Abstract
Background
Multidrug-resistant tuberculosis (MDR TB) is an important global public health threat, but accurate estimates of MDR TB burden among children are lacking.
Methods
We analyzed demographic, clinical and laboratory data for newly-diagnosed pediatric (<15 years) TB cases reported to the US National TB Surveillance System (NTSS) during 1993–2014. MDR TB was defined as culture-confirmed TB disease with resistance to at least isoniazid and rifampicin. To ascertain potential under-estimation of pediatric MDR TB, we surveyed high burden states for clinically-diagnosed cases treated for MDR TB.
Results
Of 20,789 pediatric TB cases, 5,162 (24.8%) had bacteriologically-confirmed TB. Among 4,862 (94.2%) with drug-susceptibility testing, 82 (1.7%) had MDR TB. Most pediatric MDR TB cases were female (n=51, 62%), median age was 5 years (IQR 1–12), one-third were Hispanic (n=28, 34%), and two-thirds (n=55, 67%) were born in the US. Most cases had additional resistance to ≥1 other first-line drug (n=66; 80.5%) and one-third had resistance to ≥1 second-line drug (24/73 tested). Of 77 who started treatment prior to 2013, 66 (86%) completed treatment and 4 (5%) died. Among the four high TB burden states/jurisdictions surveyed, there was 42–55% under-estimation of pediatric MDR TB cases when using only culture-confirmed case definitions.
Conclusions
Only one-quarter of pediatric TB cases had culture-confirmed TB, likely resulting in underestimation of true pediatric MDR TB burden in the US using strictly bacteriologic criteria. Better estimates of pediatric MDR TB burden in the US are needed and should include clinical diagnoses based on epidemiologic criteria.
Keywords: tuberculosis, pediatric, antimicrobial resistance, multidrug resistance
Background
Multidrug-resistant TB (MDR TB), defined as resistance to at least isoniazid and rifampicin, is an important clinical and public health threat worldwide with an estimated 480,000 cases in 2015 [1]. Accurate estimates of MDR TB burden among children are not available, in part due to the paucibacillary nature of TB in children and challenges with microbiologic confirmation of disease [2]. Globally, only 10–30% of pediatric TB cases have laboratory-confirmed TB disease; most cases are diagnosed clinically based on symptoms and chest radiograph findings [3, 4]. Confirming MDR TB requires microbiologic diagnosis, which makes detecting all cases of MDR TB among children impossible with the currently available diagnostics. Pediatric TB cases may be recommended for empiric MDR TB treatment based on clinical or epidemiologic evidence of MDR TB, such as known contact to a pulmonary MDR TB case, first-line TB treatment failure or loss to follow-up during previous TB treatment. Currently, in the US, only laboratory-confirmed cases of MDR TB are counted as MDR TB in the national TB surveillance system.
Recent modeling studies estimate that 850,000–1,000,000 children developed TB in 2010, of whom 25,000–32,000 had MDR TB [5, 6]. The World Health Organization (WHO) estimated 490,000 incident cases of TB among children in 2012, the first global TB estimate among children and about half of the modeling estimates [7]. This WHO estimate assumed that the ratio of notified to incident cases was the same for adults and children, essentially ignoring the difficulties of pediatric TB diagnosis. More recent estimates of pediatric TB by WHO have incorporated new modeling approaches that better estimate TB burden among children [1]. However, a major gap in diagnosis and treatment of pediatric TB and MDR TB still exists. Identifying pediatric MDR TB is important not only for initiating appropriate treatment in children, but also to identify where recent transmission may have occurred. Knowledge of the clinical management and outcomes of children with MDR TB is limited worldwide, consisting mainly of case reports and small case series [8–14]. However, appropriate treatment of pediatric MDR TB has favorable outcomes, even in resource-limited, high HIV prevalence settings [8, 15]. As funding for TB control activities decreases, better estimates of MDR TB burden are needed to develop accurate spending estimates and to support development of pediatric formulations of second-line anti-TB drugs.
The US has had declining TB incidence for 22 years, with 2015 representing the first year since 1993 that TB case counts increased, yet annual proportions of MDR TB have remained essentially stable [16]. Children account for approximately 5% of the TB burden in the US, with 440 cases of childhood TB reported in 2015 [16]. However, the epidemiology of MDR TB among children in the US has not previously been reported. In this study, we describe the epidemiology of pediatric MDR TB in the US over the past two decades.
Methods
All 50 states and the District of Columbia report TB cases to the US national TB surveillance system (NTSS), using a standardized case report form on which demographic, clinical, laboratory, initial treatment regimen, and treatment outcome information are collected. Inclusion in the NTSS requires the case meet one of the following verification criteria: 1) laboratory-confirmed cases have Mycobacterium tuberculosis isolated from a clinical specimen by culture or nucleic acid amplification test (NAAT) or, in the absence of these, demonstration of acid-fast bacilli in the specimen; 2) clinical cases meet the following criteria: a) positive tuberculosis skin test or interferon gamma release assay, b) other signs or symptoms compatible with TB (e.g., abnormal chest radiograph, or clinical evidence of current disease), c) treatment with ≥2 anti-TB medications, and d) completed diagnostic evaluation; or 3) provider diagnosis case is diagnosed by a healthcare provider, but does not fulfill all criteria necessary to meet laboratory or clinical case definitions.
In this analysis, we included all newly-diagnosed pediatric TB cases (age <15 years) reported to the NTSS from 1993 through 2014 [17]. Laboratory-confirmed MDR TB was defined as culture-confirmed TB disease caused by Mycobacterium tuberculosis resistant to at least isoniazid and rifampicin during initial or final DST (in contrast to standard annual reports of US national TB surveillance data that use only initial DST) [18]. US-born persons were defined as persons born in the US, Puerto Rico, or US outlying area or born abroad to US parents; all other persons were defined as foreign-born. Children were grouped into age categories which are biologically-relevant to diagnosis and disease progression [2]. HIV test results were available for each reported TB case, with the following exceptions. Through 2004, California only reported positive HIV results based on TB and AIDS registry matching; all other California TB cases were classified as “Unknown.” California did not report any HIV results from 2005–2010. HIV data are missing from Vermont during 2007–2014. Regional categorization of countries was based on the WHO Global Tuberculosis Report [1]. Anti-TB drug categories and resistance patterns are defined by WHO [19]. NTSS end-of-treatment outcomes have been previously defined [17]. Briefly, a patient either 1) completed the prescribed course of therapy as recorded by the patient’s clinician, 2) could not be located before treatment completion, 3) refused to complete therapy, 4) permanently stopped therapy because of an adverse event due to anti-TB medications, 5) died before treatment completion, or 6) moved to a different local health department jurisdiction and the final treatment outcome was unknown. Sputum culture conversion was defined as an initial culture positive sputum specimen, followed by at least one negative sputum culture without a subsequent positive culture.
To estimate potential underestimation of pediatric MDR TB in NTSS, which is based only on phenotypic drug-susceptibility test results, we contacted six states that contributed the highest number of laboratory-confirmed pediatric MDR TB cases to NTSS during the study period. Each state was asked to report all cases of pediatric TB in patients who received MDR TB treatment and, when available, the reason for this treatment decision. Empiric MDR TB was defined as a case who received MDR TB treatment based on clinical or epidemiologic evidence of MDR TB without laboratory confirmation of MDR TB. State case ID and report year were used to match the cases in NTSS for complete epidemiologic data. The proportion of underestimation was calculated per reporting state as number of empiric MDR TB cases divided by total number of cases treated for MDR TB (laboratory-confirmed and empiric MDR TB) during the same period.
Univariate analyses were used to assess trends and frequencies. Bivariate analyses were used to assess associations of demographic and clinical characteristics with age group and with empiric MDR TB using Pearson’s chi-square or Fishers exact test, where appropriate. The Kruskal-Wallis test was used to test association of continuous variables. P-value <0.05 was considered statistically significant. Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA).
This study was approved by the NTSS Analytic Steering Committee and determined to be an evaluation of routine surveillance data by the CDC.
Results
Of 20,789 children with TB disease reported from 1993–2014, 5,162 (24.8%) had culture-confirmed TB, of which 4,862 (94.2%) had phenotypic DST results available to RIF and INH (Figure 1). The annual proportion of culture-confirmed pediatric TB ranged between 23–34%. Eighty-two (1.7%) children had lab-confirmed MDR TB, of whom 78 (95%) had no previous history of TB. Annually, 1–6 pediatric MDR TB cases were reported (0.4–2.6% of pediatric TB cases with DST results) (Figure S1).
Figure 1.
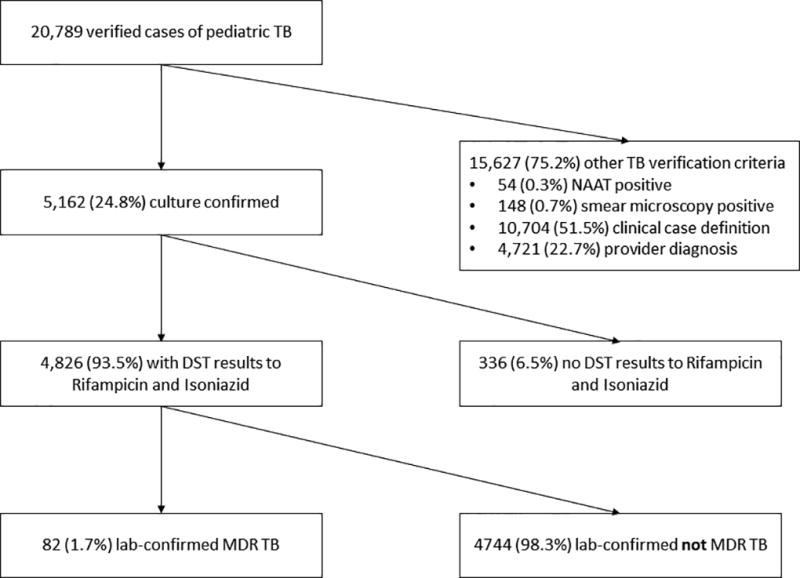
Flow chart of all verified pediatric tuberculosis cases reported from 1993 to 2014 to lab-confirmed MDR TB cases.
NAAT=nucleic acid amplification test; DST=drug susceptibility test; MDR TB=multidrug-resistant tuberculosis.
Most laboratory-confirmed pediatric MDR TB cases were female (n=51, 62%) and one-third were of Hispanic ethnicity (n=28, 34%) (Table S1). The median age was 5 years (interquartile range [IQR] 1–12). Two-thirds (n=55; 67%) were US-born persons. Twenty-two states reported ≥1 pediatric MDR TB case, but over 60% of cases occurred in five states. Most pediatric MDR TB cases had pulmonary involvement only (n=55; 67%) (Table S2).
Pediatric MDR TB by age group
Younger children aged <1 year and 1–4 years were more likely to be born in the US (88% and 92%, respectively) than older children aged 5–9 years and 10–14 years (47% and 44%, respectively; p value=0.0003) (Table 1). Most children aged 10–14 years had positive sputum culture (82%) compared to younger children (<1 year: 13%; 1–4 years: 17%; 5–9 years: 20%, respectively; p value=<0.0001). A large proportion of young children had positive cultures from gastric aspirates (<1 year: 56%; 1–4 years: 54%).
Table 1.
Sociodemographic and clinical characteristics of children with lab-confirmed MDR TB in the United States by age group, 1993–2014 (N=82)
| Characteristic | Age group (years) | p-value | ||||
|---|---|---|---|---|---|---|
| <1 | 1–4 | 5–9 | 10–14 | |||
| n (%) | n (%) | n (%) | n (%) | |||
| Sex | Male | 5 (31) | 9 (38) | 6 (40) | 11 (41) | |
| Female | 11 (69) | 15 (62) | 9 (60) | 16 (59) | 0.9 | |
| Race/Ethnicity | Asian | 0 (0) | 8 (33) | 5 (33) | 11 (41) | |
| Black | 5 (31) | 5 (21) | 5 (33) | 6 (22) | ||
| Hispanic | 9 (56) | 7 (29) | 5 (33) | 7 (26) | ||
| White | 2 (12) | 2 (8) | 0 (0) | 1 (4) | ||
| Multiplea | 0 (0) | 1 (4) | 0 (0) | 0 (0) | ||
| Unknown | 0 (0) | 1 (4) | 0 (0) | 2 (7) | 0.1b | |
| Reporting State | California | 1 (6) | 6 (25) | 3 (20) | 5 (19) | |
| New York City | 6 (37) | 2 (8) | 4 (26) | 3 (11) | ||
| Minnesota | 0 (0) | 1 (4) | 3 (20) | 3 (11) | ||
| New Jersey | 2 (13) | 2 (8) | 1 (8) | 2 (7) | ||
| Texas | 2 (13) | 2 (8) | 0 (0) | 3 (11) | ||
| North Carolina | 1 (6) | 4 (17) | 0 (0) | 0 (0) | ||
| Other (16 states ≤3 cases) | 4 (25) | 7 (30) | 4 (26) | 11 (41) | 0.3b | |
| Origin | US-born | 14 (87) | 22 (92) | 7 (47) | 12 (44) | |
| Foreign-born | 2 (13) | 2 (8) | 8 (53) | 15 (56) | 0.0003 | |
| Origin Region among foreign-bornb (n=27) | Africa | 1 (50) | 0 (0) | 3 (37) | 5 (33) | |
| Southeast Asia | 0 (0) | 1 (50) | 2 (25) | 3 (20) | ||
| Americas | 1 (50) | 0 (0) | 1 (13) | 3 (20) | ||
| Western Pacific | 0 (0) | 0 (0) | 2 (25) | 3 (20) | ||
| Europe | 0 (0) | 1 (50) | 0 (0) | 1 (7) | 0.8b | |
| Born in high MDR TB burden countryc (n=27) | 1 (50) | 1 (50) | 3 (38) | 7 (47) | 1.0b | |
| Lived in US <1 year (n=24) | 1 (100) | 2 (100) | 4 (57) | 9 (64) | 0.8d | |
| Previous Diagnosis of TB Disease | Yes | 0 (0) | 1 (4) | 0 (0) | 2 (7) | |
| No | 16 (100) | 22 (92) | 15 (100) | 25 (93) | ||
| Unknown | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0.8b | |
| HIV statuse | Positive | 1 (7) | 0 (0) | 2 (15) | 2 (9) | |
| Negative | 9 (60) | 8 (44) | 6 (46) | 16 (69) | ||
| Unknown | 5 (33) | 10 (56) | 5 (39) | 5 (22) | 0.3b | |
| Site of Disease | Pulmonary Only | 12 (75) | 15 (62) | 7 (47) | 21 (78) | |
| Extrapulmonary Only | 2 (12) | 5 (21) | 5 (33) | 3 (11) | ||
| Both | 2 (13) | 4 (17) | 3 (20) | 3 (11) | 0.5b | |
| Abnormal initial chest x-ray | 13 (81) | 19 (79) | 9 (60) | 26 (96) | 0.02b | |
| Positive culture specimen type | Sputum | 2 (13) | 4 (17) | 3 (20) | 22 (81) | |
| Gastric aspirate | 9 (56) | 13 (54) | 5 (33) | 0 (0) | ||
| Bronchial fluid | 3 (19) | 1 (4) | 2 (13) | 1 (4) | ||
| Otherf | 2 (13) | 6 (25) | 5 (33) | 4 (15) | <0.0001b | |
MDR TB = multidrug-resistant tuberculosis; IQR = interquartile range
multiple races reported
Fishers exact test
high MDR TB country based on WHOs 27 high MDR TB burden countries [1]
Kruskal-Wallis test
California HIV data missing from 2005–2010 (n=13)
other specimen types include cerebral spinal fluid, lymph node, skeletal system, soft tissue, peritoneal fluid, pus, or unknown specimen type
Drug resistance
Of the 82 laboratory-confirmed MDR TB cases, all had DST results to ≥1 first-line drug in addition to isoniazid and rifampicin, and 73 (89%) had results to ≥1 second-line drug (Table 2). Most pediatric MDR TB cases had TB with additional resistance to ≥1 other first-line drug (66; 81%). Of those tested, almost one-third had TB resistant to ≥1 second-line drug (24/73).
Table 2.
Additional drug resistance of M. tuberculosis isolates from children with lab-confirmed MDR TB in the United States, 1993–2014 (N=82)
| Drug category | Tested | Resistant | |
|---|---|---|---|
| N | n | % | |
| Other first-line drugs | 82 | 66 | 81 |
| Ethambutol | 81 | 47 | 58 |
| Pyrazinamide | 70 | 36 | 51 |
| Streptomycin | 80 | 56 | 70 |
| Second-line drugs | 73 | 24 | 33 |
| Any second-line injectable | 68 | 10 | 15 |
| Any fluoroquinolone | 57 | 5 | 9 |
| Extensive drug resistancea | 54 | 3 | 6 |
| Other second-line drugsb | 70 | 15 | 21 |
MDR TB = multidrug-resistant tuberculosis; M. tuberculosis resistant to isoniazid and rifampicin
Resistance to a fluoroquinolone and ≥ 1 second-line injectable
cycloserine, ethionamide, p-aminosalicylic acid
Initial treatment regimen, duration and treatment outcomes
Among 80 pediatric MDR TB cases alive at diagnosis, most (n=77; 96%) were initially treated with only first-line drugs (Figure S2). Median duration of treatment was 20 months (IQR 15–24) (Table 3). Among the 77 who started treatment prior to 2013, 66 (86%) completed treatment, 4 (5%) died, 5 (6%) were lost, refused treatment, or moved, and 2 (3%) had no treatment outcome documented. Among the 21 (68%) with documented culture conversion, the median time to conversion was 2 months (IQR 1–4).
Table 3.
Treatment characteristics and outcomes among children with lab-confirmed MDR TB started on treatment, 1993–2012a (N=77)
| Characteristics | n (%) | |
|
| ||
| Duration, months (n=75) | median (IQR) | 20 [15–24] |
|
| ||
| Outcome | n (%) | |
|
| ||
| Sputum culture conversion (n=31)c | Yes | 21 (68) |
| No | 7 (22) | |
| Unknown | 3 (10) | |
| Months to culture conversion (n=21) | median (IQR) | 2 [1–4] |
| Reason therapy stopped | Completed therapy | 66 (86) |
| Died | 4 (5) | |
| Lost or Refused | 2 (3) | |
| Moved, unknown outcome | 3 (3) | |
| Not documented | 2 (3) | |
MDR TB = multidrug-resistant tuberculosis
analysis limited to 1993–2012 to allow for patients to complete 2 years of MDR TB treatment
cases with initial sputum culture positive
Estimating pediatric MDR TB
Of the six highest pediatric MDR TB burden states/jurisdictions surveyed, three responded with information about empirically-treated MDR TB cases. California began recording such cases in 2002, Minnesota in 2010, and New York City (NYC) in 1993. Pediatric MDR TB was underestimated by 55% (6 empiric MDR/11 total MDR), 50% (1/2), and 42% (11/26) in California, Minnesota, and NYC, respectively. All empiric MDR TB cases received MDR TB treatment because of known contact with an MDR TB source case, in addition to clinical evidence of active TB disease. Children receiving empiric MDR TB treatment were more likely to be 1–4 years old (78% vs 14%; p-value<0.0001), have unknown HIV status (67% vs 28%; p-value=0.04), or have received ethionamide in the initial regimen (50% vs 15%; p-value=0.02) compared to laboratory-confirmed MDR TB cases (Table 4).
Table 4.
Characteristics of lab-confirmed versus empiric pediatric MDR TB, participating U.S. jurisdictions, n=39
| Characteristics | Lab-Confirmed MDR TB |
Empiric MDR TB | p-value |
|---|---|---|---|
| n=21 | n=18 | ||
| n (%) | n (%) | ||
| Female Sex | 9 (43) | 12 (67) | 0.1 |
| Age (years) | |||
| <1 | 6 (29) | 1 (6) | |
| 1–4 | 3 (14) | 14 (78) | |
| 5–9 | 7 (33) | 1 (6) | |
| 10–14 | 5 (24) | 2 (11) | <0.0001a |
| Reporting state/jurisdiction | |||
| California | 5 (24) | 6 (33) | |
| Minnesota | 1 (5) | 1 (6) | |
| New York City | 15 (71) | 11 (61) | 0.8a |
| Race/Ethnicity | |||
| Asian | 7 (33) | 2 (11) | |
| Black | 4 (19) | 8 (44) | |
| Hispanic | 9 (43) | 6 (33) | |
| White | 0 (0) | 1 (6) | |
| Multiple | 0 (0) | 1 (6) | |
| Unknown | 1 (5) | 0 (0) | 0.1a |
| US born | 17 (81) | 17 (94) | 0.3a |
| Previous TB diagnosis | 1 (5) | 0 (0) | 1a |
| Known HIV statusb (n=30) | 13 (72) | 4 (33) | 0.04 |
| HIV positive among known statusb (n=17) | 3 (23) | 0 (0) | 0.5a |
| Disease site | |||
| Pulmonary only | 13 (62) | 15 (83) | |
| Extrapulmonary only | 4 (19) | 1 (6) | |
| Both | 4 (19) | 2 (11) | 0.4a |
| Abnormal initial chest radiograph | 14 (67) | 17 (94) | 0.05a |
| Verification criteria | |||
| Lab confirmed | 21 (100) | 0 (0) | |
| Clinical case | 0 (0) | 9 (50) | |
| Provider diagnosis | 0 (0) | 9 (50) | <0.0001a |
| Initial drug regimen (n=38) | |||
| IR | 1 (5) | 0 (0) | |
| IRZ | 2 (10) | 0 (0) | |
| IRZE | 8 (38) | 6 (33) | |
| Multiple other drugs | 9 (43) | 12 (67) | 0.6a |
| Drugs/Drug groups in initial regimen (n=38) | |||
| Any first-line drug | 19 (95) | 16 (89) | 0.6a |
| Isoniazid | 14 (70) | 6 (33) | 0.02 |
| Rifampicin | 15 (75) | 7 (39) | 0.02 |
| Pyrazinamide | 16 (80) | 13 (72) | 0.7a |
| Ethambutol | 12 (60) | 12 (67) | 0.7 |
| Streptomycin | 3 (15) | 1 (6) | 0.6a |
| Any second-line drug | 7 (35) | 10 (56) | 0.2 |
| Fluoroquinolone | 3 (15) | 4 (22) | 0.7a |
| Second-line injectable | 5 (25) | 4 (22) | 1.0a |
| Ethionamide | 3 (15) | 9 (50) | 0.02 |
| Cycloserine | 6 (30) | 5 (28) | 0.9 |
| PAS | 0 (0) | 1 (6) | 0.5a |
| Treatment duration, median [IQR] months (n=35)d | 20 [16–24] | 19 [18–21] | 0.8c |
| End of treatment outcome (n=35)d | |||
| Completed | 14 (78) | 15 (88) | |
| Died | 2 (11) | 0 (0) | |
| Moved | 2 (11) | 2 (12) | 0.5a |
MDR TB=multidrug resistant tuberculosis; IR=Isoniazid and Rifampicin only; IRZ=isoniazid, rifampicin and pyrazinamide; IRZE=isoniazid, rifampicin, pyrazinamide and ethambutol; PAS=p-aminosalicylic acid; IQR=interquartile range
Fishers exact test
California HIV data missing from 2005–2010 (n=9).
Kruskal-Wallis test
Alive at diagnosis, started treatment prior to 2013 and had a documented reason therapy stopped
Discussion
This is the first nationally-representative description of the epidemiology of pediatric MDR TB in the US. The prevalence of laboratory-confirmed MDR TB among children with TB from 1993–2014 (1.7%) was lower than the estimated Americas regional (2.2%) and global (3.2%) prevalence, and similar to the overall prevalence of MDR TB in the US (1.5%) [5, 16]. Although MDR TB treatment is complicated and toxic, children in this cohort responded to treatment better than adults with MDR TB in the US and globally, and similarly to other cohorts of children receiving individualized MDR TB treatment [20–23]. Importantly, two-thirds of all pediatric MDR TB cases were US-born and nearly all younger children (<5 years) were US-born, likely reflecting transmission of MDR TB in the US. TB disease in children often represents recent transmission, with most disease manifestations in children occurring in the first 6–12 months following primary infection [2, 24]. Our findings underscore the importance of quickly starting appropriate treatment and initiating active contact investigation of all pulmonary MDR TB cases to prevent transmission to children and others in the home or in the community.
Diagnosing MDR TB in children can be challenging due to paucibacillary disease and difficulty with sputum expectoration. We found that children <5 years with confirmed MDR TB were less likely to have positive cultures from sputum specimens. Most positive cultures among this age group were from specimens that require more invasive procedures, namely gastric aspiration. Induced sputum has been shown to have similar yield to gastric aspirate and offers a safe, simple alternative that can be done in an outpatient setting with proper staff training [25]. Newer molecular diagnostics may decrease time to detection of TB and drug resistance compared to culture-based methods, but the sensitivity of these tests are less than culture and the difficulty of specimen collection among young children remains [26]. Studies are needed, and some are underway, to determine biomarkers of TB disease in specimens that are easily obtainable from young children [27].
Because MDR TB diagnosis requires laboratory confirmation, MDR TB burden among children may be underdiagnosed and, therefore, underestimated worldwide. Of all pediatric TB cases in the US since 1993, only 25% were culture-confirmed and 23% had DST results. Thus, case counts that rely solely on laboratory diagnosis are likely to represent a fraction of the actual burden. Our study sought to expand the definition of pediatric MDR TB to include clinically-diagnosed cases who were treated for MDR TB based on epidemiologic and clinical criteria, similar to “probable MDR TB disease” defined by the Sentinel Project on Pediatric Drug-Resistant Tuberculosis [28]. Our survey of several states/jurisdictions found that 42–55% of children treated for MDR TB would not be counted as MDR TB cases using current case definitions reliant on laboratory confirmation. A previous study in NYC similarly found that 13 out of 20 (65%) pediatric TB cases treated for MDR TB did not have laboratory confirmation and treatment instead relied on source case DST [22]. Considering only laboratory-confirmed MDR TB among children fails to capture the complex treatment and case management work performed by the state and local health departments. A more inclusive definition would help national, state and local health departments understand their MDR TB burden and better align program and funding needs.
All children treated empirically for MDR TB were contacts to an MDR TB case and met the clinical case definition for TB. Most were <5 years of age, reflecting the difficulty of laboratory diagnosis in young children. Whereas most laboratory-confirmed MDR TB cases received rifampicin and isoniazid in the initial regimen, most empiric MDR TB cases did not. According to national guidelines, children found to have MDR TB through contact tracing should receive treatment according to the source case’s DST results [29]. Children without a known MDR TB contact or other risk factors for MDR TB would be started on first-line therapy until DST results are available. Since DST can take weeks to months for results, the initial regimen reported likely does not reflect the effective regimen that the patient received. Treatment outcomes were good in both groups; however, quicker diagnoses and source case investigation to guide effective treatment may improve outcomes further.
We recognize that a more inclusive case definition could result in overestimation and misdiagnosis of pediatric MDR TB without clearly-defined epidemiologic and clinical criteria to guide treatment decisions. Even though children often have few side effects from second-line drugs and none of the children in this cohort stopped treatment due to adverse drug reactions, the risk of hearing loss and other adverse drug reactions is greater than with first-line treatment [30]. To lessen the risk of over-diagnosis, thorough clinical investigation is imperative. Thorough source case investigation may be more critical when the clinical investigation is not successful in determining drug-susceptibility. Even in locations with high TB transmission, the presumed source case’s DST results have been found to be highly relevant and should guide initial management [31]. Frontline health workers are a key aspect of complete source case investigations and contact tracing. For example, understanding community dynamics and childcare strategies can help identify others who spend time with the child, but may not be part of the household.
This cohort may represent an underestimation of confirmed MDR TB among children for 2013 and 2014 due to delays in DST result reporting. Initial DST results are sometimes reported months after the initial case report, and final DST results are reported once treatment has stopped. However, children often do not have many if any follow up DST results due to specimen collection difficulties, paucibaciliary nature of disease, and quick response to effective treatment. In addition, NTSS does not collect information about phenotypic DST results between initial and final DST, or any molecular assays that detect MDR TB-associated mutations. These assays have become routine in the past decade which may result in underestimation of confirmed MDR TB in the more recent years. The small sample size of this cohort limited statistical power. Our analysis of MDR TB treatment was limited to the initial regimen at the time the case was reported. This initial regimen likely does not represent the MDR TB regimen because of inherent delays in diagnosis of drug resistance. This points to the need to collect supplemental surveillance information on treatment of MDR TB cases. A study on MDR TB treatment practices in the US found a median of 7 regimen changes per patient during treatment [20]. Additional surveillance information on the treatment of MDR TB cases may improve accuracy of national estimates, particularly for pediatric MDR TB case counts.
Over the past two decades, the proportion of foreign-born individuals among all TB cases has increased to 66% and nearly all adult MDR TB cases are foreign-born [16, 20]. However, this study illustrates that there is still MDR TB transmission occuring in the US as children serve as “sentinel” cases indicating recent transmission. Analyses of pediatirc DR TB can be an additional tool to monitor changing DST profile of circulating strains. As the TB epidemic in the US changes to mirror TB epidemics elsewhere, MDR TB may become a greater concern. Providers should not only consider laboratory and clinical characteristics, but also epidemiologic characteristics, when deciding how to treat pediatric TB cases. Children who are clinically-diagnosed and treated for MDR TB most likely represent true cases of MDR TB and should be monitored for case counts, not only to help TB control programs better forecast program needs, but to more accurately determine the burden of MDR TB among children.
Until there are better laboratory diagnostics for children, pediatric MDR TB will be underestimated globally. Including empiric MDR TB diagnoses in national estimates may close the gap between the estimated burden and the reported burden.
Supplementary Material
Summary.
Between 1993 and 2014, only 25% of pediatric TB cases in the US had culture-confirmed TB. Among those with culture-based drug susceptibility test results, 82 (1.7%) had MDR-TB. Most completed treatment (88%) despite extensive first- and second-line drug resistance.
Acknowledgments
We would like to acknowledge Sapna Bamrah Morris, Dawn Tuckey, Bruce Bradley, Vern Green, and Mark Miner from the Field Services Branch in the Division of Tuberculosis Elimination at the US Centers for Disease Control and Prevention for facilitating communication with the states/jurisdictions TB programs. We would like to acknowledge Doug Proops and Felicia Dworkin from Bureau of Tuberculosis Control, New York City Department of Health and Mental Hygiene for reviewing the regimens and helping characterize clinical MDR TB cases. We also acknowledge all the state and local public health departments for their continuous efforts towards TB surveillance, control and elimination. Finally, we would like to acknowledge all the children who have had the unfortunate circumstance of living with this disease and the caretakers that helped to diagnose and treat them.
Footnotes
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
All authors have no conflicts of interest to declare.
References
- 1.World Health Organization. Global Tuberculosis Report 2015. Geneva; 2015. [Google Scholar]
- 2.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era [State of the Art] The International Journal of Tuberculosis and Lung Disease. 2004;8(4):392–402. [PubMed] [Google Scholar]
- 3.Sandgren A, Hollo V, Quinten C, Manissero D. Childhood tuberculosis in the European Union/European Economic area, 2000 to 2009. Euro Surveill. 2011;16(12):19825. [PubMed] [Google Scholar]
- 4.Mtabho CM, Irongo CF, Boeree MJ, Aarnoutse RE, Kibiki GS. Childhood tuberculosis in the Kilimanjaro region: lessons from and for the TB programme. Tropical Medicine & International Health. 2010;15(5):496–501. doi: 10.1111/j.1365-3156.2010.02481.x. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins HE, Tolman AW, Yuen CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. The Lancet. 2014;383(9928):1572–9. doi: 10.1016/S0140-6736(14)60195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. The Lancet Global Health. 2014;2(8):e453–e9. doi: 10.1016/S2214-109X(14)70245-1. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Global tuberculosis report 2012. Geneva: The World Health Organization; 2012. [Google Scholar]
- 8.Thomas T, Shenoi S, Heysell S, et al. Extensively drug-resistant tuberculosis in children with human immunodeficiency virus in rural South Africa. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2010;14(10):1244. [PMC free article] [PubMed] [Google Scholar]
- 9.Seddon JA, Hesseling AC, Willemse M, Donald PR, Schaaf HS. Culture-confirmed multidrug-resistant tuberculosis in children: clinical features, treatment, and outcome. Clinical infectious diseases. 2012;54(2):157–66. doi: 10.1093/cid/cir772. [DOI] [PubMed] [Google Scholar]
- 10.Schaaf H, Shean K, Donald P. Culture confirmed multidrug resistant tuberculosis: diagnostic delay, clinical features, and outcome. Archives of disease in childhood. 2003;88(12):1106–11. doi: 10.1136/adc.88.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Méndez EA, Baquero AF, García MM, et al. Anales de pediatria. Barcelona, Spain: 2003: 2007. Multidrug-resistant tuberculosis in the pediatric age group; pp. 206–11. [DOI] [PubMed] [Google Scholar]
- 12.Fairlie L, Beylis NC, Reubenson G, Moore DP, Madhi SA. High prevalence of childhood multi-drug resistant tuberculosis in Johannesburg, South Africa: a cross sectional study. BMC infectious diseases. 2011;11(1):28. doi: 10.1186/1471-2334-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drobac PC, Mukherjee JS, Joseph JK, et al. Community-based therapy for children with multidrug-resistant tuberculosis. Pediatrics. 2006;117(6):2022–9. doi: 10.1542/peds.2005-2235. [DOI] [PubMed] [Google Scholar]
- 14.Condos R, Hadgiangelis N, Leibert E, Jacquette G, Harkin T, Rom WN. Case series report of a linezolid-containing regimen for extensively drug-resistant tuberculosis. CHEST Journal. 2008;134(1):187–92. doi: 10.1378/chest.07-1988. [DOI] [PubMed] [Google Scholar]
- 15.Coninx R, Mathieu C, Debacker M, et al. First-line tuberculosis therapy and drug-resistant Mycobacterium tuberculosis in prisons. The Lancet. 1999;353(9157):969–73. doi: 10.1016/s0140-6736(98)08341-x. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2015. Atlanta, GA: US Department of Health and Human Services; 2016. [Google Scholar]
- 17.Centers for Disease Control and Prevention. CDC Tuberculosis Surveillance Data Training: Report of Verified Case of Tuberculosis (RVCT) Services USDoHaH; 2009. [Google Scholar]
- 18.Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2015. Oct, 2015. [Google Scholar]
- 19.World Health Organization. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis: Emergency Update 2008. Geneva: World Health Organization; 2008. [Google Scholar]
- 20.Marks SM, Flood J, Seaworth B, et al. Treatment Practices, Outcomes, and Costs of Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis, United States, 2005–2007. Emerging Infectious Disease journal. 2014;20(5):812. doi: 10.3201/eid2005.131037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahuja SD, Ashkin D, Avendano M, et al. Multidrug Resistant Pulmonary Tuberculosis Treatment Regimens and Patient Outcomes: An Individual Patient Data Meta-analysis of 9,153 Patients. PLoS Med. 2012;9(8):e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feja K, McNelley E, Tran CS, Burzynski J, Saiman L. Management of Pediatric Multidrug-Resistant Tuberculosis and Latent Tuberculosis Infections in New York City From 1995 to 2003. The Pediatric Infectious Disease Journal. 2008;27(10):907–12. doi: 10.1097/INF.0b013e3181783aca. [DOI] [PubMed] [Google Scholar]
- 23.Ettehad D, Schaaf HS, Seddon JA, Cooke GS, Ford N. Treatment outcomes for children with multidrug-resistant tuberculosis: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2012;12(6):449–56. doi: 10.1016/S1473-3099(12)70033-6. [DOI] [PubMed] [Google Scholar]
- 24.Schaaf HS, Gie RP, Beyers N, Sirgel FA, de Klerk PJ, Donald PR. Primary drug-resistant tuberculosis in children. The International Journal of Tuberculosis and Lung Disease. 2000;4(12):1149–55. [PubMed] [Google Scholar]
- 25.Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. The Lancet. 2005;365(9454):130–4. doi: 10.1016/S0140-6736(05)17702-2. [DOI] [PubMed] [Google Scholar]
- 26.Detjen AK, DiNardo AR, Leyden J, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. The Lancet Respiratory Medicine. 2015;3(6):451–61. doi: 10.1016/S2213-2600(15)00095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Rie A, Moultrie H. Novel biomarkers for paediatric tuberculosis. The Lancet Infectious Diseases. 2014;14(10):900–1. doi: 10.1016/S1473-3099(14)70898-9. [DOI] [PubMed] [Google Scholar]
- 28.Seddon JA, Perez-Velez CM, Schaaf HS, et al. Consensus Statement on Research Definitions for Drug-Resistant Tuberculosis in Children. Journal of the Pediatric Infectious Diseases Society. 2013;2(2):100–9. doi: 10.1093/jpids/pit012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blumberg H, Burman W, Chaisson R, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. American Journal of Respiratory and Critical Care Medicine. 2003;167(4):603–62. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 30.Schaaf HS, Thee S, van der Laan L, Hesseling AC, Garcia-Prats AJ. Adverse effects of oral second-line antituberculosis drugs in children. Expert Opinion on Drug Safety. 2016:1–13. doi: 10.1080/14740338.2016.1216544. [DOI] [PubMed] [Google Scholar]
- 31.Schaaf HS, Marais BJ, Whitelaw A, et al. Culture-confirmed childhood tuberculosis in Cape Town, South Africa: a review of 596 cases. BMC infectious diseases. 2007;7(1):1. doi: 10.1186/1471-2334-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


