Abstract
Two species of the genus Borrelia, Borrelia bissettiae sp. nov. and Borrelia californiensis sp. nov., were first described by Postic and co-workers on the basis of genetic analyses of several loci. Multilocus sequence analysis of eight housekeeping loci confirmed that these two Borrelia genomospecies are distinct members of the Borrelia burgdorferi sensu lato complex. B. bissettiae sp. nov. was initially described in transmission cycles involving Neotoma fuscipes wood rats and Ixodes pacificus ticks in California, and Neotoma mexicana and Ixodes spinipalpis in Colorado. The preferred host of B. californiensis sp. nov. appears to be the California kangaroo rat, Dipodomys californicus; Ixodes jellisoni, I. spinipalipis and I. pacificus ticks are naturally infected with it. Thus, the ecological associations of the two genomospecies and their genetic distance from all other known Borrelia genomospecies species justify their description as separate genomospecies: B. bissettiae sp. nov. (type strain DN127T = DSM 17990T = CIP 109136T) and B. californiensis (type strain CA446T = DSM 17989T = ATCC BAA-2689T).
Lyme borreliosis (LB) is caused by several species of bacteria belonging to the LB group of spirochaetes, also referred to as Borrelia burgdorferi sensu lato. B. burgdorferi sensu lato is a heterogeneous species complex that currently consists of at least 20 recognized or proposed genomospecies. These bacteria are maintained in natural transmission cycles among vertebrate reservoir hosts and ticks of the Ixodes persulcatus species complex or other species of the genus Ixodes, such as Ixodes spinipalpis (Brown & Lane, 1992, 1996; Kurtenbach et al., 2006).
The strain designated the Borrelia bissettiae sp. nov. type strain, DN127T, was isolated from a questing Ixodes pacificus tick collected in Del Norte County, California, during the 1980s (Bissett & Hill, 1987). Additional strains of this genomospecies have been isolated from I. pacificus or Ixodes neotomae (now I. spinipalpis) in California and Colorado (Bissett & Hill, 1987; Brown & Lane, 1992; Maupin et al., 1994; Schneider et al., 2000) and from rodents captured in the Chicago area of Illinois (Picken & Picken, 2000). Postic et al. (1998) proposed that these strains constitute a distinct genomospecies within the B. burgdorferi sensu lato complex and named it B. bissettii sp. nov. In the USA, B. bissettiae enzootic transmission cycles were also found in some southern states involving Ixodes affinis and various rodent-host species (Oliver et al., 2003). In the far west of the USA, B. bissettiae was associated with the dusky-footed woodrat, Neotoma fuscipes Baird (Brown et al., 2006). In the same geographical region, B. bissettiae was detected in a host-seeking avian tick, Ixodes auritulus, co-infected with B. burgdorferi (Padgett & Bonilla, 2011) and, more recently, in the blood of several bird species and I. pacificus immatures infesting birds (Newman et al., 2015). In the latter study, the infection prevalence in I. pacificus larvae was much lower for B. bissettiae than it was for B. burgdorferi; thus, the role of birds as either primary or secondary reservoir hosts for B. bissettiae remains to be established.
Of the tick species known to transmit B. bissettiae sp. nov. in the USA, i.e. I. pacificus and I. spinipalpis in the far west and south-west, and I. affinis in the south-east (Bissett & Hill, 1987; Lin et al., 2001, 2003; Maupin et al., 1994), only I. pacificus attaches to humans with any frequency. This may partly explain why B. bissettiae is not considered to be a human pathogen in the USA (Maupin et al., 1994). On the other hand, B. bissettiae occasionally infects humans in northern California as demonstrated by the presence of its DNA in a few serum specimens, but signs or symptoms suggestive of clinical Lyme disease are lacking in this region (Girard et al., 2011).
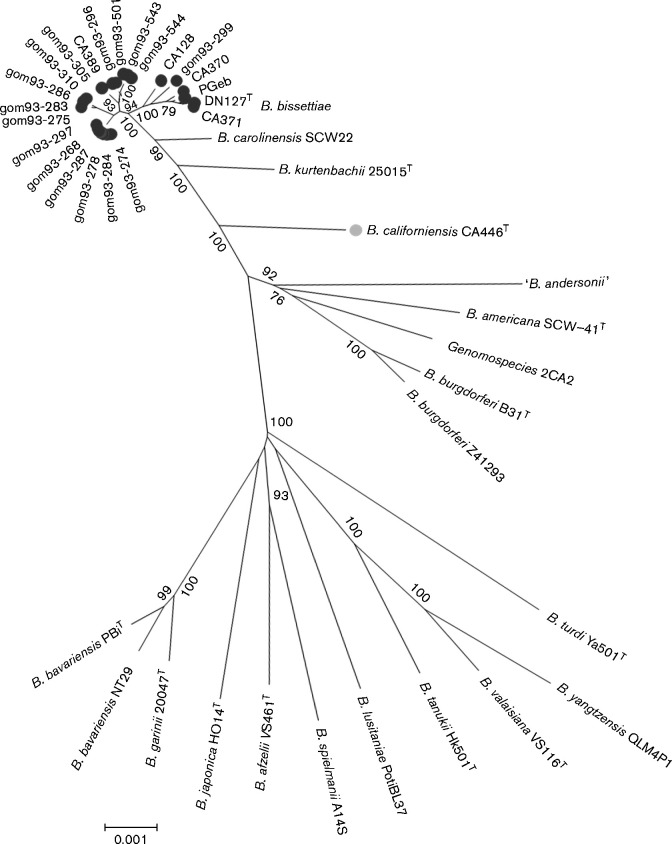
In Europe, B. bissettiae sp. nov. DNA has been detected in human patients (Picken et al., 1996a, b; Rudenko et al., 2008, 2009; Strle et al., 1997), and in questing Ixodes ricinus ticks (Hulínská et al., 2007; Tappe et al., 2014). One human isolate of B. bissettiae (PGeb) was obtained from a German patient without a history of travel, providing direct evidence that B. bissettiae occurs in Europe (Fingerle et al., 2008) (Fig. 1).
Fig. 1.
Molecular phylogenetic analysis of Borrelia bissettiae sp. nov. (dark grey dots) and Borrelia californiensis sp. nov. (light grey dot) strains. The evolutionary history was inferred by using the maximum-likelihood method based on the Tamura–Nei model (Tamura & Nei, 1993). The tree with the highest log-likelihood ( − 21015.8975) is shown. Bootstrap values (500 replications) are shown next to nodes. Initial tree(s) for the heuristic search were obtained by applying the neighbour-joining method to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach. A discrete Gamma distribution was used to model evolutionary rate differences among sites [5 categories (+G, parameter = 0.2602)]. All positions containing gaps and missing data were eliminated. There were a total of 4779 positions in the final dataset. Evolutionary analyses were conducted in mega6 (Tamura et al., 2013). Bar, 0.001 substitutions per site.
In phylogenetic analyses of the rrf-rrl intergenic spacer (IGS) region of B. burgdorferi sensu lato, strains CA443 and CA446 from northern California fell into a clade well separated from all other known genomospecies, a finding consistent with them representing a distinct genomospecies for which the name Borrelia californiensis sp. nov. was proposed (Postic et al., 2007). Twenty-three Borrelia strains mainly isolated from the California kangaroo rat (Dipodomys californicus) clustered together with strains CA404, CA443 and CA446 (Postic et al., 2007). Those data suggest that all such strains belong to the genomospecies B. californiensis sp. nov., and that D. californicus is a primary reservoir host of this genomospecies. Strains CA443 and CA446 investigated by multilocus sequence analysis (MLSA) with eight housekeeping genes formed a distinct clade that differed from all other species of the genus Borrelia (Margos et al., 2010) (Fig. 1). Genetic-distance analysis confirmed the distinctness of these strains from other described species of the genus Borrelia (Margos et al., 2010).
The samples used for studies of the two genomospecies are listed in Table 1.
Table 1.
Borrelia bissettiae sp. nov. and B. californiensis sp. nov. isolates from California and Colorado, USA, evaluated in previous studies
| ST* | Strain ID | Country of origin | Region | Genomospecies | Biological source of isolate | Year of collection | Typed with: | pubMLST ID/GenBank accession no. |
|---|---|---|---|---|---|---|---|---|
| 156 | CA128 | USA | Mendocino County, CA | B. bissettiae | I. neotomae (now I. spinipalpis) ex N. fuscipes | 1991 | MLSA | 1002 |
| 282 | CA370 | USA | Alameda County, CA | B. bissettiae | N. fuscipes ear biopsy | 1992 | MLSA | 1003 |
| 283 | CA371 | USA | Alameda County, CA | B. bissettiae | N. fuscipes ear biopsy | 1992 | MLSA | 1004 |
| 270 | CA389 | USA | Alameda County, CA | B. bissettiae | I. pacificus | 1993 | MLSA | 1005 |
| 272 | DN127-Cl9-2/p7 | USA | Del Norte County, CA | B. bissettiae | I. pacificus | 1985 | MLSA | 1006 |
| 273 | gom93-268 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex Neotoma mexicana | 1993 | MLSA | 1007 |
| 273 | gom93-274 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1008 |
| 273 | gom93-275 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1009 |
| 273 | gom93-278 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1010 |
| 160 | gom93-283 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1011 |
| 273 | gom93-284 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1012 |
| 274 | gom93-286 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1013 |
| 273 | gom93-287 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1014 |
| 271 | gom93-296 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1015 |
| 273 | gom93-297 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1016 |
| 158 | gom93-299 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1017 |
| 275 | gom93-305 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex Peromyscus difficilis | 1993 | MLSA | 1018 |
| 276 | gom93-310 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1019 |
| 277 | gom93-501 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1020 |
| 277 | gom93-543 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1021 |
| 277 | gom93-544 | USA | Larimer County, CO | B. bissettiae | I. spinipalpis ex N. mexicana | 1993 | MLSA | 1022 |
| 667 | PGeb | Germany | Baden-Württemberg | B. bissettiae | Human | 1996 | MLSA | 1874 |
| 447 | CA443 | USA | Mendocino County, CA | B. californiensis | D. californicus ear biopsy | 1995 | MLSA | 1450 |
| 447 | CA446 | USA | Mendocino County, CA | B. californiensis | D. californicus ear biopsy | 1995 | MLSA | 1277 |
| na | CA552 | USA | Mendocino County, CA | B. californiensis | Ixodes jellisoni ex D. californicus | 1998 | rrf-rrl IGS | AY182059 |
| na | CA507 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1997 | rrf-rrl IGS | AY182056 |
| na | CA504 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1997 | rrf-rrl IGS | AY182055 |
| na | CA502 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1997 | rrf-rrl IGS | AY182054 |
| na | CA462 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1996 | rrf-rrl IGS | AY182053 |
| na | CA448 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1995 | rrf-rrl IGS | AY182052 |
| na | CA442 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1995 | rrf-rrl IGS | AF073254 |
| na | CA411 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1994 | rrf-rrl IGS | AY182048 |
| na | CA31 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1990 | rrf-rrl IGS | AJ006372 |
| na | CA22 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1990 | rrf-rrl IGS | AY177631 |
| na | CA134 | USA | Mendocino County, CA | B. californiensis | I. pacificus ex D. californicus | 1991 | rrf-rrl IGS | AY182042 |
| na | CA468 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1996 | rrf-rrl IGS | AY177641 |
| na | CA404 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1993 | rrf-rrl IGS | AJ006371 |
| na | CA33 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1990 | rrf-rrl IGS | AY177632 |
| na | CA20 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1990 | rrf-rrl IGS | AY180239 |
| na | CA142 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1991 | rrf-rrl IGS | AY182043 |
| na | CA409 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1993 | rrf-rrl IGS | AF073255 |
| na | CA547 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1998 | rrf-rrl IGS | AY177642 |
| na | CA445 | USA | Mendocino County, CA | B. californiensis | D. californicus | 1995 | rrf-rrl IGS | AF073256 |
na, Not applicable.
st, sequence type.
Description of Borrelia bissettiae sp. nov.
Borrelia bissettiae [bis.set′ti.ae. N.L. gen. n. bissettiae of Bissett, proposed in honour of Dr Marjorie L. Bissett, who isolated and described this spirochaete along with her co-worker Warren Hill (Bissett & Hill, 1987)].
Cells are helical, approximately 0.2 μm by 20 μm, and stain well with Giemsa stain. Unstained cells can be visualized by dark-field microscopy. Flexible and motile with rotational and forward/backwards movement. Cells can be cultured in vitro under microaerophilic conditions (Johnson et al., 1984b) using liquid media such as Barbour–Stoenner–Kelly (BSK) medium. Optimal growth occurs at 33–34 °C.
The type strain, DN127T, was isolated from a questing I. pacificus tick in the late 1980s. It has been deposited in the German Microbial Strain Collection ( = DSM 17990T) and at the Institut Pasteur, Paris, France ( = CIP 109136T). B. bissettiae can be distinguished from other genomospecies of the genus Borrelia via sequences of the 5S–23S IGS, the rrs locus and by MLSA (Margos et al., 2010; Postic et al., 1998). The B. bissettiae group is heterogeneous as shown by 5S–23S rRNA IGS (Postic et al., 1998), MLSA analyses and by the size of the 16S–23S rRNA IGS fragment (Bunikis et al., 2004) that approximates 1000 or 1100 bp (unpublished data). This bacterium is maintained in nature in diverse transmission cycles involving various rodent reservoir hosts and certain tick species of the genus Ixodes. Strains of this species have been found in the USA and Europe. The mean DNA G+C content of the type strain is 27 mol%.
Description of Borrelia californiensis sp. nov.
Borrelia californiensis (ca.li.for.ni.en′sis N.L. fem. adj. californiensis belonging to California, from where the type strain was isolated) was proposed by Postic et al. (2007).
Cells are helical, approximately 0.2 μm by 20 μm, and stain well with Giemsa stain. Unstained cells can be visualized by dark-field microscopy. Flexible and motile with rotational and forward/backwards movement. Cells can be cultured in vitro under microaerophilic conditions (Johnson et al., 1984a) using liquid media such as BSK medium. Optimal growth occurs at 33–34 °C.
The type strain, CA446T, was isolated from an ear-punch biopsy excised from a male D. californicus captured in November 1995 by Kerry A. Padgett at the University of California Hopland Research and Extension Center in Mendocino County, California. It has been deposited in the American Type Culture Collection ( = ATCC BAA-2689T) and the German Microbial strain collection ( = DSM 17989T). Sequence analysis of the rrf-rrl intergenic spacer and the rrs and flagellin genes differentiates B. californiensis from B. bissettiae (Postic et al., 1998). B. californiensis strains are also distinguishable from all other LB species by using two different MLSA schemes (Margos et al., 2010; Postic et al., 2007). B. californiensis seems a rather homogeneous species. So far, it is restricted in distribution to northern California where its primary vertebrate host is the California kangaroo rat, Dipodomys californicus (Brown & Lane, 1992, 1996; Lane & Brown, 1991). Known vectors include Ixodes jellisoni, I. pacificus and I. spinipalpis. The mean DNA G+C content of the type strain is 27 mol%.
Acknowledgements
The authors gratefully acknowledge Richard N. Brown, Kerry A. Padgett and Joyce E. Kleinjan who conducted ecologic studies at the University of California Hopland Research and Extension Center (formerly the Hopland Field Station) in Mendocino County that resulted in the detection and isolation of both spirochaetes at that facility. Those studies were supported in large part by funding to R. S. L. from the US National Institutes of Health (grant AI22501) and the Centers for Disease Control and Prevention (cooperative agreement U50/CCU906594).
Footnotes
Abbreviations: IGS, intergenic spacer; LB, Lyme borreliosis; MLSA, multilocus sequence analysis.
The GenBank/EMBL/DDBJ accession numbers for the sequences obtained in this study are KT709291–KT709458 and KT709517–KT709532. Sequence data are also available at the Borrelia MLST website at http://www.pubMLST.org/borrelia, hosted at the University of Oxford, UK (ID numbers 1002–1022, 1277 and 1450).
References
- Bissett M. L., Hill W. Characterization of Borrelia burgdorferistrains isolated from Ixodes pacificusticks in California. J Clin Microbiol. 1987;25:2296–2301. doi: 10.1128/jcm.25.12.2296-2301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. N., Lane R. S. Lyme disease in California: a novel enzootic transmission cycle of Borrelia burgdorferi . Science. 1992;256:1439–1442. doi: 10.1126/science.1604318. [DOI] [PubMed] [Google Scholar]
- Brown R. N., Lane R. S. Reservoir competence of four chaparral-dwelling rodents for Borrelia burgdorferi in California. Am J Trop Med Hyg. 1996;54:84–91. doi: 10.4269/ajtmh.1996.54.84. [DOI] [PubMed] [Google Scholar]
- Brown R. N., Peot M. A., Lane R. S. Sylvatic maintenance of Borrelia burgdorferi (Spirochaetales) in Northern California: untangling the web of transmission. J Med Entomol. 2006;43:743–751. doi: 10.1603/0022-2585(2006)43[743:SMOBBS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bunikis J., Garpmo U., Tsao J., Berglund J., Fish D., Barbour A. G. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology. 2004;150:1741–1755. doi: 10.1099/mic.0.26944-0. [DOI] [PubMed] [Google Scholar]
- Fingerle V., Schulte-Spechtel U. C., Ruzic-Sabljic E., Leonhard S., Hofmann H., Weber K., Pfister K., Strle F., Wilske B. Epidemiological aspects and molecular characterization of Borrelia burgdorferi s.l. from southern Germany with special respect to the new species Borrelia spielmanii sp. nov. Int J Med Microbiol. 2008;298:279–290. doi: 10.1016/j.ijmm.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Girard Y. A., Fedorova N., Lane R. S. Genetic diversity of Borrelia burgdorferi and detection of B. bissettii-like DNA in serum of north-coastal California residents. J Clin Microbiol. 2011;49:945–954. doi: 10.1128/JCM.01689-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulínská D., Votýpka J., Kríz B., Holínková N., Nováková J., Hulínský V. Phenotypic and genotypic analysis of Borrelia spp. isolated from Ixodes ricinus ticks by using electrophoretic chips and real-time polymerase chain reaction. Folia Microbiol (Praha) 2007;52:315–324. doi: 10.1007/BF02932085. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Hyde F. W., Rumpel C. M. Taxonomy of the Lyme disease spirochetes. Yale J Biol Med. 1984a;57:529–537. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Schmid G. P., Hyde F. W., Steigerwalt A. G., Brenner D. J. Borrelia burgdorferi sp. nov.: etiological agent of Lyme disease. Int J Syst Bacteriol. 1984b;34:496–497. doi: 10.1099/00207713-34-4-496. [DOI] [Google Scholar]
- Kurtenbach K., Hanincová K., Tsao J. I., Margos G., Fish D., Ogden N. H. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol. 2006;4:660–669. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Brown R. N. Wood rats and kangaroo rats: potential reservoirs of the Lyme disease spirochete in California. J Med Entomol. 1991;28:299–302. doi: 10.1093/jmedent/28.3.299. [DOI] [PubMed] [Google Scholar]
- Lin T., Oliver J. H., Jr, Gao L., Kollars T. M., Jr, Clark K. L. Genetic heterogeneity of Borrelia burgdorferi sensu lato in the southern United States based on restriction fragment length polymorphism and sequence analysis. J Clin Microbiol. 2001;39:2500–2507. doi: 10.1128/JCM.39.7.2500-2507.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T., Oliver J. H., Jr, Gao L. Comparative analysis of Borrelia isolates from southeastern USA based on randomly amplified polymorphic DNA fingerprint and 16S ribosomal gene sequence analyses. FEMS Microbiol Lett. 2003;228:249–257. doi: 10.1016/S0378-1097(03)00763-8. [DOI] [PubMed] [Google Scholar]
- Margos G., Hojgaard A., Lane R. S., Cornet M., Fingerle V., Rudenko N., Ogden N., Aanensen D. M., Fish D., Piesman J. Multilocus sequence analysis of Borrelia bissettii strains from North America reveals a new Borrelia species, Borrelia kurtenbachii . Ticks Tick Borne Dis. 2010;1:151–158. doi: 10.1016/j.ttbdis.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin G. O., Gage K. L., Piesman J., Montenieri J., Sviat S. L., VanderZanden L., Happ C. M., Dolan M., Johnson B. J. Discovery of an enzootic cycle of Borrelia burgdorferi in Neotoma mexicana and Ixodes spinipalpis from northern Colorado, an area where Lyme disease is nonendemic. J Infect Dis. 1994;170:636–643. doi: 10.1093/infdis/170.3.636. [DOI] [PubMed] [Google Scholar]
- Newman E. A., Eisen L., Eisen R. J., Fedorova N., Hasty J. M., Vaughn C., Lane R. S. Borrelia burgdorferi sensu lato spirochetes in wild birds in northwestern California: associations with ecological factors, bird behavior and tick infestation. PLoS One. 2015;10:e0118146. doi: 10.1371/journal.pone.0118146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. H., Jr, Lin T., Gao L., Clark K. L., Banks C. W., Durden L. A., James A. M., Chandler F. W., Jr An enzootic transmission cycle of Lyme borreliosis spirochetes in the southeastern United States. Proc Natl Acad Sci U S A. 2003;100:11642–11645. doi: 10.1073/pnas.1434553100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett K. A., Bonilla D. L. Novel exposure sites for nymphal Ixodes pacificus within picnic areas. Ticks Tick Borne Dis. 2011;2:191–195. doi: 10.1016/j.ttbdis.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Picken R. N., Picken M. M. Molecular characterization of Borrelia spp. isolates from greater metropolitan Chicago reveals the presence of Borrelia bissettii. Preliminary report. J Mol Microbiol Biotechnol. 2000;2:505–507. [PubMed] [Google Scholar]
- Picken R. N., Cheng Y., Strle F., Cimperman J., Maraspin V., Lotric-Furlan S., Ruzic-Sabljic E., Han D., Nelson J. A., other authors Molecular characterization of Borrelia burgdorferi sensu lato from Slovenia revealing significant differences between tick and human isolates. Eur J Clin Microbiol Infect Dis. 1996a;15:313–323. doi: 10.1007/BF01695664. [DOI] [PubMed] [Google Scholar]
- Picken R. N., Cheng Y., Strle F., Picken M. M. Patient isolates of Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities of strain 25015. J Infect Dis. 1996b;174:1112–1115. doi: 10.1093/infdis/174.5.1112. [DOI] [PubMed] [Google Scholar]
- Postic D., Ras N. M., Lane R. S., Hendson M., Baranton G. Expanded diversity among Californian borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127) J Clin Microbiol. 1998;36:3497–3504. doi: 10.1128/jcm.36.12.3497-3504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic D., Garnier M., Baranton G. Multilocus sequence analysis of atypical Borrelia burgdorferi sensu lato isolates—description of Borrelia californiensis sp. nov., and genomospecies 1 and 2. Int J Med Microbiol. 2007;297:263–271. doi: 10.1016/j.ijmm.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Rudenko N., Golovchenko M., Mokrácek A., Piskunová N., Ruzek D., Mallatová N., Grubhoffer L. Detection of Borrelia bissettii in cardiac valve tissue of a patient with endocarditis and aortic valve stenosis in the Czech Republic. J Clin Microbiol. 2008;46:3540–3543. doi: 10.1128/JCM.01032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko N., Golovchenko M., Ru˚zek D., Piskunova N., Mallátová N., Grubhoffer L. Molecular detection of Borrelia bissettii DNA in serum samples from patients in the Czech Republic with suspected borreliosis. FEMS Microbiol Lett. 2009;292:274–281. doi: 10.1111/j.1574-6968.2009.01498.x. [DOI] [PubMed] [Google Scholar]
- Schneider B. S., Zeidner N. S., Burkot T. R., Maupin G. O., Piesman J. Borrelia isolates in Northern Colorado identified as Borrelia bissettii . J Clin Microbiol. 2000;38:3103–3105. doi: 10.1128/jcm.38.8.3103-3105.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle F., Picken R. N., Cheng Y., Cimperman J., Maraspin V., Lotric-Furlan S., Ruzic-Sabljic E., Picken M. M. Clinical findings for patients with Lyme borreliosis caused by Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities to strain 25015. Clin Infect Dis. 1997;25:273–280. doi: 10.1086/514551. [DOI] [PubMed] [Google Scholar]
- Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;5:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. mega6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe J., Jordan D., Janecek E., Fingerle V., Strube C. Revisited: Borrelia burgdorferi sensu lato infections in hard ticks (Ixodes ricinus) in the city of Hanover (Germany) Parasit Vectors. 2014;7:441. doi: 10.1186/1756-3305-7-441. [DOI] [PMC free article] [PubMed] [Google Scholar]