Abstract
The cancer community continues to search for an efficient and cost-effective technique to isolate and characterize circulating cells (CTCs) as a ‘real-time liquid biopsy’. Existing methods to isolate and analyze CTCs require various transfer, wash, and staining steps that can be time consuming, expensive, and led to the loss of rare cells. To overcome the limitations of existing CTC isolation strategies, we have developed an inexpensive ‘lab on a chip’ device for the enrichment, staining, and analysis of rare cell populations. This device utilizes immunomagnetic positive selection of antibody-bound cells, isolation of cells through an immiscible interface, and filtration. The isolated cells can then be stained utilizing immunofluorescence or used for other downstream detection methods. We describe the construction and initial preclinical testing of the device. Initial tests suggest that the device may be well suited for the isolation of CTCs and could allow the monitoring of cancer progression and the response to therapy over time.
Keywords: Micro-fluidic device, Magnetophoresis, Rare cells, Circulating tumor cells, Prostate cancer
Introduction
Circulating tumor cells (CTCs) found in the peripheral blood originate from solid tumors and are involved in hematogenous metastatic spread [1]. Research on using CTCs as biomarkers has become a hot topic in the cancer community, and the use of peripheral blood as a ‘real-time liquid biopsy’ for the detection, isolation, and characterization of CTC’s continues to be developed as an alternative to standard biopsies [2–6]. Liquid biopsies can be performed repeatedly with low risk of side effects, making them an attractive approach for monitoring cancer progression and response to therapy over time [7–10]. A disadvantage of existing methods to isolate and analyze CTCs is that they often require various transfer, wash, and staining steps, which are time consuming, expensive, and led to the loss of these rare cells [11–14]. To overcome the limitations of existing CTC isolation strategies, we have developed an inexpensive ‘lab on a chip’ device for the enrichment, staining, and analysis of rare cell populations. Our device combines three different principles for the isolation of CTCs, namely immunomagnetic positive selection of antibody-bound cells, isolation of cells through an immiscible interface, and filtration. This combination makes it possible to integrate rare cell isolation with downstream molecular detection methods on a single platform.
Methods and materials
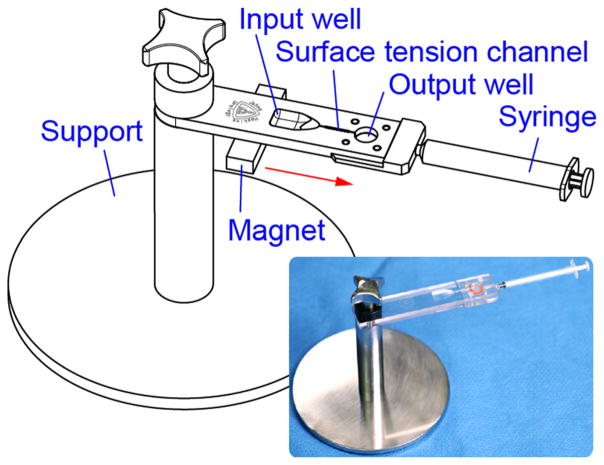
The surface tension and magnetophoretic device is depicted in Fig. 1. The overall device is supported with a base that sits on the laboratory bench. The device consists of a base plate, a sieve, a sieve gasket, and a cap plate that is guided by dowel pins and attaches to the plate. The cap mounts and seals the sieve on the plate.
Fig. 1.
Surface tension and magnetophoretic device for rare cell isolation
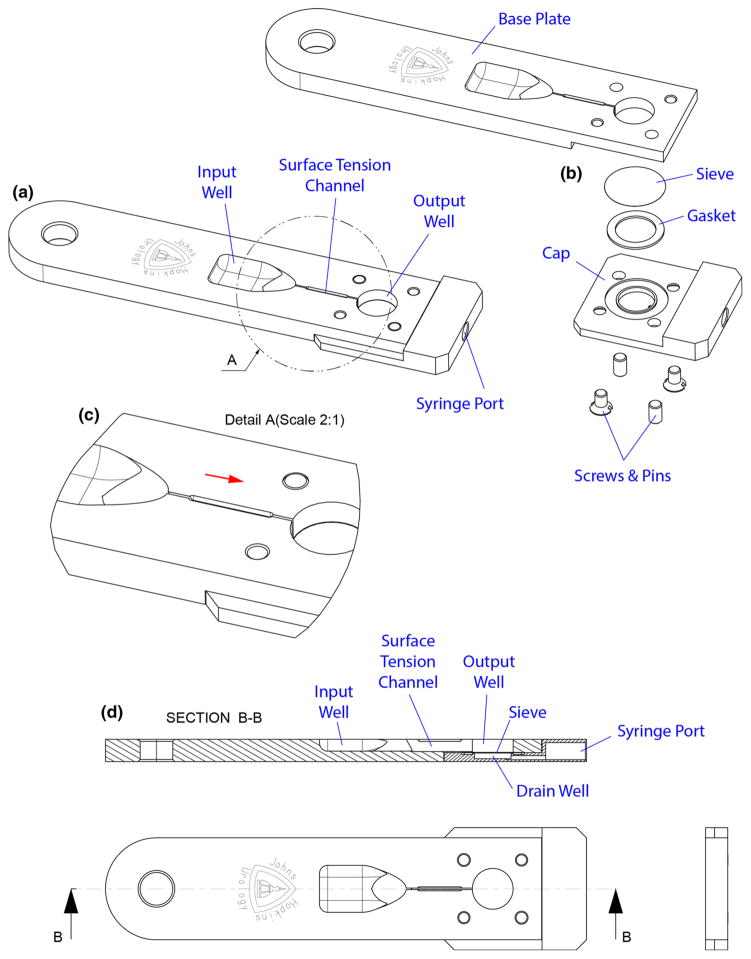
Details of the device design are shown in Fig. 2. The base plate includes an input well with a tapered funnel-like surface into a surface tension channel. The surface tension channel links the input and the output well. The well height and widths of both wells are constrained such that the input well has a 500 μL capacity and the output well has a 400 μL capacity. The output well is filled with a washing solution such as phosphate buffered saline (PBS). Furthermore, the output well has a 8.0 μm polycarbonate microporous membrane sieve (PCT8025100 Polycarbonate, Sterlitech Corporation, USA), which is chosen for a pore size that is capable of trapping antibody-bound cells. This membrane sieve is sealed with a gasket under the output well. Furthermore, a syringe attaches to the end of the device, and a drain well located under the channel communicates to the port of this syringe to extract the washing solution by passing it through the filter.
Fig. 2.
Details of the device: a view, b exploded view, c details, d cross section
During operation, the surface tension channel is filled with 10 μL of olive oil, a high surface tension solution. Previous studies have shown that immiscible phase filtration (IPF) holds great promise for the isolation of, e.g., proteins [15], cells [16], and nucleic acids [17]. As the surface tension is more dominant then gravity, the oil acts as a virtual wall between the immiscible phase (channel with olive oil) and both aqueous phases (input and output well with PBS) [18]. As both fluids have a different density, they will not mix and it is thereby not possible for cells or contaminants to move on their own from the input to the output well. By labeling the cells of interest (cancer cells) with magnetic beads and simply passing a magnet underneath the device (from input to output well), only the cells labeled with magnetic beads will be able to overcome this dominant surface tension and will be pulled through the surface channel into the output well. Thereby, it is possible to filter ‘unwanted cells’ in one single step and maintaining the viability of the cells of interest, without the need for centrifugation and multiple washing steps [19].
Cell preparation
Immortalized LNCaP and DU145 prostate cancer cells were utilized to test the ability of the device to isolate cancer cells. Cell lines are maintained at 37 °C and 5% CO2 in RPMI culture media (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco, USA). Before running experiments, cells were incubated for 15 min at 37 °C with enzyme free, PBS-based Cell Dissociation Buffer (Gibco, USA) and subsequently re-suspended in 2 mL of RPMI. Next, this sample is stained with (1 μL/mL sample) CellTracker Green CMFDA (Life Technologies, Oregon, USA) and incubated for 30 min at 37 °C. This contributed to the visualization of the cytosol of the cancer cell.
Paramagnetic particle preparation
The paramagnetic particles (PMPs) used for the enrichment of the prostatic epithelial tumor cells were 4.5 μm ‘CELLection Epithelial Enrich DynaBeads’ that were coated with anti-EpCAM antibody (ThermoFisher Scientific, Waltham, MA). Furthermore, in separate experiments, 4.5 μm Dynabeads coated with anti-CD45 antibody (ThermoFisher Scientific, Waltham, MA) were used as a negative control to account for nonspecific cell capture. Before using the beads, the stock buffer solution had to be removed from the antibody-labeled PMPs by washing and re-suspending them twice in PBS. In order to attract these PMPs and implicitly the antibody-bound cells, a magnet was manually and freely moved under the device. This motion induced by a magnetic field on a particle of magnetic material in a fluid is called magnetophoresis.
Experimental setup
A known number of stained cancer cells (1000 cells, measured with the Cellometer System (Nexcelom, Bioscience, USA) was spiked into vials filled with 500 μL RPMI. Subsequently, different quantities (0.5, 5.0, 25 or 50 μL) of anti-EpCAM or anti-CD45 PMPs were added to these samples. Next, these cell suspensions were admixed on a shaker at 4 °C for 30 min, while gently twisting and shaking to allow binding.
After 30 min, the prepared cell solution was placed in the input well, and the magnet was placed under this well. With slow advancement of the magnet under the channel toward the output well, bound cells of interest traversed the immiscible oil channel and entered the output well. The syringe was then used to extract the washing solution by passing it through the filter. Once these processes were complete, the filter was removed and handled over on a glass slide (VWR Superfrost Plus, USA), and Diamond anti-fade mounting media containing DAPI (Life Technologies, Grand Island, NY) was used to coverslip the slides.
Slide scanning and analysis
After mounting the membrane on a glass slide, the slides were imaged with Metasystems (Newton, MA) Metafer5—MetaCyte scanning software. Images of the cells were generated by the software based on fluorescence [20]. As we conducted spiking experiments of solely cancer cells (with a fluorescent green cytosol) in RPMI, there was no need to stain for epithelial markers [9, 21]. Therefore, the cell selection algorithm was based on the following criteria: having a nucleus (DAPI+), a green cytosol (Cell-Tracker Green), and a cellular shape (roundness). Extracted image data were then transferred to the MATLAB R2016a program (Mathworks, Natick, MA), which counted the number of captured cells.
Results
Cell-spiking experiments were conducted to determine the recovery rate of the device. Before starting the experiments, EpCAM expression in both cell lines was determined to be high by flow cytometry. These experiments showed an EpCAM expression of nearly 100% in both cell lines.
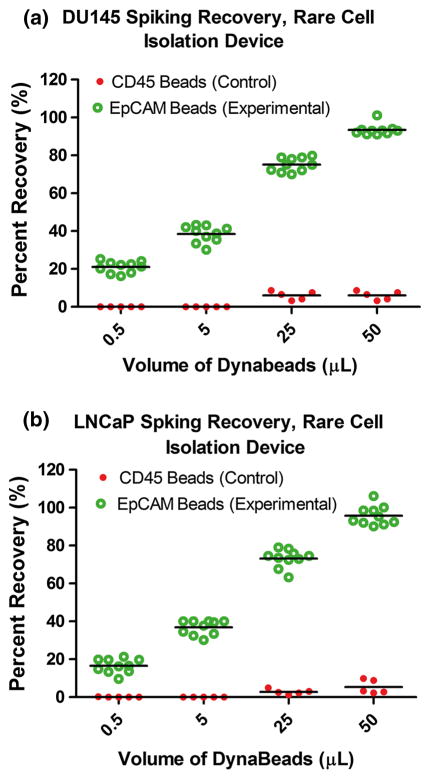
The initial preclinical spiking experiments showed that both cell types could be captured (Fig. 3) with an increase in recovery up to approximately 95% using 50 μL of anti-EpCAM Dynabeads (n = 10 per concentration for both cell lines) (Fig. 4). Anti-CD45 Dynabeads were also tested as a negative control to account for nonspecific cell capture (n = 5 per concentration for both cell lines). This demonstrated a low rate of recovery across tested concentrations [22].
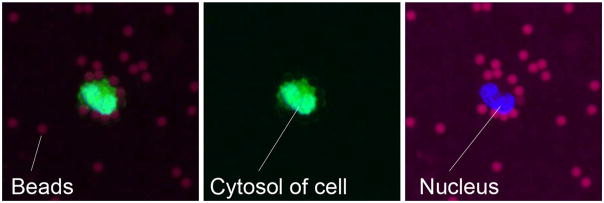
Fig. 3.
Example of a prostate cancer cell (DU145) captured and visualized on the membrane. Red, magnetic beads; green, cytosol; and blue, nucleus
Fig. 4.
Percent recovery (%) of: a DU145 prostate cancer cells and b LNCaP prostate cancer cells, using different volumes of anti-EpCAM Dynabeads (n = 10 per concentration), as well as a negative control using anti-CD45 Dynabeads (n = 5 per concentration)
Discussion and future directions
Results demonstrated a recovery percentage above 100% in two experiments (Fig. 4). This is likely due to the fact that the Cellometer (which estimates the number of cancer cells in 1 mL of sample) is not 100% accurate, with the result that the number of spiked cancer cells unfortunately could never have been exactly 1000 cells. To increase the reliability of future spiking experiments, a single-cell manipulator can be used by which the preferred number of cells from the plate can be aspirated, whereupon these can be added to the sample. In addition, the software for counting the number of captured cells did not always recognize clusters, adding to potential inaccuracies in counting.
Another potential problem in using anti-EpCAM labeled magnetic beads to select CTCs is the fact that during disease progression the expression of EpCAM and other epithelial cell surface markers may change or decrease [22–25]. Furthermore, several studies have shown that CTCs are not the only cells in peripheral blood on which EpCAM is expressed, as it could also be expressed on cells of hematopoietic lineage [23–25]. Nevertheless, EpCAM was utilized here as a ‘proof of principle’ phenotypic cell marker as this is currently the most common cell surface marker utilized in CTC detection [7].
Furthermore, as patient samples are utilized in future studies, PMPs capable of binding to all the rare cells of interest (e.g., via a linking antibody/streptavidin-labeled Dynabeads) will be utilized. These beads can bind to ‘disease-specific’ markers of interest, which may be expressed during the progression of a patients’ disease [21]. Lastly, we used CellTracker Green as a proof of principle for the sensitivity of the device. When switching to patient samples, this dye will no longer be used for the visualization of the cells, because all cells, including white blood cells, would be labeled. However, following EpCAM-mediated isolation of tumor cells, future strategies for visualization of the cells could include immunofluorescence methods or fluorescence in situ hybridization directly on the membrane of the device.
In this paper, a microfluidic device is introduced with which it is possible to isolate tumor cells with a high sensitivity. Future experiments will be conducted to ascertain specificity. The first step will be switching to a cell suspension derived from a biologic fluid. Tumor cells will be spiked into blood (circulating tumor cells) or bone marrow (disseminated tumor cells) and processed as described previously [26–28].
Conclusion
There continues to be a need for simple, noninvasive, and inexpensive tests for the isolation and characterization of circulating tumor cells. This device combines three different methods, namely isolation of cells through an immiscible interface, using the surface tension channel; immunomagnetic positive selection, using the magnetophoretic property of the magnetic-bead-bound cancer cells; and filtration, using the microporous membrane sieve. This combination makes it possible to integrate rare cell isolation with downstream molecular detection methods in a single platform. Initial tests suggest that the device may be well suited for the isolation of CTCs. Processing on one device saves time and reagents and reduces the risk of sample loss. Further development of the presented technology could potentially allow the monitoring of cancer progression and the response to therapy over time and thereby facilitate personalized treatment strategies and improved outcomes.
Acknowledgments
Funding This work is supported by NCI Grant Nos. U54CA143803, CA163124, CA093900, CA143055 to K.J.P. as well as the Prostate Cancer Foundation, the Patrick C. Walsh Fund, and a gift from the Stutt family. E.E.vdT. is supported by the Cure for Cancer Foundation.
Footnotes
Compliance with ethical standards
Conflict of interest Author E.E.vdT. declares that she has no conflict of interest. Author J.E.V. declares that he has no conflict of interest. Author C.J. declares that he has no conflict of interest. Author D.P. declares that he has no conflict of interest. Author S.L. declares that he has no conflict of interest. Author J.J.M.C.H.dlR. declares that he has no conflict of interest. Author T.M.dR. declares that he has no conflict of interest. Author M.A.G. declares that he has no conflict of interest. Author K.J.P. is a consultant for Celsee Diagnostics and is on the board of directors for Curis, Inc. Author D.S. declares that he has no conflict of interest.
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent No humans were involved.
References
- 1.Pienta KJ, Robertson BA, Coffey DS, Taichman RS. The cancer diaspora: metastasis beyond the seed and soil hypothesis. Clin Cancer Res. 2013;19(21):5849–55. doi: 10.1158/1078-0432.CCR-13-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pantel K, Alix-Panabieres C. Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res. 2013;73(21):6384–8. doi: 10.1158/0008-5472.CAN-13-2030. [DOI] [PubMed] [Google Scholar]
- 3.Friedlander TW, Ngo VT, Dong H, Premasekharan G, Weinberg V, Doty S, Zhao Q, Gilbert EG, Ryan CJ, Chen WT, Paris PL. Detection and characterization of invasive circulating tumor cells derived from men with metastatic castration-resistant prostate cancer. Int J Cancer. 2014;134(10):2284–93. doi: 10.1002/ijc.28561. [DOI] [PubMed] [Google Scholar]
- 4.Lindemann F, Schlimok G, Dirschedl P, Witte J, Riethmuller G. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet. 1992;340(8821):685–9. doi: 10.1016/0140-6736(92)92230-d. [DOI] [PubMed] [Google Scholar]
- 5.Alix-Panabieres C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14(9):623–31. doi: 10.1038/nrc3820. [DOI] [PubMed] [Google Scholar]
- 6.Barradas AM, Terstappen LW. Towards the biological understanding of CTC: capture technologies, definitions and potential to create metastasis. Cancers (Basel) 2013;5(4):1619–42. doi: 10.3390/cancers5041619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lianidou ES, Strati A, Markou A. Circulating tumor cells as promising novel biomarkers in solid cancers. Crit Rev Clin Lab Sci. 2014;51(3):160–71. doi: 10.3109/10408363.2014.896316. [DOI] [PubMed] [Google Scholar]
- 8.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 9.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 10.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213–21. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 11.Casavant BP, Guckenberger DJ, Berry SM, Tokar JT, Lang JM, Beebe DJ. The VerIFAST: an integrated method for cell isolation and extracellular/intracellular staining. Lab Chip. 2013;13(3):391–6. doi: 10.1039/c2lc41136a. [DOI] [PubMed] [Google Scholar]
- 12.Wolff A, Perch-Nielsen IR, Larsen UD, Friis P, Goranovic G, Poulsen CR, Kutter JP, Telleman P. Integrating advanced functionality in a microfabricated high-throughput fluorescent-activated cell sorter. Lab Chip. 2003;3(1):22–7. doi: 10.1039/b209333b. [DOI] [PubMed] [Google Scholar]
- 13.Easley CJ, Karlinsey JM, Bienvenue JM, Legendre LA, Roper MG, Feldman SH, Hughes MA, Hewlett EL, Merkel TJ, Ferrance JP, Landers JP. A fully integrated microfluidic genetic analysis system with sample-in-answer-out capability. Proc Natl Acad Sci USA. 2006;103(51):19272–7. doi: 10.1073/pnas.0604663103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin HK, Zheng S, Williams AJ, Balic M, Groshen S, Scher HI, Fleisher M, Stadler W, Datar RH, Tai YC, Cote RJ. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res. 2010;16(20):5011–8. doi: 10.1158/1078-0432.CCR-10-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sur K, McFall SM, Yeh ET, Jangam SR, Hayden MA, Stroupe SD, Kelso DM. Immiscible phase nucleic acid purification eliminates PCR inhibitors with a single pass of paramagnetic particles through a hydrophobic liquid. J Mol Diagn. 2010;12(5):620–8. doi: 10.2353/jmoldx.2010.090190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moussavi-Harami SF, Annis DS, Ma W, Berry SM, Coughlin EE, Strotman LN, Maurer LM, Westphall MS, Coon JJ, Mosher DF, Beebe DJ. Characterization of molecules binding to the 70 K N-terminal region of fibronectin by IFAST purification coupled with mass spectrometry. J Proteome Res. 2013;12(7):3393–404. doi: 10.1021/pr400225p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry SM, Alarid ET, Beebe DJ. One-step purification of nucleic acid for gene expression analysis via immiscible filtration assisted by surface tension (IFAST) Lab Chip. 2011;11(10):1747–53. doi: 10.1039/c1lc00004g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atencia J, Beebe DJ. Controlled microfluidic interfaces. Nature. 2005;437(7059):648–55. doi: 10.1038/nature04163. [DOI] [PubMed] [Google Scholar]
- 19.Zhao B, Moore JS, Beebe DJ. Surface-directed liquid flow inside microchannels. Science. 2001;291(5506):1023–6. doi: 10.1126/science.291.5506.1023. [DOI] [PubMed] [Google Scholar]
- 20.Valkenburg KC, Amend SR, Verdone JE, van der Toom EE, Hernandez JR, Gorin MA, Pienta KJ. A simple selection-free method for detecting disseminated tumor cells (DTCs) in murine bone marrow. Oncotarget. 2016;7(43):69794–803. doi: 10.18632/oncotarget.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Toom EE, Verdone JE, Gorin MA, Pienta KJ. Technical challenges in the isolation and analysis of circulating tumor cells. Oncotarget. 2016;7(38):62754–66. doi: 10.18632/oncotarget.11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Toom E, Gorin MA, Verdone JE, Jun C, Petrisor D, Stoianovici D, Pienta KJ. A device for rare cell isolation and characterization. American Urological Association, Engineering and Urology Society, 31th annual meeting; San Diego. 2016; p. 25. [Google Scholar]
- 23.Adams DL, Martin SS, Alpaugh RK, Charpentier M, Tsai S, Bergan RC, Ogden IM, Catalona W, Chumsri S, Tang CM, Cristofanilli M. Circulating giant macrophages as a potential biomarker of solid tumors. Proc Natl Acad Sci USA. 2014;111(9):3514–9. doi: 10.1073/pnas.1320198111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenwort G, Jurkin J, Yasmin N, Bauer T, Gesslbauer B, Strobl H. Identification of TROP2 (TACSTD2), an EpCAM-like molecule, as a specific marker for TGF-beta1-dependent human epidermal Langerhans cells. J Invest Dermatol. 2011;131(10):2049–57. doi: 10.1038/jid.2011.164. [DOI] [PubMed] [Google Scholar]
- 25.Shetye JD, Liljefors ML, Emdin SO, Frodin JE, Strigard K, Mellstedt HT, Porwit A. Spectrum of cytokeratin-positive cells in the bone marrows of colorectal carcinoma patients. Anticancer Res. 2004;24(4):2375–83. [PubMed] [Google Scholar]
- 26.Cho EH, Wendel M, Luttgen M, Yoshioka C, Marrinucci D, Lazar D, Schram E, Nieva J, Bazhenova L, Morgan A, Ko AH, Korn WM, Kolatkar A, Bethel K, Kuhn P. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys Biol. 2012;9(1):016001. doi: 10.1088/1478-3975/9/1/016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krivacic RT, Ladanyi A, Curry DN, Hsieh HB, Kuhn P, Bergsrud DE, Kepros JF, Barbera T, Ho MY, Chen LB, Lerner RA, Bruce RH. A rare-cell detector for cancer. Proc Natl Acad Sci USA. 2004;101(29):10501–4. doi: 10.1073/pnas.0404036101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marrinucci D, Bethel K, Kolatkar A, Luttgen MS, Malchiodi M, Baehring F, Voigt K, Lazar D, Nieva J, Bazhenova L, Ko AH, Korn WM, Schram E, Coward M, Yang X, Metzner T, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol. 2012;9(1):016003. doi: 10.1088/1478-3975/9/1/016003. [DOI] [PMC free article] [PubMed] [Google Scholar]