Abstract
Background
Despite extensive study of the impact of stroke on muscle and functional performance, questions remain regarding the extent to which changes are due to the neurological injury vs. age-related loss of morphology and force production.
Objectives
To synthesize available evidence describing post-stroke changes in lower extremity muscle size and strength compared to healthy adults.
Methods
Scientific literature was searched up to April 2016 to identify studies that included lower extremity muscle size and strength measures in individuals with chronic stroke. Lower extremity muscle size and strength data from healthy controls were sought for comparison. Relative differences were calculated between paretic, nonparetic, and control limbs.
Results
Fifteen studies with 375 participants (61% male; age = 62 ± 5 years; time since stroke = 60 ± 42 months) were included. The paretic limb exhibited deficits of ~13% in thigh muscle size, ~5% in lower leg muscle size, and ~8% in lean leg mass compared to the nonparetic limb. Paretic plantarflexor and knee extensor strength were 52 and 36% lower, respectively, compared to the nonparetic limb. When compared to age-matched control data, both paretic and nonparetic limbs showed deficits in muscle size and strength.
Conclusions
Age-related differences support the impact of stroke-related sarcopenia as a contributor to hemiparetic muscle dysfunction. Understanding these muscular changes is necessary for designing appropriate exercise interventions aimed at restoring muscle function.
Keywords: Stroke, rehabilitation, muscle, muscle mass, strength, sarcopenia, systematic review
Introduction
Post-stroke muscular dysfunction is likely a multi-factorial phenomenon that includes contributions from decreased descending drive and disuse (reduced physical activity and compensatory motor patterns) that lead to muscle atrophy and weakness. The fact that stroke is often associated with advanced age has also brought recent attention to the potential impact of aging on hemiparetic muscle. These post-stroke skeletal muscle adaptations have even been referred to as “stroke-induced sarcopenia.”1 Sarcopenia, the age-related loss of muscle mass and function, is recognized as a diagnosable and treatable condition in aging adults, while it has more recently become a focus in the evaluation and treatment of individuals following stroke. Given the nature of neurological insult, the prevailing notion is that muscle recruitment (i.e. central activation) are predominately responsible for weakness post-stroke. However, muscle atrophy has also been shown to be a contributing mechanism underlying hemiparetic weakness.2–4 In a clinical point of view, paretic muscle atrophy strongly correlates with decreased gait speeds and reduced fitness levels in individuals following stroke.4 Despite this information, current clinical practice guidelines fail to adequately address the peripheral muscle adaptations post-stroke, thus clinicians often fail to emphasize attenuation of muscle atrophy in rehabilitation.5,6
Normative data to describe age-related changes in skeletal muscle exist in healthy adults, while the concept of stroke-related sarcopenia is relatively new. Across all individuals, both muscle power and strength decline around the age of 40 years, with power decreasing earlier and more rapidly.7 In older adults, normative data reveal that muscle mass decreases ~0.1 kg per year.8 A previous systematic review9 demonstrated that individuals following stroke experience loss of muscle mass in both the paretic and nonparetic limbs, but little is known about how these losses compare to those of neurologically healthy age-matched adults. The lack of available knowledge regarding changes in muscle mass and their relationship to weakness and function, all of which are criteria in the diagnosis of sarcopenia,10 represents a significant deficiency in the scientific literature. Therefore, the purpose of this systematic review is to synthesize available evidence describing post-stroke changes in lower extremity muscle size and strength compared to healthy adults.
Methods
Literature search methods
This systematic review is based on a search of scientific literature from their inception up to April 2016 of the following databases: PubMed/Ovid, CINAHL, Scopus, and Cochrane. Medical subject headings and CINAHL headings, as well as appropriate search teams, were applied. The following is the search used in PubMed: (“stroke” [Mesh]) AND (“muscle strength” [Mesh] OR “muscle weakness” [Mesh] OR “muscle strength dynamometer” [Mesh]) AND (“muscles” [Mesh] OR “muscle mass” OR “sarcopenia” [Mesh] OR “muscular atrophy” [Mesh]). The search was further limited to the English language and human subjects.
Eligible studies
Once duplicate articles were deleted, titles and abstracts were screened by two independent reviewers. Full-text articles were retrieved and reviewed by two independent reviewers for selection reliability. After identifying articles, references were checked for additional relevant studies. Inclusion criteria were the following: (1) Studies included participants of any age who were in the chronic (>6 months) phase of stroke. (2) Studies reported a muscle size (i.e. muscle mass, cross sectional area, volume, thickness) measure of the lower extremity. (3) Studies reported a strength measure of the lower extremity. Any study design was considered, and intervention studies were included if they contained baseline measures of a treatment group and/or a stroke control group.
Data extraction and analysis
Study characteristics, patient demographics, muscle size measures, and muscle strength measures were extracted from each study. For continuous variables, the means and standard deviations were extracted. Muscle size and strength data from healthy control subjects were also extracted in order to make comparisons to the stroke participants. When control data was not available for comparisons, normative data from the literature for muscle size8,11–15 and strength8,16 were sought in order to compare paretic and non-paretic muscle size and strength measures to reference data. Muscle size and strength reference data of younger17 and older8 non-stroke adults were compared to the stroke data of this systematic review to examine the concept of stroke-related sarcopenia. Finally, correlations between muscle size and strength measures were also extracted from studies. The strength of correlations was categorized as low (<0.25), fair (0.25–0.49), moderate to good (0.50–0.74), and good to excellent (>0.75).18 Corresponding authors of the included studies were contacted in cases when relevant data could not be extracted.
To assess risk of bias in individual studies, we used two tools, both recommended by the Cochrane Handbook. The Risk of Bias Assessment Tool19 was used for randomized trials and the Newcastle–Ottawa Quality Assessment Scale20 was used for observational studies. With the Newcastle–Ottawa Scale, two items (“case definition” and “ascertainment of exposure”) were customized to the stroke population and the research question at hand. For the item, “Is the case definition adequate?” a star was given when studies included data on type and location of stroke (i.e. more information than just the participants self-reporting occurrence of stroke). For the item “ascertainment of exposure,” a star was given if there was any blinding to study aim(s), cohort allocation, or side of paresis. Level of evidence was reported for each of the included studies, using the Oxford Centre of Evidence- Based Medicine (CEBM) table.21 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used throughout the development of this review.22
Results
Inclusion of studies
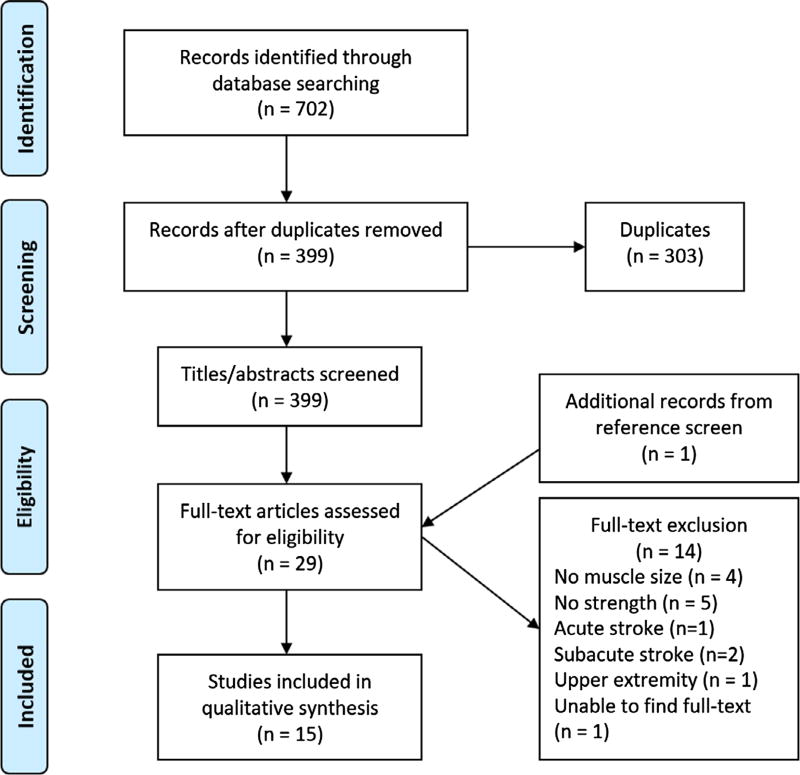
The database search revealed 702 references. After removing duplicates, screening titles/abstracts and full-texts, and searching references, 15 studies were identified for inclusion in this review. (Figure 1) Upon screening titles and abstracts, the primary and secondary reviewers had a 99% agreement. These reviewers achieved a 100% consensus for full-text. Two studies23,24 in this review utilized the same subjects, whose demographic data are represented only once in this systematic review.
Figure 1.
Flow of studies through the review.
Quality of studies
Based on the criteria from the Centre of Evidence-Based Medicine, one study (randomized controlled trial) represents Level 1, eight studies (case-controls) represent Level 3, and six studies (five cross-sectional [no controls] and one non-controlled trial) represent Level 4. (Table 1) Muscle size measures are primary outcomes in 14 of the included studies. Due to the inclusion of various study designs, risk of bias is high. (Tables 2 and 3) The lack of blinding is also a limitation across several studies, and many25–28 have incomplete outcome data specific to the research questions of this review. Control subjects were adequately defined in all case-control studies and matched to stroke subjects by one or more factor (i.e. age, sex, BMI) in most case-control studies.
Table 1.
Characteristics of included studies.
| Author (year) | Study design/level of evidence |
n | Subject characteristics | Muscle size measure | Muscle size outcome | Strength measure | Muscle strength outcome | Muscle size & strength relation- ship |
|---|---|---|---|---|---|---|---|---|
| Durand, et al.37 (2015) | Case-control (with age- and sex-matched controls)/level 3 | 10 | 6 men, 4 women | DEXA-lean muscle mass of lower limbs (kg) | P: 7.8 ± 2.3 kg | MVIC of knee extensors on dynamometer (Nm) | P: 50.6 ± 31.3 Nm | No correlations described between these outcomes |
| 63 ± 7 years | NP: 9.0 ± 2.7 kg | NP: 87.2 ± 53.0 Nm | ||||||
| TSS: 14.3 ± 7.1 years | ||||||||
| FMA-LE: 23 ± 7 | ||||||||
| Ambulate 30 ft with or without assistive device | ||||||||
| Frohlich-Zwahlen, et al.25 (2014) | Case-control (with age- and sex-matched controls)/level 3 | 20 | 11 men, 9 women | US- CSA of knee extensors and flexors, and ankle plantarflexors and dorsiflexors (cm2) | Cannot extract | MVIC of knee and ankle muscles on dynamometer (Nm/kg) | P knee ext: 1.39 ± 0.80 Nm/kg | P limb knee ext strength significantly correlated with VL (r = 0.40) and RF (r = 0.64) thickness. P dorsiflex strength significantly correlated with TA thickness (r = 0.77). P plantarflex strength significantly correlated with GM thickness (r = 0.49) |
| 52 ± 11 years | NP knee ext: 1.86 ± 0.72 Nm/kg | |||||||
| 78 ± 23 kg | P knee flex: 0.53 ± 0.37 Nm/kg | |||||||
| TSS: 1.9 ± 0.7 years | NP knee flex: 0.84 ± 0.31 Nm/kg | |||||||
| Walk 10 m independently | P dorsiflex: 0.20 ± 0.13 Nm/kg | |||||||
| NP dorsiflex: 0.34 ± 0.09 Nm/kg | ||||||||
| P plantarflex: 0.46 ± 0.39 Nm/kg | ||||||||
| NP plantarflex: 0.79 ± 0.30 Nm/kg | ||||||||
| Kim, et al.26 (2012) | Cross-sectional (no controls)/level 4 | 30 | 15 men, 15 women | US- thickness of medial gastrocnemius (mm) | P: 9.7 ± 05 mm | MVIC of medial gastrocnemius on dynamometer | Cannot extract | Not described |
| 68 years | ||||||||
| TSS: 17.4 mo | ||||||||
| Klein, et al.23 (2010) | Cross-sectional (controls used only to compare gait measures)/level 4 | 7 | 5 men, 2 women | MRI-CSA (cm2) and volume (cm3) of plantarflexors | CSA | MVIC of plantarflex on custom dynamometer (Nm) | P: 56.7 ± 57.4 Nm | No significant correlations between MVIC and plantarflexor (r = 0.49) or gastrocnemii (r = 0.42) volumes |
| 56 ± 4 years | P: 49.5 ± 13.5 cm2 | NP: 147.0 ± 35.7 Nm | Gait speed correlated with MVIC of P limb (r = 0.75) | |||||
| TSS: 38 mo NP | NP: 55.6 ± 14.2 cm2 | |||||||
| Independent walkers with hemiparesis | Volume | |||||||
| Gait speed: 0.83 ± 0.33 m/s | P: 1,019 ± 297 cm3 | |||||||
| 2-min walk test: 95.7 ± 37.7 m | NP: 1,154 ± 319 cm3 | |||||||
| Klein, et al.24 (2013) | Case-control (controls not matched)/level 3 | 7 | (Same subjects from Klein, 2010) | MRI- CSA (cm2) and volume (cm3) of dorsiflexors | CSA | MVIC of dorsiflexors on custom dynamometer (Nm) | P: 29.8 ± 21.3 Nm | Not described |
| 5 men, 2 women | P: 13.2 ± 3.0 cm2 | NP: 42.5 ± 12.0 Nm | ||||||
| 57 ± 9 years NP | NP: 12.9 ± 4.1 cm2 | |||||||
| TSS: 38 mo | Volume | |||||||
| Independent walkers with hemiparesis | P: 260 ± 69 cm3 | |||||||
| Gait speed: 0.83 ± 0.33 m/s | NP: 250 ± 82 cm3 | |||||||
| Knarr, et al.29 (2013) | Cross-sectional (no controls)/level 4 | 17 | 15 men, 2 women | MRI- volume of plantarflexors (cm3) | P limb 80 ± 10% of NP | MVIC of plantarflexors on dynamometer (Nm) | P limb 41% of NP | No significant correlations for volume and MVIC between limbs |
| 61 ± 9 years | ||||||||
| TSS: > 6 mo | Cannot extract specific values | Cannot extract specific values | ||||||
| MacIntyre, et al.35 (2010) | Case-Control (age- and sex-matched controls)/level 3 | 11 | 6 men, 5 women | CT- mass (mg/mm) and muscle density (mg/cm3) of calf musculature | Muscle mass | Isokinetic contraction of plantarflexors and knee extensors on dynamometer (Nm/kg) | Plantarflexors | Side-to-side differences in muscle density not significantly related to side-to-side differences in plantarflexor (r = 0.37) or knee extensor (r = 0.00) strength |
| 72 ± 12 years | P: 456.8 ± 92.4 | P: 0.30 ± 0.16 Nm/kg | ||||||
| TSS: 60.0 ± 35.8 mo NP | NP: 460.5 ± 83.4 | NP: 0.55 ± 0.19 Nm/kg | ||||||
| 71 ± 17 kg | Muscle density | Knee extensors | ||||||
| Berg balance scale: 29 ± 14 | P: 70.2 ± 5.68 | P: 0.55 ± 0.21 Nm/kg | ||||||
| 8/11 subjects use walking aid | NP: 70.9 ± 5.11 | NP: 0.72 ± 0.21 Nm/kg | ||||||
| Marin, et al.36 (2013) | Randomized controlled trial/level 1 | 20 | 11 men, 9 women | US- thickness of rectus femoris, vastus lateralis, medial gastrocnemius (cm) | Rectus femoris | MVIC of knee extensors on dynamometer (Nm) | P: 68.0 Nm | Not described |
| 63 years | P: 1.23 cm | NP: 92.6 Nm | ||||||
| TSS: 4.3 years | NP: 1.28 cm | |||||||
| Berg balance scale: 46 ± 9 | Vastus lateralis | |||||||
| NIH stroke scale: 1.25 | P: 1.28 cm | |||||||
| NP: 1.42 cm | ||||||||
| Medial gastrocnemius | ||||||||
| P: 1.38 cm | ||||||||
| NP: 1.41 cm | ||||||||
| Pang, et al.33 (2005) | Cross-sectional (no controls)/level 4 | 58 | 35 men, 23 women | DEXA- leg lean mass (g) | P: 7578.5 g | MVIC of knee extensor strength w/handheld dynamometer (N) | P: 188.7 ± 71.3 N | Significant correlation (r = 0.50) between leg lean mass and strength of P limb |
| 66 ± 9 years | NP: 7952.5 g | NP: 256.9 ± 86.4 N | ||||||
| TSS: 5.6 ± 5.1 years | ||||||||
| 6MWT: 312 ± 132 m | ||||||||
| Patterson, et al.29 (2007) | Cross-sectional (no controls)/level 4 | 74 | 43 men, 31 women | DEXA- leg lean mass (kg) | P: 7.62 ± 2.03 kg | Isokinetic eccentric knee extension on dynamometer (Nm) | P: 66.3 ± 38.1 Nm | P limb lean mass significantly correlated with gait speed (r = 0.25). |
| 64 ± 10 years | NP: 7.98 ± 1.97 kg | NP: 117.1 ± 42.2 Nm | Gait speed significantly correlated with P (r = 0.60) & NP (r = 0.38) limb strength | |||||
| TSS: 48 ± 59 mo | ||||||||
| NIH stroke scale: 3 ± 3 | (n = 65) | (n = 62) | ||||||
| Berg balance scale: 38 ± 8 | ||||||||
| Gait speed: 0.51 ± 0.26 m/s | ||||||||
| 6MWT: 216 ± 120 m | ||||||||
| Prado-Medeiros, et al.28 (2012) | Case-control (age- and sex-matched controls)/level 3 | 13 | 9 men, 4 women | MRI- volume of quadriceps (cm3) | Cannot extract specific values | MVIC knee flexion and extension on dynamometer (Nm/kg) | Cannot extract | Significant correlation of quadriceps volume and knee extensor strength at 60°/s (r = 0.70) |
| 54 ± 8 years | No correlation for hamstrings volume and strength | |||||||
| TSS: 47.4 ± 29.9 mo | 24 ± 11% deficit of P to NP for quadricep volume | 53 ± 19% deficit of P to NP for conc KE at 60°/s | ||||||
| 70.5 ± 13.7 kg | ||||||||
| Modified ashworth scale <3 | ||||||||
| Functional ambulation categories: level 2, 3, or 4 | ||||||||
| Ryan, et al.34 (2011) | Cross-sectional (no controls)/level 4 | 70 | 39 men, 31 women | CT- CSA (cm2) and volume (cm3) of midthigh | Area | Isokinetic knee extension at 90°/s on dynamometer (Nm) | Concentric | Eccentric strength of P & NP correlated w/volume in P (r = 0.40) & NP (r = 0.50) |
| 63 ± 1 years | P: 59.4 ± 2.5 cm2 | P: 21.9 ± 2.3 Nm | Concentric strength of NP correlated w/volume in NP (r = 0.28) | |||||
| TSS: 39 ± 7 mo | NP: 74.6 ± 2.7 cm2 | NP: 54.0 ± 5.2 Nm | ||||||
| 6MWT: 633 ± 46 m | Volume | Eccentric | ||||||
| P: 1,245 cm3 | P: 70.6 ± 5.1 Nm | |||||||
| NP: 1,545 cm3 | NP: 120.9 ± 5.7 Nm | |||||||
| Ryan, Ivey, et al.30 (2011) | Non-controlled trial/level 4 | 15 | 10 men, 5 women | CT- CSA (cm2) and volume (cm3) of midthigh | Area | 1RM- leg extension and leg press (lbs) | Leg extension | Not described |
| 65 ± 2 years | P: 68.7 ± 5.0 cm2 | P: 53 ± 8 lb | ||||||
| TSS: 8 ± 2\,years | NP: 88.1 ± 6.8 cm2 | NP: 105 ± 8 lb | ||||||
| Gait speed: 0.71 m/s | Volume | Leg Press | ||||||
| P: 460 ± 44 cm3 | P: 282 ± 36 lb | |||||||
| NP: 454 ± 37 cm3 | NP: 422 ± 33 lb | |||||||
| Silva-Couto, et al.31 (2014) | Case-control (controls matched by age, sex, and BMI)/level 3 | 14 | 12 men, 2 women | MRI- quadricep and hamstring volume (cm3) | Quadriceps | Isokinetic knee extension and flexion on dynamometer (Nm) | Concentric extension | Not described |
| 61 ± 8 years | P: 999.8 ± 247.5 cm3 | P: 87.2 ± 33.9 Nm | ||||||
| TSS: 7.3 ± 6.0 years | Hamstrings | NP: 169.9 ± 62.5 Nm | ||||||
| Fugl-Meyer: 63.5 ± 6.6 | P: 541.8 ± 138.9 cm3 | Concentric flexion | ||||||
| Gait speed: 0.80 ± 0.53 m/s | P: 42.3 ± 13.8 Nm | |||||||
| Berg balance scale: 47 ± 5 | NP: 91.7 ± 20.9 Nm | |||||||
| Eccentric extension | ||||||||
| P: 104.8 ± 48.4 Nm | ||||||||
| NP: 163.2 ± 67.0 Nm | ||||||||
| Eccentric flexion | ||||||||
| P: 135.4 ± 28.5 Nm | ||||||||
| NP: 153.9 ± 40.0 Nm | ||||||||
| Sunnerhagen, et al.32 (1999) | Case-control (compared to age-matched historical control group)/level 4 | 16 | 11 men, 5 women | CT- CSA of thigh musculature (cm2) | P: 127.2 ± 8.86 cm2 | Isokinetic knee extension and flexion on dynamometer (Nm) | Extension at 60°/s | Strong correlation of CSA and strength in P limb (r = 0.81) |
| 59 years | NP: 133.4 ± 7.57 cm2 | P: 95.3 Nm | Moderate correlation of CSA and strength in NP limb (r = 0.57) | |||||
| TSS: 14 (8–22) mo | NP: 110.0 Nm | |||||||
| Independent walkers | Flexion at 60°/s | |||||||
| Gait speed: 1.07 (0.39–1.50) m/s | P: 39.0 Nm | |||||||
| NP: 46.1 Nm |
Notes: TSS = time since stroke, FMA-LE = Fugl-Meyer lower extremity motor score, 6MWT = 6-min walk test, DEXA = dual-energy xray absorptiometry, US = ultrasonography, CT = commuted tomography, MRI = magnetic resonance imaging, CSA = cross-sectional area, MVIC = maximum voluntary isometric contraction, P = paretic, NP = non-paretic.
Table 2.
Risk of bias in experimental studies, using cochrane tool19.
| Study, Year | Random sequence generation |
Allocation concealment |
Blinding of participant/ personnel |
Blinding of outcome assessment |
Incomplete outcome data |
Selective outcome reporting |
Other sources of bias |
|---|---|---|---|---|---|---|---|
| Marin, 2013 | Low | Low | Low | Low | Low | Low | High |
| Ryan, Ivey, 2011 | High | High | High | High | Low | Low | High |
Table 3.
Risk of bias in observational studies, using Newcastle–Ottawa scale20.
| Selection | Exposure | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Study, year | Case definition adequate |
Representative cases |
Selection of controls |
Definition of controls |
Comparability | Ascertainment of exposure |
Same method for controls |
Non-response rate |
| Durand, 2015 | * | * | – | * | ** | – | – | – |
| Frohlich-Zwahlen, 2014 | * | – | – | * | ** | – | – | – |
| Kim, 2012 | * | * | n/a | n/a | n/a | – | n/a | n/a |
| Klein, 2010 | * | * | n/a | n/a | n/a | – | n/a | n/a |
| Klein, 2013 | * | – | – | * | – | – | – | – |
| Knarr, 2013 | – | – | n/a | n/a | n/a | – | n/a | n/a |
| MacIntyre, 2010 | – | * | * | * | ** | – | * | * |
| Pang, 2005 | – | * | n/a | n/a | n/a | – | n/a | n/a |
| Patterson, 2007 | – | – | n/a | n/a | n/a | – | n/a | n/a |
| Prado-Medeiros, 2012 | – | * | – | * | ** | * | – | – |
| Ryan, 2011 | * | * | n/a | n/a | n/a | – | n/a | n/a |
| Silva-Couto, 2014 | * | * | * | * | ** | * | – | * |
| Sunnerhagen, 1999 | * | * | – | * | ** | – | – | – |
Notes: Studies were given “*” if they achieved the description in each item. Under the Comparability item, studies with control groups were given “*” if they controlled on one factor and “**” if they controlled on two factors. Cross-sectional studies without control groups were given “n/a” for items related to controls.
Participant characteristics
In total, there were 375 stroke participants (sample size range: 7–74), representing 61% male and 39% female. The average age of the participants was 62 years (range: 52–72 years), and time since stroke was 60 months (range: 14–172 months).
There were also 84 healthy controls in the studies with a mean age of 56 years (range: 38–71 years). The subjects of the included studies represented many different countries to include Brazil, Canada, Spain, Sweden, Switzerland, and the United States. All post-stroke subjects had hemiparesis and were in the chronic phase, as indicated by time since stroke values. Participant level of function was not reported in a consistent, comparable manner across studies. Most studies described stroke participants as mid-to high-functioning, independent walkers, which is evident given gait speeds averaging 0.78 m/s from five included studies,23,29–32 6-Min Walk Tests averaging 387 m in three of the included studies,29,33,34 and Berg Balance Scale averaging a total score of 40/56 in four of the included studies.29,31,35,36
Muscle size measures
Several different tools were used in the studies to measure muscle size including: CT, MRI, ultrasonography, and DEXA scans. The midthigh and lower leg musculature were most often measured. Additionally, the lower extremity as a whole was examined in three studies.29,33,37 Relative differences were calculated as the muscle size of the paretic limb divided by the muscle size of the nonparetic limb, then multiplied by 100%. The lean mass of the paretic leg was an average of 92% (range: 87–95%) of the lean mass of the nonparetic leg. The paretic thigh muscle size was an average of 87% (range: 76–101%) and the paretic lower leg muscle size was an average of 95% (range: 80–104%) of the nonparetic muscle size. Specific muscle size values could not be extracted in three25,27,28 of the 15 studies, two of which used MRI and one ultrasound. (Table 1).
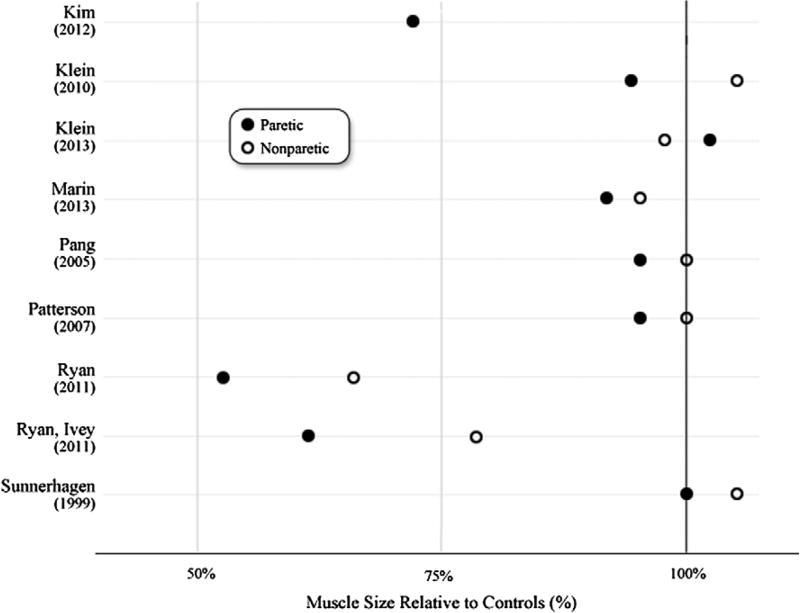
Post-stroke muscle size measures were also compared to control data. (Figure 2) When no control data from included studies were available/extractable, reference data from the literature were found. Subjects from reference literature were age-matched (±10 years) to subjects in the studies of this systematic review. This reference data come from studies with similar methods to the included studies in this systematic review. (CT,8,12,38 MRI,13,14 US11,15) Relative differences were calculated as stroke limb muscle size divided by control limb muscle size, then multiplied by 100%. (Figure 2).
Figure 2.
Paretic and nonparetic muscle size relative to control muscle.
Notes: Paretic and nonparetic muscle size values were divided by control data from included studies or normative data from the literature (CT,8,12,38 MRI,13,14 US11,15) to calculate relative differences. Different muscle groups in the same set of subjects are represented in the two studies by Klein, et al.23,24
Strength measures
Dynamometry, used in 14 out of the 15 included studies, was the primary tool used to measure muscle strength. The most examined muscle group was the knee extensors for both isometric and isokinetic dynamometry, measured in 10 of the included studies.25,28,29,31–37 Other muscle groups measured include knee flexors and ankle plantarflexors and dorsiflexors. Maximal voluntary isometric contractions were executed in the protocols of nine of the 15 included studies. Relative differences were calculated as paretic limb strength divided by the nonparetic limb strength, then multiplied by 100%. The paretic knee extensor strength was an average of 64% (51–87%) of the nonparetic knee extensor strength. Paretic knee flexor strength was an average of 65% (46–85%), paretic dorsiflexor strength was an average of 65% (59–70%), and paretic plantarflexor strength was an average of 48% (39–58%) of the nonparetic strength. Despite similar tools, researchers used many different methods and muscle groups to attain strength values, which prevented combination of data across all studies. In addition, units of measurement varied between studies. For example, force (N) is often reported, instead of torque (Nm). (Table 1).
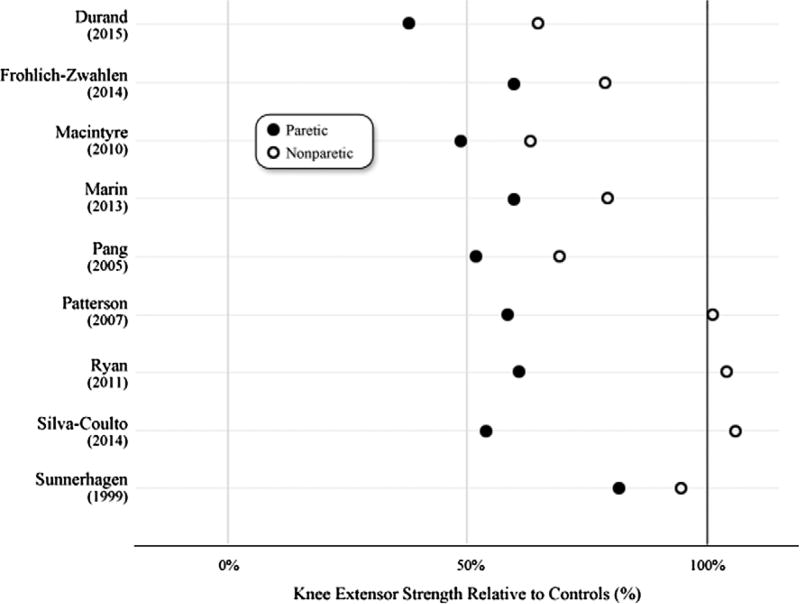
Knee extensor strength values were compared to control data (when available) or age-matched (±10 years) reference values. (isometric16 and isokinetic8) The reference data comes from studies with similar methods to those used in the included studies of this systematic review. Relative to the age-matched control data, paretic and nonparetic knee extensor strength exhibited deficits. (Figure 3).
Figure 3.
Paretic and nonparetic knee extensor strength relative to control muscle.
Notes: Paretic and nonparetic knee extensor strength were divided by control data from included studies or age-matched normative data8,16 to calculate relative differences.
Although muscle power generation was not one of the primary outcomes of this systematic review, it was measured in two of the included studies,28,31 and the paretic knee extensor power generation was an average of 57% of the nonparetic side (56% from Silva-Couto, et al.31 and 58% in Prado-Medeiros, et al.30).
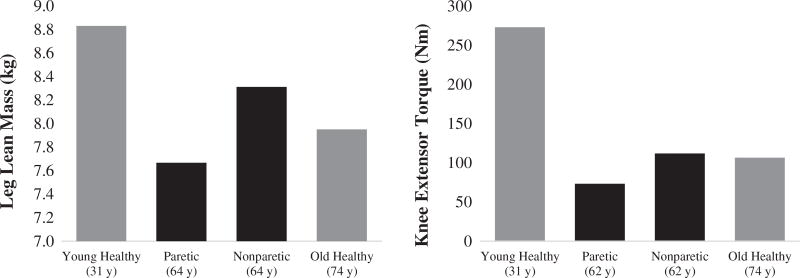
Age-related comparisons
The paretic limb exhibits significant deficits in muscle size and strength when compared to data from adults that are ≥10 years older than the sample of stroke subjects included in this analysis. (Figure 4) For strength, the nonparetic limb exhibits values near that of the older adults. Additional normative data of young adults (age: 31 years) was included to illustrate the deficits of stroke muscle size and strength in both limbs.
Figure 4.
Age-related comparisons. Notes: Black bars represent stroke data analyzed within this systematic review leg lean mass29,33,37 and knee extensor torque.25,29,32,34–37 Gray bars represent normative data from the literature for healthy muscle of young (31 years)17 and older (74 years)8 adults.
Relationships between muscle size and strength
Correlations between muscle size and strength were reported in nine of the 15 studies, with r-values widely ranging from 0.25 to 0.81. (Table 1) More specifically, the correlation coefficients averaged 0.58 for relationships between knee extensor strength and thigh muscle size, indicating a moderate relationship between these two variables. This r-value is larger than reported (r = 0.37) in a study of 2,647 non-stroke adults averaged 62 years of age,16 the same average age of the stroke participants included in this study. For the relationship between plantarflexor size and strength, correlation coefficients averaged 0.49, indicating a fair relationship.
Discussion
The purpose of this systematic review was to present data from current literature on lower extremity muscle size and strength in individuals post-stroke and to compare the data to age-matched muscle. The results revealed that the paretic limb undergoes substantial reductions in muscle size and strength. Importantly, the nonparetic limb also adapts following stroke when compared to age-matched muscle. The results of this study support the implementation of exercise interventions to reverse the muscle size and strength losses that occur in both the paretic and non-paretic limbs.
Interestingly, both limbs exhibit deficits in muscle size and strength in the included sample that is an average of 60 months post-stroke. This is considered to be the chronic phase of stroke. By only including studies with participants in the chronic phase of stroke, the chance that neurological recovery is continuing to occur has been decreased. In clinical and scientific settings, the nonparetic limb is often considered to have similar size and strength to healthy muscle, however, the results of this systematic review suggest this is not the case. A systematic review by English et al.9 revealed similar results of stroke subjects experiencing significantly less regional muscle mass bilaterally compared to healthy adults. The weakness and atrophy observed in both limbs are likely contributed to by both disuse and decreased descending drive via ipsilateral and contralateral pathways. It is often accepted that hemiparetic weakness is largely attributed to impaired central (cortical) activation, however Miller et al.39 demonstrated that central activation deficits could not entirely explain bilateral weakness. With the use of higher resolution imaging techniques, there is more valid support for muscle atrophy as a mechanism underlying hemiparetic weakness,40 though its importance in explaining recovery remains unknown. It is imperative to include age-matched controls in stroke rehabilitation research trials rather than comparing inter-limb differences, which have the potential to underestimate the impact of stroke on muscle.
An often stated goal of individuals with stroke is to increase walking speed.41 The correlations of paretic muscle atrophy29,34 and bilateral weakness29 with slower gait speeds validates them as appropriate targets in gait interventions. More specifically, deficits in plantarflexor function explain approximately 67% of variance in gait speeds42 and limit propulsion of the body forward during walking. Further, strength of the paretic plantarflexors more strongly correlates to gait dysfunction than the paretic knee extensors.43 The results of this systematic review reveal greater declines in plantarflexor size and strength than knee extensor size and strength. The post-stroke participants in the included studies had paretic plantarflexor strength deficits that averaged of 52% (much greater than the paretic knee extensor deficits that averaged 36%). Knowing that a 1–2% deficit in strength between dominant and non-dominant limbs is normal in healthy middle-aged adults,44,45 individuals with stroke show excessive imbalances between limbs. These imbalances present clinically concerning consequences that may manifest as asymmetric motor patterns (e.g. gait).46 Clinicians often strive to improve symmetry through gait interventions, however, significant strengthening (such as in evidence-based review by Pak and Patten40) to address bilateral muscle atrophy and weakness may need to precede standard functional training if optimal gains are desired.
Although muscle power was not one of the primary outcomes in the search criteria, this systematic review made apparent the lack of data on muscle power following stroke. Only two studies28,31 had extractable muscle power data. The relative power deficits (43%) of the knee extensors are greater than their strength deficits (36%), which is consistent with previous literature.47 Just as with muscle size and strength, asymmetries of muscle power between limbs were present in the data of this systematic review. Dawes et al.41 found that leg extensor power asymmetry post-stroke was more strongly correlated to decreased gait speeds and step lengths. Literature of the older adult population demonstrates that power accounts for more of the variance in functional ability than muscular strength.48–50 Although few longitudinal data exist to describe loss of muscle power after stroke, we do know that it declines at a greater and faster rate than strength in aging adults.7 In non-stroke older adults, training to improve muscle power generation appears to enhance functional outcomes (gait speed, timed-up-and-go, etc.) to a greater extent than traditional strength training.51 Initial studies with stroke participants have shown the potential for a power training intervention to improve not only muscle power generation, but also strength and gait speed.52–55 Power training may provide a more appropriate alternative to strength training to prevent age-related declines in both muscle power and strength.
This systematic review is not without limitations: (1) the lack of concordant methods across studies prevented inclusion of all muscle size and strength values in the analyses, therefore, relative differences were calculated in effort to provide quantitative results. (2) The included studies had an overall high risk of bias. Many studies had missing outcome data, specific to the research question. In other cases, muscle size and strength were not the primary outcome measures of the included studies, thus the results should be interpreted with this in mind. (3) The participants included in the studies of this systematic review represent the mid- to higher functioning stroke population as evidenced by demographic data. Inclusion of only high-functioning individuals is a common theme in stroke rehabilitation research that limits generalizability. (4) None of the included studies measured muscle size as total body skeletal muscle mass, which is the outcome recommended by the European Sarcopenia Working Group.10 This hinders the comparison of muscle size in stroke participants to large-scale sarcopenia studies, such as the study by Janssen et al.56 that designated sarcopenia cut-off points in a cohort of 14,818 older adults. (5) Lastly, by including “AND” between morphological and strength parameters in the search strategy, we may have missed some research studies. However, the intent of this review was to capture studies that evaluated both muscle size and strength, since both are included in the definition of sarcopenia.
Conclusions
The changes in muscle size and function following stroke have previously been termed “stroke-induced sarcopenia,”1 or more recently “stroke-related sarcopenia.”57 Awareness of sarcopenia, in general, has increased in older adults, as the population’s life expectancy continues to rise. More recent attention has been given to sarcopenic-like effects after stroke, with more and more individuals surviving strokes and living longer.58 The hope is that these survivors will live long and functionally independent lives. In summary, this systematic review reveals that both limbs exhibit deficits in muscle size and strength when compared to age-matched non-stroke data. Comparisons to normative data of older and younger adults without stroke expose the severe deficits in muscle size and strength in the paretic limb. As individuals with stroke get older, muscular function in both limbs may continue to decline, perhaps at greater rates than individuals without stroke. Future research should compare stroke subjects to age-matched controls across the age continuum to differentiate the cumulative impact of the neurological insult and aging on skeletal muscle. Implications for resistance training are suggested throughout this review given the potential to reverse these muscular declines.
Acknowledgments
The authors would like to thank Dr. Heather S. Bonilha, PhD, CCC-SLP for her significant contribution to this systematic review through early idea development and manuscript editing. Authors would also like to thank librarian Candace Moorer for her help and guidance in the literature search strategies.
References
- 1.Scherbakov N, von Haehling S, Anker SD, Dirnagl U, Doehner W. Stroke induced sarcopenia: Muscle wasting and disability after stroke. Int J Cardiol. 2013;170(2):89–94. doi: 10.1016/j.ijcard.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen L, Jacobsen BK. Changes in muscle mass, fat mass, and bone mineral content in the legs after stroke: A 1 year prospective study. Bone. 2001;28(6):655–659. doi: 10.1016/s8756-3282(01)00434-3. [DOI] [PubMed] [Google Scholar]
- 3.Metoki N, Sato Y, Satoh K, Okumura K, Iwamoto J. Muscular atrophy in the hemiplegic thigh in patients after stroke. Am J Phys Med Rehabil. 2003;82(11):862–865. doi: 10.1097/01.PHM.0000091988.20916.EF. [DOI] [PubMed] [Google Scholar]
- 4.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83(12):1703–1707. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- 5.VA/DOD. Clinical practice guideline for the management of stroke rehabilitation. J Rehabil Res Dev. 2010;47(9):1–43. [PubMed] [Google Scholar]
- 6.Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2016;47(6):e98–e169. doi: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 7.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American college of sports medicine position stand. exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: The health, aging and body composition study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 9.English C, McLennan H, Thoirs K, Coates A, Bernhardt J. Loss of skeletal muscle mass after stroke: A systematic review. Int J Stroke. 2010;5(5):395–402. doi: 10.1111/j.1747-4949.2010.00467.x. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dias CP, Freire B, Goulart NB, et al. Muscle architecture and torque production in stroke survivors: An observational study. Top Stroke Rehabil. 2016;0:1–8. doi: 10.1080/10749357.2016.1210873. [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The health ABC study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 13.Hasson CJ, Kent-Braun JA, Caldwell GE. Contractile and non-contractile tissue volume and distribution in ankle muscles of young and older adults. J Biomech. 2011;44(12):2299–2306. doi: 10.1016/j.jbiomech.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morse CI, Thom JM, Birch KM, Narici MV. Changes in tricep surae muscle architecture with sarcopenia. Acta Physiol Scand. 2005;183:291–298. doi: 10.1111/j.1365-201X.2004.01404.x. [DOI] [PubMed] [Google Scholar]
- 15.Strasser EM, Draskovits T, Praschak M, Quittan M, Graf A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age (Dordr) 2013;35(6):2377–2388. doi: 10.1007/s11357-013-9517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Nelson DR, Zhao Y, Cui Z, Johnston JA. Relationship between muscle mass and muscle strength, and the impact of comorbidities: A population-based, cross-sectional study of older adults in the United States. BMC Geriatrics. 2013;13:990S. doi: 10.1186/1471-2318-13-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch NA, Metter EJ, Lindle RS, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol (1985) 1999;86(1):188–194. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- 18.Portney LG, Watkins MP. Foundations of Clincal Research: Applications to Clinical Practice. 3. NJ: Pearson Prentice Hall; 2009. Systematic reviews and meta-analysis; pp. 357–381. [Google Scholar]
- 19.Higgins JPT, Green S. Cochrane Handbook for Systematic Review of Interventions Version 5.1.0. The Cochrane Collaboration. 2011 http://handbook.cochrane.org2011.
- 20.Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Accessed 2013]; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp2013.
- 21.OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2. Oxford Centre for Evidence-Based Medicine. [Accessed Februray 16, 2017]; http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Published March 2009.
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Klein CS, Brooks D, Richardson D, McIlroy WE, Bayley MT. Voluntary activation failure contributes more to plantar flexor weakness than antagonist coactivation and muscle atrophy in chronic stroke survivors. J Appl Physiol. 2010;109(5):1337–1346. doi: 10.1152/japplphysiol.00804.2009. [DOI] [PubMed] [Google Scholar]
- 24.Klein CS, Power GA, Brooks D, Rice CL. Neural and muscular determinants of dorsiflexor weakness in chronic stroke survivors. Motor Control. 2013;17(3):283–297. doi: 10.1123/mcj.17.3.283. [DOI] [PubMed] [Google Scholar]
- 25.Frohlich-Zwahlen AK, Casartelli NC, Item-Glatthorn JF, Maffiuletti NA. Validity of resting myotonometric assessment of lower extremity muscles in chronic stroke patients with limited hypertonia: A preliminary study. J Electromyogr Kinesiol. 2014;24(5):762–769. doi: 10.1016/j.jelekin.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Kim TG, Bae SH, Kim GY, Kim YE, Kim KY. Analysis of ultrasonographic architectural properties of muscles of chronic stroke patients during different muscle activities. J Phys Ther Sci. 2012;24(10):1059–1062. [Google Scholar]
- 27.Knarr BA, Ramsay JW, Buchanan TS, Higginson JS, Binder-Macleod SA. Muscle volume as a predictor of maximum force generating ability in the plantar flexors post-stroke. Muscle Nerve. 2013;48(6):971–976. doi: 10.1002/mus.23835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prado-Medeiros CL, Silva MP, Lessi GC, et al. Muscle atrophy and functional deficits of knee extensors and flexors in people with chronic stroke. Phys Ther. 2012;92(3):429–439. doi: 10.2522/ptj.20090127. [DOI] [PubMed] [Google Scholar]
- 29.Patterson SL, Forrester LW, Rodgers MM, et al. Determinants of walking function after stroke: Differences by deficit severity. Arch Phys Med Rehabil. 2007;88(1):115–119. doi: 10.1016/j.apmr.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Ryan AS, Ivey FM, Prior S, Li GH-M, Hafer-Macko C. Skeletal muscle hypertrophy and muscle myostatin reduction after resistive training in stroke survivors. Stroke. 2011;42(2):416–420. doi: 10.1161/STROKEAHA.110.602441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva-Couto M, Prado-Medeiros CL, Oliveira AB, et al. Muscle atrophy, voluntary activation disturbances, and low serum concentrations of IGF-1 and IGFBP-3 are associated with weakness in people with chronic stroke. Phys Ther. 2014;94(7):957–967. doi: 10.2522/ptj.20130322. [DOI] [PubMed] [Google Scholar]
- 32.Sunnerhagen KS, Svantesson U, Lonn L, Krotkiewski M, Grimby G. Upper motor neuron lesions: Their effect on muscle performance and appearance in stroke patients with minor motor impairment. Arch Phys Med Rehabil. 1999;80(2):155–161. doi: 10.1016/s0003-9993(99)90113-2. [DOI] [PubMed] [Google Scholar]
- 33.Pang MYC, Eng JJ, McKay HA, Dawson AS. Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporos Int. 2005;16(12):1769–1779. doi: 10.1007/s00198-005-1925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan AS, Buscemi A, Forrester L, Hafer-Macko CE, Ivey FM. Atrophy and intramuscular fat in specific muscles of the thigh: Associated weakness and hyperinsulinemia in stroke survivors. Neurorehabil Neural Repair. 2011;25(9):865–872. doi: 10.1177/1545968311408920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacIntyre NJ, Rombough R, Brouwer B. Relationships between calf muscle density and muscle strength, mobility and bone status in the stroke survivors with subacute and chronic lower limb hemiparesis. J Musculoskelet Neuronal Interact. 2010;10(4):249–255. [PubMed] [Google Scholar]
- 36.Marin PJ, Ferrero CM, Menendez H, Martin J, Herrero AJ. Effects of whole-body vibration on muscle architecture, muscle strength, and balance in stroke patients: A randomized controlled trial. Am J Phys Med Rehabil. 2013;92(10):881–888. doi: 10.1097/PHM.0b013e318292336c. [DOI] [PubMed] [Google Scholar]
- 37.Durand MJ, Murphy SA, Schaefer KK, et al. Impaired hyperemic response to exercise post stroke. PLoS One. 2015;10(12):e0144023. doi: 10.1371/journal.pone.0144023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochi M, Tabara Y, Kido T, et al. Quadriceps sarcopenia and visceral obesity are risk factors for postural instability in the middle-aged to elderly population. Geriatr Gerontol Int. 2010;10(3):233–243. doi: 10.1111/j.1447-0594.2010.00610.x. [DOI] [PubMed] [Google Scholar]
- 39.Miller M, Flansbjer UB, Lexell J. Voluntary activation of the knee extensors in chronic poststroke subjects. Am J Phys Med Rehabil. 2009;88(4):286–291. doi: 10.1097/PHM.0b013e318198b569. [DOI] [PubMed] [Google Scholar]
- 40.Pak S, Patten C. Strengthening to promote functional recovery poststroke: An evidence-based review. Top Stroke Rehabil. 2008;15(3):177–199. doi: 10.1310/tsr1503-177. [DOI] [PubMed] [Google Scholar]
- 41.Dawes H, Smith C, Collett J, et al. A pilot study to investigate explosive leg extensor power and walking performance after stroke. J Sports Sci Med. 2005;4(4):556–562. [PMC free article] [PubMed] [Google Scholar]
- 42.Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin Biomech. 1999;14(2):125–135. doi: 10.1016/s0268-0033(98)00062-x. [DOI] [PubMed] [Google Scholar]
- 43.Kim CM, Eng JJ. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys Ther. 2003;83(1):49–57. [PubMed] [Google Scholar]
- 44.Bohannon RW, Magasi SR, Bubela DJ, Wang YC, Gershon RC. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve. 2012;46(4):555–558. doi: 10.1002/mus.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neder JA, Nery LE, Shinzato GT, Andrade MS, Peres C, Silva AC. Isokinetic strength and power in nonathletic men and women from 20 to 80 years old. J Orthop Sports Phys Ther. 1999;29(2):116–126. doi: 10.2519/jospt.1999.29.2.116. [DOI] [PubMed] [Google Scholar]
- 46.Lauziere S, Mieville C, Betschart M, Aissaoui R, Nadeau S. Plantarflexor weakness is a determinant of kinetic asymmetry during gait in post-stroke individuals walking with high levels of effort. Clin Biomech (Bristol, Avon) 2015;30(9):946–952. doi: 10.1016/j.clinbiomech.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Clark DJ, Condliffe EG, Patten C. Reliability of concentric and eccentric torque during isokinetic knee extension in post-stroke hemiparesis. Clin Biomech. 2006;21(4):395–404. doi: 10.1016/j.clinbiomech.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Bean JF, Kiely DK, LaRose S, Goldstein R, Frontera WR, Leveille SG. Are changes in leg power responsible for clinically meaningful improvements in mobility in older adults? J Am Geriatr Soc. 2010;58(12):2363–2368. doi: 10.1111/j.1532-5415.2010.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rice J, Keogh JWL. Power training: Can it improve functional performance in older adults? A systematic review. Int J Exerc Sci. 2009;2(2):131–151. [Google Scholar]
- 50.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: Which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003;58(8):M728–M733. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 51.Tschopp M, Sattelmayer MK, Hilfiker R. Is power training or conventional resistance training better for function in elderly persons? A meta-analysis. Age Ageing. 2011;40(5):549–556. doi: 10.1093/ageing/afr005. [DOI] [PubMed] [Google Scholar]
- 52.Mehrholz J, Rutte K, Pohl M. Jump training is feasible for nearly ambulatory patients after stroke. Clin Rehabil. 2006;20(5):406–412. doi: 10.1191/0269215506cr954oa. [DOI] [PubMed] [Google Scholar]
- 53.Hunnicutt JL, Aaron SE, Embry AE, et al. The effects of power training in young and older adults after stroke. Stroke Res Treat. 2016:1–5. doi: 10.1155/2016/7316250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan P, Embry A, Perry L, Holthaus K, Gregory CM. Feasibility of lower-limb muscle power training to enhance locomotor function poststroke. J Rehabil Res Dev. 2015;52(1):77–84. doi: 10.1682/JRRD.2014.04.0109. [DOI] [PubMed] [Google Scholar]
- 55.Williams G, Clark RA, Hansson J, Paterson K. Feasibility of ballistic strengthening exercises in neurologic rehabilitation. Am J Phys Med Rehabil. 2014;93(9):828–833. doi: 10.1097/PHM.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 56.Janssen I, Haymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 57.Scherbakov N, Sandek A, Doehner W. Stroke-related sarcopenia: specific characteristics. J Am Med Dir Assoc. 2015;16(4):272–276. doi: 10.1016/j.jamda.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: A report from the American heart association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]