Abstract
Objective
The purpose of this study was to comprehensively evaluate the effect of short-term exercise intervention on the cardiovascular functions and quality of life (QoL) of patients with chronic heart failure (CHF).
Methods
This meta-analysis was analyzed using RevMan5.3 and Stata 13.0. The parameters of cardiovascular functions and QoL were assessed. Weighted mean differences and their corresponding 95% confidence intervals (CIs) were computed for continuous variables.
Results
Data from 2533 CHF patients enrolled in 28 published studies of randomized controlled trials (RCTs) were collated. There were significant differences in VO2 max prior to and after exercise intervention in CHF patients who are 50–55 years old (5 RCTs; 95% CI, −4.86 to −2.29; I2 = 50.5%), 60–65 years old (10 RCTs; 95% CI, −2.66 to −2.04; I2 = 0%), and 69–75 years old (5 RCTs; 95% CI, −1.88 to −0.34; I2 = 38.5%). VO2 max was significantly increased by aerobic exercise (9 RCTs; 95% CI, −3.45 to −1.92; I2 = 37.7%) and combined aerobic resistance exercise (4 RCTs; 95% CI, −4.41 to −0.26; I2 = 76.6%). There were significant differences in cardiac output (n = 303; 95% CI, −0.25 to −0.02; I2 = 12%) and QoL (n = 299; 95% CI, 3.19 to 9.70; I2 = 17%) prior to and after short-term exercise.
Conclusion
Aerobic exercise and aerobic with resistance exercise can significantly improve the aerobic capacity of CHF patients, whereas resistance exercise cannot. The improvement in aerobic capacity caused by aerobic exercise and aerobic with resistance exercise decreases with age. Systolic blood pressure and ventricle structures and functions of CHF patients show no significant changes after the short-term exercise intervention.
Keywords: cardiovascular functions, meta-analysis, patients with chronic heart failure, short-term exercise intervention
Introduction
Chronic heart failure (CHF) is a progressive syndrome with debilitating symptoms and a poor prognosis. Severe exercise intolerance with marked dyspnea and fatigue at rest or exertion is one of the most prominent symptoms of CHF. It results in a poor quality of life (QoL) for the patients and an economic burden on healthcare systems. The estimated costs related to CHF have been steadily rising in the United States and other developed countries.1 With the aging of the population and improvement of medical management, the prevalence of CHF and studies of CHF are gradually spreading worldwide.2
CHF can be comprehensively evaluated using the cardiovascular functions and QoL. Aerobic capacity, blood pressure, ventricular structures, and functions are the important parameters to assess the cardiovascular functions of CHF. Aerobic capacity, which reflects the cardiorespiratory fitness of CHF patients, is assessed by VO2 max.3 It is reported that systolic blood pressure (SBP) is the most relevant component of blood pressure for the prognosis of people suffering from arterial hypertension.4 Ventricular structures and functions manifest the ejection capacity of heart and ventricular filling capacity.5 They were quantified by such parameters as cardiac output (CO), left ventricular ejection fraction (LVEF), heart rate (HR), and heart rate variability (HRV). Minnesota Living with Heart Failure Questionnaire (MLHFQ) is a professional method developed by Rector to assess the QoL of patients with CHF, including the patient's perception of the effects of CHF and its treatment on her or his daily life.6
Medication was more commonly chosen by a vast number of patients with CHF to improve their cardiovascular functions and QoL compared with exercise intervention. Although exercise intervention cannot take the place of medication, it was highly recommended by the Agency for Health Care Policy and Research Guidelines on Cardiac Rehabilitation, and it was regarded as adjunctive therapy in the treatment of selected patients with CHF.7 Exercise intervention is also recommended by the American College of Cardiology/American Heart Association as a safe and effective treatment for patients with CHF, and studies on exercise intervention mainly focus on short-term follow-up exercise intervention.8 Short-term exercise intervention is the most common method used by exercise rehabilitation in patients with CHF because it is easy to observe the effects of exercise on patients with CHF and to control other intervention factors.5 In addition, there have been numerous studies on the effects of short-term exercise intervention on the cardiovascular system in patients with CHF.5
Short-term aerobic exercise rehabilitation programs improve the aerobic capacity and QoL of CHF patients.5 Endurance exercise and continuous exercise have beneficial effects in CHF patients.5, 9 However, the review study of *Piña et al5 reported that it was not clear whether short-term exercise could improve the cardiovascular system of CHF patients because there were several study limitations. Hedback and Perk10 found that 4 months of exercise training could improve the exercise tolerance of CHF patients; however, there was no significant difference between the exercise and control groups in ventricular changes. It thus remains controversial whether short-term exercise intervention can benefit the whole cardiovascular functions and QoL of CHF patients.
Therefore, this study aimed to quantitatively explore and assess the effect of short-term exercise intervention on the cardiovascular system and QoL of CHF patients through meta-analytic techniques. It was hypothesized that short-term exercise intervention would improve the cardiovascular functions and QoL of patients with CHF.
Methods
Search strategy
A search of PubMed, Google Scholar, Cochrane Controlled Trials Registry, Yahoo, and Springer database was performed using these key terms: Aerobic, Aerobic exercise, resistance exercise, exercise training, endurance exercise, short-term exercise, long-term exercise, sports, swimming, football, jogging, heart failure, CHF patients, and randomized controlled trials (RCTs). The published time of references that we used was from 2004 to 2014 in this study.
Inclusion criteria
The following inclusion criteria were used:
-
1.
Only RCTs examining the effect of exercise intervention on CHF patients were included in this study.
-
2.
The participants were patients with CHF (50–75 years old), and participants with CHF evoked by specific conditions or pathologies were excluded.
-
3.
The exercise intervention was selected for short-term duration (8–24 weeks). The exercise types (aerobic exercise or resistance exercise or combination aerobic and resistance exercise), exercise frequency (more than 3 times per week), exercise intensity (40–80% VO2 reserve) were included in this study. Studies on participants who previously had exercise-based rehabilitations were excluded. The exercise intervention group should receive short-term exercise intervention. The control group must not do any exercise training.
-
4.
There were several outcome measures in the study: (1) VO2 max; (2) SBP; (3) CO, LVEF, HR, and SDNN; and (4) MLHFQ.
Study selection
The prespecified criteria were used by two reviewers to screen the titles, abstracts, and full text of relevant articles. An article was excluded if it did not meet the inclusion criteria and selection standard. Any disagreements about the included studies were discussed and then resolved by a third author.
Data collation
The following information was extracted independently by two reviewers in these studies: design of the study, participant characteristics, selection of follow-up and outcome measures, detailed information of the intervention group (i.e., type of exercise, intensity, and duration of exercise intervention), and nature of the control group. Similarly, a third author was asked to make the final decision if any disagreement persisted.
Quality assessment
The Cochrane Collaboration recommendations were used to assess the risk of bias for all the included articles in this study.11 The following information was assessed: selection bias, performance bias, detection bias, and attrition bias.
Statistical analysis
Stata MP. 13.0 and RevMan5.3 were used for the meta-analysis. The between-studies heterogeneity was evaluated using chi-square test and I2 statistic. The fixed-effects model was used if the heterogeneity test did not show significant difference (p > 0.1, I2 < 50%). Otherwise, the random-effects model was adopted in this study. The mean difference (MD) and 95% confidence interval (CI) were used to analyze the continuous variables. A p value less than 0.05 was considered statistically significant. The potential heterogeneity was explored by metaregression and subgroup analysis in this study.
Results
Search results
A total of 7251 records were identified through PubMed, Google Scholar, Yahoo, and other sources. Twenty-eight published reports, which analyzed exercise intervention in 2533 CHF patients, met the criteria for included RCTs studies (Figure 1). A total of 28 RCTs12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 with exercise durations of 8–24 weeks were enrolled. In addition, the characteristics of patients with CHF enrolled in RCTs are listed in Table 1.
Figure 1.
Flow diagram for inclusion of studies in meta-analysis.
Table 1.
Baseline characteristics in CHF patients and intervention of included literature.
| Reference | Experimental group | Control group | Methods | Follow-up time | Exercise intervention |
|---|---|---|---|---|---|
| Blumenthal et al (2005)12 | 48 | 42 | RCT | 16 weeks | Aerobic exercise training for 35 minutes 3 times/week for 16 weeks |
| Huang et al (2014)13 | 33 | 33 | RCT | 12 weeks | High-intensity interval aerobic training at ventilatory anaerobic threshold for 50 min/day, 3 days/week for 4 weeks, and then 3-min intervals at 40 and 80% VO2 reserve for 50 min/day, 3 days/week for 8 weeks |
| Servantes et al (2011)14 | 18 | 14 | RCT | 3 months | Home-based aerobic exercise 3 supervised exercise sessions |
| Maria Sarullo et al (2006)15 | 30 | 30 | RCT | 3 months | 3 months of supervised physical training program using a bicycle ergometer for 30 min three times a week at a load corresponding to 60–70% of their oxygen consumption (VO2) |
| Servantes et al (2011)14 | 18 | 14 | RCT | 3 months | 3 months of home-based aerobic with strength exercise of three supervised exercise sessions |
| Edelmann et al (2011)16 | 44 | 20 | RCT | 3 months | Supervised endurance or resistance exercise |
| Chrysohoou et al (2014)17 | 33 | 39 | RCT | 12 weeks | High-intensity intermittent endurance training 30s at 100% of max workload, 30s at rest, for 45 min/day, 3 days/week by 12 weeks) |
| Westhoff-Bleck et al (2013)18 | 24 | 24 | RCT | 24 weeks | 24 weeks of structured exercise training |
| Ricca-Mallada et al (2012)19 | 10 | 10 | RCT | 24 weeks | Physical training 3 times a week for 24 weeks |
| Fu et al (2011)20 | 15 | 15 | RCT | 12 weeks | Aerobic interval training (3-minute intervals at 40% and 80% VO2, sustained 60% VO2 max) for 30 min/day, 3 days/week for 12 weeks |
| Nishi et al (2011)21 | 33 | 12 | RCT | 3 months | Walking, bicycling on an ergometer, and calisthenics of 40–60 min/session 3–5 sessions/week for 3 months |
| Murad et al (2012)22 | 31 | 35 | RCT | 16 weeks | Aerobic exercise 3 times per week for 16 weeks |
| Osbak et al (2011)23 | 240 | 23 | RCT | 12 weeks | Aerobic exercise training 3 times weekly for 12 weeks |
| Yeh et al. (2011)24 | 50 | 50 | RCT | 12 weeks | Tai Chi exercise program twice weekly for 12 weeks |
| Maiorana et al (2011)25 | 12 | 12 | RCT | 12 weeks | Aerobic exercise 3 supervised sessions per week for 12 weeks |
| Kitzman et al (2010)26 | 26 | 27 | RCT | 16 weeks | ET exercised 3 times per week for 16 weeks for a total of 48 sessions in a dedicated facility under medical supervision |
| Pullen et al (2009)27 | 21 | 19 | RCT | 3 months | One-hour yoga sessions were conducted twice per week |
| Brubaker et al (2009)28 | 30 | 29 | RCT | 16 weeks | Sixteen-week supervised ET program of endurance exercise (walking and stationary cycling) three times per week for 30 to 40 minutes at moderate intensity |
| Collins et al (2003)29 | 15 | 16 | RCT | 12 weeks | Aerobic exercise program 3 times per week for 12 weeks |
| Belardinelli et al (2008)30 | 44 | 42 | RCT | 8 weeks | Aerobic dancing 3 times a week |
| Andersen et al (2006)31 | 21 | 20 | RCT | 5 months | Aerobic and resistance training program twice a week for 5 months |
| Selig et al (2004)32 | 19 | 20 | RCT | 3 months | Resistance training 3 sessions per week for 3 months |
| Fu et al (2011)20 | 15 | 15 | RCT | 12 weeks | Moderate continuous training for 30 min/day, 3 days/week |
| Conraads et al (2007)33 | 8 | 9 | RCT | 4 months | Undergo standard pharmacological therapy plus a 4-month-long endurance exercise training program consisting of 3 sessions/week |
| Pozehl et al (2010)34 | 22 | 20 | RCT | 12 weeks | Aerobic exercise 3 times weekly in a hospital-based rehabilitation setting and resistance training twice weekly at home |
| Jurgens et al (2013)35 | 48 | 51 | RCT | 5 stages | Training intervention |
| Maiorana et al (2011)25 | 12 | 12 | RCT | 12 weeks | Resistance exercise 3 supervised sessions per week for 12 weeks |
| Belardinelli et al (2008)30 | 44 | 42 | RCT | 8 weeks | Aerobic exercise training at 70% of peak VO2, 3 times a week |
CHF = chronic heart failure; ET = exercise training; RCT = randomized controlled trial.
Risk of bias of included studies
Every included study had a high risk according to the Cochrane Collaboration recommendations. Accordingly, the evidence in this meta-analysis had a high overall risk of bias. Quality assessment of included studies was performed using the risk of bias table of RevMan 5.3, including random sequence generation, blinding of outcome assessments, allocation concealment, incomplete outcome data, blinding of participants and personnel, selective reporting, and other bias. The detailed risk of bias is illustrated in Table 2.
Table 2.
Risk of bias assessment of included studies.
| Author (year) | Adequate sequence generation | Allocation concealment | Blinding of participants and personal | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Blumenthal et al. (2005)12 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Huang et al. (2014)13 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? |
| Maria Sarullo et al. (2006)15 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? |
| Servantes et al. (2011)14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Edelmann et al. (2011)16 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Chrysohoou et al. (2014)17 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? |
| Westhoff-Bleck et al. (2013)18 | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ? |
| Ricca-Mallada et al. (2012)19 | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✕ |
| Fu et al. (2011)20 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Nishi et al. (2011)21 | ✓ | ✓ | ✕ | ? | ✓ | ✓ | ? |
| Murad et al. (2012)22 | ✓ | ✓ | ? | ? | ✓ | ✓ | ✓ |
| Osbak et al. (2011)23 | ✓ | ✕ | ✕ | ✓ | ✓ | ✓ | ? |
| Yeh et al. (2011)24 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ |
| Kitzman et al. (2010)26 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Pullen et al. (2009)27 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Brubaker et al. (2009)28 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Collins et al. (2003)29 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? |
| Andersen et al. (2006)31 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? |
| Selig et al. (2004)32 | ✓ | ✓ | ? | ✓ | ? | ✓ | ? |
| Conraads et al. (2007)33 | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✕ |
| Pozehl et al. (2010)34 | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✕ |
| Jurgens et al. (2013)35 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Maiorana et al. (2011)25 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Belardinelli et al. (2008)30 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
✕ = high risk; ✓ = low risk; ? = unclear risk.
Effect of short-term exercise intervention on aerobic capacity
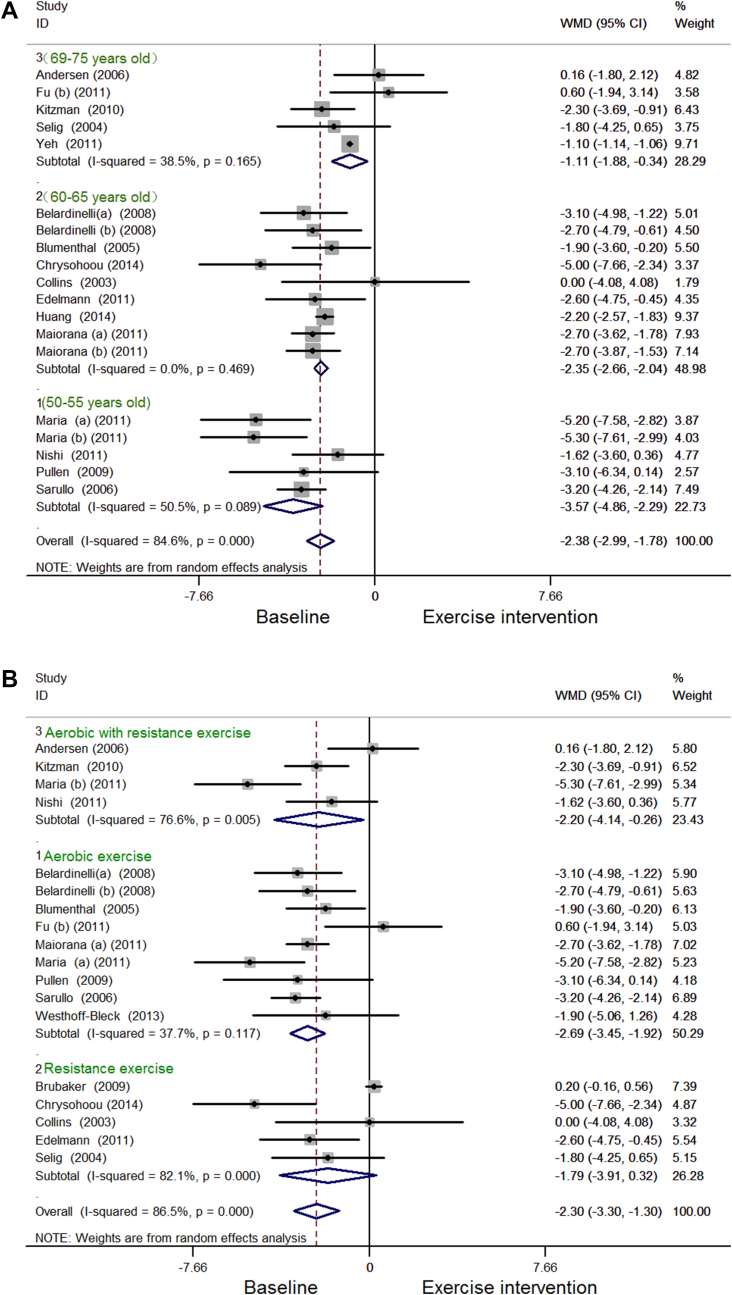
Among the 527 patients enrolled in 20 RCTs, the VO2 max of patients with CHF had significant differences prior to and after short-term exercise intervention. The meta-analysis of VO2 max showed high heterogeneity, so that it was analyzed by subgroup analysis according to the covariate of ages with a high proportion of adjusted R2 of covariates (39.60%) and a significant difference (p = 0.005) by metaregression (Table 3). VO2 max was significantly increased for CHF patients who were 50–55 years old [5 RCTs, random-effects weighted (WMD), −3.57; 95% CI, −4.86 to −2.29; I2 = 50.5%, p = 0.089; Figure 2Ai], 60–65 years old (10 RCTs; random-effects WMD, −2.35; 95% CI, −2.66 to −2.04; I2 = 0.0%, p = 0.469; Figure 2Aii), and 69–75 years old (5 RCTs; random-effects WMD, −1.11; 95% CI, −1.88 to −0.34; I2 = 38.5%, p = 0.165; Figure 2Aiii). The parameter of VO2 max was also analyzed by subgroup analysis according to the covariate of type of exercise. VO2 max was significantly increased by aerobic exercise (9 RCTs; random-effects SMD, −2.69; 95% CI, −3.45 to −1.92; I2 = 37.7%, p = 0.117; Figure 2Bi) and aerobic with resistance exercise (4 RCTs; random-effects SMD, −2.20; 95% CI, −4.41 to −0.26; I2 = 76.6%, p = 0.005; Figure 2Biii), whereas VO2 max had no significant change after resistance exercise (5 RCTs; random-effects SMD, −1.79; 95% CI, −3.91 to 0.32; I2 = 82.1%, p = 0.000; Figure 2Bii).
Table 3.
Univariate metaregression of the covariates of ages, and duration in the meta-analysis in parameter of VO2 max.
| Coef. | p | Adj R2 | 95% CI | |
|---|---|---|---|---|
| Ages | 0.129 | 0.005 | 39.60% | 0.045 to 0.214 |
| Duration | 0.746 | 0.183 | 8.24% | −0.381 to 1.872 |
| Class of HF | 0.016 | 0.671 | −7.16% | −0.614 to 0.093 |
Adj R2 = adjusted R2, proportion of between-study variance explained; CI = confidence interval; Coef. = coefficient of metaregression; HF = heart failure.
Figure 2.
(A) Forest plot showing the effect of short-term exercise intervention on VO2 max in CHF patients who were 50–55 years old (i), 60–65 years old (ii), and 69–75 years old (iii). (B) Forest plot showing the effect of short-term exercise intervention on VO2 max in CHF patients: (i) aerobic exercise; (ii) resistance exercise; (iii) aerobic with resistance exercise. CHF = chronic heart failure.
Effect of short-term exercise intervention on SBP
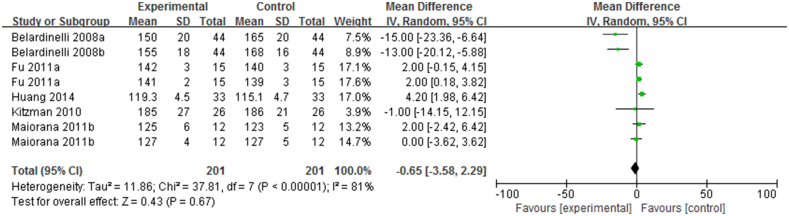
The SBP, reported in 201 CHF patients enrolled in six RCTs, was not significantly decreased after short-term exercise intervention (n = 201, I2 = 81%; 95% CI, −3.85 to 2.99; p = 0.67; Figure 3), and there appears to be some heterogeneity.
Figure 3.
Forest plot showing the effect of short-term exercise intervention on SBP of CHF patients in randomized controlled trials (RCTs).
Effect of short-term exercise intervention on ventricular structures and functions
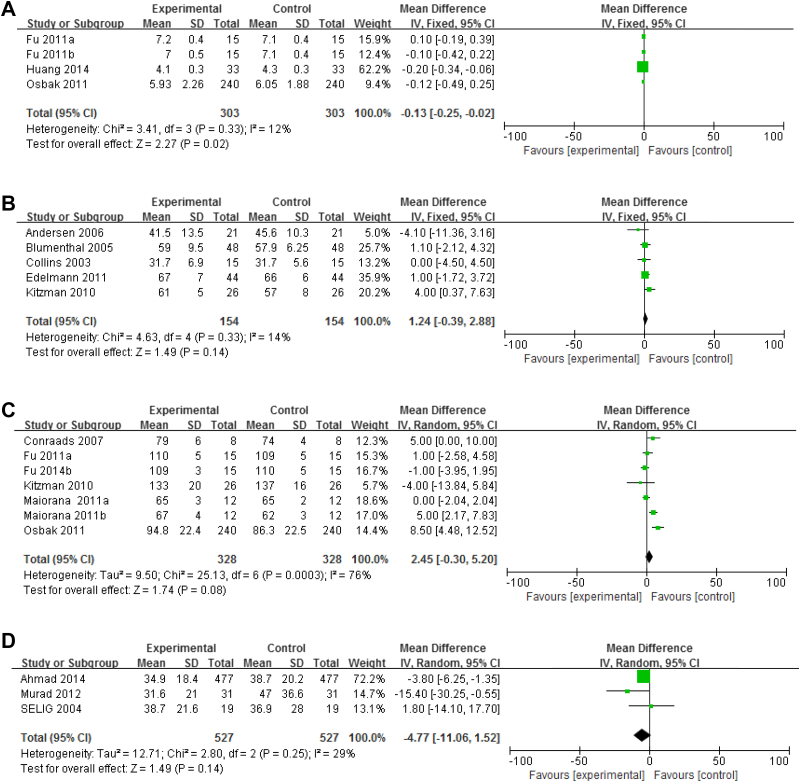
CO, LVEF, HR, and HRV were assessed using the ventricular structures and functions. CO, reported in 303 CHF patients enrolled in four RCTs, was significantly increased after short-term exercise intervention (n = 303; I2 = 12%; 95% CI, −0.25 to −0.02; p = 0.02; Figure 4A). However, LVEF showed no significant difference prior to and after short-term exercise (LVEF; n = 154, I2 = 14%; 95% CI, −0.39 to 2.88; p = 0.14; Figure 4B). A total of 127 patients with CHF were enrolled in six RCTs, and HR showed no significant difference prior to and after short-term exercise intervention (HR: n = 328, I2 = 76%; 95% CI, −0.30 to 5.20; p = 0.08; Figure 4C). There appears to be some heterogeneity. SDNN is a parameter of HRV in the time domain. HRV was not significantly improved after short-term exercise intervention (SDNN: 3 RCTs; n = 527, I2 = 29%; 95% CI, −11.06 to 1.52; p = 0.14; Figure 4D).
Figure 4.
(A) Forest plot showing the effect of short-term exercise intervention on CO of CHF patients in RCTs. (B) Forest plot showing the effect of short-term exercise intervention on LVEF of CHF patients in RCTs. (C) Forest plot showing the effect of short-term exercise intervention on HR of CHF patients in RCTs. (D) Forest plot showing the effect of short-term exercise intervention on HRV of CHF patients in RCTs. CHF = chronic heart failure; CO = cardiac output; LVEF = left ventricular ejection fraction; RCTs = randomized controlled trials.
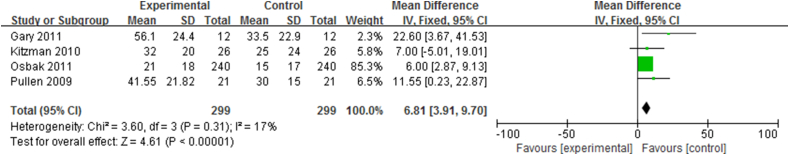
Effect of short-term exercise intervention on QoL of CHF patients
The QoL, reported in 299 CHF patients enrolled in four RCTs, was significantly improved after short-term exercise intervention (MLHFQ: n = 299, I2 = 17%; 95% CI, 3.19 to 9.70; p < 0.00001; Figure 5).
Figure 5.
Forest plot showing the effect of short-term exercise intervention on QoL of CHF patients in RCTs. CHF = chronic heart failure; QoL = quality of life; RCTs = randomized controlled trials.
Discussion
There have been relatively few review studies on the effects of exercise on CHF patients in comparison with the numerous review studies on medication, even though there are several reports on exercise intervention of CHF patients.36, 37 Although exercise intervention cannot take the place of medication for CHF patients, it is an effective method for the cardiac rehabilitation of patients with CHF. In addition, there numerous studies on short-term exercise intervention in patients with CHF, and short-term exercise intervention is recommended as an effective treatment for CHF patients. However, it is not clear whether short-term exercise intervention has effects on whole cardiovascular functions and QoL. This meta-analysis study comprehensively assessed the effect of short-term exercise interventions on whole cardiovascular functions and QoL in patients with CHF. In this systematic review and meta-analysis, the data of 2533 CHF patients in 28 published studies were studied to assess the cardiovascular systems and QoL.
Effect of short-term exercise intervention on cardiovascular parameters
The systematic review of RCTs confirms a favorable effect of short-term exercise intervention on CHF patients who were 50–55, 60–65, and 69–75 years old. VO2 max was significantly improved by short-term exercise intervention at different ages. Meanwhile, the change of VO2 max decreased with age. VO2 max is the most important parameter to describe aerobic capacity, and it is a good predictor of prognosis for CHF patients; in addition, some studies reported that aerobic power, muscle strength, and skeletal muscle metabolism could be improved after short-term exercise intervention.5 In addition, VO2 max showed different changes with different types of exercise. VO2 max was significantly increased by aerobic exercise and aerobic with resistance exercise. Some studies found that aerobic and resistance exercise showed different changes in the cardiovascular system.38
There was a significant difference in CO prior to and after exercise intervention. However, there were no significant differences in SBP, HR, and LVEF prior to and after exercise intervention. These findings agree in part with a previous study that explored the effects of modified high-intensity interval training on peak cardiac power output in patients with CHF. The study found that CO significantly increased after exercise training, whereas SBP, HR, and LVEF were not significantly improved.13 CO was significantly increased whereas SBP was not improved after exercise intervention because of the elevated peripheral vascular conductance peak.13 CO was mainly determined by stroke volume and HR at rest or during exercise. The change in HR was not only relevant to CO, but also to stroke volume, maximum HR, exercise intensity, and LVEF.8 Some studies reported that exercise training can increase the maximum CO, but this change was not paralleled by alterations in LVEF or end-systolic or end-diastolic volumes at rest.8, 39 In addition, in the present study, these changes of parameters may be related to the disease status of patients with CHF or numbers of included studies. HRV analysis is a noninvasive method used to evaluate autonomic regulation.40 The reduction of HRV in patients with cardiovascular diseases is related to high cardiovascular mortality and is considered a risk factor for the occurrence of cardiac arrhythmias.41, 42 HRV was assessed by SDNN, which indicates the standard deviation of all normal RR intervals in the time domain. In this study, there was no significant difference in HRV prior to and after short-term exercise intervention. Reboredo et al43 found that HRV both in time and frequency domains had no significant difference for 12-week exercise training. In the present study, there were just three included studies analyzing the HRV of patients with CHF. Therefore, the change of HRV may relate to the numbers of studies. A condition-specific measure of MLHFQ was used to assess the effect of short-term exercise intervention on QoL.44 Some studies have shown that the MLHFQ is responsive to the QoL of CHF patients.6 The MLHFQ have a significantly lower (improved) physical score after short-term exercise in this study because of the improvement of cardiovascular functions.44
Study limitations
In this analysis, we spent a considerable amount of time on retrieval and evaluation of the related literature, and there were a few RCT studies on exercise and cardiovascular disease in contrast to the RCT studies on drug therapy. However, those articles still have some risk of bias according to the Cochrane Collaboration recommendations, and some methods of literature quality evaluation had a high subjectivity. Therefore, methodologically rigorous articles were still limited. The judgment of publication bias in funnel plot asymmetry showed unclear risk and small study bias. Future RCTs need to give detailed information between control and intervention groups. In addition, the outcome measures varied considerably in the studies, and some studies just assessed smaller outcome measures, such as SDNN. Finally, further studies on the relation between exercise and cardiovascular disease are needed because of the complicated physiology and pathology of exercise, such as different ages, long-term durations, and different sports.
Conclusions
The results of this meta-analysis regarding exercise, cardiovascular functions, and QoL confirm that short-term exercise intervention can significantly improve the aerobic capacity, CO, and QoL of CHF patients. Aerobic exercise and combined aerobic and resistance exercise can significantly improve the aerobic capacity of CHF patients, whereas resistance exercise cannot. In addition, the improvement of aerobic capacity caused by aerobic exercise and aerobic with resistance exercise may decrease with age. SBP, HR, LVEF, and HRV are not improved after short-term exercise intervention. In summary, the effect of short-term exercise intervention on cardiovascular functions and QoL of CHF patients is associated with age, exercise intensity, exercise duration, and type of exercise.
Conflicts of interest
The authors have no conflicts of interest or financial disclosures to report.
Funding/support
This work was supported by the National Natural Science Foundation of China (No. 61374015, No. 61202258), the PhD Programs Foundation of Ministry of Education of China (No. 20110042120037), and the Fundamental Research Funds for the Central Universities (No. N110219001).
References
- 1.Holland D.J., Kumbhani D.J., Ahmed S.H. Effects of treatment on exercise tolerance, cardiac function, and mortality in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2011;16:1676–1686. doi: 10.1016/j.jacc.2010.10.057. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D., Adams R.J., Brown T.M. Heart disease and stroke statistics—2010 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2010;121:948. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 3.Tong T.K., Chung P.K., Leung R.W. Effects of non-Wingate-based high-intensity interval training on cardiorespiratory fitness and aerobic-based exercise capacity in sedentary subjects: a preliminary study. J Exerc Sci Fit. 2011;9:75–81. [Google Scholar]
- 4.Kannel W.B., Gordon T., Schwarz M.J. Systolic versus diastolic blood pressure and risk of coronary heart disease: Framingham Study. Am J Cardiol. 1971;27:335–346. doi: 10.1016/0002-9149(71)90428-0. [DOI] [PubMed] [Google Scholar]
- 5.Piña I.L., Apstein C.S., Balady G.J. Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 6.Rector T.S., Kubo S.H., Cohn J.N. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol. 1993;71:1106–1107. doi: 10.1016/0002-9149(93)90582-w. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services. Agency for Health Care Policy and Research . Agency for Health Care Policy and Research; Washington, DC: October 1995. Clinical Practice Guideline No. 17: Cardiac Rehabilitation. AHCPR Publication No. 96–0672. [Google Scholar]
- 8.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA Guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;15:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Hughes D.C., Lenihan D.J., Harrison C.A. Exercise intervention for cancer survivors with heart failure: two case reports. J Exerc Sci Fit. 2011;9:65–73. doi: 10.1016/s1728-869x(11)60009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedback B., Perk J. Can high-risk patients after myocardial infarction participate in comprehensive cardiac rehabilitation? Scand J Rehabil Med. 1990;22:15–20. [PubMed] [Google Scholar]
- 11.Higgins J.P., Altman D.G., Gøtzsche P.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blumenthal J.A., Sherwood A., Babyak M.A. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease. JAMA. 2005;293:1626–1634. doi: 10.1001/jama.293.13.1626. [DOI] [PubMed] [Google Scholar]
- 13.Huang S.C., Wong M.K., Lin P.J. Modified high-intensity interval training increases peak cardiac power output in patients with heart failure. Eur J Appl Physiol. 2014;114:1853–1862. doi: 10.1007/s00421-014-2913-y. [DOI] [PubMed] [Google Scholar]
- 14.Servantes D.M., Pelcerman A., Salvetti X.M. Effects of home-based exercise training for patients with chronic heart failure and sleep apnoea: a randomized comparison of two different programs. Clin Rehabil. 2011;26:45–57. doi: 10.1177/0269215511403941. [DOI] [PubMed] [Google Scholar]
- 15.Maria Sarullo F., Gristina T., Brusca I. Effect of physical training on aerobic capacity, gas-exchange and N-terminal pro-brain natriuretic peptide levels in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2006;13:812–817. doi: 10.1097/01.hjr.0000238396.42718.61. [DOI] [PubMed] [Google Scholar]
- 16.Edelmann F., Gelbrich G., Düngen H.D. Exercise training improves aerobic capacity and diastolic function in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 17.Chrysohoou C., Tsitsinakis G., Vogiatzis I. High intensity-interval exercise improves quality of life of patients with chronic heart failure: a randomized controlled trial. Q J Med. 2014;107:25–32. doi: 10.1093/qjmed/hct194. [DOI] [PubMed] [Google Scholar]
- 18.Westhoff-Bleck M., Schieffer B., Tegtbur U. Aerobic training in adults after atrial switch procedure for transposition of the great arteries improves aerobic capacity without impairing systemic right ventricular function. Int J Cardiol. 2013;170:24–29. doi: 10.1016/j.ijcard.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Ricca-Mallada R., Migliaro E.R., Piskorski J. Exercise training slows down heart rate and improves deceleration and acceleration capacity in patients with heart failure. J Electrocardiol. 2012;45:214–219. doi: 10.1016/j.jelectrocard.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Fu T.C., Wang C.H., Lin P.S. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int J Cardiol. 2013;167:41–50. doi: 10.1016/j.ijcard.2011.11.086. [DOI] [PubMed] [Google Scholar]
- 21.Nishi I., Noguchi T., Iwanaga Y. Effects of exercise training in patients with chronic heart failure and advanced left ventricular systolic dysfunction receiving β-blockers. Circ J. 2011;75:1649–1655. doi: 10.1253/circj.cj-10-0899. [DOI] [PubMed] [Google Scholar]
- 22.Murad K., Brubaker P.H., Fitzgerald D.M. Exercise training improves heart rate variability in older patients with heart failure: a randomized controlled single-blinded trial. Congest Heart Fail. 2012;18:192–197. doi: 10.1111/j.1751-7133.2011.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osbak P.S., Mourier M., Kjaer A. A randomized study of the effects of exercise training on patients with atrial fibrillation. Am Heart J. 2011;162:1080–1087. doi: 10.1016/j.ahj.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Yeh G.Y., McCarthy E.P., Wayne P.M. Tai Chi exercise in patients with chronic heart failure. Arch Intern Med. 2011;171:750–757. doi: 10.1001/archinternmed.2011.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiorana A.J., Naylor L.H., Exterkate A. The impact of exercise training on conduit artery wall thickness and remodeling in chronic heart failure patients. Hypertension. 2011;57:56–62. doi: 10.1161/HYPERTENSIONAHA.110.163022. [DOI] [PubMed] [Google Scholar]
- 26.Kitzman D.W., Brubaker P.H., Morgan T.M. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pullen P.R., Thompson W.R., Benardot D. Benefits of yoga for African American heart failure patients. Med Sci Sports Exerc. 2010:651–657. doi: 10.1249/MSS.0b013e3181bf24c4. [DOI] [PubMed] [Google Scholar]
- 28.Brubaker P.H., Moore J.B., Stewart K.P. Endurance exercise training in older patients with heart failure: Results from a randomized controlled single-blind trial. J Am Geriatr Soc. 2009;57:1982–1989. doi: 10.1111/j.1532-5415.2009.02499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins E., Langbein W.E., Dilan-Koetje J. Effects of exercise training on aerobic capacity and quality of life in individuals with heart failure. Heart Lung. 2004;5:154–161. doi: 10.1016/j.hrtlng.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Belardinelli R., Lacalaprice F., Ventrella C. Waltz dancing in patients with chronic heart failure new form of exercise training. Circ Heart Fail. 2008;1:107–114. doi: 10.1161/CIRCHEARTFAILURE.108.765727. [DOI] [PubMed] [Google Scholar]
- 31.Andersen K., Jónsdóttir S., Sigurethsson A.F. The effect of physical training in chronic heart failure. Eur J Heart Fail. 2006;8:97–101. doi: 10.1016/j.ejheart.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Selig S.E., Carey M.F., Menzies D.G. Moderate-intensity resistance exercise training in patients with chronic heart failure improves strength, endurance, heart rate variability and forearm blood flow. J Card Fail. 2004;11:21–29. doi: 10.1016/s1071-9164(03)00583-9. [DOI] [PubMed] [Google Scholar]
- 33.Conraads V.M., Vanderheyden M., Paelinck B. The effect of endurance training on aerobic capacity following cardiac resynchronization therapy in chronic heart failure patients: a pilot trial. Eur J Cardiovasc Prev Rehabil. 2007;14:99–106. doi: 10.1097/HJR.0b013e32801164b3. [DOI] [PubMed] [Google Scholar]
- 34.Pozehl B., Duncan K., Hertzog M. Heart failure exercise and training camp: effects of a multicomponent exercise training intervention in patients with heart failure. Heart Lung. 2010;6:S1–S13. doi: 10.1016/j.hrtlng.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurgens C.Y., Lee C.S., Reitano J.M. Heart failure symptom monitoring and response training. Heart Lung. 2013:1–8. doi: 10.1016/j.hrtlng.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Recommendations for exercise training in chronic heart failure patients. Eur Heart J. 2001;22:125–135. doi: 10.1053/euhj.2000.2440. [DOI] [PubMed] [Google Scholar]
- 37.Clark A.L., Poole-Wilson P.A., Coats A.J. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol. 1996;28:1092–1102. doi: 10.1016/S0735-1097(96)00323-3. [DOI] [PubMed] [Google Scholar]
- 38.Gossard D., Haskell W.L., Taylor B. Effects of low- and high-intensity home-based exercise training on functional capacity in healthy middle-age men. Am J Cardiol. 1986;57:446–449. doi: 10.1016/0002-9149(86)90770-8. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan M.J., Higginbotham M.B., Cobb F.R. Exercise training in patients with severe left ventricular dysfunction: hemodynamic and metabolic effects. Circulation. 1988;78:506–515. doi: 10.1161/01.cir.78.3.506. [DOI] [PubMed] [Google Scholar]
- 40.Reed M.J., Robertson C.E., Addison P.S. Heart rate variability measurements and the prediction of ventricular arrhythmias. QJM. 2005;98:87–95. doi: 10.1093/qjmed/hci018. [DOI] [PubMed] [Google Scholar]
- 41.Hayano J., Takahashi H., Toriyama T. Prognostic value of heart rate variability during long-term follow-up in chronic haemodialysis patients with end-stage renal disease. Nephrol Dial Transplant. 1999;14:1480–1488. doi: 10.1093/ndt/14.6.1480. [DOI] [PubMed] [Google Scholar]
- 42.Fukuta H., Hayano J., Ishihara S. Prognostic value of heart rate variability in patients with end-stage renal disease on chronic haemodialysis. Nephrol Dial Transplant. 2003;18:318–325. doi: 10.1093/ndt/18.2.318. [DOI] [PubMed] [Google Scholar]
- 43.Reboredo Mde M., Pinheiro Bdo V., Neder J.A. Effects of aerobic training during hemodialysis on heart rate variability and left ventricular function in end-stage renal disease patients. J Bras Nefrol. 2010;32:367–373. [PubMed] [Google Scholar]
- 44.Kitzman D.W., Little W.C., Brubaker P.H. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]