Abstract
Objective
To describe epidemiology of bacterial meningitis in the World Health Organization Eastern Mediterranean Region countries and assist in introduction of new bacterial vaccines.
Study design
A laboratory-based sentinel surveillance was established in 2004, and up to 10 countries joined the network until 2010. Personnel at participating hospitals and national public health laboratories received training in surveillance and laboratory methods and used standard clinical and laboratory-confirmed case definitions.
Results
Over 22 000 suspected cases of meningitis were reported among children ≤5 years old and >6600 among children >5 years old. In children ≤5 years old, 921 of 13 125 probable cases (7.0%) were culture-confirmed. The most commonly isolated pathogens were S pneumoniae (27% of confirmed cases), N meningitidis (22%), and H influenzae (10%). Among culture-confirmed case-patients with known outcome, case-fatality rate was 7.0% and 12.2% among children ≤5 years old and those >5 years old, respectively. Declining numbers of Haemophilus influenzae type b meningitis cases within 2 years post-Haemophilus influenzae type b conjugate vaccine introduction were observed in Pakistan.
Conclusions
Bacterial meningitis continues to cause significant morbidity and mortality in the Eastern Mediterranean Region. Surveillance networks for bacterial meningitis ensure that all sites are using standardized methodologies. Surveillance data are useful to monitor impact of various interventions including vaccines, but maintaining data quality requires consistent reporting and regular technical support.
Bacterial meningitis, which is associated with high morbidity and mortality, continues to be an important public health priority in the Eastern Mediterranean Region (EMR). Among children beyond the neonatal period, the majority of bacterial meningitis is caused by Haemophilus influenzae type b (Hib), Streptococcus pneumoniae (Sp), and Neisseria meningitidis (Nm), for which effective vaccines are currently available. In addition, the EMR has experienced multiple outbreaks of meningococcal meningitis over the last 3 decades, especially in Saudi Arabia, where the Hajj takes place,1 and in Sudan,2 which is part of the “meningococcal meningitis belt”. Adequate laboratory-based surveillance is critical to generate data on disease burden, monitor the impact of vaccines postintroduction, and detect outbreaks early.
Pathogen-specific burden of disease data for bacterial meningitis are still limited in the EMR.3 Over the last decade, many countries in the region introduced Hib conjugate vaccines into routine childhood vaccination schedules which has led to a significant decrease in burden of disease.4 However, only a few countries in the region have introduced pneumococcal conjugate vaccines to date. The support from the GAVI Alliance has made it possible for low-income countries to introduce Hib and pneumococcal conjugate vaccines; however, most countries in the region, in particular low-middle income countries, have been struggling to introduce these vaccines, and meningococcal vaccine has just been introduced by the first country (Sudan) in the region. In addition to the financial constraints, the lack of adequate data on disease burden and the distribution of the various etiologies as well as serotypes/serogroups are often cited as important reasons for the delay in making evidence-based decisions for vaccine introduction. Obtaining adequate data on meningitis burden requires a strong surveillance system and microbiology laboratory infrastructure, but it can also be affected by the high proportion of children who receive antibiotics before cultures are collected. Standardized procedures for surveillance and adequately trained laboratories can lead to a significant improvement in the quality of the data collected, however. The recent introduction of polymerase chain reaction has also helped increase the yield of tests from cerebrospinal fluid (CSF) specimens.
In January 2004, World Health Organization (WHO)/Eastern Mediterranean Regional Office (EMRO) sponsored a workshop to develop guidelines for establishing laboratory-based bacterial meningitis surveillance (BMS) in the region. This workshop resulted in 8 countries becoming part of the network and developing their individual work plans. Countries started conducting surveillance in 2005–2006. The principal objectives of the surveillance are to monitor disease trends and detect outbreaks, provide data necessary for evidence-based decision making regarding vaccine introduction, monitor impact of vaccines and other interventions, and build national laboratory capacity.
In this article, we describe the results of the first few years of enhanced laboratory-based surveillance for bacterial meningitis, as well as the challenges associated with managing such a network.
Methods
The BMS network in the EMR is supported by the WHO/EMRO and partners. Launched in early 2004, the 10 EMR countries currently participating in the network include Afghanistan, Iran, Iraq, Egypt, Libya, Morocco, Pakistan, Sudan, Syria, and Yemen. Table I lists the participating hospitals and years for which data are provided for this analysis. Selection of hospitals participating in the enhanced BMS network was based on existing epidemiologic and laboratory capacity of each site along with the representativeness of the proposed surveillance population. Table II lists the years of introduction of the pediatric Hib and pneumococcal conjugate vaccines by EMR countries. One country (Syria) introduced Hib conjugate vaccine before surveillance was initiated, and 6 introduced Hib conjugate vaccine following surveillance initiation. Morocco was the first country to introduce pneumococcal conjugate vaccine (2010).
Table I.
Participating countries, sentinel hospitals, and years* of data collection and contribution
| Country | Sentinel hospitals | Years of data contributed |
|---|---|---|
| Afghanistan | Indera Gandi Hospital | 2008–2010 |
| Egypt | Abbassia Fever Hospital | 2009–2010 |
| Alexandria Fever Hospital | ||
| Imbaba Fever Hospital | ||
| Mahala Fever Hospital | ||
| Qena Fever Hospital | ||
| Iran | Abouza Khouzestan | 2007–2008 |
| Besat Kordestan | ||
| Bou Ali Sina Mazandaran | ||
| Iraq | Al-Kadhymia | 2007–2010 |
| Central Child | ||
| Children Welfare | ||
| Tikrit | ||
| Babil Pediatric Hospital | ||
| Basrah General Hospital | ||
| Elwiyah Pediatric Hospital | ||
| Libya | Benghazi Pediatric Hospital | 2006–2008 |
| Batnan Medical Center | ||
| Zliten General Hospital | ||
| Tripoli Pediatric Hospital | ||
| Morocco | Ibn Ruchd University Hospital, Casablanca | National meningitis surveillance since 2004; enrollment in network in 2007 |
| Rabat Pediatric University Hospital | ||
| Beni Malal General Hospital, Beni Mallal | ||
| Mohamed Elkhamis General Hospital, Tanger | ||
| Pakistan | National Institute of Child Health, Karachi | 2005–2010 |
| Mayo hospital, King Edward Medical University, Lahore | ||
| Childrens Hospital | ||
| Sudan | Umdorman Pediatric Hospital | 2004–2010 |
| ElObayed Hospital | ||
| Khartoum Pediatric Hospital | ||
| Madani Pediatric Hospital | ||
| Dongola Hospital | ||
| Gadaref Hospital | ||
| Niyala Hospital | ||
| Port Sudan Hospital | ||
| Syria | Al Asad Hospital, Hama | 2005–2010 |
| Al Basel Hospital, Tartous | ||
| Pediatric Hospital, Aleppo | ||
| Al Mogtahed Hospital, Damascus | ||
| National Hospital, Lattakia | ||
| Al Asad Hospital, Lattakia | ||
| Pediatric Hospital, Damascus | ||
| Yemen | El Saudi Hospital, Hajjeh | 2004–2010 |
| El Swedy Hospital, Taiz | ||
| El Wehda Hospital, Aden | ||
| El Sabe’en Hospital, Sana’a | ||
| El Thawra, Sana’a | ||
| El Thawra, Ebb |
For each country, not all hospitals contributed data for all years.
Table II.
Introduction of the pediatric Hib and pneumococcal conjugate vaccines by EMR countries
| Country | Year of Hib introduction | Year of PCV introduction |
|---|---|---|
| Afghanistan | January 2009 | Conditional GAVI approval for introduction |
| Iran | No current plans for introduction | No current plans for introduction |
| Iraq | January 2012 | No current plans for introduction |
| Libya | 2007 | No current plans for introduction |
| Morocco | 2007 | October 2010 |
| Pakistan | October 2008–January 2009 | Phased introduction began October 2012 |
| Sudan | January 2008 | Introduction planned for 2013 |
| Syria | 2001 | No current plans for introduction |
| Yemen | 2005 | January 2011 |
PCV, pneumococcal conjugate vaccine; GAVI, Global Alliance for Vaccines and Immunization.
Participating hospitals apply consistent case definitions and standard operating procedures for all related clinical, epidemiologic, and laboratory procedures (http://www.who.int/nuvi/surveillance/resources/en/index.html). Data collected using standardized case report forms are transmitted monthly to EMR regional coordinator for aggregation and analysis. Institutional review board review of BMS network was not needed as this was considered an ongoing national surveillance activity; all procedures were part of standard case management.
Case Definitions
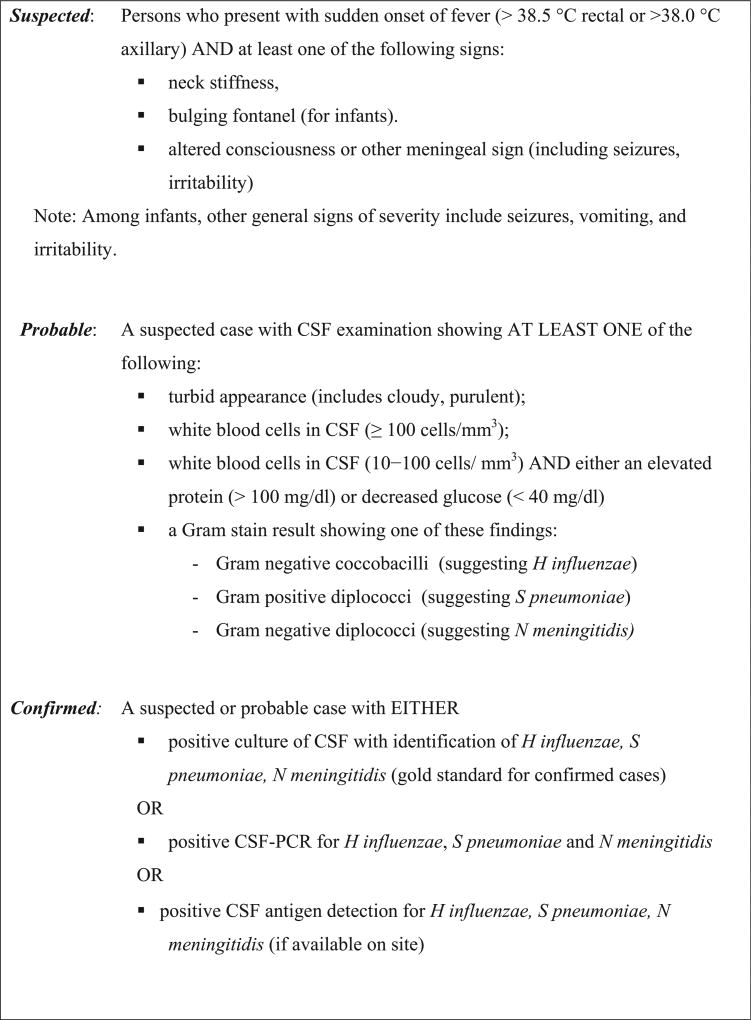
The BMS network enrolled all persons older than 30 days; the neonatal period was excluded from surveillance because of the differing epidemiology and microbiology of bacterial meningitis in this age group. Case definitions used (suspected, probable, and confirmed bacterial meningitis) are described in Figure 1.
Figure 1.
Case definitions for acute BMS. PCR, polymerase chain reaction.
Specimen Collection and Laboratory Testing
Once a case of suspected meningitis was identified, CSF was collected and tested for glucose, protein, cell counts, gram stain, and culture. Where possible, CSF was also tested for presence of Sp, Hib, or Nm by latex agglutination. Standard testing methodologies used are described elsewhere.5
Data Collection, Aggregation, and Analyses
Using a standardized case report form, information collected included patient demographics, clinical presentation of disease, vaccination status, laboratory results, and clinical outcome. Country data are securely transmitted to the WHO/EMRO for aggregation and analysis each month. Summary data are fed back to contributing countries and WHO.
For this study, we analyzed aggregated multisite data by 2 age groups: (1) ≤5 years old; and (2) >5 years old. We compared the distribution of clinical symptoms and discharge diagnoses by the 3 case definitions and by age group. In calculations of case-fatality ratios, we excluded cases with unknown outcome.
To evaluate trends over time, we restricted our analyses to the countries that had 2 or more years of surveillance data collected both before and after introduction of Hib or pneumococcal vaccine: Pakistan (King Edward Medical University, National Institute of Children Health) and Sudan (Khartoum General and Pediatric Hospital, Omdurman General and Pediatric Hospital). However, Sudan had incomplete reporting in 2006 because of interruption of financial support, thus, leaving only Pakistan for the trend analysis. Trends of bacterial meningitis prior to and following Hib conjugate vaccine introduction in Morocco have been presented previously.6
Results
Descriptive Epidemiology of Meningitis
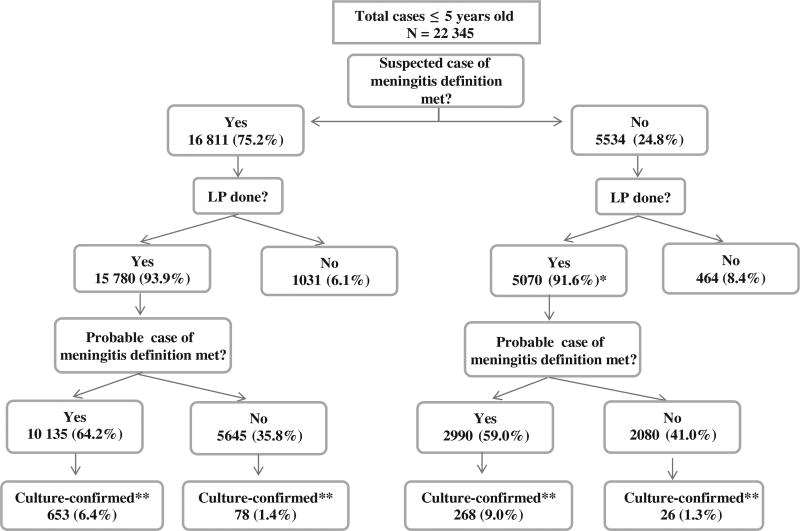
Among the 10 participating EMR countries, 48 sentinel hospitals contributed surveillance data covering a reporting range of 4 months to 7 years (Table I). In total, almost 30 000 cases were enrolled, of which 22 345 (77%) were in children aged 1 month-5 years old. The distribution of suspected, probable, and culture-confirmed cases among children ≤5 years old is presented in Figure 2. Of the 22 345 cases of children ≤5 years old who were suspected to have meningitis, 75% met the strict suspected case definition. The remaining 25% were clinically suspected to have meningitis and enrolled into surveillance without meeting the criteria for a suspected case, mainly because fever was not present or information on fever was unavailable.
Figure 2.
Distribution of suspected, probable, and culture-confirmed cases among children <5 years old. *Cases were classified as not meeting suspected case definition due to missing clinical data. †Culture-confirmed: Any case with positive culture of CSF with identification of Haemophilus influenzae, Sp, Nm (gold standard for confirmed cases). Total confirmed includes other bacteria or nonspecified bacteria. Note: PCR and antigen detection data not included. LP, lumbar puncture
Lumbar puncture was performed in 20 850 (94%) cases of children ≤5 years old, of whom 13 125 (63%) met the definition for probable meningitis. Culture confirmation of 1 of the 3 vaccine-preventable bacteria was obtained for 653 (5%) of the probable cases in this age group.
Epidemiology of Meningitis by Bacterial Etiology
The distribution of confirmed bacterial pathogens isolated from CSF by the 2 age groups is shown in Table III (including all cases undergoing lumbar puncture regardless of whether or not they met the suspected or probable case definition). The most common pathogen identified in children ≤5 years old was Sp (27%) followed by Nm (22%) and Hib (10%). In children >5 years old, the most common pathogen identified was Nm (50%). As a single country, Sudan contributed the largest number of Nm cases (n = 130). In 2 BMS network countries (Morocco, Syria), Hib conjugate vaccine was introduced prior to surveillance initiation; in 4 additional countries (Pakistan, Yemen, Libya, Iraq), Hib conjugate vaccine introduction occurred during the reported surveillance period. In Pakistan, the only country with uninterrupted data available before and after Hib conjugate vaccine introduction in 2008, the percent of Haemophilus influenzae among cases of confirmed meningitis reported in 2010 (4.0%) had declined by 72% compared with the average for the 3 years prior to vaccine introduction (2005–2007; 14.2%). In comparison, the percent of Sp among confirmed cases of meningitis declined by 17% (45.8% [average, 2005–2007] vs 38.0%) (Table IV).
Table III.
Culture-confirmed* cases by age-group and pathogen among all cases undergoing lumbar puncture
| Age group |
Total no. of specimens tested, N |
Total confirmed, n |
Hi, n (% of confirmed) |
Sp, n (% of confirmed) |
Nm, n (% of confirmed) |
|---|---|---|---|---|---|
| ≤5 y old | 16 646 | 1025 | 97 (10%) | 279 (27%) | 248 (22%) |
| >5 old | 4596 | 418 | 14 (3%) | 124 (30%) | 210 (50%) |
| Total | 21 242 | 1443 | 111 (8%) | 403 (28%) | 458 (32%)† |
Hi, Haemophilus influenza; PCR, polymerase chain reaction.
Culture-confirmed: Any case with positive culture of CSF with identification of Hi, Sp, Nm (gold standard for confirmed cases). Note: PCR and antigen detection data not included. Total confirmed includes other bacteria or nonspecified bacteria.
n = 130 cases of Nm meningitis were reported by Sudan, including 79 cases among children ≤5 years old.
Table IV.
| Calendar year | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 |
|---|---|---|---|---|---|---|
| Case definition | ||||||
| Probable | 684 | 1828 | 1070 | 319 | 904 | 495 |
| Confirmed by culture only‡ | 29 | 56 | 39 | 16 | 122 | 50 |
| S pneumoniae (% of confirmed) | 19 (65.5%) | 23 (41.1%) | 12 (30.8%) | 1 (6.3%) | 20 (16.4) | 19 (38.0) |
| H influenzae (% of confirmed) | 2 (6.9%) | 10 (17.9%) | 7 (17.9%) | 0 (0%) | 3 (2.5%) | 2 (4.0%) |
| N meningitidis (% of confirmed) | 2 (6.9%) | 2 (3.6%) | 2 (5.1%) | 1 (6.3%) | 1 (0.8%) | 0 (0%) |
Participating sentinel hospitals: King Edward University, National Institute of Children Health.
Hib conjugate vaccine was introduced in October 2008–January 2009. Anecdotally, efforts to improve meningitis surveillance were increased in 2009.
Includes confirmed cases that did not meet probable case definition. Total confirmed includes other bacteria and nonspecified bacteria.
Among the 1263 culture-positive cases for which outcome information was available, 7.0% and 12.2% of children ≤5 years old and those >5 years old, respectively, died. The case fatality ratio was generally higher in the older age group (Table V). The distribution of clinical symptoms and discharge diagnoses—including case fatality ratios—for suspected, probable, and confirmed cases are shown in Tables VI and VII. In general, older cases (>5 years old) were more likely to present with headache, neck rigidity, and vomiting and less likely with seizures and convulsions than young children. Fifty percent to 75% of survivors (all ages) were reported as having neurologic complications.
Table V.
CFRs* by age group and pathogen
| Age group |
Confirmed total† | Hi | Sp | Nm | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||
| Deaths | CFR (%) |
Deaths | CFR (%) |
Deaths | CFR (%) |
Deaths | CFR (%) |
|
| ≤5 y old | 70 | 7.0% | 13 | 13.4% | 22 | 7.9% | 14 | 5.7% |
| >5 y old | 51 | 12.2% | 2 | 14.3% | 26 | 21.0% | 13 | 6.2% |
| Total | 121 | 8.4% | 15 | 13.5% | 48 | 11.9% | 27 | 5.9% |
CFR, case fatality-ratio.
CFR: proportion of deaths out of cases with known outcome (unknown or missing outcome excluded).
Confirmed: suspected or probable case with positive CSF culture (excluding contaminants or cultures with no growth) or cases that did not meet suspected or probable definition but had positive CSF cultures. Totals included other bacteria.
Table VI.
Frequency* of clinical symptoms, prior use of antibiotics, and disease sequelae for suspected, probable, and confirmed cases ≤5 years old
| Case definition | Suspected N = 16 811 |
Probable† N = 13 125 |
Confirmed cases meeting probable case definition N = 921 |
Confirmed cases that did not meet probable case definition N = 104 |
|---|---|---|---|---|
| Clinical presentations: signs and symptoms | ||||
| Fever | 86% | 89% | 90% | 83% |
| Headache | 11% | 12% | 17% | 9% |
| Neck rigidity | 23% | 26% | 36% | 13% |
| Vomiting | 41% | 40% | 51% | 39% |
| Skin rash | 3% | 3% | 4% | 4% |
| Petechiae | 2% | 2% | 5% | 3% |
| Seizures/convulsions | 68% | 69% | 59% | 65% |
| Altered consciousness | 26% | 26% | 31% | 28% |
| Bulging fontanelle | 14% | 15% | 23% | 14% |
| Coma | 8% | 7% | 8% | 6% |
| Antibiotics received prior to lumbar puncture | 30% | 24% | 27% | 43% |
| Disease sequelae | ||||
| Outcome (death) | 3% | 3% | 9% | 3% |
| Neurologic complications (any) | 75% | 75% | 54% | 77% |
Proportion of cases with a given symptom. Cases with missing responses are excluded.
Includes cases that did not meet probable case definition because fever was absent at presentation or the presence of fever was unknown.
Table VII.
Frequency* of clinical symptoms, prior use of antibiotics, and disease sequelae for suspected, probable, and confirmed cases >5 years of age
| Case definition | Suspected N = 5331 |
Probable† N = 4898 |
Confirmed cases meeting probable case definition n = 398 |
Confirmed cases that did not meet probable case definition n = 20 |
|---|---|---|---|---|
| Clinical presentations: signs and symptoms | ||||
| Fever | 82% | 85% | 87% | 80% |
| Headache | 70% | 72% | 73% | 56% |
| Neck rigidity | 64% | 68% | 82% | 58% |
| Vomiting | 67% | 69% | 70% | 60% |
| Skin rash | 5% | 4% | 7% | 13% |
| Petechiae | 5% | 5% | 9% | 0 |
| Seizures/convulsions | 38% | 36% | 39% | 54% |
| Altered consciousness | 33% | 33% | 46% | 17% |
| Coma | 14% | 13% | 24% | 8% |
| Antibiotics received prior to lumbar puncture | 31% | 29% | 25% | 36% |
| Disease sequelae | ||||
| Outcome (death) | 6% | 7% | 19% | 5% |
| Neurological complications (any) | 70% | 69% | 50% | 74% |
Proportion of cases with a given symptom. Cases with missing responses were excluded.
Includes cases that did not meet probable case definition because fever was absent at presentation or the presence of fever was unknown.
Discussion
This article describes the results of the first 6 years of surveillance for bacterial meningitis in a network of selected countries in the WHO EMR, first established in 2004. With thousands of cases of bacterial meningitis identified, this article highlights the burden of bacterial meningitis in the region, indicating its continuing public health importance. Though the network required significant efforts to be built, it enabled countries in the region to develop adequate bacteriologic laboratory capacity and to conduct surveillance using standard methods and procedures, thus, improving the quality of the data. This report also demonstrates how surveillance data can be useful to monitor the impact of public health interventions, such as the recent introduction of Hib conjugate vaccines, and also can help with policy decisions regarding the introduction of other new vaccines such as pneumococcal vaccines.
Morbidity and Mortality
Sp is the most important cause of bacterial meningitis currently among children ≤5 years old in the region, an expected finding given that most countries in the EMR have introduced Hib conjugate vaccines. These data support the fact that conjugate pneumococcal vaccines may further reduce morbidity and mortality from bacterial meningitis, in addition to its known impact on severe pneumonia.7,8 During this surveillance period, only a small proportion of available Sp isolates was serotyped. However, other reports have recently shown that either the 10-valent or 13-valent pneumococcal conjugate vaccine would provide adequate protection for the EMR countries.9
Vaccine Impact
In addition to supporting decisions for various interventions such as new vaccine introduction, surveillance allows countries to monitor the impact of such interventions. However, for surveillance data to be used for this purpose, efforts should be made to ensure surveillance methods and population covered, as well as data reporting, remain constant over time. In the absence of these essential requirements, it is very difficult to interpret the data and be confident when using the data for trend analyses, as illustrated in the case of Sudan and Pakistan.
Challenges Establishing a Network for Bacterial Disease Surveillance
Establishing bacterial surveillance for meningitis is challenging and requires significant support and capacity building both at the laboratory and the clinical levels. These challenges include providing adequate training initially and through regular follow-up, ensuring a continuity in specimen processing even when the laboratory is not open at all times, and maintaining adequate laboratory supplies and management. For example, as seen in this article, we found that many confirmed cases did not meet the “suspected” case definition, indicating that clinicians were still using their clinical judgment to enroll meningitis cases. However, when these patients underwent lumbar puncture, a large proportion of them met the probable case definition, and the rate of culture confirmation was similar to those who met the suspected case definition (Figure 2). If we had excluded these cases early on, we would have missed a significant proportion of disease. In addition, the suspected case definition might not have been consistently implemented, resulting in inaccurate or incomplete collection of information on the presenting signs and symptoms. It is also possible that use of antibiotics and antipyretics prior to admission and evaluation at the hospital may have both changed the clinical presentation—as indicated by signs and symptoms collected on the case report form—of some patients who are still suspected of having possible meningitis by the physicians evaluating them, and also resulted in lower rates of culture-confirmed infections.
In addition, the variability of performance between countries and between hospitals within the same country, personnel turnover and the requirement of regular training, and the need for continuous monitoring added to the difficulties encountered. Challenges in coordinating a surveillance network for Hib and pneumococcal disease had been discussed earlier.10 Also, the network sought to establish meningitis surveillance in countries with inadequate surveillance infrastructure. WHO worked closely with the ministries of health and other partners in the various countries to strengthen the national public health laboratories and enable them to play a leadership role in supporting the sentinel surveillance sites. In addition to the expected challenges, this region has experienced multiple political conflicts, often resulting in difficulties in visiting some of the sites, as well as shipping materials and specimens across borders.
Despite the variety of challenges encountered, the benefits of this regional meningitis surveillance network are multifold. Member countries have already used the data for public health decision making such as introduction of Hib conjugate vaccine in Iraq and development of applications for Global Alliance for Vaccines and Immunization support of pneumococcal vaccine introduction in Afghanistan, Yemen, and Sudan. In some major hospitals, bacterial diseases surveillance was established for the first time. Development of capacity for laboratory surveillance of bacterial diseases in public hospitals has benefited other programs, which use the same microbiologic platform to identify other causes of meningitis such as typhoid and salmonella, in addition to improving laboratory practices overall such as reducing contamination rates.
Starting in 2010, the EMR joined the WHO Invasive Bacterial Diseases Surveillance network to become part of a global network that can benefit from additional technical support. Many lessons can be learned from this surveillance network that will be useful for the next phase in the region. These include the benefits of capacity building, value of site visits, the importance of consistent reporting using standardized case definitions and operating procedures, and the need for regional meetings to encourage collaboration and to motivate poorly performing surveillance sites. As countries gain more experience, and as the political situation in the EMR continues to evolve, decisions need to be made at the regional and country level about the optimal surveillance model, depending on how countries plan to use the data to support public health decisions regarding various interventions, such as the introduction of life saving conjugate pneumococcal vaccines.
Acknowledgments
The authors acknowledge the efforts of multiple persons who have contributed to the development of the meningitis network in all participating hospitals and in the following institutions: the US Naval Medical Research Unit 3 (in particular, Drs Guillermo Pimental, Mumtaz Wasfy, and Salma Afifi), located in Cairo, Egypt, which served as the regional reference lab until 2007; the Central Public Health Laboratory, Cairo, Egypt, which is the current regional reference laboratory; and Centers for Disease Control and Prevention’s Global Reference Laboratory, Atlanta, Georgia, which provides tremendous support to the region and all countries and hospitals contributing to the network hospitals.
Funded by the World Health Organization, the GAVI Hib Initiative, and Pneumococcal Vaccines Accelerated Development and Introduction Plan consortiums, funded by the GAVI Alliance. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Glossary
- BMS
Bacterial meningitis surveillance
- CSF
Cerebrospinal fluid
- EMR
Eastern Mediterranean Region
- EMRO
Eastern Mediterranean Regional Office
- Hib
Haemophilus influenzae type b
- Nm
Neisseria meningitidis
- Sp
Streptococcus pneumoniae
- WHO
World Health Organization
Footnotes
Author Disclosures
The authors declare no conflicts of interest, real or perceived.
References
- 1.Lingappa JR, Al-Rabeah AM, Hajjeh R, Mustafa T, Fatani A, Al-Bassam T, et al. Serogroup W-135 Meningococcal disease during the Hajj, 2000. Emerg Infect Dis. 2003;9:665–71. doi: 10.3201/eid0906.020565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afifi S, Karsany MS, Wasfy M, Pimental G, Marfin A, Hajjeh R. Laboratory-based surveillance for patients with acute meningitis in Sudan, 2004–2005. Eur J Clin Microbiol Infect Dis. 2009;28:429–35. doi: 10.1007/s10096-008-0643-y. [DOI] [PubMed] [Google Scholar]
- 3.Hausdorff WP, Hajjeh R, Al-Mazrou A, Shibl A, Soriano-Gabarro M. The epidemiology of pneumococcal, meningococcal, and Hib disease in North Africa and the Eastern Mediterranean Region (EMR)—current status and needs. Vaccine. 2007;25:1935–44. doi: 10.1016/j.vaccine.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Gessner BD. Haemophilus influenzae type b vaccine impact in resource-poor settings in Asia and Africa. Expert Rev Vaccines. 2009;8:91–102. doi: 10.1586/14760584.8.1.91. [DOI] [PubMed] [Google Scholar]
- 5.CDC and WHO. Laboratory methods for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. [Accessed September 12, 2012]; Available at: http://whqlibdoc.who.int/hq/2011/WHO_IVB_11.09_eng.pdf.
- 6.Braikat M, Barkia A, El Mdaghri N, Rainey JJ, Cohen AL, Teleb N. Vaccination with Haemophilus influenzae type b conjugate vaccine reduces bacterial meningitis in Morocco. Vaccine. 2012;30:2594–9. doi: 10.1016/j.vaccine.2012.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 8.Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomized, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–46. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 9.Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Hance LF, Reithinger R, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the Pneumococcal Global Serotype Project. PLoS Med. 2010;7:e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine OS, Cherian T, Hajjeh R, Knoll MD. Progress and future challenges in coordinated surveillance and detection of pneumococcal and Hib disease in developing countries. Clin Infect Dis. 2009;48(Suppl 2):S33–6. doi: 10.1086/596479. [DOI] [PubMed] [Google Scholar]