Abstract
Background
Naegleria fowleri is a thermophilic ameba found in freshwater that causes primary amebic meningoencephalitis (PAM) when it enters the nose and migrates to the brain. Patient exposure to water containing the ameba typically occurs in warm freshwater lakes and ponds during recreational water activities. In June 2016, an 18-year-old woman died of PAM after traveling to North Carolina, where she participated in rafting on an artificial whitewater river.
Methods
We conducted an epidemiologic and environmental investigation to determine the water exposure that led to the death of this patient.
Results
The case-patient's most probable water exposure occurred while rafting on an artificial whitewater river during which she was thrown out of the raft and submerged underwater. The ∼11.5 million gallons of water in the whitewater facility were partially filtered, subjected to UV light treatment, and occasionally chlorinated. Heavy algal growth was noted. Eleven water-related samples were collected from the facility; all were positive for N. fowleri. Of 5 samples collected from the nearby natural river, 1 sediment sample was positive for N. fowleri.
Conclusions
This investigation documents a novel exposure to an artificial whitewater river as the likely exposure causing PAM in this case. Conditions in the whitewater facility (warm, turbid water with little chlorine and heavy algal growth) rendered the water treatment ineffective and provided an ideal environment for N. fowleri to thrive. The combination of natural and engineered elements at the whitewater facility create a challenging environment to control the growth of N. fowleri.
Keywords: Naegleria fowleri, primary amebic meningoencephalitis, environmental
Introduction
Swimming and other recreational water activities are enjoyed by millions of Americans every year during the warm summer months. While these activities provide a health benefit in the form of physical activity, infection from waterborne pathogens can occur. An infrequent but severe waterborne infection is caused by Naegleria fowleri (N. fowleri), a free-living ameba that thrives in warm freshwater. This ameba causes primary amebic meningoencephalitis (PAM) when water containing the ameba enters the nose, allowing it to gain access to the brain via the cribriform plate. The resulting infection is fulminant, causing death in 97% of U.S. cases in a median of 5 days (1). PAM, as a type of meningitis, is often mistaken for other, more common types of meningitis and diagnosis is often made post-mortem.
Since 1962, 143 PAM cases have been reported in the United States, predominantly among males and children, with most reporting recreational water exposure such as swimming, diving, waterskiing, or wakeboarding in lakes in the week prior to illness onset (2). Whitewater rafting has not been previously documented as a water exposure leading to PAM, and was not considered to be a risk factor because natural whitewater rivers and streams move swiftly and many are cooler water sources originating in the mountains. This contrasts with the warm, slow-moving or stagnant lakes, ponds, and rivers where most infections occur.
Artificial whitewater courses gained popularity with the introduction of whitewater slalom as an Olympic sport in 1972. Some artificial whitewater courses worldwide are built in or are diversions from natural streambeds. More recently, whitewater courses have been built that are completely manufactured as concrete channels with closed loop systems using recirculated water (Scott Shipley, personal communication). There are currently three closed loop concrete channel whitewater courses in the United States open to the general public.
Case Report
On the morning of June 17, 2016, a previously healthy 18-year-old woman presented to a Columbus, Ohio emergency department with a headache that began 3 days prior associated with fever and lethargy. She had been seen by her primary care physician who diagnosed possible sinusitis and prescribed amoxicillin. However, following that visit, she started having high fevers with progressive lethargy. In the emergency department, she had altered mental status, responded only to noxious stimuli, and was febrile (103°F) with a pulse of 107 beats per minute, blood pressure of 115/69 mmHg, and respirations of 22/minute. In order to evaluate for meningitis, a lumbar puncture was performed revealing an opening pressure of 36 cmH2O. Cerebrospinal fluid (CSF) analysis showed a white blood cell count of 3808 cells/μL with 78% neutrophils, 18% monocytes, and 4% lymphocytes, a red blood cell count of 516 cells/ μL, protein 410 mg/dL, and glucose <10 mg/dL. A computed tomography (CT) scan of the brain was interpreted as normal. The patient was admitted and treated empirically for bacterial and viral etiologies of meningitis with ceftriaxone, vancomycin, ampicillin, doxycycline, and acyclovir. Over the next 36 hours, she rapidly declined despite antimicrobial therapy and became obtunded requiring intubation and critical care management. A repeat CT scan of the brain showed interval increased effacement of cerebral sulci and decreased ventricular size compatible with diffuse cerebral edema. A right frontal extraventricular drain was placed revealing an intracranial pressure of 90 cmH2O at 3:00 PM on 18 June. Strategies to try to manage the patient's elevated intracranial pressure included the administration of mannitol, hypertonic saline, and dexamethasone, hyperventilation, pentobarbital, and vasopressors to increase mean arterial pressure and generate cerebral perfusion pressure. Therapeutic hypothermia was also initiated. At this time, CSF Gram stain showed no organisms; herpes simplex and varicella zoster virus PCR were negative, and the diagnosis of PAM was considered given the patient's rapid decline and nonresponse to appropriate coverage for bacterial and viral meningitis. CDC was consulted on 18 June and conventional amphotericin B, fluconazole, azithromycin, and rifampin were added based on treatment protocols used in PAM survivors (3). Miltefosine, which is also part of the recommended treatment for PAM, was administered once it arrived from CDC, approximately 8 hours later. A wet mount of the CSF revealed possible motile trophozoites. Despite multiple aggressive measures, the patient's ICP remained > 50 cmH2O. At approximately 9 PM on 18 June, the patient had a sudden change in hemodynamics and an acute drop in ICP suspicious for brain death. Cardiac death occurred the following morning on 19 June. She received one dose of miltefosine prior to death. CSF arrived at CDC on 21 June and a real-time polymerase chain reaction (PCR) test that simultaneously detects N. fowleri, Balamuthia mandrillaris, and Acanthamoeba spp. was positive for N. fowleri, which was further characterized as genotype 1.
On initial suspicion of PAM, the patient's family and friends were asked about the patient's freshwater exposures in the 2 weeks prior to her illness onset. The patient had recently returned from an 8-day youth choir trip to West Virginia and North Carolina. The patient's only freshwater exposure on that trip occurred during a visit to the U.S. National Whitewater Center (USNWC) on 8 June during which the patient fell out of the raft and was submerged under the water. The patient's mother reported that the patient had described the incident to her and reported a large volume of water entered her nose while she was submerged. No other freshwater exposures that resulted in water entering the nose were reported during the incubation period.
Environmental Investigation
The USNWC is located in Charlotte, NC on 1,100 acres near the Catawba River. Activities offered include whitewater rafting and kayaking, flatwater kayaking, stand-up paddle boarding, rock climbing, zip lines, ropes courses, a canopy tour, and mountain biking. Whitewater activities take place in a recirculating artificial whitewater facility. The channel structures within the whitewater facility are poured concrete with a geobarrier and water barrier membrane below the concrete to prevent mixing with groundwater. At the time of the investigation, water for filling and maintaining water levels in the whitewater facility was obtained from onsite wells and county municipal water. At no place is river water introduced into the system. Storm drains and ground drainage around the site are graded to minimize runoff from entering the whitewater channels. However, the channels are open, so water levels do increase during rain storms. Additionally, there was an active construction site directly adjacent to the lower pond section of the whitewater channels at the time the case-patient rafted and during this investigation.
The whitewater facility had the capacity to hold 12 million gallons of water. Multiple pumps were used to lift the water approximately 21 feet to an upper pond, and water then flowed by gravity down one of two channels. A portion of the water in the channels was withdrawn from the lower pond for filtration. The filtration system (filter pore size = 200 microns) was operated to treat a volume corresponding to the total volume of the system once every 24 hours. After filtration, the water passed through a low-pressure ultraviolet (UV) treatment unit. After treatment, the water was discharged to the upper pond. Operators manually added liquid chlorine as a disinfectant to the upper pond only when fecal coliform counts were trending upward or algal growth visibly increased; however, chlorine concentrations were not monitored. Each evening when the facility was closed, pumps were turned off and most of the water drained to the lower pond; however, low levels of water always remained in the channels.
Sediment and algal growth were removed from the upper pond by vacuuming on an as-needed basis when the pumps were turned off. The system was drained and cleaned annually, during which scrubbing and pressure washing was performed to clean the concrete and rock surfaces. The system was left dry for approximately two months annually. The facility had a contract with a commercial laboratory to perform weekly water testing for fecal coliform bacteria, turbidity, pH, temperature and heavy metals. On the date of inspection, the pumps were turned off, revealing a layer of algae and biofilm in the upper pond and channel surfaces.
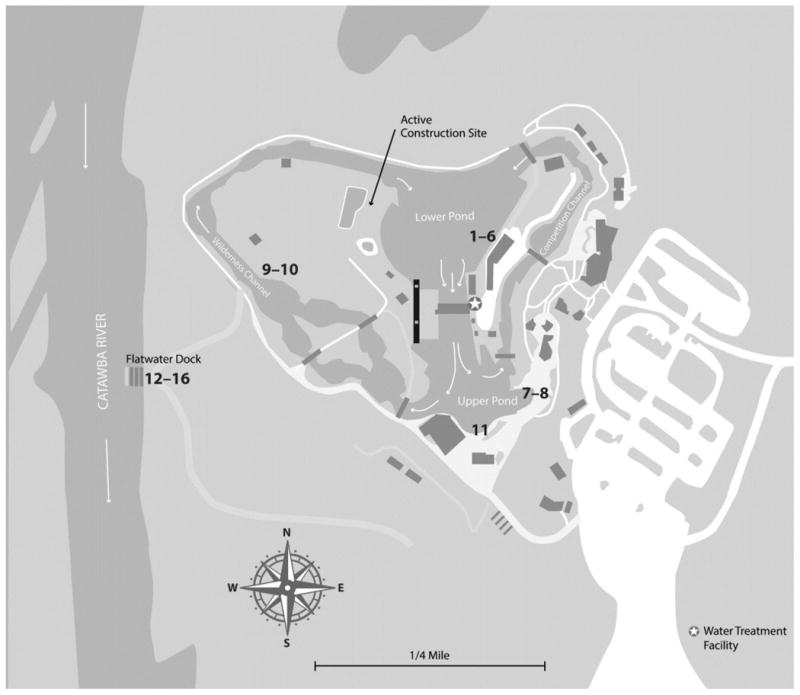
Physical and chemical parameters were measured in water at the USNWC lower pond on 22 June during an onsite visit by health officials. Water, facility filter backwash, submerged plant material, and surface swab samples were collected within the channels and the upper and lower ponds of the USNWC. Water, sediment, and surface swab samples were also collected from or near the adjacent Flatwater Dock in the Catawba River (Figure) for comparison. Large volume water samples (33–50 L) were collected using dead-end ultrafiltration (DEUF) (4); 1-L grab samples were collected in parallel. Samples were transported and stored in the laboratory at ambient temperature to maintain N. fowleri viability.
Figure. Map of Whitewater Center with Water and Environmental Sampling Locations.
Sample processing began on the morning of 23 June. All ultrafilters and grab samples collected from the Catawba River and USNWC and facility filter backwash samples were processed using a concentration procedure; sediment, plant material, and surface swab samples were processed using an elution and concentration procedure (Centers for Disease Control and Prevention, Division of Healthcare Quality Promotion, Environmental Surface Sampling Methods, 2014). Concentrates were divided; one aliquot was submitted to a N. fowleri real-time polymerase chain reaction (PCR) assay (“direct”) and a second aliquot was submitted to a culture assay (“culture”)(5, 6). Aliquots of one top pond sample and the Catawba River sediment sample were also submitted directly (i.e., without prior concentration) to the culture assay. If trophozoites or cysts were observed on a culture plate within 7 days of incubation at 44°C, material from the plate was harvested and submitted to real-time PCR for confirmation of culturable N. fowleri. Cultured organisms were genotyped by sequencing the 5.8S ribosomal RNA gene and internal transcribed spacers 1 and 2 (ITS1 and ITS2)(7).
Total chlorine residual concentration measured in the lower pond of the whitewater facility was 0.15 mg/L and the free chlorine residual was 0.05 mg/L. The turbidity in the lower pond was 6.7 NTU and the temperature was 30°C. The turbidity near the Flatwater Dock in the Catawba River was 4.1 NTU and the temperature was 28°C.
All 11 samples collected within the whitewater facility (i.e., small and large volume water, facility filter backwash, and surface swab) were positive for N. fowleri by direct testing and by culture. Additionally, the cycle threshold (Ct) value for the direct testing performed on the top pond sample was comparable to those seen for culture and did not require concentration to detect amebae. Of the 5 samples taken from the Flatwater Dock area on the Catawba River (i.e., small and large volume, sediment, and surface swab), all were negative for N. fowleri by direct testing and one sediment sample was positive by culture (Table 1). All positive N. fowleri cultures were identified as genotype I.
Table 1. N. fowleri testing results for samples collected within the U.S. National Whitewater Center (USNWC) and from or near the Flatwater Dock on the Catawba River.
| Sample Number | Sample Type | Direct Results* | Culture Results* |
|---|---|---|---|
| USNWC Samples | |||
| 1 | Pod 1 backwash (0.75 L) | Positive | Positive |
| 2 | Pod 2 backwash (0.75 L) | Positive | Positive |
| 3 | Pod 3 backwash (0.75 L) | Positive | Positive |
| 4 | Pod 4 backwash (0.75 L) | Positive | Positive |
| 5 | Bottom pond small-volume water (0.75 L) | Positive | Positive |
| 6 | Bottom pond large-volume water (50 L) | Positive | Positive |
| 7 | Top pond small volume water (∼0.7 L) | Positive | Positive |
| Un-concentrated | NA | Positive | |
| 8 | Top pond small volume water, un-concentrated (∼0.5 L) | Positive | NA |
| 9 | Wilderness channel surface swab (4″ × 4″)§ | Positive | Positive |
| 10 | Wilderness channel submerged plant material | Positive | Positive |
| 11 | Boat loading ramp surface swab (4″ × 4″)§ | Positive | Positive |
| Flatwater Dock at Catawba River (CR) Samples | |||
| 12 | CR small-volume water | Negative | Negative |
| 13 | CR large-volume water | Negative | Negative |
| 14 | CR sediment | Negative | Negative |
| Un-concentrated | NA | Positive | |
| 15 | Flatwater Dock sub-surface swab (4″ × 4″) | Negative | Negative |
| 16 | Flatwater Dock above surface swab (4″ × 4″) | Negative | Negative |
A sample was considered positive for N. fowleri when Ct <40
Location swabbed is below water level when system pumps are running
NA: not analyzed
With these findings and in consultation with local, state, and federal public health officials, the USNWC decided to voluntarily close their whitewater facility on 24 June until they could assess their water treatment operations and develop a remediation strategy.
Discussion
Each summer in the United States, 0–8 PAM cases are reported, typically in patients who report warm freshwater exposure in lakes, ponds, and reservoirs located in southern tier U.S. states. In recent years, new locations and types of water exposures have been documented in PAM cases including the first cases from the northern states of Minnesota, Indiana, Maryland, and Kansas and cases associated with the use of tap water in neti pots, for ritual nasal rinsing, and on backyard water slides (8-11). The case reported here represents yet another novel type of water exposure associated with PAM. Based on the epidemiologic investigation, the most likely water exposure leading to PAM in this case-patient was falling out of the raft at the USNWC during which a large volume of water entered the patient's nasal cavity. This conclusion is supported by environmental sampling that found all samples taken from the whitewater channels to be positive for N. fowleri genotype 1 by culture and direct testing.
During the site visit, health officials documented several water conditions that likely allowed for the presence of N. fowleri in the whitewater channels. First, the water temperature was 30 °C, offering an ideal temperature for a thermophilic organism like N. fowleri to thrive. Second, the pore size of the filtration system in use at the facility was inadequate for removal of microorganisms. Additionally, the water in the channels was turbid and the water treatment operations in use at the USNWC (i.e., ultraviolet light, occasional addition of chlorine) were likely inadequate to control the growth of N. fowleri in turbid water. When chlorine is added to turbid water with a high load of organic content, it is rapidly consumed and does not remain in the water to provide ongoing disinfection. While UV light disinfection can inactivate N. fowleri, it is ineffective when water turbidity is high (12). A number of factors likely contributed to the elevated turbidity in the whitewater channels and ponds, including the fact that it is a closed-loop system with continuous input of particulate matter and it utilizes a filtration system unable to remove most of these particulates. As a result, there was no effective water treatment occurring in the whitewater channels. Lastly, the biofilm growth noted on the bottom and sides of the whitewater channels also contributed to growth of N. fowleri in this facility. Free-living amebae, including N. fowleri, are frequent inhabitants of biofilms, which provide a source of nutrients as well as protection from disinfectants (13, 14).
While the whitewater channels provided an ideal environment for N. fowleri to thrive, it is not clear how the pathogen might have initially entered the water. The whitewater channels are drained each year and refilled annually with water primarily from the county utility (with supplementation from onsite wells, as needed) that meets drinking water standards, indicating it contains adequate residual chlorine levels, to which N. fowleri is sensitive; therefore, the source water is unlikely to contain N fowleri. However, as a common water and soil inhabitant, N. fowleri could have entered the whitewater channels through storm water run-off or soil blown into the channels, wildlife such as turtles or birds, or on a kayak that had previously been used in a natural water body.
The USNWC poses a difficult challenge in the control and prevention of N. fowleri. While it is an artificial water venue, it was designed to mimic the features of a natural whitewater river with the addition of rocks and use of uneven surfaces. Natural water bodies often contain N. fowleri as a natural constituent of freshwater (15-17). However, facilities can be designed, constructed, or maintained in ways that unintentionally encourage the proliferation of N. fowleri. A review of CDC surveillance data for PAM documented a number of water exposures that occurred in recreational water venues that had been altered in ways that might have increased the risk of N. fowleri infection (Table 2). Some venues added sand or concrete to the bottom and sides of the venue, potentially increasing surfaces for biofilm growth. Other venues minimally treated the water (e.g., for water clarity or aesthetics) which altered the microbial ecosystem and provided a perception of good water quality, but did not inactivate N. fowleri. In addition, some venues added water slides and tow cables (i.e., to pull inflatable tubes and water skiers) which altered the way humans interacted with the venue, resulting in an increased risk of nasal exposure to water.
Table 2. Recreational Water Venues with Artificial Features Associated with U.S. PAM Cases, 1962–2016.
| Venue | Year | County, State | Bottom composition | Water Treatment | Water Source | Size of water body | Other Features | Number of associated PAM cases |
|---|---|---|---|---|---|---|---|---|
| Artificial whitewater river (this report) | 2016 | Mecklenburg, NC | Concrete | 200-μm particulate filter, UV, manual, intermittent chlorination | Municipal drinking water and wells | ∼11.5 million gallons | Closed-loop, recirculated system; whitewater kayaking | 1 |
| Water Park | 2013 | Pulaski, AR | Sand | Chlorine, pH balanced, and chemicals | Spring and well | 3.5 acres | Slides | 2 |
| Campground Lake | 2009 | Madison, FL | Sand | Chlorine | Deep well | 1.5 acres | Slide | 1 |
| Watersports Complex | 2007,2009 | Orange, FL | Sand | Untreated, monthly testing | Rain | Waterskiing and wakeboarding | 2 | |
| Water Park | 1979,1980 | Orange, FL | Sand | “Unique filtration system” | Lake | 6 acres | Slides | 2 |
| Lake | 1977 | Richmond, GA | Sand | Untreated | Cement retaining walls | 1 | ||
| Lake | 1950s and 60s | Chesterfield, VA | Likely Sand | Unknown | Unknown | Slides, diving boards, fountains | 4 |
In response to this PAM case and investigation, the USNWC worked to reduce the risk of future infections by improving the current water treatment systems. In addition to the existing UV light treatment, the USNWC modified the filtration systems and added ozonation and an automated chlorine injection system with the goal of reducing the organics and maintaining a level of 0.5 ppm of free chlorine. Maintaining a chlorine residual of 0.5 ppm has proven to be effective for preventing infections associated with drinking water systems that previously had PAM cases associated with them (11, 18).
A challenge for reducing the risk of PAM at this venue was that state recreational water regulations, as written, did not apply to this venue. Regulations exist for swimming pools and water parks as well as for swimming areas of lakes and reservoirs. However, facilities like the USNWC do not fall into either of these categories and at the time this case occurred, the USNWC was only required, through a lease agreement, to perform weekly testing for fecal coliform bacteria and meet minimum standards (<200 colonies/100 ml). These standards are not designed for N. fowleri control and do not account for the complex engineering challenges, biofilm growth, warm temperatures, and forceful nasal water entry in this venue. As more recreational water venues are constructed that do not meet traditional definitions of swimming pools or water parks, public health authorities and other state and local regulatory bodies will be required to make difficult decisions in the absence of scientific data to determine how to best regulate these venues in order to protect the public's health. In response to this PAM case, the local Board of Health adopted rules governing recreational whitewater systems for the purpose of creating an environment that is not hospitable to potentially pathogenic microorganisms. The primary focus of the rules includes disinfection (i.e., to maintain a free chlorine concentration of 0.5 ppm when a secondary disinfection such as ozone or ultraviolet light is active), daily water quality monitoring of pH and water temperature, and the documentation and removal of organic accumulation.
While tap water and the water in pools, waterparks, and other artificial water venues can be treated to reduce the growth of N. fowleri, there are also personal actions that can be taken to reduce the risk of PAM. These include keeping the head above water, holding the nose shut, or using nose clips when taking part in water-related activities to limit the amount of water going up the nose. When using water for nasal rinsing (e.g., using a neti pot or practicing nasal rinsing as part of ritual ablution), water should be boiled, filtered, or bought as sterile or distilled water. Water should not be used directly from the tap for these activities. (http://www.cdc.gov/parasites/naegleria/prevention.html).
Since 2010, each summer in the United States has brought reports of PAM cases with newly identified transmission routes, new geographic areas of infection, and new types of water venues where exposures can occur. Once limited primarily to 15 southern-tier states, PAM cases have recently been reported from Minnesota (2010 and 2012), Kansas (2011), Indiana (2012), and Maryland (2016). PAM cases have also been reported from other countries and in U.S. patients who have traveled abroad(19, 20). As a thermophilic ameba, predictions of a warming climate have implications for the ecology of Naegleria fowleri and for infections, which warrants further research, monitoring, and awareness of this pathogen. Clinicians in all regions of the United States and internationally should be aware of this infection and recognize that not all patients will have the traditional exposure to warm recreational freshwater.
Summary.
Naegleria fowleri is a thermophilic ameba found in freshwater that causes primary amebic meningoencephalitis (PAM). This article describes a PAM case in an 18-year-old woman who was exposed to N. fowleri while rafting on an artificial whitewater river.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention.
The authors wish to acknowledge Zack Moore, Michael Beach, Jonathan Yoder, Katie Fullerton, and John Rossow for their contributions to this investigation.
Financial support. The authors completed this work in the course of their regular duties for their affiliated institutions and received no additional funding.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts.
References
- 1.Capewell LG, Harris AM, Yoder JS, Cope JR, Eddy BA, Roy SL, et al. Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937-2013. J Pediatric Infect Dis Soc. 2015;4:e68–75. doi: 10.1093/jpids/piu103. [DOI] [PubMed] [Google Scholar]
- 2.Yoder JS, Eddy BA, Visvesvara GS, Capewell L, Beach MJ. The epidemiology of primary amoebic meningoencephalitis in the USA, 1962-2008. Epidemiol Infect. 2010;138:968–75. doi: 10.1017/S0950268809991014. [DOI] [PubMed] [Google Scholar]
- 3.Linam WM, Ahmed M, Cope JR, Chu C, Visvesvara GS, da Silva AJ, et al. Successful treatment of an adolescent with Naegleria fowleri primary amebic meningoencephalitis. Pediatrics. 2015;135:e744–8. doi: 10.1542/peds.2014-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mull B, Hill VR. Recovery of diverse microbes in high turbidity surface water samples using dead-end ultrafiltration. J Microbiol Methods. 2012;91:429–33. doi: 10.1016/j.mimet.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mull BJ, Narayanan J, Hill VR. Improved method for the detection and quantification of Naegleria fowleri in water and sediment using immunomagnetic separation and real-time PCR. J Parasitol Res. 2013;2013:608367. doi: 10.1155/2013/608367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol. 2006;44:3589–95. doi: 10.1128/JCM.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou L, Sriram R, Visvesvara GS, Xiao L. Genetic variations in the internal transcribed spacer and mitochondrial small subunit rRNA gene of Naegleria spp. J Eukaryot Microbiol. 2003;50(Suppl):522–6. doi: 10.1111/j.1550-7408.2003.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 8.Kemble SK, Lynfield R, DeVries AS, Drehner DM, Pomputius WF, 3rd, Beach MJ, et al. Fatal Naegleria fowleri infection acquired in Minnesota: possible expanded range of a deadly thermophilic organism. Clin Infect Dis. 2012;54:805–9. doi: 10.1093/cid/cir961. [DOI] [PubMed] [Google Scholar]
- 9.Yoder JS, Straif-Bourgeois S, Roy SL, Moore TA, Visvesvara GS, Ratard RC, et al. Primary amebic meningoencephalitis deaths associated with sinus irrigation using contaminated tap water. Clin Infect Dis. 2012;55:e79–85. doi: 10.1093/cid/cis626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Notes from the field: primary amebic meningoencephalitis associated with ritual nasal rinsing--St. Thomas, U.S. Virgin islands, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:903. [PMC free article] [PubMed] [Google Scholar]
- 11.Cope JR, Ratard RC, Hill VR, Sokol T, Causey JJ, Yoder JS, et al. The first association of a primary amebic meningoencephalitis death with culturable Naegleria fowleri in tap water from a US treated public drinking water system. Clin Infect Dis. 2015;60:e36–42. doi: 10.1093/cid/civ017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobsey M. Inactivation of health-related microorganisms in water by disinfection processes. Water Science and Technology. 1989;21:179–95. [Google Scholar]
- 13.Miller HC, Wylie J, Dejean G, Kaksonen AH, Sutton D, Braun K, et al. Reduced efficiency of chlorine disinfection of Naegleria fowleri in a drinking water distribution biofilm. Environ Sci Technol. 2015;49:11125–31. doi: 10.1021/acs.est.5b02947. [DOI] [PubMed] [Google Scholar]
- 14.Sheehan KB, Fagg JA, Ferris MJ, Henson JM. PCR detection and analysis of the free-living amoeba Naegleria in hot springs in Yellowstone and Grand Teton National Parks. Appl Environ Microbiol. 2003;69:5914–8. doi: 10.1128/AEM.69.10.5914-5918.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wellings FM, Amuso PT, Chang SL, Lewis AL. Isolation and identification of pathogenic Naegleria from Florida lakes. Appl Environ Microbiol. 1977;34:661–7. doi: 10.1128/aem.34.6.661-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Painter SM, Pfau RS, Brady JA, McFarland AM. Quantitative assessment of Naegleria fowleri and Escherichia coli concentrations within a Texas reservoir. J Water Health. 2013;11:346–57. doi: 10.2166/wh.2013.162. [DOI] [PubMed] [Google Scholar]
- 17.Sifuentes LY, Choate BL, Gerba CP, Bright KR. The occurrence of Naegleria fowleri in recreational waters in Arizona. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2014;49:1322–30. doi: 10.1080/10934529.2014.910342. [DOI] [PubMed] [Google Scholar]
- 18.Robinson BaC, P E. Disinfection of water for control of amoebae. Water. 1984:21–4. [Google Scholar]
- 19.Booth PJ, Bodager D, Slade TA, Jett S. Primary amebic meningoencephalitis associated with hot spring exposure during international travel - Seminole County, Florida, July 2014. MMWR Morb Mortal Wkly Rep. 2015;64:1226. doi: 10.15585/mmwr.mm6443a5. [DOI] [PubMed] [Google Scholar]
- 20.Shakoor S, Beg MA, Mahmood SF, Bandea R, Sriram R, Noman F, et al. Primary amebic meningoencephalitis caused by Naegleria fowleri, Karachi, Pakistan. Emerg Infect Dis. 2011;17(2):258–61. doi: 10.3201/eid1702.100442. [DOI] [PMC free article] [PubMed] [Google Scholar]


