Abstract
Background
The carotid intima media thickness (cIMT) and carotid plaque score (cPS) are respective markers of early and late stage subclinical atherosclerosis. Relationships between some laboratory parameters and subclinical atherosclerosis are not yet clear in community dwelling individuals and non-diabetic subjects, so we try to elucidate these relationships and find a model to predict early and late stage subclinical atherosclerosis.
Methods
We examined relationships of the cIMT and cPS with different laboratory and demographic data of 331 subjects from a community-based prospective cohort study, using univariate and multivariate analyses.
Results
In regression models and after multiple adjustments, only systolic blood pressure (SBP), age, glycated hemoglobin (HBA1c), and waist circumference (WC) were determinants of the cIMT, and only age, SBP, HBA1c, and blood urea nitrogen (BUN) were determinants of a cPS of > 2 in all individuals. Only HBA1c lost its association with regard to predicting the cIMT in non-diabetic subjects.
Conclusions
HBA1c at > 5.9% can determine early and late stage subclinical atherosclerosis in community dwelling individuals, but only late stage subclinical atherosclerosis in non-diabetic subjects.
Keywords: Carotid Intima media thickness, Atherosclerosis, Hemoglobin A, Glycosylated, Blood urea nitrogen
Background
Carotid intima media thickness (cIMT) is a measure of the intima and media layers of the carotid artery. It is performed by B mode ultrasound in clinical practice. Hypertrophy of these intima or media (or both) layers results in a thicker cIMT. Factors that are responsible for this hypertrophy also develop or lead to progression of atherosclerosis [1]. The cIMT is considered to represent asymptomatic and subclinical atherosclerosis. This was studied and found to be a reliable and consistent marker of cardiovascular (CV) events in previous studies [1, 2]. It has been available to evaluate atherosclerosis and CV events since 2002 [3].
Studies also suggest that the carotid plaque score (cPS) alongside the cIMT is a valid marker of subclinical atherosclerosis and a strong predictor of CV disease (CVD) [4, 5]. Thickening of the cIMT reflects early stages of atherosclerosis, but plaque formation indicates later stages [6].
Glycosylated hemoglobin (HBA1c) was proposed as a screening and diagnostic marker for type 2 diabetes mellitus (T2DM) [7], as it can be used to determine the level of blood sugar over a long period of time and is also highly correlated with long-term complications of T2DM [8] and is associated with CVD in diabetic patients [9, 10]. It was found to be an important determinant of subclinical atherosclerosis, such as carotid atherosclerosis in T2DM [11]. Although it is widely used in diabetic patients, some epidemiological studies suggested an association between HBA1c and CVD in non-diabetic populations [12, 13], but other studies failed to reach this conclusion [14, 15]. Thus, there is uncertainty if HBA1c is correlated with subclinical atherosclerosis in non-diabetic patients or if it can be used to predict the cIMT.
To date, only a few studies have investigated relationships of the Uric acid level (URCA), HBA1c, and other tests with factors of subclinical atherosclerosis, such as the cIMT and cPS [16–19]. As there is controversy regarding these relationships in the general population and also in non-diabetic patients, the main objectives of this study were to elucidate these correlations and try to find a model to predict the cIMT and a high cPS based on patient characteristics and laboratory results and identify patients at high risk for developing CVD, both in community dwelling and non-diabetic individuals. Not all the community dwelling individual has the opportunity or necessity of performing a carotid duplex ultrasound to find out the status of subclinical atherosclerosis, so a model based on the characteristics and routine laboratory results may help to identify high risk patients for subclinical atherosclerosis to send out for further analysis.
Methods
Data of individuals from a community-based prospective cohort study investigating CV and cerebrovascular risk factors on residents of 6 Villages in Shihlin District, and 6 villages in Wenshan District that are in the coverage of Shin Kong Wu Ho-Su Memorial Hospital and Wan Fang Hospital respectively in 2005 and 2006 were used in this study. Six thousand phone calls were made using the area codes of these districts. Respondents who consented to enter the study, asked about the demographic information and they were asked for the permission of keeping their contact information and to call upon them to come to the respective hospitals for further examination. Exclusion criteria were: age ≤ 30, incomplete questionnaire, prior history of cancer, chronic kidney disease, refusing to blood draw or duplex ultrasound. Individuals that had both duplex records and laboratory blood test results at the same visit were accepted in our study (Fig. 1). All study subjects signed a consent form in order to enter the original study, and no names were published in the results.
Fig. 1.
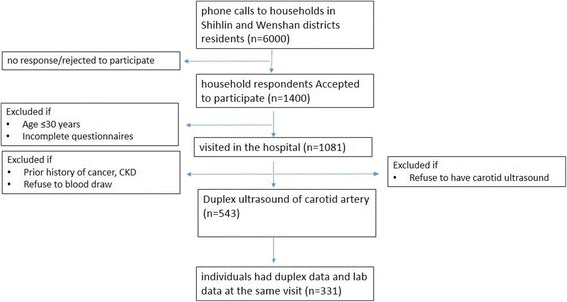
Flowchart for selection of the study participants. CKD: Chronic Kidney Disease
Simple and descriptive statistics (numbers and percentages for categorical variables and the mean and standard deviation (SD) for continuous variables) and analysis of categorical variables based on age group (< 45, 45~ 60, and > 60 years) and sex were carried out with Fisher’s exact test, and continuous variables were analyzed with an analysis of variance (ANOVA) and a post-hoc (least significant difference) test. Data including demographic characteristics, such as age, sex, age (years), height (cm), weight (kg), waist circumference (WC; cm), body-mass index (BMI; kg/m2), a history of stroke (STR), history of T2DM, and CVD, systolic (SBP) and diastolic blood pressure (DBP) (mmHg), pulse (beats/min), and also laboratory data including fasting blood sugar (FBS, mg/dl), uric acid level (URCA, mg/dl), blood urea nitrogen (BUN, mg/dl), creatinine (Cr, mg/dl), aspartate aminotransferase (AST, units/L), alanine transaminase (ALT, units/L), triglyceride (TRG, mg/dl), cholesterol (CHOL, mg/dl), low-density lipoprotein (LDL, mg/dl), high-density lipoprotein (HDL, mg/dl), C-reactive protein (CRP, mg/L), and glycated hemoglobin (HBA1c; %) were gathered using standard methods (using an X-1500-Sysmex, Deckman AU5800 and Tosho HLC-723G8 Automated glycol-hemoglobin analyzer).
Ultrasound of the carotid and vertebral arteries was done with a B-mode Duplex ultrasound (SONO 5500, HP, USA) and the following measurements were obtained for every individual: flow (ml), end diastolic velocity (EDV; cm/s), and peak systolic velocity (PSV; cm/s) measured in one cardiac cycle. The mean velocity (MV) and resistance index (RI) were calculated using these respective formulas: MV = (PSV + 2*EDV)/3 and RI = (PSV-MV)/PSV. The diameter of the cervical portion of the common carotid artery (CCA), the internal carotid artery (ICA) beyond the carotid bulb, and the external carotid artery (ECA) and vertebral artery (VA) sequentially on both sides and the number of plaques in the carotid arteries were measured by a cardiologist. The cPS in each person was calculated by adding the numbers of plaques on the right and left sides.
The cIMT was assessed at 1 cm from the carotid bulb on the left and right sides. The mean flow and RI were determined for every subject by taking the average of all flows and RIs of the left and right carotid and vertebral arteries. The mean cIMT and diameter of each artery were calculated by taking the average of the left and right sides in each individual.
Cutoff points for the cPS, cIMT, HBA1c, and SBP were set at the level of the 75% quartile of all subjects’ data. A simple Pearson’s correlation was calculated, and variables with significant correlations with the cIMT were included in a multiple linear regression to predict the cIMT. Two logistic regressions were done using variables with a significant correlation with the cIMT and cPS to calculate the odds ratio (OR) and 95% confidence interval (CI) for predicting a thick cIMT and a high cPS (using 75% percentiles as the cutoff points). Receiver operating characteristic (ROC) curves were drawn, and the Mann-Whitney test, ORs, and CIs were used to determine the significance. Multiple and logistic regressions of non-diabetic individuals were used to clarify the role of T2DM in determining the cIMT and high cPS. Observations were deleted due to missing values for the response or explanatory variables in all models. The alpha error was set to 0.05, and we used SAS version 9.4 (SAS, Cary, NC, USA) for all data analyses.
Results
There were 150 (46.9%) women and 170 (53.1%) men with a mean age of 57.06 (range 32~ 85) years and a mean BMI of 23.55 kg/m2. The mean SBP and DBP were 123.57 and 78.02 mmHg, respectively. They had 0, 1, 2 (25%, 50%, and 75% quartiles, respectively) cPSs, with a minimum of 0 and a maximum of 17. The mean cIMT was 0.67 (range 0.43~ 1.125) cm. The 25%, 50%, and 75% quartiles were 0.59, 0.66, and 0.75 cm, respectively. Demographic, duplex, and laboratory data of subjects are presented in Table 1 categorized based on sex and age groups.
Table 1.
Characteristics of community dwelling individuals, stratified by sex and age group
| Variable | N | Mean ± SD Number (%) |
Age group (years) | Sex | |||||
|---|---|---|---|---|---|---|---|---|---|
| < 45 | 45~ 60 | > 60 | p | Male | Female | p | |||
| Total | – | – | 40 | 150 | 129 | < 0.0001 | 150 (46.9%) | 170 (53.1%) | 0.26 |
| Age (years) | 320 | 57.06 ± 10.85 | 38.7 ± 3.6ab | 52.8 ± 3.9c | 67.7 ± 5.5 | < 0.0001 | 58.89 ± 11.73 | 55.43 ± 9.75 | 0.0044 |
| Height (cm) | 320 | 160.99 ± 8.49 | 165.4 ± 9.0 ab | 160.5 ± 7.8 | 160.2 ± 8.7 | 0.0019 | 167.20 ± 6.77 | 155.52 ± 5.61 | < 0.0001 |
| Weight (kg) | 320 | 61.28 ± 10.78 | 64.9 ± 12.4a | 59.7 ± 10.9 | 61.9 ± 9.8 | 0.0182 | 65.54 ± 9.60 | 55.75 ± 8.52 | < 0.0001 |
| WC (cm) | 320 | 79.46 ± 9.74 | 78.9 ± 10.0b | 76.9 ± 9.7c | 82.6 ± 8.8 | < 0.0001 | 85.00 ± 8.10 | 74.56 ± 8.35 | < 0.0001 |
| BMI (kg/cm2) | 320 | 23.55 ± 3.10 | 23.6 ± 3.3 | 23.1 ± 3.3c | 24.0 ± 2.7 | 0.0451 | 24.13 ± 2.88 | 23.03 ± 3.21 | 0.0014 |
| FBS (mg/dl) | 320 | 95.37 ± 21.86 | 91.7 ± 28.9 | 93.0 ± 19.2c | 99.3 ± 21.9 | 0.0314 | 99.23 ± 24.33 | 91.96 ± 18.83 | 0.0029 |
| URCA (mg/dl) | 320 | 5.59 ± 1.44 | 5.6 ± 1.6 | 5.4 ± 1.4 c | 5.9 ± 1.4 | 0.0140 | 6.37 ± 1.30 | 4.91 ± 1.18 | < 0.0001 |
| BUN(mg/dl) | 296 | 14.13 ± 4.05 | 11.4 ± 3.4 ab | 13.6 ± 3.4c | 15.8 ± 4.4 | < 0.0001 | 15.38 ± 4.06 | 13.23 ± 3.87 | < 0.0001 |
| Creatinine(mg/dl) | 296 | 0.92 ± 0.63 | 0.8 ± 0.2b | 0.8 ± 0.2c | 1.1 ± 1 | 0.0046 | 1.13 ± 0.90 | 0.75 ± 0.14 | < 0.0001 |
| AST(units/L) | 320 | 24.45 ± 15.38 | 20.3 ± 4.8 | 25.4 ± 20.7 | 24.7 ± 8.8 | 0.170 | 25.94 ± 20.96 | 23.14 ± 7.44 | 0.104 |
| ALT(units/L) | 320 | 23.99 ± 16.75 | 20.37.6 | 25.3 ± 19.9 | 23.7 ± 14.6 | 0.242 | 27.30 ± 21.78 | 21.07 ± 9.65 | 0.0008 |
| TGL(mg/dl) | 320 | 118.23 ± 76.60 | 125.2 ± 110.2 | 111.1 ± 64.3 | 124.5 ± 77.4 | 0.288 | 128.31 ± 87.56 | 109.34 ± 64.40 | 0.0269 |
| CHOL (mg/dl) | 320 | 203.66 ± 33.21 | 196.8 ± 37.6 | 204.8 ± 32.7 | 204.5 ± 32.4 | 0.372 | 200.25 ± 30.94 | 206.68 ± 34.90 | 0.0840 |
| LDL(mg/dl) | 320 | 135.81 ± 34.08 | 129.6 ± 36.8 | 135.6 ± 33.8 | 138.2 ± 33.5 | 0.371 | 136.08 ± 31.55 | 135.56 ± 36.26 | 0.891 |
| HDL(mg/dl) | 320 | 47.47 ± 13.63 | 45.7 ± 12.6 | 49.7 ± 13.6c | 45.3 ± 13.6 | 0.0161 | 42.48 ± 12.73 | 51.88 ± 12.88 | < 0.0001 |
| CRP(mg/L) | 320 | 0.188 ± 0.626 | 0.15 ± 0.2 | 0.13 ± 0.2 | 0.26 ± 0.9 | 0.256 | 0.196 ± 0.39 | 0.181 ± 0.77 | 0.823 |
| HBA1c (%) | 296 | 5.75 ± 0.959 | 5.7 ± 1.6 | 5.6 ± 0.6c | 6 ± 1.0 | 0.0243 | 5.88 ± 1.11 | 5.69 ± 0.82 | < 0.0001 |
| Stroke | 316 | 8 (2.5%) | 0 | 2 (1.3%) | 6 (4.8%) | 0.109 | 5 (3.4%) | 3 (1.8%) | 0.50 |
| HTN | 320 | 105 (32.8) | 2 (5%) | 37 (24.7%) | 66 (51.2%) | < 0.0001 | 61 (40.7) | 44 (25.9%) | 0.00 |
| DM | 320 | 38 (11.5%) | 2 (5%) | 12 (8%) | 24 (18%) | 0.0086 | 27 (18%) | 11 (6.5%) | 0.00 |
| CVD | 313 | 49 (14.8%) | 3 (7.5%) | 21 (14.6%) | 25 (19.5%) | 0.166 | 24 (16.2%) | 25 (15.1%) | 0.87 |
| SBP(mmHg) | 320 | 123.57 ± 18.95 | 112.2 ± 10.8ab | 119.9 ± 17.8c | 131.5 ± 19.2 | < 0.0001 | 128.10 ± 16.15 | 119.58 ± 20.34 | < 0.0001 |
| DBP(mmHg) | 320 | 78.02 ± 10.67 | 74.4 ± 8ab | 78.2 ± 11.5 | 79.0 ± 10.2 | 0.057 | 80.25 ± 10.10 | 76.05 ± 10.80 | 0.0004 |
| Pulse (beats/min) | 320 | 72.21 ± 32.39 | 73.9 ± 8.5 | 73.2 ± 30.4 | 70.5 ± 38.9 | 0.747 | 74.78 ± 46.18 | 69.95 ± 9.48 | 0.183 |
| Mean flow (ml) | 329 | 219.80 ± 35.91 | 232.4 ± 28.3b | 226.6 ± 37.2c | 207.8 ± 33.9 | < 0.0001 | 223.59 ± 35.89 | 216.33 ± 36.19 | 0.0742 |
| Mean RI | 322 | 0.656 ± 0.042 | 0.66 ± 0.037a | 0.64 ± 0.042c | 0.67 ± 0.045 | < 0.0001 | 0.668 ± 0.042 | 0.646 ± 0.040 | < 0.0001 |
| Mean ECA DIA (cm) | 331 | 0.357 ± 0.034 | 0.353 ± 0.029b | 0.352 ± 0.037c | 0.365 ± 0.033 | 0.0036 | 0.370 ± 0.033 | 0.346 ± 0.032 | < 0.0001 |
| Mean ICA DIA (cm) | 331 | 0.429 ± 0.030 | 0.419 ± 0.0348b | 0.424 ± 0.286c | 0.439 ± 0.028 | < 0.0001 | 0.443 ± 0.028 | 0.416 ± 0.026 | < 0.0001 |
| Mean CCA DIA (cm) | 331 | 0.577 ± 0.056 | 0.554 ± 0.0382b | 0.566 ± 0.0506c | 0.599 ± 0.0614 | < 0.0001 | 0.599 ± 0.055 | 0.558 ± 0.506 | < 0.0001 |
| Mean VA DIA (cm) | 325 | 0.316 ± 0.030 | 0.313 ± 0.0323 | 0.314 ± 0.0306 | 0.321 ± 0.0296 | 0.104 | 0.324 ± 0.030 | 0.309 ± 0.0285 | < 0.0001 |
| cPS | 331 | 1.86 ± 2.90 | 0.175 ± 0.594b | 0.893 ± 1.663c | 3.659 ± 3.596 | < 0.0001 | 2.55 ± 3.45 | 1.35 ± 2.25 | 0.0002 |
| Plaque presence | 331 | 178(53.8%) | 4(10%) | 63(42%) | 107(82.9%) | < 0.0001 | 95(63.3%) | 79(46.5%) | 0.003 |
| cIMT (cm) | 309 | 0.673 ± 0.110 | 0.571 ± 0.0762ab | 0.647 ± 0.0849c | 0.740 ± 0.1093 | < 0.0001 | 0.698 ± 0.11 | 0.654 ± 0.103 | 0.0006 |
| cIMT > 0.75 | 309 | 75(24.3%) | 2(5.3%) | 17(12.14%) | 54(45%) | < 0.0001 | 42 (30.4%) | 31 (19.2%) | 0.03 |
SD standard deviation, WC waist circumference, BMI body-mass index, DM diabetes melitus, HTN hypertension, CVD cardio vascular disease, SBP systolic blood pressure, DBP diastolic blood pressure, FBS fast blood sugar, URCA uric acid level, BUN blood urea nitrogen, AST aspartate aminotransferase test, ALT alanine aminotransferase test, TGL triglyceride, CHOL cholesterol, LDL low density lipoprotein, HDL high density lipoprotein, CRP C-reactive protein, HBA1c, glycated hemoglobin, cIMT carotid intima media thickness, RI resistance index, ECA external carotid artery, ICA internal carotid artery, CCA common carotid artery, VA vertebral artery, cPS carotid plaque score
Results of Pearson correlations between the cIMT and different variables and also between the cPS and different variables are presented in Table 2. We entered each variable with a significant correlation with the cIMT in a linear regression model, and variables with high co-linearity were removed from the model using their variance inflation factor (VIF). Models were run for the logarithmic scale of the cIMT, as the cIMT was not normally distributed (Table 3).
Table 2.
Pearson correlations of variables of interest with the carotid intima media thickness (cIMT) and carotid plaque score (cPS)
| Variable | cIMT | cPS | ||
|---|---|---|---|---|
| r | p | r | p | |
| Sex | 0.196 | 0.001* | 0.204 | 0.000* |
| Age | 0.569 | 0.000* | 0.538 | 0.000* |
| Height | 0.18 | 0.759 | 0.052 | 0.356 |
| Weight | 0.168 | 0.004* | 0.049 | 0.384 |
| WC | 0.333 | 0.000* | 0.190 | 0.001* |
| BMI | 0.229 | 0.000* | 0.031 | 0.582 |
| FBS | 0.273 | 0.000* | 0.120 | 0.031* |
| URCA | 0.122 | 0.034* | 0.134 | 0.017* |
| BUN | 0.319 | 0.000* | 0.323 | 0.000* |
| Cr | 0.086 | 0.153 | 0.153 | 0.008* |
| AST | 0.063 | 0.281 | −0.019 | 0.740 |
| ALT | 0.091 | 0.117 | −0.044 | 0.431 |
| TGL | 0.132 | 0.023* | 0.072 | 0.197 |
| CHOL | 0.089 | 0.125 | 0.043 | 0.440 |
| LDL | 0.101 | 0.82 | 0.085 | 0.131 |
| HDL | − 0.155 | 0.007* | −0.148 | 0.008* |
| CRP | 0.084 | 0.145 | 0.022 | 0.698 |
| HBA1c | 0.332 | 0.000* | 0.174 | 0.003* |
| Stroke | 0.168 | 0.004* | 0.114 | 0.043* |
| HT | 0.423 | 0.000* | 0.376 | 0.000* |
| DM | 0.220 | 0.000* | 0.159 | 0.004* |
| CVD | 0.096 | 0.102 | 0.094 | 0.096 |
| SBP | 0.435 | 0.000* | 0.316 | 0.000* |
| DBP | 0.280 | 0.000* | 0.069 | 0.216 |
| Pulse | −0.006 | 0.917 | −0.032 | 0.564 |
| Mean flow | −0.146 | 0.011 | −0.248 | 0.000* |
| Mean RI | 0.167 | 0.004* | 0.266 | 0.000* |
| Mean ECA DIA | 0.215 | 0.000* | 0.061 | 0.270 |
| Mean ICA DIA | 0.228 | 0.000* | 0.200 | 0.000* |
| Mean CCA DIA | 0.322 | 0.000* | 0.330 | 0.000* |
| Mean VA DIA | 0.016 | 0.785 | 0.084 | 0.129 |
| Plaque score | 0.542 | 0.000* | 1 | – |
| Plaque presence | 0.485 | 0.000* | 0.597 | 0.000* |
| cIMT | 1 | – | 0.542 | 0.000* |
r, Pearson correlation; p, p value; * p < 0.05
WC waist circumference, BMI body-mass index, DM diabetes melitus, HTN hypertension, CVD cardio vascular disease, SBP systolic blood pressure, DBP diastolic blood pressure, FBS fast blood sugar, URCA uric acid level, BUN blood urea nitrogen, AST aspartate aminotransferase test, ALT alanine aminotransferase test, TGL triglyceride, CHOL cholesterol, LDL low density lipoprotein, HDL high density lipoprotein, CRP C-reactive protein, HBA1c glycated hemoglobin, cIMT carotid intima media thickness, RI resistance index, ECA external carotid artery, ICA internal carotid artery, CCA common carotid artery, VA vertebral artery, cPS carotid plaque score
Table 3.
Multiple linear regression for predicting Log (intima media thickness; cIMT)
| Variable | Beta | Standarderror | t Value | Pr > |t| |
|---|---|---|---|---|
| Intercept | −1.34196 | 0.11238 | −11.94 | < 0.0001 |
| Sex | 0.01090 | 0.02033 | 0.54 | 0.5924 |
| Age | 0.00586 | 0.00080079 | 7.32 | < 0.0001 |
| WC | 0.00228 | 0.00113 | 2.01 | 0.0452 |
| URCA | −0.01027 | 0.00700 | −1.47 | 0.1435 |
| BUN | 0.00326 | 0.00207 | 1.57 | 0.1166 |
| HDL | − 0.00004201 | 0.00063481 | −0.07 | 0.9473 |
| HbA1c | 0.03971 | 0.01228 | 3.23 | 0.0014 |
| Stroke | 0.02649 | 0.07207 | 0.37 | 0.7136 |
| SBP | 0.00165 | 0.00046219 | 3.58 | 0.0004 |
| DM | −0.03006 | 0.03215 | −0.93 | 0.3508 |
Linear model for predicting Log (cIMT), F value = 21.31, p < 0.0001, adjusted r squared = 0.413
WC waist circumference, DM diabetes melitus, SBP systolic blood pressure, URCA uric acid level, BUN blood urea nitrogen, HDL high density lipoprotein, HBA1c glycated hemoglobin
We used the third quartiles of WC (85.5 cm), HBA1c (5.9%), age (65 years), and SBP (135 mmHg) as cutoff points to run a logistic regression, and found the OR of the variables for predicting a thick cIMT (Table 4). We then ran a logistic regression model to predict a high cPS, and significantly correlated variables of age, sex, HDL, BUN, creatinine, HBA1c, URCA, T2DM and stroke history, SBP, and WC were included in the model; p values, ORs, and CIs of this model are also presented in Table 4. The ROC curves were drawn using the logistic regression to predict a thick cIMT and a high cPS in two models separately for each significant variable in previous logistic models (Figs. 2 and 3).
Table 4.
Logistic regression model odds ratio for predicting a thick carotid intima media thickness (cIMT; of > 0.75 cm) and high plaque score (> 2) in all the individuals and in non-diabetic subjects
| cIMT> 0.75 in all the individuals | cPS > 2 in all the individuals | cPS > 2 in non-diabetic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Odds ratio | 95% Wald confidence interval | Odds ratio | 95% Wald confidence interval | Odds ratio | 95% Wald confidence interval | |||
| Aged > 65 years | 3.182 | 1.611 | 6.285 | 3.232 | 1.626 | 6.425 | 2.607 | 1.191 | 5.708 |
| HBA1c > 5.9% | 2.396 | 1.224 | 4.690 | 2.237 | 1.058 | 4.729 | 2.714 | 1.273 | 5.787 |
| SBP > 135 mmHg | 2.432 | 1.231 | 4.802 | 2.672 | 1.408 | 5.072 | 3.893 | 1.942 | 7.801 |
| WC > 85 cm | 3.458 | 1.766 | 6.770 | 0.972 | 0.932 | 1.013 | – | – | – |
| Sex | – | – | – | 0.919 | 0.389 | 2.169 | 1.235 | 0.499 | 3.055 |
| Bun | – | – | – | 1.103 | 1.013 | 1.201 | 1.115 | 1.014 | 1.226 |
| Creatinine | – | – | – | 3.846 | 0.564 | 26.234 | 1.965 | 0.259 | 14.903 |
| HDL | – | – | – | 0.982 | 0.958 | 1.006 | 0.986 | 0.962 | 1.010 |
| Stroke | – | – | – | 3.898 | 0.301 | 50.529 | 2.622 | 0.112 | 61.164 |
| URCA | – | – | – | 1.169 | 0.905 | 1.510 | 1.040 | 0.789 | 1.370 |
| DM | – | – | – | 1.406 | 0.494 | 3.998 | – | – | – |
HBA1c glycated hemoglobin, SBP systolic blood pressure, WC waist circumference, BUN blood urea nitrogen, URCA uric acid, DM diabetes mellitus
Fig. 2.
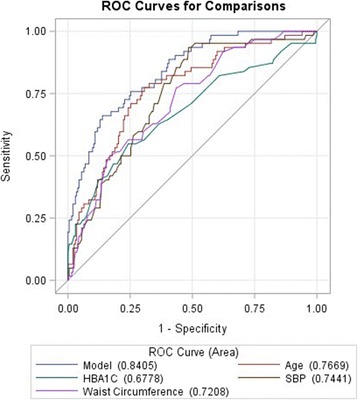
ROC curve for the logistic model for predicting a thick carotid intima media thickness (cIMT of > 0.75 cm) based on four independent variable of age, glycated hemoglobin (HBA1c), waist circumference, and systolic blood pressure (SBP)
Fig. 3.
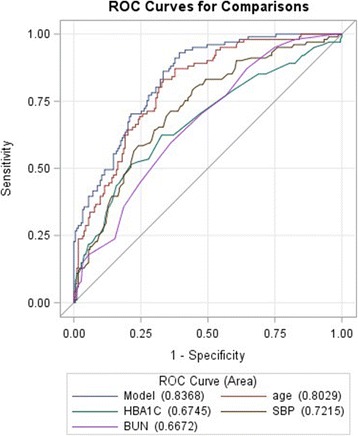
ROC curve for the logistic model for predicting a high plaque score (of > 2) based on four independent variable of age, glycated hemoglobin (HBA1C), systolic blood pressure (SBP), and blood urea nitrogen (BUN)
The ICA RI and CCA RI had significant correlations with the cIMT (r = 0.18748 and 0.22010, respectively, both p < 0.001), and the mean RI was correlated with the cIMT (Pearson r = 0.16738, p = 0.0036), but their relationship was not independent of age and sex, as this relationship disappeared after controlling for age and sex.
To further clarify associations of different variables in non-diabetic subjects, we ran second sets of Pearson correlations of HBA1c with cIMT and cPS and models to predict cIMT and cPS only in non-diabetic subjects. A significant correlation of HBA1c with the cIMT but not the cPS was present in T2DM subjects, and with both in non-diabetic subjects. After controlling for age, sex, SBP, and WC, however, HBA1c was not correlated with the cIMT in non-diabetic subjects (Table 5). However HBA1c could predict high cPS in non-diabetic individuals after controlling for age, SBP, sex, BUN, CR, HDL, stroke, and URCA (Table 4).
Table 5.
Pearson correlation of glycated hemoglobin (HBA1c) with the carotid intima media thickness (cIMT) and plaque score (PS), and multiple regression for log (cIMT) in diabetic and non-diabetic individuals
| cIMT | cPS | Multivariate linear regression for log(cIMT) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WC | Sex | Age | SBP | HBA1c | ||||||||||
| r | p | r | p | b | p | b | p | b | p | b | p | b | p | |
| Diabetic | 0.35 | 0.049 | −0.06 | 0.69 | – | – | – | – | – | – | – | – | – | – |
| non-diabetic | 0.196 | 0.002 | 0.158 | 0.012 | 0.0018 | 0.1 | 0.01 | 0.5 | 0.0059 | < 0.0001 | 0.0005 | 0.0002 | 0.021 | 0.27 |
Adjusted r-squared for non-diabetic =0.365; WC waist circumference, SBP systolic blood pressure
Discussion
Data of carotid duplex parameters and certain laboratory tests of 331 individuals from the general population were examined for relationships. Sex, age, weight, WC, BMI, FBS, URCA, BUN, TGL, HDL, HBA1C, a history of stroke, hypertension, and diabetes, SBP, DBP, the mean RI of CCA, ICA, ECA, VA, diameters of the ECA, ICA, and CCA, and the cPS were correlated with the cIMT. But among them, only age, HBA1C, WC, and SBP remained significantly associated with the cIMT after controlling for other correlated factors. Sex, age, WC, FBS, URCA, BUN, creatinine, HDL, HBA1C, a history of stroke, hypertension, and diabetes, SBP, the mean RI, and diameters of ICA and CCA were correlated with the cPS. But among them only age, HBA1C, and SBP remained significantly associated with the cPS after controlling for other factors. Results also showed that HBA1c cannot serve as an independent factor for predicting the cIMT in non-diabetic subjects but could predict a high cPS in non-diabetic subjects.
We found a significant difference in cIMTs between men and women, and the cIMT was thicker in men. Our results are consistent with other studies [16, 17, 20]; however, sex does not independently predict the cIMT as confirmed by other studies [20]. On the other hand, we found a robust correlation between age and the cIMT, as it remained significant after controlling for other factors. Figure 4 shows that the mean cIMT was thicker in each age group compared to younger groups, and this difference was significant between some age groups. These findings are in accordance with other studies [16, 17, 20–22].
Fig. 4.
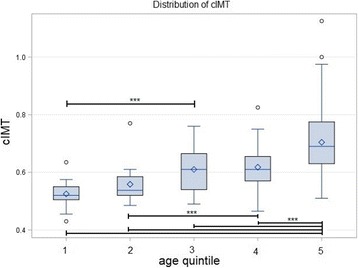
Box plots of the distribution of the carotid intima media thickness (cIMT) in different quintiles of age group. A significant difference is indicated by line and asterisks
We found the BMI, TGL, HDL, FBS, and histories of CVD and T2DM to be factors correlated with the cIMT, and these results were consistent with those of Abbasi et al. [16], but we also found WC to be a stronger factor and independent determinant of the cIMT. Although some studies mentioned that LDL and TGL were correlated with the cIMT [20, 21], another study questioned this association, as there were no correlations of TGL and LDL with the cIMT [17]. We found no significant association of CRP with the cIMT or cPS. Although most studies confirmed this association [17, 21–25], Gao et al. [26] named it as an indirect determinant of the cIMT. We also found BUN to be an independent factor to identify individuals with a high cPS. Although Zhu et al. [27] did not find it to be a determining factor for thickening of the cIMT, it was correlated with the cIMT before running the multiple regression. Our results are consistent with theirs, except that we found a mild but significant association with a high cPS, even after adjusting for other variables.
URCA was found to determine subclinical atherosclerosis, as it independently predicted the cIMT in some studies [17], but other studies found no association [16, 28]. It was correlated with the cIMT in our study, but it failed to predict the cIMT or cPS after controlling for other variables.
Some studies found a correlation between HBA1c and the cIMT in diabetic patients [22, 24, 29]. Other studies [30, 31], on the other hand, found an independent association between these two factors, although Shah et al. [30] reported that this had a low prediction of variance (of < 20%). In studies of non-diabetic individuals [18, 19], no association was found, but Marini et al. [32] in a study of pre-diabetic patients versus non-diabetic controls proved otherwise. Some studies showed a strong association between the cIMT and HBA1c in people with normal glucose tolerance [33, 34]. And Huang et al. [33], in a study of a non-diabetic Chinese population, had similar results. Kowall et al. [35] found no association of glycemic measures such as FBS, HBA1c, and 2-h plasma glucose with the cIMT. Our study showed a significant correlation between the cIMT and HBA1c, and it remained significant even after controlling for many factors including the T2DM status. We also saw an increasing trend in cIMT levels in each HBA1c tertile, showing the strong association (Fig. 5).
Fig. 5.
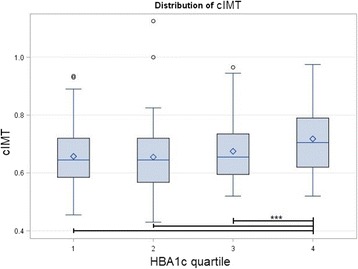
Box plots of the distribution of the carotid intima media thickness (cIMT) in different quartile of glycated hemoglobin (HBA1c). A significant difference is indicated by line and asterisks
Few studies have investigated the relationship between HBA1c and carotid plaque in non-diabetic individuals, and there are non-consistent results in the literature [33, 36]. Lee et al. [18] and Huang et al. [33] found a significant association between HBA1c and carotid plaque in non-diabetic people, but this relationship did not remain significant after adjusting for other factors. But Jorgensen et al. [35] found an independent relationship between these two measures. We also stratified subjects by the T2DM status and found correlations of the cIMT and cPS with HBA1c. We could not run a regression model on T2DM individuals due to the small sample size of T2DM subjects, but regression models on non-diabetic subjects showed that HBA1c could not independently predict the cIMT. However, this relationship was more robust regarding predicting a high cPS, as it was significant even after controlling for many variables (Tables 4 and 5). When we want to see the relationship between HBA1c and cIMT in non-diabetic subjects, our sample size decreases due to this stratification, so it is also probable that lack of association is because of the limited number of individuals in non-diabetic group. Our sample size in T2DM group is much smaller (n = 38) and it is probable that lack of correlation between HBA1c and high cPS is also due to this fact.
Our results suggest that HBA1c cannot predict the cIMT, but it can predict it with a high cPS in non-diabetic subjects. But as a general criterion, we can still use HBA1C to identify high-risk patients due to the ROC curves, and we can combine different variables to further increase the accuracy (Figs. 2 and 3).
Although it is rational to accept that carotid luminal enlargement can be used as a marker of atherosclerosis, as it should increase to preserve the space after hardening of the lumen and plaque formation, there is no proof whether these changes occur before the onset of diabetes [18], and more studies are needed to clearly discern this association.
Our study used no scoring system to compare the individuals for detection of subclinical atherosclerosis. So studies that can use the proposed variables with significant determination of early or late stage subclinical atherosclerosis to generate a scoring model would be recommended to further quantify the level of association. Additionally, status of the diabetes diagnosis, whether it is newly diagnose or not, and also the duration of diabetes, can potentially have some effects on our results and should be considered as a limitation in our study.
Conclusions
In conclusion, HBA1c can be used alongside age, SBP, WC and BUN to identify individuals at high risk of early or late stage subclinical atherosclerosis from the community. HBA1c should be removed from our prediction model if one is dealing with early stage subclinical atherosclerosis in non-diabetic subjects, although HBA1c is useful in predicting late stage subclinical atherosclerosis in non-diabetic subjects.
Acknowledgments
We should thank Dr. Li-Ming Lien and Dr. Wen-Harn Chen for their contributions. We also should thank Dr. Jerald Medway for his kind contribution for the manuscript editing.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- ANOVA
Analysis of variance
- BMI
Body mass index
- BUN
Blood urea nitrogen
- CCA
Common carotid artery
- CHOL
Cholesterol
- cIMT
Carotid intima media thickness
- cPS
Carotid plaque score
- CV
Cardiovascular
- CVD
Cardio vascular disease
- DBP
Diastolic blood pressure
- ECA
External carotid artery
- FBS
Fasting blood sugar
- HBA1c
Glycated hemoglobin
- HDL
High-density lipoprotein
- HTN
Hypertension
- ICA
Internal carotid artery
- LDL
Low-density lipoprotein
- Log
Logarithm with base 10
- RI
Resistance index
- SBP
Systolic blood pressure
- SD
Standard deviation
- T2DM
Type 2 diabetes mellitus
- URCA
Uric acid level
- VA
Vertebral artery
- WC
Waist circumference
Authors’ contributions
JA and BCH contributed to data analysis and manuscript writing, study design and data collection. JA contributed to manuscript revision and approval of the final submission. All authors contributed to the drafting and editing of this manuscript and approved the final version submitted for publication.
Ethics approval and consent to participate
The study received ethical approval from Taipei Medical University. Written consent was obtained from all the participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Javad Alizargar, Phone: +886-(2)-2736-1661, Email: jaz.tmu@gmail.com.
Chyi-Huey Bai, Phone: +886-(2)-2736-1661, Email: baich@tmu.edu.tw.
References
- 1.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography carotid Intima media thickness task force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Society of atherosclerosis imaging and prevention developed in collaboration with the international atherosclerosis Society Appropriate use criteria for carotid intima media thickness testing. Atherosclerosis. 2011;214:43–46. doi: 10.1016/j.atherosclerosis.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 3.Kasliwal RR, Bansal M, Desai D, Sharma M. Carotid intima-media thickness: current evidence, practices, and Indian experience. Indian J Endocrinol Metab. 2014;18(1):13–22. doi: 10.4103/2230-8210.126522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prati P, Tosetto A, Vanuzzo D, Bader G, Casaroli M, Canciani L, et al. Carotid intima media thickness and plaques can predict the occurrence of ischemic cerebrovascular events. Stroke. 2008;39:2470–2476. doi: 10.1161/STROKEAHA.107.511584. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 6.Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep. 2009;11:21–27. doi: 10.1007/s11886-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 7.Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–2206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 8.Lippi G, Targher G. Glycated hemoglobin (HbA1c): old dogmas, a new perspective? Clin Chem Lab Med. 2010;48(5):609–614. doi: 10.1515/cclm.2010.144. [DOI] [PubMed] [Google Scholar]
- 9.Elley CR, Kenealy T, Robinson E, Drury PL. Glycated haemoglobin and cardiovascular outcomes in people with type 2 diabetes: a large prospective cohort study. Diabet Med. 2008;25:1295–1301. doi: 10.1111/j.1464-5491.2008.02581.x. [DOI] [PubMed] [Google Scholar]
- 10.Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjörnsdóttir S, et al. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR) J. Intern Med. 2010;268:471–482. doi: 10.1111/j.1365-2796.2010.02265.x. [DOI] [PubMed] [Google Scholar]
- 11.Choi S-W, Shin M-H, Yun W-J, Kim H-Y, Lee Y-H, Kweon S-S, et al. Association between hemoglobin A 1c, carotid atherosclerosis, arterial stiffness, and peripheral arterial disease in Korean type 2 diabetic patients. J Diabetes Complicat. 2011;25:7–13. doi: 10.1016/j.jdiacomp.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Garg N, Moorthy N, Kapoor A, Tewari S, Kumar S, Sinha A, et al. Hemoglobin A(1c) in nondiabetic patients: an independent predictor of coronary artery disease and its severity. Mayo Clin Proc. 2014;89:908–916. doi: 10.1016/j.mayocp.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pradhan AD, Rifai N, Buring JE, Ridker PM. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med. 2007;120:720–727. doi: 10.1016/j.amjmed.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cederberg H, Saukkonen T, Laakso M, Jokelainen J, Härkönen P, Timonen M, et al. Postchallenge glucose, A1C, and fasting glucose as predictors of type 2 diabetes and cardiovascular disease: a 10-year prospective cohort study. Diabetes Care. 2010;33:2077–2083. doi: 10.2337/dc10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbasi MR, Abbaszadeh SH, Rokni-Yazdi H, Lessan-Pezeshki M, Khatami MR, Mahdavi-Mazdeh M, et al. Carotid intima-media thickness as a marker of atherosclerosis in hemodialysis patients. Indian J Nephrol. 2016;26(2):97–101. doi: 10.4103/0971-4065.161544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassols J, Martínez-Calcerrada JM, Prats-Puig A, Carreras-Badosa G, Díaz-Roldán F, Osiniri I, et al. Uric acid, carotid intima-media thickness and body composition in prepubertal children. Pediatr Obes. 2016;11(5):375–382. doi: 10.1111/ijpo.12074. [DOI] [PubMed] [Google Scholar]
- 18.Lee SW, Kim HC, Lee YH, Song BM, Choi H, Park JH, Rhee Y, Kim CO. Association between HbA1c and carotid atherosclerosis among elderly Koreans with normal fasting glucose. PLoS One. 2017;12(2):e0171761. doi: 10.1371/journal.pone.0171761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doruk H, Mas MR, Ateşkan U, Isik AT, Sağlam M, Kutlu M. The relationship between age and carotid artery intima-media thickness, hemoglobin A1c in nondiabetic, healthy geriatric population. Arch Gerontol Geriatr. 2005;41:113–119. doi: 10.1016/j.archger.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Jarauta E, Mateo-Gallego R, Bea A, Burillo E, Calmarza P, Civeira F. Carotid intima-media thickness in subjects with no cardiovascular risk factors. Rev Esp Cardiol. 2010;63(1):97–102. doi: 10.1016/S0300-8932(10)70014-2. [DOI] [PubMed] [Google Scholar]
- 21.Saha A, Sinha PK, Paul R, Bandyopadhyay R, Biswas K, Banerjee AK. Study of carotid intima media thickness and its correlation with novel risk factors in ischemic stroke. Neurol Asia. 2011;16:25–31. [Google Scholar]
- 22.Hayashi Y, Okumura K, Matsui H, et al. Impact of low-density lipoprotein particle size on carotid intima-media thickness in patients with type 2 diabetes mellitus. Metabolism. 2007;56:608–613. doi: 10.1016/j.metabol.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Osiniri I, Sitjar C, Soriano-Rodriguez P, Prats-Puig A, Casas-Satre C, Mayol L, et al. Carotid intima-media thickness at 7 years of age: relationship to C-reactive protein rather than adiposity. J Pediatr. 2012;160:276–280. doi: 10.1016/j.jpeds.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Lee YH, Shin MH, Choi JS, Rhee JA, Nam HS, Jeong SK, et al. HbA1c is significantly associated with arterial stiffness but not with carotid atherosclerosis in a community-based population without type 2 diabetes: the dong-gu study. Atherosclerosis. 2016;247:1–6. doi: 10.1016/j.atherosclerosis.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Boaz M, Chernin G, Schwartz I, Katzir Z, Schwartz D, Agbaria A, et al. C-reactive protein and carotid and femoral intima media thickness: predicting inflammation. Clin Nephrol. 2013;80(6):449–455. doi: 10.5414/CN108067. [DOI] [PubMed] [Google Scholar]
- 26.Gao Z, Khoury PR, McCoy CE, Shah AS, Kimball TR, Dolan LM, Urbina EM. Adiposity has no direct effect on carotid intima-media thickness in adolescents and young adults: use of structural equation modeling to elucidate indirect & direct pathways. Atherosclerosis. 2016;246:29–35. doi: 10.1016/j.atherosclerosis.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Zhang HP, Wang YC, Ren TT, Li J, Xu ML, et al. Serum cystatin C level is associated with carotid intima-media thickening and plaque. Scand J Clin Lab Invest. 2015;75(3):265–272. doi: 10.3109/00365513.2015.1006137. [DOI] [PubMed] [Google Scholar]
- 28.Fukui M, Tanaka M, Shiraishi E, Harusato I, Hosoda H, Asano M, Kadono M, et al. Serum uric acid is associated with microalbuminuria and subclinical atherosclerosis in men with type 2 diabetes mellitus. Metabolism. 2008;57(5):625–629. doi: 10.1016/j.metabol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Vasilkova V, Mokhort T, Naumenko E. Relationship between carotid artery intima-media thickness and cardiovascular risk factors in patients with diabetes. Athersclerosis. 2016;252:138–139. doi: 10.1016/j.atherosclerosis.2016.07.696. [DOI] [Google Scholar]
- 30.Shah AS, Urbina EM, Khoury PR, Kimball TR, Dolan LM. Lipids and lipoprotein ratios: contribution to carotid intima media thickness in adolescents and young adults with type 2 diabetes mellitus. J Clin Lipidol. 2013;7:441–445. doi: 10.1016/j.jacl.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gayathri R, Chandni R, Udayabhaskaran V. Carotid artery intima media thickness in relation with atherosclerotic risk factors in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2012;60:20–24. [PubMed] [Google Scholar]
- 32.Marini MA, Fiorentino TV, Andreozzi F, Mannino GC, Succurro E, Sciacqua A, Perticone F, Sesti G. Hemorheological alterations in adults with prediabetes identified by hemoglobin A1c levels. Nutr Metab Cardiovasc Dis. 2017;27(7):601–608. doi: 10.1016/j.numecd.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Bi Y, Wang W, Xu M, Xu Y, Li M, et al. Glycated hemoglobin A1c, fasting plasma glucose, and two-hour postchallenge plasma glucose levels in relation to carotid intima-media thickness in chinese with normal glucose tolerance. J Clin Endocrinol Metab. 2011;96:E1461–E1465. doi: 10.1210/jc.2010-2697. [DOI] [PubMed] [Google Scholar]
- 34.Venkataraman V, Amutha A, Anbalagan VP, Deepa M, Anjana RM, Unnikrishnan R, et al. Association of glycated hemoglobin with carotid intima medial thickness in Asian Indians with normal glucose tolerance. J Diabetes Complicat. 2012;26:526–530. doi: 10.1016/j.jdiacomp.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Kowall B, Ebert N, Then C, Thiery J, Koenig W, Meisinger C, et al. Associations between blood glucose and carotid intima-media thickness disappear after adjustment for shared risk factors: the KORA F4 study. PLoS One. 2012;7:e52590. doi: 10.1371/journal.pone.0052590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jørgensen L, Jenssen T, Joakimsen O, Heuch I, Ingebretsen OC, Jacobsen BK. Glycated hemoglobin level is strongly related to the prevalence of carotid artery plaques with high echogenicity in nondiabetic individuals: the Tromsø study. Circulation. 2004;110:466–470. doi: 10.1161/01.CIR.0000136809.55141.3B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.


