Abstract
Background
Abdominal aortic calcium (AAC) and coronary artery calcium (CAC) independently and similarly predict cardiovascular disease (CVD) events. The standard AAC and CAC score, the Agatston method, up-weights for greater calcium density, thus models higher calcium density as a CVD hazard.
Methods and Results
Computed tomography scans were used to measure AAC and CAC volume and density in a multi-ethnic cohort of community dwelling individuals, and Cox-proportional hazard was used to determine their independent association with incident coronary heart disease (CHD, defined as myocardial infarction, and resuscitated cardiac arrest, or CHD death), cardiovascular disease (CVD, defined as CHD plus stroke and stroke death), and all-cause mortality (ACM). In 997 participants with Agatston AAC and CAC scores > 0, mean age was 66 ± 9 years and 58% were men. Over an average follow-up of 9 years, there were 77 CHD, 118 CVD, and 169 ACM events. In mutually adjusted models, additionally adjusted for CVD risk factors, an increase in ln(AAC volume) per standard deviation (SD) was significantly associated with increased ACM (HR=1.25, 95% CI 1.38, 1.42, P<0.01), and an increase ln(CAC volume) per SD was significantly associated with CHD (HR=1.17, 95% CI 1.04, 1.59, P=0.02) and CVD (HR=1.20, 95% CI 1.05, 1.36, P<0.01). In contrast, both AAC and CAC density were not significantly associated with CVD events.
Conclusions
The Agatston method of up-weighting calcium scores for greater density may be inappropriate for CVD risk prediction in both the abdominal aorta and coronary arteries.
Keywords: coronary artery calcium, cardiovascular disease, abdominal aortic calcium, density calcium score, volume calcium score, agatston calcium score
Research suggests abdominal aortic calcium (AAC) predicts future cardiovascular disease (CVD) events similar to, and independent of coronary artery calcium (CAC). In the Multi-Ethnic Study of Atherosclerosis (MESA), we have previously reported that while CAC was a stronger predictor for coronary heart disease (CHD) and CVD than AAC, AAC was a stronger predictor for CVD mortality and all-cause mortality (ACM).1 These findings are noteworthy because to date, CAC is the most significant subclinical CVD marker in terms of CVD risk prediction.2 That is CAC predicts future CVD events and improves CVD risk stratification beyond traditional CVD risk factors.3,4
The standard method used to score AAC and CAC, the Agatston, up-weights plaque for higher calcium density. Thus, at any given level of CAC volume, a more dense plaque is modeled to predict higher risk of CVD. However, the appropriateness of up-weighting CAC for density is controversial. Hou et al. reported in 1889 patients with plaque measured from coronary computed tomography angiography (CTA) scans, that the incidence of CVD events was lower among patients with only calcified plaques (5.5%), compared to those with both calcified and non-calcified (37.7%), and those with only non-calcified plaques (22.7%).5 Similarly, in 1,102 patients with non-obstructive plaque, receiving CTA for evaluation of chest pain, Ahmadi et al. reported a higher risk of ACM in those with non-calcified plaques (7.4, 95% CI: 2.7, 20.1), and mixed plaques (3.2, 95% CI: 1.3, 8.0), compared to those with calcified plaques.6 Finally, we have recently reported that for a given CAC volume, higher CAC density was associated with lower, rather than higher, CVD risk.7 The impact of AAC volume vs. density in CVD is understudied.
We set out to determine the independent associations of AAC volume and density, compared to CAC volume and density for CVD events. We hypothesized that AAC and CAC volume would be positively associated with increased CVD risk, but the association for AAC and CAC density would be opposite. We also hypothesized that compared to CAC volume and density, AAC volume and density would have the strongest associations with CVD events.
Methods
Study sample
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multi-center, prospective cohort study designed to investigate prevalence, correlates, and progression of subclinical atherosclerosis and their associations with incident clinical events. A detailed description of the study design has been published.8 In brief, MESA recruited 6,814 persons age 45–84 of European-, Hispanic-, African-, and Chinese-American descent, free from clinical manifest CVD at baseline. Participants were recruited between July 2000 and August 2002 at 6 U.S field centers; New York, NY; Baltimore, MD; Winston-Salem, NC; St Paul, MN; Chicago IL; and Los Angeles, CA. Signed informed consent was obtained for all participants, and institutional review board approval was obtained for all participating institutions.
During follow up visits between August 2002 and September 2005, a randomly selected subsample of 2,202 MESA participants were invited to participate in an ancillary study that aimed to determine the presence and extent of AAC. Of these, 2,172 agreed to participate. Individuals were excluded if they were pre-menopausal, or had a recent (within 6 months) abdominal computed tomography (CT) scan. This left 1,970 participants who underwent abdominal CT scans to measure AAC, and at the same time received cardiac scans to measure CAC. For these analyses the study population was limited to 997 participants with both AAC and CAC prevalent (Agatston scores > 0), since a density score only has meaning in those with non-zero calcium volume.
Calcium measurement
The methodology for acquisition and interpretation for AAC has previously been described.9 Abdominal images were obtained using multi-detector CT scanners at Columbia University, Wake Forest University, and University of Minnesota field centers (Sensation 64 [Siemens, Malvern, Pennsylvania] and GE Lightspeed [GE Healthcare, Waukesha, Wisconsin], Siemens S4 Volume Zoom, and Siemens Sensation 16, respectively). Electron-beam CT scanners were utilized at Northwestern University and University of California, Los Angeles (Imatron C-150, Imatron Inc., South San Francisco, California). Images were reconstructed in a 35cm field of view with a slice thickness of 3mm (EBCT scanners) or 2.5mm (multi-detector scanners). An 8cm segment proximal to the aortic bifurcation was used to quantify AAC. At the same exam, each participant was scanned twice for CAC using the same site-specific scanners. The methodology for acquisition and interpretation of the scans as well as reproducibility of the readings, has been previously reported.10
Agatston11 scores were calculated for both AAC and CAC. Abdominal and cardiac CT slices were used to identify an area of plaque defined by density Hounsfield Units (HU) greater than 130.11 The plaque area (mm2) was then multiplied by 1, 2, 3, or 4, depending on the plaque’s maximum density. Plaques with maximum density of 130 to 199 HU were multiplied by 1, those with 200 to 299 HU were multiplied by 2, those with 300 to 399 HU were multiplied by 3 and those with 400 HU or greater were multiplied by 4. Both the AAC and the CAC scores for all CT slices were then summed to produce the total plaque-specific scores for the abdominal aorta and coronary arteries. The AAC volume (mm3) and CAC volume (mm3) scores were the sum of all plaque areas multiplied by the CT slice thickness. The AAC density and CAC density scores were calculated as: density = [Agatston] / [Area, in mm2]. Where Area, mm2 = [Volume mm3] / [CT scan slice thickness, in mm]. Thus density from these calculations represents a participant’s average multiplicative density factor (1–4 scale) derived from their original Agatston score.
Risk factor assessments
Participants were given standardized questionnaires at baseline, which were used to obtain information on demographics, medical history, and smoking history. A medication inventory was also performed, and medications were grouped based on use to treat high blood pressure, or elevated blood glucose. Blood pressure was measured 3 times in the seated position with a Dinamap model Pro 100 automated oscillometric sphygmomanometer after at least 5 minutes of rest. The average of the last 2 measurements was used. Blood samples were obtained after a 12h fast for measurements of total cholesterol, high-density lipoprotein (HDL) cholesterol, and glucose. Diabetes was defined as fasting plasma glucose ≥ 126 mg/dL, or use of hypoglycemic medications.
CVD events and mortality follow-up
A detailed description of the adjudication process has been previously published.12 Briefly, participants and their next of kin (if participants were unavailable) were contacted at intervals of 9–12 months by telephone, and trained interviewers inquired about interim hospital admissions, cardiovascular outpatient diagnoses, and death. Medical records and death certificates were requested for verification. Two physicians blinded to participants’ risk factors reviewed, classified CVD events, and assigned incidence dates. If disagreements persisted after adjudication, a full mortality and morbidity review committee made the final classification. For this current study, CVD events will include: 1) CHD (defined as myocardial infarction, resuscitated cardiac arrest, or CHD death); 2) CVD (defined as CHD plus non-fatal or fatal stroke); and 3) ACM.
Statistical Analysis
Descriptive statistics for the study cohort were presented as means (SD) and medians (25-th, 75-th percentiles) for continuous variables that were normally distributed and skewed (respectively), and frequencies for categorical variables across AAC density quartiles. Pearson’s correlation coefficients were used to determine the univariate associations of the AAC and CAC Agatston, volume, and density scores, and we plotted a scatter plot of AAC volume and density. Multivariable Cox proportional hazards were used to determine the independent associations of AAC and CAC volume and density scores for incident CHD, CVD, and ACM. Model 1 represents the associations of AAC volume and AAC density, adjusted for each other, but not for CVD risk factors. Model 2 represents the associations of AAC volume, AAC density, CAC volume, and CAC density, all mutually adjusted for each other, but not for CVD risk factors. Model 3 is Model 2 but now additionally adjusted for Global Framingham Risk Score13 (GFRS, a composite of age, sex, smoking status, diabetes status, total and HDL cholesterol, systolic blood pressure and hypertension treatment), ethnicity, and statin therapy. Separate sensitivity analysis were conducted for participants with AAC and CAC > 0. As AAC and CAC Agatston and volume scores were skewed, natural logs (ln) were used. All analyses were conducted using PSAW Statistics 20 (IBM, Corp., 2011 Amronk, NY). P-value ≤ 0.05 (two-sided) was considered significant for all analyses.
Results
Among 997 participants, the mean age was 66 ± 9 years, the mean AAC density was 3.10 ± 0.58 and the mean CAC density was 2.75 ± 0.72 (out of a density range between 1 to 4). The median (25–75th percentiles) AAC volume was 1,021 (288–2,641 mm3), and median (25–75th percentiles) CAC volume was 116 (34–356 mm3). Over an average follow-up of 9 years, there were 77 CHD, 118 CVD, and 169 ACM events. Compared to the first quartile, participants in the fourth AAC density quartile were less likely to be male, less likely to have European, Hispanic, and African Ancestry, but more likely to have Chinese ancestry. Participants in the fourth AAC density quartile also had more prevalent CVD risk factors, and higher calcium scores (Table 1). AAC density was positively correlated with ln(AAC volume), ln(Agatston AAC score), CAC density, ln(CAC volume), and ln(Agatston CAC score) (Table 2). Participants with higher ln(AAC volume), also had higher AAC density scores (Figure).
Table 1.
Cohort Characteristics for Participants with Nonzero Abdominal Aortic Calcium (AAC) and Coronary Artery Calcium (CAC) Scores by Quartiles of AAC Density Scores
| AAC Density Score, Quartile | |||||
|---|---|---|---|---|---|
| Q1 [0.8–2.9] N=249 |
Q2 [2.9–3.2] N=254 |
Q3 [3.2–3.5] N=247 |
Q4 [3.5–4.0] N=247 |
Cohort N=997 |
|
| Age, years | 63 ± 9 | 67 ± 10 | 67 ± 9 | 69 ± 9 | 66 ± 9 |
| Male, sex, n (%) | 157 (63) | 155 (61) | 141 (57) | 127 (51) | 580 (58) |
| Ethnicity, n (%) | |||||
| European | 101 (41) | 146 (58) | 129 (52) | 89 (36) | 465 (47) |
| Hispanic | 78 (31) | 53 (21) | 51 (21) | 62 (25) | 244 (24) |
| African | 50 (20) | 41 (16) | 37 (15) | 34 (14) | 162 (16) |
| Chinese | 20 (8) | 14 (6) | 30 (12) | 62 (25) | 126 (13) |
| Clinical characteristics | |||||
| Total cholesterol, mg/dL | 194 ± 32 | 195 ± 35 | 197 ± 37 | 197 ± 35 | 196 ± 34 |
| HDL cholesterol, mg/dL | 47 ± 13 | 48 ± 13 | 49 ± 14 | 53 ± 15 | 49 ± 14 |
| Systolic blood pressure, mm Hg | 130 ± 22 | 131 ± 22 | 133 ± 22 | 133 ± 20 | 132 ± 22 |
| Blood pressure treatment, n (%) | 105 (42) | 113 (45) | 116 (47) | 127 (51) | 461 (46) |
| Ever smoking, n (%) | 140 (56) | 155 (61) | 136 (55) | 137 (56) | 568 (57) |
| Diabetes, n (%) | 35 (14) | 38 (15) | 35 (14) | 34 (14) | 142 (14) |
| Statin therapy, n (%) | 38 (15) | 53 (21) | 47 (19) | 69 (28) | 207 (21) |
| Scores | |||||
| General FRS, 10 year % CVD risk | 17 ± 9 | 19 ± 9 | 19 ± 9 | 19 ± 9 | 19 ± 9 |
| AAC Agatston | 152 (37–462) | 1426 (571–2962) | 1795 (666–4227) | 2499 (1163–4471) | 1176 (314–3137) |
| AAC volume, mm3 | 171 (59–454) | 1266 (502–2448) | 1478 (614–3325) | 2064 (928–3662) | 1021 (288–2641) |
| AAC density | 2.30 ± 0.51 | 3.05 (0.08) | 3.31 ± 0.09 | 3.72 ± 0.13 | 3.10 ± 0.58 |
| CAC Agatston | 53 (19–173) | 169 (28–495) | 156 (45–428) | 145 (40–446) | 118 (29–387 |
| CAC volume, mm3 | 58 (21–176) | 151 (35–425) | 135 (50–382) | 142 (48–407) | 116 (34–356) |
| CAC density | 2.54 (0.76) | 2.64 (0.68) | 2.77 ± 0.67 | 3.07 ± 0.65 | 2.75 ± 0.72 |
| Events | |||||
| Coronary heart disease, n (%) | 14 (6) | 22 (9) | 19 (8) | 22 (9) | 77 (8) |
| Cardiovascular disease, n (%) | 23 (9) | 38 (15) | 26 (11) | 31 (13) | 118 (12) |
| All-cause mortality, n (%) | 31 (12) | 52 (21) | 45 (18) | 41 (17) | 169 (17) |
Data are [ranges], means ± SD, median (25–75th), and frequencies (%). HDL = high density lipoprotein, FRS = Framingham risk score, a composite of age, sex, smoking, diabetes, systolic blood pressure, blood pressure treatment, total, and HDL cholesterol used to predict an individual's 10 year risk for future cardiovascular disease.
Table 2.
Pearson's Correlation Coefficients of Abdominal Aortic Calcium (AAC) and Coronary Artery Calcium (CAC) scores in Participants with Nonzero AAC and CAC
| AAC density | ln(AAC volume) | ln(Agatston AAC) | CAC density | ln(CAC volume) | ln(Agatston CAC) | |
|---|---|---|---|---|---|---|
| AAC density | 1 | 0.65* | 0.70* | 0.25* | 0.21* | 0.21* |
| ln(AAC volume) | 1 | 0.99* | 0.21* | 0.36* | 0.36* | |
| ln(Agatston AAC) | 1 | 0.21* | 0.36* | 0.36* | ||
| CAC density | 1 | 0.64* | 0.71* | |||
| ln(CAC volume) | 1 | 0.99* | ||||
| ln(Agatston CAC) | 1 | |||||
Two-tailed p-value for significance < 0.01, ln = natural log.
Figure.
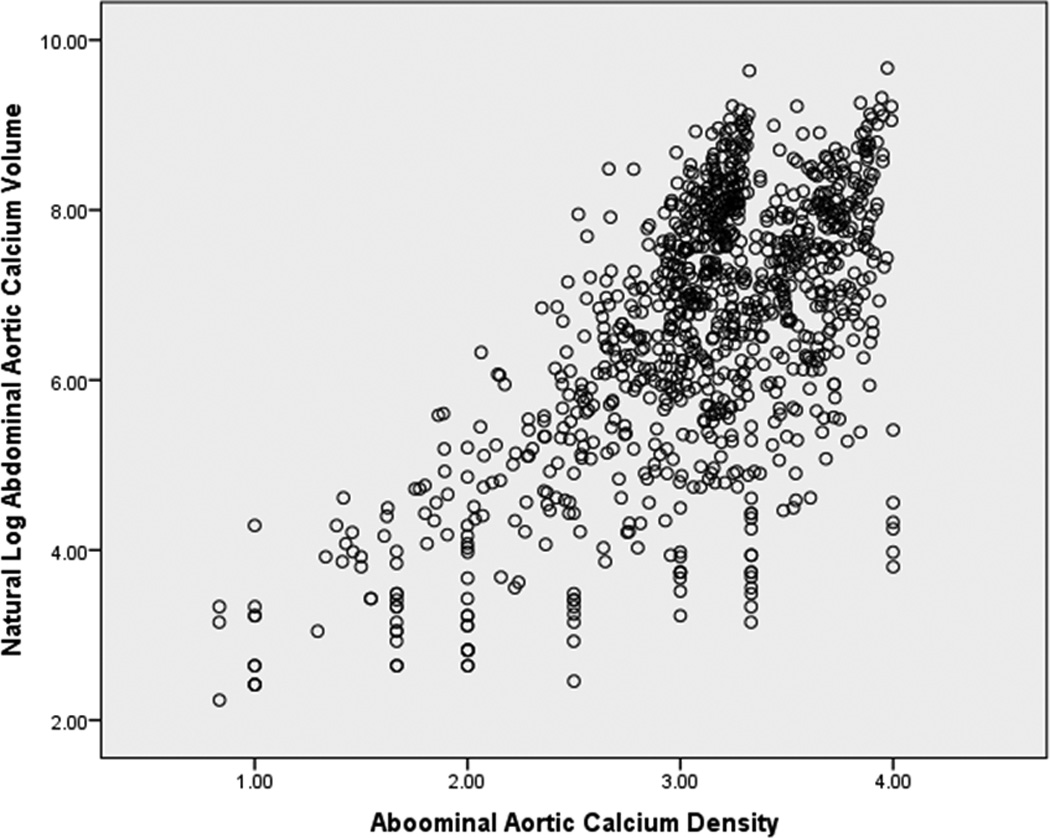
Scatter plot of abdominal aortic calcium density vs. volume
In models adjusted for ln(AAC volume), a SD higher in AAC density was not significantly associated with CHD [0.70; 95% CI 0.26, 1.86), or CVD [0.41; 95% CI 0.16, 1.04), but was associated with lower risk of ACM [0.43; 95% CI 0.19, 0.95) (Table 3 – model 1). In contrast, a SD higher in ln(AAC volume) was associated with higher risk of CHD [1.27; 95% CI 1.10, 1.45), CVD [1.34; 95% CI 1.17, 1.53), and ACM [1.33; 95% CI 1.19, 1.49) after adjustment for AAC density. After inclusion of both CAC density and ln(CAC volume), mutually adjusted, ln(AAC volume) remained significantly associated with higher risk for CHD, CVD and ACM, while AAC density trended towards reduced risk (Table 3 – model 2). After further adjustments for GFRS, ethnicity, and statin therapy, no significant associations were observed for AAC or CAC density with incident CHD, CVD, and ACM (Table 3 – model 3), although inspection of point estimates demonstrated that in general, they were consistently below 1. In contrast, after adjusting for AAC density, CAC density, and CVD risk factors, greater ln(AAC volume) remained strongly associated with ACM [1.25; 95% CI 1.36, 1.42) and greater ln(CAC volume) remained strongly associated with CHD [1.17; 95% CI 1.04, 1.59) and CVD [1.20; 95% CI 1.05, 1.36). Separate sensitivity analysis conducted for participants with AAC and CAC > 0 yielded similar associations of volume and density of AAC and CAC (respectively) with CVD events (not shown).
Table 3.
Multivariable Hazard Ratios (HR) for Abdominal Aortic Calcium (AAC) Density, ln(AAC volume), Coronary Artery Calcium (CAC) Density, and ln(CAC Volume) for Incident Coronary Heart Disease (CHD), Cardiovascular Disease (CVD), and All-cause Mortality (ACM)
| CHD (N=77) | CVD (N=117) | ACM (N=168) | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | Pvalue | HR (95% CI) | Pvalue | HR (95% CI) | Pvalue | |
| Model 1 | ||||||
| AAC density, per SD | 0.70 (0.26, 1.86) | 0.47 | 0.41 (0.16, 1.04) | 0.06 | 0.43 (0.19, 0.95) | 0.04* |
| ln(AAC volume), per SD | 1.27 (1.10, 1.45) | < 0.01* | 1.34 (1.17, 1.53) | < 0.01* | 1.33 (1.19, 1.49) | < 0.01* |
| Model 2 | ||||||
| AAC density, per SD | 0.97 (0.34, 2.74) | 0.95 | 0.66 (0.24, 1.80) | 0.42 | 0.46 (0.20, 1.07) | 0.07 |
| ln(AAC volume), per SD | 1.15 (1.002, 1.33) | 0.05 | 1.21 (1.06, 1.38) | < 0.01* | 1.30 (1.15, 1.48) | < 0.01* |
| CAC density, per SD | 0.65 (0.33–1.26) | 0.20 | 0.47 (0.25–0.90) | 0.02 | 0.92 (0.56, 1.53) | 0.76 |
| ln(CAC volume), per SD | 1.23 (1.08, 1.41) | < 0.01* | 1.25 (1.10, 1.41) | < 0.01* | 1.04 (0.94, 1.15) | 0.44 |
| Model 3 - Adjusted for GFRS, ethnicity, and statin therapy |
||||||
| AAC density, per SD | 1.07 (0.36, 3.14) | 0.90 | 0.93 (0.33, 2.57) | 0.89 | 0.57 (0.24, 1.35) | 0.20 |
| ln(AAC volume), per SD | 1.10 (0.95, 1.28) | 0.20 | 1.14 (1.00, 1.32) | 0.06 | 1.25 (1.36, 1.42) | < 0.01* |
| CAC density, per SD | 0.74 (0.38, 1.47) | 0.08 | 0.55 (0.29, 1.04) | 0.07 | 1.12 (0.66, 1.88) | 0.68 |
| ln(CAC volume), per SD | 1.17 (1.04, 1.59) | 0.02* | 1.20 (1.05, 1.36) | < 0.01* | 0.99 (0.90, 1.10) | 0.91 |
CHD = myocardial infarction, resuscitated cardiac arrest, or CHD death. CVD = CHD plus non-fatal or fatal stroke.
Global Framingham risk score (GFRS, composite of age, sex, smoking and diabetes status, total and HDL cholesterol, systolic blood pressure and blood pressure treatment). Two-tailed p-value < 0.05 considered significant, ln = natural log.
Discussion
In individuals with calcified plaque, in mutually adjusted models, we observed that AAC and CAC density scores were not significantly associated with CVD events after accounting for AAC and CAC volume, and other CVD risk factors. In contrast, both higher AAC and CAC volume scores were associated with CVD risk independent of AAC and CAC density.
To date, few studies have investigated the impact for calcified plaque composition (calcium density) independently from plaque burden (calcium volume) on CVD risk. Most studies measuring calcified plaque have used the Agatston score, which models calcium density as though it were associated with higher risk of CVD, independent of and multiplicative to plaque area (also measure of plaque burden). However, in MESA, at any level of CAC volume, CAC density was associated with lower CVD risk.7 These results were supported by prior research with studies using CTA showing lower rates of adverse CVD in persons with primarily calcified plaque, compared to those with non-calcified plaque, and mixed (calcified and non-calcified) plaque.5,14 In contrast to prior studies, Bellasi et al recently reported that both higher CAC density and volume were associated with increased hazard for ACM.15 However, this study was conducted in hemodialysis patients with advanced chronic kidney disease (CKD). Calcification in patients without advanced CKD is thought to occur primarily in the intimal layer and lead to vascular obstruction, while advanced CKD is associated with increased calcification of the medial artery layer an important risk factor for vascular stiffness.16 In our study, and in unadjusted models, for a given AAC volume, AAC density was also inversely associated with ACM. Our current study along with prior research calls into question the Agatston method of modeling higher calcium content in atherosclerotic plaque as a CVD risk independent of, and multiplicative to plaque burden. Though not significant we generally observed HR of AAC and CAC density for CVD events below 1. More significant findings may be observed with use of the full range of density values (130 – 3000+ HU) which were unavailable in our cohort. To our knowledge, this study is the first to investigate the impact of plaque composition, independent of plaque burden in a non-coronary vascular bed, by evaluating AAC.
The mechanism by which higher calcium density in atherosclerotic plaques may be associated with reduced CVD risk is unclear. Studies investigating plaque “vulnerability” most frequently identify thin-cap fibroatheroma (TCFA), characterized by a large lipid or necrotic core separated from the coronary arterial lumen by a thin membrane cap.17 TCFA may rupture and undergo thrombosis, which can lead to arterial occlusive crisis. It is likely that plaque with thicker membrane cap, smaller lipid or necrotic core and higher calcium content may be less vulnerable to rupture. It is also uncertain whether the same mechanism is responsible for the accumulation of calcified plaque in the aorta and coronaries. The modest correlations observed in our study between AAC density and volume with CAC density and volume suggest differential accumulation of AAC and CAC over time.
Compared to CAC, AAC forms denser plaque, accumulates at a younger age, and is more easily measured. AAC is associated with adverse CVD outcomes similar to CAC.18,19 These calcified plaques are associated with deleterious changes in the anatomy of the aorta including increasing abdominal aortic diameter, which can lead to ruptured aortic aneurysms.20 Furthermore the presence of AAC predicts greater CAC levels, as well as atherosclerosis in other vascular beds like the carotids and lower extremities.21 Thus, AAC may provide an opportunity to measure the extent of atherosclerosis in other vascular beds including in the coronary arteries.
The strengths of our study include a community-living, ethnically-diverse sample including both sexes, and an average of 9 years follow-up with adjudicated CVD events. Also, prevalent CVD was excluded at baseline. Our study, however, also has important limitations. First, for unbiased comparisons among participants with anatomical differences, AAC was measured using an 8 cm segment superior to the aorto-iliac bifurcation. Since most AAC accumulates at or near the bifurcation, an 8 cm segment proximal to the bifurcation is a good representation of a person’s total AAC burden. Also our study sample was limited to participants with both prevalent AAC and CAC, which may have reduced power. Several associations of AAC and CAC density with CHD, CVD, and ACM had strongly inverse hazard ratios but failed to reach statistical significance in fully adjusted models. Whether this represents a chance finding or limited statistical power is uncertain and will require larger studies with longer-term follow-up in the future. Second, calcium density was assessed using 4-point scale rather than a continuous HU scale (130 – 3000), with the highest score on the 4-point scale (4) representing densities on the HU scale greater than 400. Studying the full density range may have provided stronger associations. Additionally, for each plaque, the maximum density was used to characterize the entire plaque. Thus plaques that were primarily high risk (mostly low calcium density) may have been misclassified as low risk due to relatively few high calcium densities within the plaque. This misclassification would bias our results towards the null, limiting the strength of the associations for AAC and CAC density.
Conclusion
In mutually adjusted models, additionally adjusted for CVD risk factors, AAC density and CAC density were not associated with higher risk for CVD events, and their trend was protective. Thus the Agatston method of modeling calcium density as a CVD hazard by up-weighting plaque area for higher calcium density may be inappropriate for the abdominal aorta and coronary arteries. Further studies are needed to assess the impact of calcium density in CVD, and how to best utilize calcium density in CVD risk prediction.
Clinical Perspective.
The standard method used to score calcified plaque in arteries, the Agatston, up-weights plaque for higher calcium density, thus modeling density as a cardiovascular disease hazard. While the Agatston score is widely used in CVD risk prediction, the appropriateness of up-weighting calcium scores for density is controversial. Several recent studies have reported reduced risk of CVD events with higher calcium density in atherosclerotic plaques, though most studies have focused on the coronary arteries. We observed that while both higher abdominal aortic calcium (AAC) and coronary artery calcium (CAC) volume similarly increased CVD risk, greater AAC and CAC density did not, and trended towards risk reduction. Our study suggests that the standard calcium score, Agatston method, which models calcium density as a CVD hazard by up-weighting plaque for higher calcium density may be flawed for both the aorta and the coronary arteries, both primary vascular beds used to assess plaque burden. We hypothesize that the higher calcium density content of atherosclerotic plaque, in contrast to its overall volume, may reflect reduced plaque vulnerability. Higher calcium density may improve plaque stability, reducing risk for thrombotic occlusive events. Further studies are needed to better understand the epidemiology of calcium density in atherosclerotic plaque, the associations with modifiable risk factors, outcomes and how best to incorporate calcium density in CVD risk prediction.
Acknowledgments
Source of Funding
This study was supported by grant HL072403 and contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Institutes of Health, National Heart, Lung, and Blood Institute, Bethesda, MD and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Abbreviations
- AAC
Abdominal aortic calcium
- ACM
All-cause mortality
- CAC
Coronary artery calcium
- CHD
Coronary heart disease
- CKD
Chronic kidney disease
- CVD
Cardiovascular disease
Footnotes
Disclosures
None.
References
- 1.Criqui MH, Denenberg JO, McClelland RL, Allison MA, Ix JH, Guerci A, Cohoon KP, Srikanthan P, Watson KE, Wong ND. Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:1574–1579. doi: 10.1161/ATVBAHA.114.303268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 4.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary Artery Calcium Score and Risk Classification for Coronary Heart Disease Prediction: The Multi-Ethnic Study of Atherosclerosis. JAMA J Am Med Assoc. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou Z, Lu B, Gao Y, Jiang S, Wang Y, Li W, Budoff MJ. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990–999. doi: 10.1016/j.jcmg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Ahmadi N, Nabavi V, Hajsadeghi F, Flores F, French WJ, Mao SS, Shavelle D, Ebrahimi R, Budoff M. Mortality incidence of patients with non-obstructive coronary artery disease diagnosed by computed tomography angiography. Am J Cardiol. 2011;107:10–16. doi: 10.1016/j.amjcard.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA J Am Med Assoc. 2014;311:271–278. doi: 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 9.Criqui MH, Kamineni A, Allison MA, Ix JH, Carr JJ, Cushman M, Detrano R, Post W, Wong ND. Risk factor differences for aortic versus coronary calcified atherosclerosis: the multiethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2289–2296. doi: 10.1161/ATVBAHA.110.208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 11.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 12.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General Cardiovascular Risk Profile for Use in Primary Care The Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadi N, Nabavi V, Hajsadeghi F, Flores F, French WJ, Mao SS, Shavelle D, Ebrahimi R, Budoff M. Mortality incidence of patients with non-obstructive coronary artery disease diagnosed by computed tomography angiography. Am J Cardiol. 2011;107:10–16. doi: 10.1016/j.amjcard.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 15.Bellasi A, Ferramosca E, Ratti C, Block G, Raggi P. The density of calcified plaques and the volume of calcium predict mortality in hemodialysis patients. Atherosclerosis. 2016;250:166–171. doi: 10.1016/j.atherosclerosis.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz U, Buzello M, Ritz E, Stein G, Raabe G, Wiest G, Mall G, Amann K. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2000;15:218–223. doi: 10.1093/ndt/15.2.218. [DOI] [PubMed] [Google Scholar]
- 17.Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol. 2015;65:846–855. doi: 10.1016/j.jacc.2014.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 19.Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, Wright CM, Criqui MH. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32:140–146. doi: 10.1161/ATVBAHA.111.235234. [DOI] [PubMed] [Google Scholar]
- 20.Laughlin GA, Allison MA, Jensky NE, Aboyans V, Wong ND, Detrano R, Criqui MH. Abdominal aortic diameter and vascular atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2011;41:481–487. doi: 10.1016/j.ejvs.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong ND, Lopez VA, Allison M, Detrano RC, Blumenthal RS, Folsom AR, Ouyang P, Criqui MH. Abdominal Aortic Calcium and Multi-Site Atherosclerosis: The Multiethnic Study of Atherosclerosis. Atherosclerosis. 2011;214:436–441. doi: 10.1016/j.atherosclerosis.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


