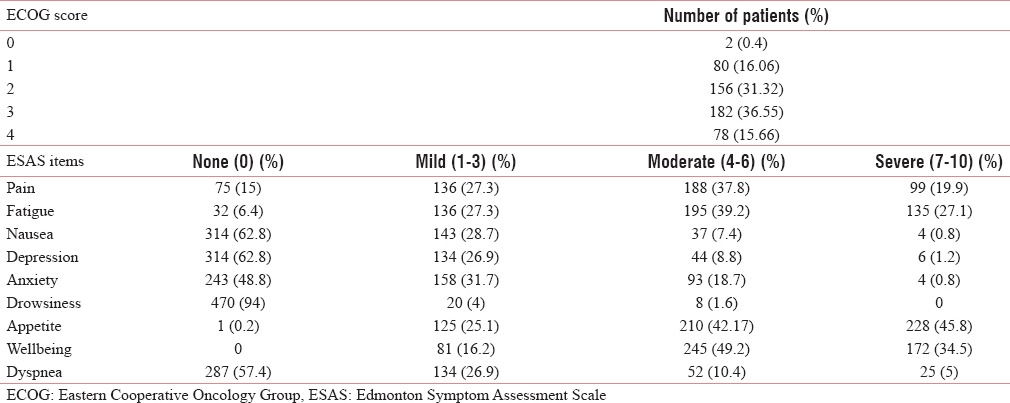
Table 3.
Baseline scoring of Dyspnea (CDS) and Qol (EORTC QLQ-C15-Pal) (n=498)
Factors influencing dyspnea
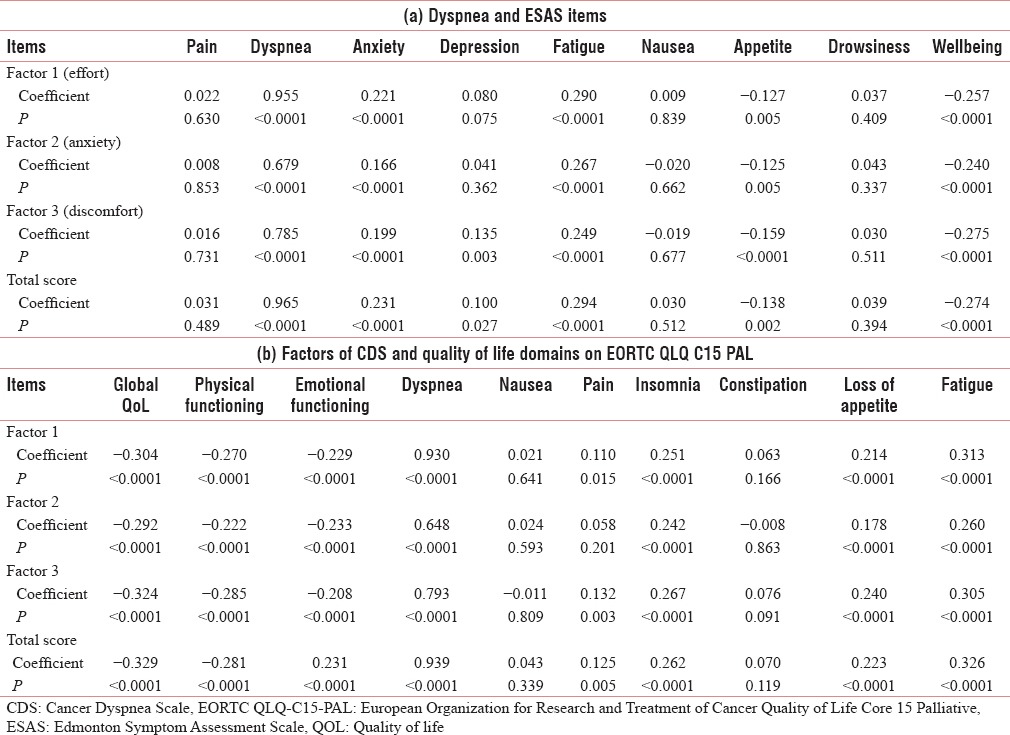
The associations between dyspnea on CDS scale and ESAS items was calculated. Significant correlations were found with ESAS items such as dyspnea (r = 0.965), anxiety (r = 0.231), fatigue (r = 0.294), appetite (r = −0.138), and sense of wellbeing (r = −0.274) items on ESAS. Depression had a significant correlation of 0.135 with factor 3 (sense of discomfort). Associations between the factors of CDS and QoL domains on EORTC QLQ C15 PAL were also calculated. Significant correlations were found with global Qol score (r = −0.329), physical functioning (r = −0.281), emotional functioning (r = 0.231), dyspnea (r = 0.939), pain (r = 0.125), insomnia (r = 0.262), loss of appetite (r = 0.223), and fatigue (r = 0.326) [Table 4]. These results signify that dyspneic patients have increased symptom burden with an increase in other symptoms such as insomnia, fatigue and pain and decrease in appetite, physical and emotional functioning with a deterrent effect on overall QoL of patients. This concept of symptom cluster in palliative care as a surrogate for symptom burden has been reported in literature previously.[15,16,17,18,19]
Table 4.
Correlation statistics
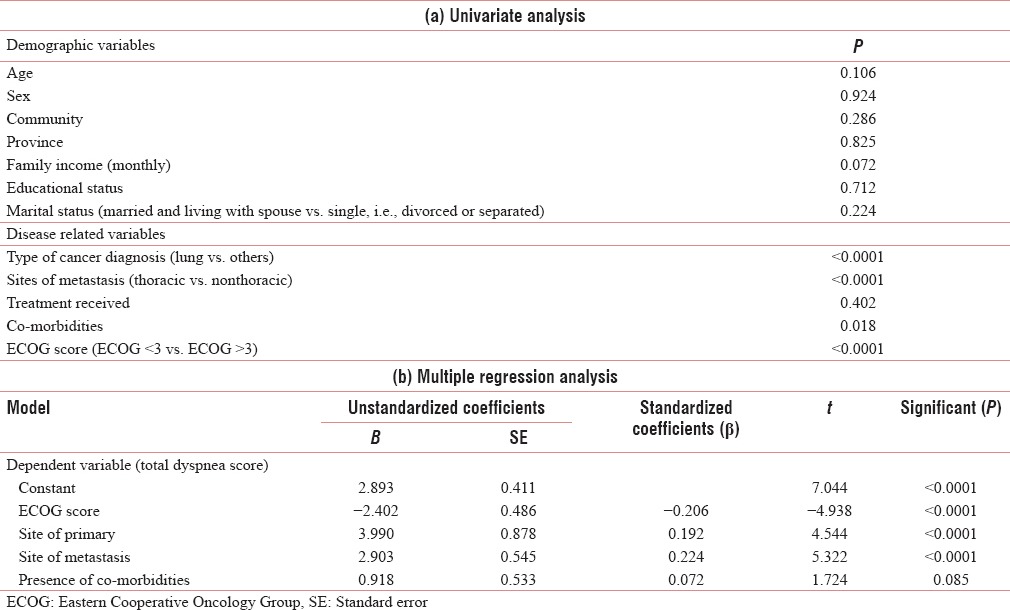
For a better understanding of the multidimensional nature of dyspnea, a linear regression model was constructed at baseline, with dyspnea as the dependent variable and other factors as independent variables. There was no significant univariate association between dyspnea and age (P = 0.106), gender (P = 0.924), community (P = 0.286), province (states) (P = 0.825), family income (P = 0.072), educational status (P = 0.712), marital status (P = 0.224), and cancer therapy received (P = 0.402). Significant univariate associations were found between dyspnea and primary lung cancers (P < 0.0001), cancers with secondaries in lung (P < 0.0001), patients with comorbidities (P = 0.018), and ECOG score of patients (P < 0.0001). Next, a multiple regression analysis was performed for those parameters which significantly correlated to dyspnea in the univariate analysis. The presence of comorbidities did not correlate significantly in the multiple regression model [Table 5].
Table 5.
Associations between dyspnea and various demographic and disease-related parameters
DISCUSSION
In advanced cancer, the goals of care change from cure to optimizing the QoL by reducing the symptom burden. Hence, it is important to assess and relieve the overall distress associated with these symptoms. In this context, CDS aptly measures the overall distress associated with dyspnea. CDS is simple and self-reporting, measures multiple aspects of dyspnea including physical effort and psychological distress, can be filled in very short duration, and has been validated for using in cancer patients in India by the authors elsewhere.[20]
This study shows that dyspnea is a common symptom in patients with advanced cancer (seen in 44.37%). These findings are consistent with previous studies where the prevalence of dyspnea in advanced cancer was from 29%–74%.[6,7,18,21] This wide range can be attributed to differences in patient selection, lack of clear symptom definition and variation in dyspnea measurement techniques. In our study, patients (94.6%) reported the perceived sense of effort in dyspnea as most bothersome. This was also accompanied by multiple symptoms, a sense of discomfort (62%) and lower QoL in the patients. This was consistent with a study done by Walsh et al.[3] Delving deeper in the data, univariate analysis showed that dyspnea worsens with worsening Performance status, preexisting lung disease, and presence of comorbidities such as diabetes, hypertension, ischemic heart disease, chronic obstructive pulmonary disease, and asthma. These results are consistent with findings from previous studies.[6,15,17] Interestingly, in this study, we did not found any significant relationship of dyspnea with demographic parameters. This is in contrast to the results of the study by Walsh et al.[3] where specific symptoms were affected by age and gender.
This is the first study on the prevalence of dyspnea in patients with advanced cancers in India. Major strengths of this study lie in its prospective design, usage of validated tools, and large heterogeneous sample of 498 patients with no missing data. There are few limitations, which need careful acknowledgement. Certain factors that may influence the course of dyspnea such as chest infections, anemia, pleural effusions, pulmonary embolism, previous chest irradiation, use of specific chemotherapeutic agents such as bleomycin and carmustine (which may cause pneumonitis as a side effect), and any lung surgeries such as pneumonectomy, interstitial tube drainage, or thoracotomy were not recorded in detail.[22] The use of single item tool (ESAS) to measure anxiety and depression might be inadequate; instead, a more specific tool like Hospital Anxiety Depression Scale would have been better.[23] Temporal relationship between dyspnea and QoL cannot be evaluated from this study because both were assessed at the same time. A longitudinal analysis would have been better.[24]
Future studies should consider longitudinal designs on wider populations, experience of dyspnea at the end of life and implications for palliative sedation. Qualitative research is needed to gain a deeper understanding in the psychosocial and spiritual issues affecting the perception of dyspnea. Clinical trials are needed to investigate available interventions and their effectiveness in mitigating the suffering from dyspnea.
CONCLUSIONS
The prevalence of dyspnea in advanced cancer patients as measured by CDS is high. It is associated with multiple symptoms and lowers the QoL. Dyspnea is higher in patients with comorbidities, lung cancer or lung metastasis, and worsening ECOG scores.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dyspnea Mechanisms, assessment, and management: A consensus statement. American thoracic society. Am J Respir Crit Care Med. 1999;159:321–40. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- 2.Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2000;19:357–62. doi: 10.1016/s0885-3924(00)00126-3. [DOI] [PubMed] [Google Scholar]
- 3.Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: Relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer. 2000;8:175–9. doi: 10.1007/s005200050281. [DOI] [PubMed] [Google Scholar]
- 4.Smith EL, Hann DM, Ahles TA, Furstenberg CT, Mitchell TA, Meyer L, et al. Dyspnea, anxiety, body consciousness, and quality of life in patients with lung cancer. J Pain Symptom Manage. 2001;21:323–9. doi: 10.1016/s0885-3924(01)00255-x. [DOI] [PubMed] [Google Scholar]
- 5.Muers MF, Round CE. Palliation of symptoms in non-small cell lung cancer: A study by the Yorkshire regional cancer organisation thoracic group. Thora×. 1993;48:339–43. doi: 10.1136/thx.48.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest. 1986;89:234–6. doi: 10.1378/chest.89.2.234. [DOI] [PubMed] [Google Scholar]
- 7.Heyse-Moore LH, Ross V, Mullee MA. How much of a problem is dyspnoea in advanced cancer? Palliat Med. 1991;5:20–6. [Google Scholar]
- 8.O’Driscoll M, Corner J, Bailey C. The experience of breathlessness in lung cancer. Eur J Cancer Care (Engl) 1999;8:37–43. doi: 10.1046/j.1365-2354.1999.00129.x. [DOI] [PubMed] [Google Scholar]
- 9.Toscani P, Brunelli C, Miccinesi G, Costantini M, Gallucci M, Tamburini M, et al. Predicting survival in terminal cancer patients: Clinical observation or quality-of-life evaluation? Palliat Med. 2005;19:220–7. doi: 10.1191/0269216305pm1000oa. [DOI] [PubMed] [Google Scholar]
- 10.Vigano A, Donaldson N, Higginson IJ, Bruera E, Mahmud S, Suarez-Almazor M, et al. Quality of life and survival prediction in terminal cancer patients: A multicenter study. Cancer. 2004;101:1090–8. doi: 10.1002/cncr.20472. [DOI] [PubMed] [Google Scholar]
- 11.Gupta D, Lis CG, Grutsch JF. The relationship between dyspnea and patient satisfaction with quality of life in advanced cancer. Support Care Cancer. 2007;15:533–8. doi: 10.1007/s00520-006-0178-7. [DOI] [PubMed] [Google Scholar]
- 12.Dudgeon D. Dyspnoea in Advanced Disease. Oxford University Press; 2005. [Last accessed on 2017 May 25]. Breathlessness in advanced cancer; pp. 75–95. Available from: http://www.oxfordscholarship.com/view/10.1093/acprof: oso/9780198530039.001.0001/acprof=9780198530039-chapter-5 . [Google Scholar]
- 13.IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp; 2011. [Google Scholar]
- 14.Kuppuswamy T, Consumer A, Index P, Workers I. Letter to the editor updating income ranges for Kuppuswamy's socio-economic status scale for the year 2014. [Last accessed on 2017 May 26];Indian J Public Health. 2015 59:2015–6. doi: 10.4103/0019-557X.157540. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26021657 . [DOI] [PubMed] [Google Scholar]
- 15.Vainio A, Auvinen A. Prevalence of symptoms among patients with advanced cancer: An international collaborative study. Symptom prevalence group. J Pain Symptom Manage. 1996;12:3–10. doi: 10.1016/0885-3924(96)00042-5. [DOI] [PubMed] [Google Scholar]
- 16.Stapleton SJ, Holden J, Epstein J, Wilkie DJ. Symptom clusters in patients with cancer in the hospice/palliative care setting. Support Care Cancer. 2016;24:3863–71. doi: 10.1007/s00520-016-3210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aktas A, Walsh D, Rybicki L. Symptom clusters and prognosis in advanced cancer. Support Care Cancer. 2012;20:2837–43. doi: 10.1007/s00520-012-1408-9. [DOI] [PubMed] [Google Scholar]
- 18.Higginson I, McCarthy M. Measuring symptoms in terminal cancer: Are pain and dyspnoea controlled? J R Soc Med. 1989;82:264–7. doi: 10.1177/014107688908200507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong ST, Butow PN, Costa DS, Lovell MR, Agar M. Symptom clusters in patients with advanced cancer: A systematic review of observational studies. J Pain Symptom Manage. 2014;48:411–50. doi: 10.1016/j.jpainsymman.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Damani A, Ghoshal A, Muckaden M. Validation of cancer dyspnea scale for advanced cancer patients in a tertiary cancer centre (FR441C) [Last accessed on 2017 May 26];Pain Symptom Manage. 2016 51:364–5. doi: 10.1016/j.jpainsymman.2017.06.004. Available from: http://www.linkinghub.elsevier.com/retrieve/pii/S0885392415008623 . [DOI] [PubMed] [Google Scholar]
- 21.Ahmedzai S. Palliation of respiratory symptoms. In: Doyle D, editor. The Oxford Textbook of Palliative Medicine. London: Oxford University Press; 1993a. pp. 349–78. [Google Scholar]
- 22.Williams CM. Dyspnea. Cancer J. 2006;12:365–73. doi: 10.1097/00130404-200609000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. Longitudinal assessment of symptom severity among hospitalized elders diagnosed with cancer, heart failure, and chronic obstructive pulmonary disease. J Hosp Med. 2012;7:567–72. doi: 10.1002/jhm.1925. [DOI] [PubMed] [Google Scholar]


