Abstract
Electroencephalography (EEG) and magnetoencephalography (MEG) are non-invasive neuroimaging techniques that have been used extensively to study various resting state and cognitive processes in the brain. The purpose of this review is to highlight a number of recent studies that have investigated the alpha band (8–12 Hz) oscillatory activity present in MEG and EEG, to provide new insights into the maladaptive network activity underlying attentional impairments in attention-deficit/hyperactivity disorder (ADHD). Studies reviewed demonstrate that event-related decrease in alpha is attenuated during visual selective attention, primarily in ADHD inattentive type, and is often significantly associated with accuracy and reaction time during task performance. Furthermore, aberrant modulation of alpha activity has been reported across development and may have abnormal or atypical lateralization patterns. Modulations in the alpha band thus represent a robust, relatively unexplored putative biomarker of attentional impairment in ADHD, a strong prospect for future studies aimed at examining underlying neural mechanisms and treatment response among individuals with ADHD. Potential limitations of its use as a diagnostic biomarker and directions for future research are discussed.
Keywords: EEG, MEG, alpha, spectral power, neurophysiology, biomarker
Over the last decade, cognitive neuroscience has made much gain in understanding the engagement and interactions of multiple brain networks that underlie cognitive processes (1–3). Electroencephalography (EEG) and magnetoencephalography (MEG) are extensively used techniques that capture, on millisecond time scales, brain oscillatory activity present in electrophysiological signals; this allows for the study of cognitive processes via quantification of brain network interactions as they occur, ostensibly in real time (4–7). The object of this review is to highlight recent studies that have used task-related modulations of alpha band (8–12 Hz) oscillatory activity to offer new insights into maladaptive network activity underlying attentional impairments in attention-deficit/hyperactivity disorder (ADHD). ADHD is one of the most prevalent disorders in childhood, affecting an estimated 5–11% of children (8), with longitudinal studies indicating that 30–70% of individuals continue to meet diagnostic criteria into adulthood (9). In addition to highly variable rates of diagnostic persistence and treatment response, the need to further understand the neural mechanisms underlying ADHD is underscored by extremely poor outcomes in adulthood such as frequent psychiatric co-morbidity, substance abuse, incarceration, divorce, poor health, and high societal cost ($143–$266B annually; 10). In this review, we suggest that studies of oscillatory activity may address this need. We begin with a historical overview of oscillatory studies in ADHD, then focus on task-related modulation of alpha band activity, which have emerged more recently as promising indicators of the neurophysiological underpinnings of the cognitive deficits present in ADHD. Finally, we discuss oscillatory power as a potential biomarker in ADHD and consider possible directions, and challenges, for future research.
Resting State EEG and ADHD
There is a long history of EEG studies in ADHD, with the first study of resting state brain oscillations in children with behavioral problems consistent with ADHD reported in 1938 by Jasper et al (11). The earliest observations were described as frontocentral “slowing” in the EEG of affected children (11), which means an increase in the power expressed within slower frequency oscillations (theta band, 4–7 Hertz [Hz]) over frontal and central scalp (12, 13). This led to a sustained 40-years and counting!) focus of research on elevated theta power (“slowing” of brain activity) and diminished power in “faster” frequencies (i.e., beta band, 13–25 Hz), as well as the corresponding ratio of theta- to beta-band power, also known as the theta to beta ratio (TBR) (14). Efforts to validate the TBR as a biomarker of ADHD diagnosis seemed promising until fairly recently (pre-2010). Previous research studies and meta-analysis of the TBR reported high accuracy (89%; 15, 16) and large effect size (ES=3.1; 13) for ADHD diagnosis. However, recent independent replication studies and meta-analysis (17–21) reported low accuracy (range: 38 to 63%; 22) and significant heterogeneity among study results, suggesting that even though the overall ES of 0.62 is significant for ADHD diagnosis, it is misleading and potentially an overestimation of the true ES (23). While a sufficient number of individuals with ADHD (20–30%) have elevated TBR, which drives a significant group effect, the TBR is not a valid discriminator of ADHD diagnosis.
Findings for alpha band spectral power at rest in ADHD have been mixed and may depend on developmental level, ADHD subtype, and psychiatric comorbidities. Overall, higher levels (21, 24–26), no significant differences (27–31), and lower levels (30, 32–39) of alpha spectral power between samples with and without ADHD have been reported; however, no clear pattern has emerged according to age or ADHD subtype, the latter of which is not often reported. Recent studies suggest significant heterogeneity in resting state EEG spectral power characteristics within ADHD (36, 40) and at the population level (41) (see Fig. 1a). Furthermore, while spectral power is the predominant metric used to reflect alpha band activity, there are other measures that have been reported such as power density, mean frequency, peak frequency, coherence, and laterality (see Table 1 for summary and definitions). While the plurality of results may reflect poor control over what participants are actually doing during ‘resting’ state, this also suggests that there may not be a resting state electrophysiological profile that accurately discriminates between those with and without ADHD. The purpose of the present paper is to suggest that other EEG/MEG signals, and in particular task-related suppression of alpha-band activity, may provide a more fruitful avenue for future research. These effects are more closely tied to specific neural systems and to cognitive functions, such as attention and working memory.
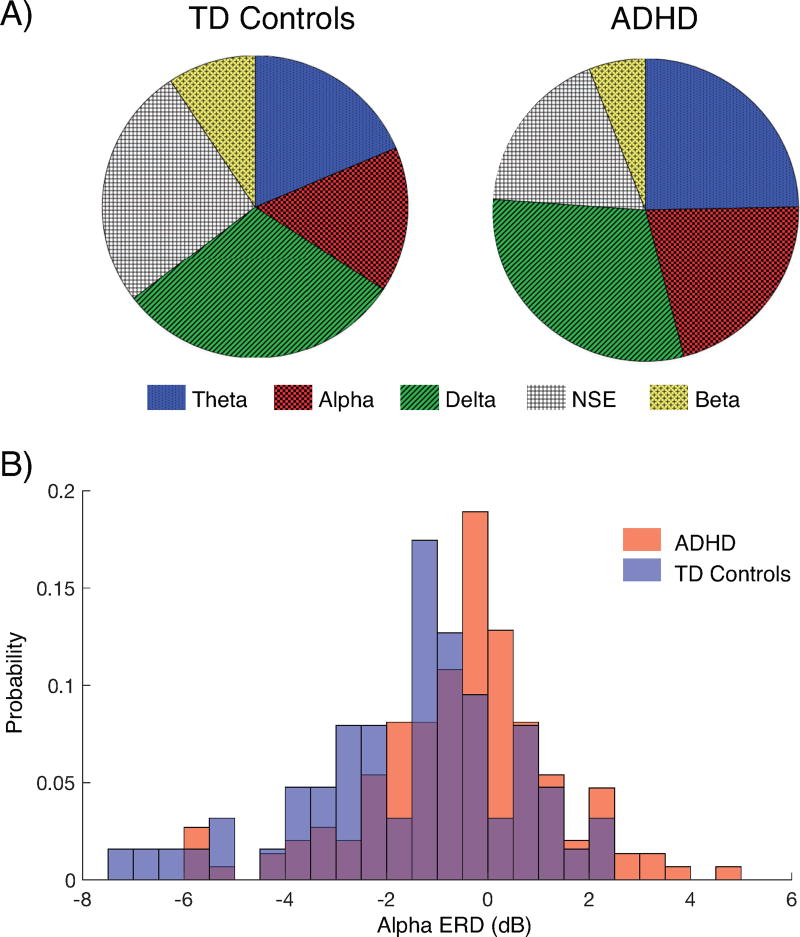
Figure 1. Heterogeneity within and between groups limits potential of existing EEG metrics as biomarkers of ADHD.
(A) Population-level EEG heterogeneity is evident in the presence of five clusters within both ADHD and typically developing (TD) control groups. Each cluster is defined by elevations in oscillatory power within a frequency band (delta 1–3 Hertz [Hz] theta, 4–7 Hz, alpha 8–12 Hz, beta 13–20 Hz) and no spectral elevation [NSE]). There is no cluster or spectral power profile characteristic of either ADHD or TD group, suggesting resting EEG spectral power measures are insufficient to serve as a biomarker of ADHD. Figure reproduced with permission from (41). (B) The distribution of alpha ERD during the encoding interval in Fig. 2c. The image illustrates that ADHD and TD controls, despite a significant difference in group mean, have largely overlapping distributions of alpha ERD. These data, argue for the unsuitability of a single EEG metric as a diagnostic biomarker of ADHD.
Table 1.
Alpha band power findings in ADHD
| First Author | Year | N (ADHD, Con) |
Age Grp |
Task | Frequency band (Hz) |
Measure | Region | Alpha in ADHD |
|---|---|---|---|---|---|---|---|---|
| Resting State | ||||||||
| Poil (20) | 2014 | 48, 68 | CH, AD | EC | 8–13 | S, MF | F, F/P | CH: higher pow; AD: lower MF |
| Koehler (25) | 2009 | 34, 34 | AD | EC | 7.5–12.5 | PD | C, P | Higher PD |
| Bresnahan (23) | 2002 | 50, 100 | AD | EO | 8–12 | S | P | Higher pow |
| Chabot (24) | 1996 | 407, 310 | CH | EC | 8–12 | S, Coh, MF | F, C | Higher pow w/ 1. normal MF (46%), 2. lower MF (30%) |
| van Dongen-Boomsma (30) | 2010 | 24, 24 | AD | EO, EC | 8–12 | S, PF | P | ND in pow or PF. Greater decrease from EC to EO. |
| Bresnahan (27) | 2006 | 50, 50 | AD | EO | 8–12 | S | -- | ND |
| Hermens (28) | 2004 | 36, 36 | AD | EC | 8–12 | S | -- | ND |
| Bresnahan (26) | 1999 | 75, 75 | CH, AD | EO | 8–12 | S | -- | ND |
| Pomoerov (38) | 2014 | 96, 376 | AD | EO, EC | 8–12 | S | F, C | Lower pow |
| Woltering (37) | 2012 | 18, 17 | AD | EO, EC | 8–12 | S | F, C, P | Lower pow |
| Barry (31) | 2009 | 30, 30 | CH | EC | 8–12 | S | G | Lower pow |
| Magee (35) | 2005 | 253, 67 | CH | EC | 8–12 | S | F, P | Lower pow |
| Clarke (32) | 2001 | 160, 80 | CH | EC | 8–12 | S | P | Low pow in boys and older CH, ND is girls and younger CH |
| Nazari (36) | 2011 | 16, 16 | CH | EO, CPT | 8–12 | S | G | Lower pow resting, higher pow CPT |
| Loo (29) | 2010 | 384, 147 | CH, AD | EO, EC, CPT | 8–12 | S | P | Lower pow in adults ADHD-C vs ADHD-I & Cons. CH: ND |
| Loo (34) | 2009 | 38, 42 | AD | EO,EC, CPT | 8–12 | S | F,P | Lower pow all conditions |
| El Sayed (33) | 2002 | 36, 63 | CH | EO, CPT | 8–12.5 | S | G | Lower pow all conditions |
| Hale (68) | 2009 | 29, 62 | AD | EC, CPT | 8–10, 10–12 | Lat | P | Greater R lat all conditions |
| Baving (150) | 1999 | 47, 70 | CH | EO | 8–10 | Lat | F | Greater R lat-boys, L lat-girls |
| Task | ||||||||
| Lenartowicz (53) | 2016 | 8, 13 | CH | SWM | 8–12 | ERD/ERI | P | Less ERD |
| Hasler (64) | 2016 | 21, 20 | AD | Flanker | 8–13 | ERD/ERI | P | Less ERD to cue/target |
| Lenartowicz (120) | 2014 | 52, 47 | CH | SWM | 8–12 | ERD/ERI | P | Less ERD for LL, not HL |
| Mazaheri (58) | 2014 | 34, 23 | CH | Cued flanker | 8–12 | ERD/ERI, CP | P | Less ERD in ADHD-I; weak CP with frontal TH |
| Missonnier (65) | 2013 | 15, 15 | AD | N-back | 9–15 | ERD/ERI | F | Less ERD then higher ERI, esp LL vs HL |
| Yordanova (61) | 2013 | 14, 14 | CH | EC, aud sel attn | 8–12 | ERD/ERI, S | MC | Greater ERD in left MC to non-target; Resting: ND |
| Mazaheri (57) | 2010 | 14, 11 | CH | Cued vis attn | 8–12 | ERD/ERI, CP | P | Less ERD in ADHD; no FP CP |
| Gomarus (63) | 2009 | 15, 15, 15 PDD | CH | Vis sel mem | 8–12 | ERD/ERI | P | ND in ERD; ADHD is Hyp/Imp type |
| Heinrich (62) | 2014 | 24, 19 | CH | Flanker | 7.5–12.5 | S | P | Higher pow on no cue trials |
| ter Huurne (67) | 2017 | 17, 18 | AD | VS attn | 8–12 | Lat | MC | MEG. No typical lateralization |
| Vollebregt (60) | 2016 | 30, 30 | CH | VS attn | 8–12 | Lat | P | MEG. No typical lateralization |
| ter Huurne (66) | 2013 | 17, 18 | AD | VS atten | 9–12 | Lat | P | MEG. Initial lat not sustained |
Note. All studies are EEG results unless noted. ADHD=Attention-deficit hyperactivity disorder; ADHD-I=Inattentive; ADHD-C=Combined; Hyp/Imp=Hyperactive/ Impulsive; Con=control; Grp=group; Hz=hertz; CH=child; AD=adult; EC=eyes closed; EO=eyes open; CPT=continuous performance test; SWM=spatial working memory; Aud=auditory; Vis=visual; VS=visual-spatial; sel=selective; attn=attention; S=spectrum (power); MF=mean frequency i.e., frequency above and below which half the alpha band power lies); PD=power density; Coh=coherence (i.e., correspondence of alpha phase or magnitude between two channels or regions); PF=peak frequency (i.e., frequency between 8–12 Hz with the highest power); Lat=laterality (i.e., power difference between hemispheres); ERD=event related decrease; ERI=event related increase; CP=coupling; F=Frontal; C=Central; P=Posterior; MC=motor cortex; G=Global; Pow=power; ND=No difference; R=right; L=left; LL=low load; HL=high load, TH=theta; MEG=Magnetoencephalography
Task-related Modulation of Alpha Power
The observation of a coupling between the power of oscillations in electrophysiological signals and cognitive processing was first reported by Hans Berger (42). He noted 8–12 Hz oscillations (alpha) in patients, resting with eyes closed, that disappeared when the eyes were opened, a phenomenon later referred to as alpha “blocking”. In 1934, Adrian & Matthews (43) reported that while alpha generation is most strongly modulated by visual inputs (and was abolished by blindness), it is also linked with cognitive processing of visual inputs, or attention. For instance, they noted that alpha increased in the presence of light when the participant was not expecting to see a stimulus, and, conversely, it is attenuated when the eyes were closed but the subject was mentally searching for something. Based on these findings, alpha oscillations were thought to represent the brain in an “idling” state (44), a view that has now been replaced by the consensus that alpha oscillations functionally inhibit specific regions, which serves to route information by blocking task-irrelevant pathways (2, 45–47). This has been demonstrated in a variety of experiments of attention and working memory, and using a spectrum of methods including MEG, spike-field animal data, concurrent EEG-fMRI, and neuromodulation. For instance, anticipation of visual targets decreases visual cortex alpha activity, whereas anticipation of visual distractors increases it (48–50). Similarly, alpha-band activity increases with attention and working memory load to selectively suppress external inputs and task-irrelevant information (51–55). In sum, the picture emerging is that alpha oscillations are associated with top-down executive control in attention and working memory tasks by selectively inhibiting (when alpha increases) or disinhibiting (when alpha decreases) specific brain regions (i.e., serving a gating function in the visual cortex; (46, 53, 56, 57). Given that children with ADHD have problems in these domains, it is natural to examine if they also have reduced abilities to modulate their alpha oscillations.
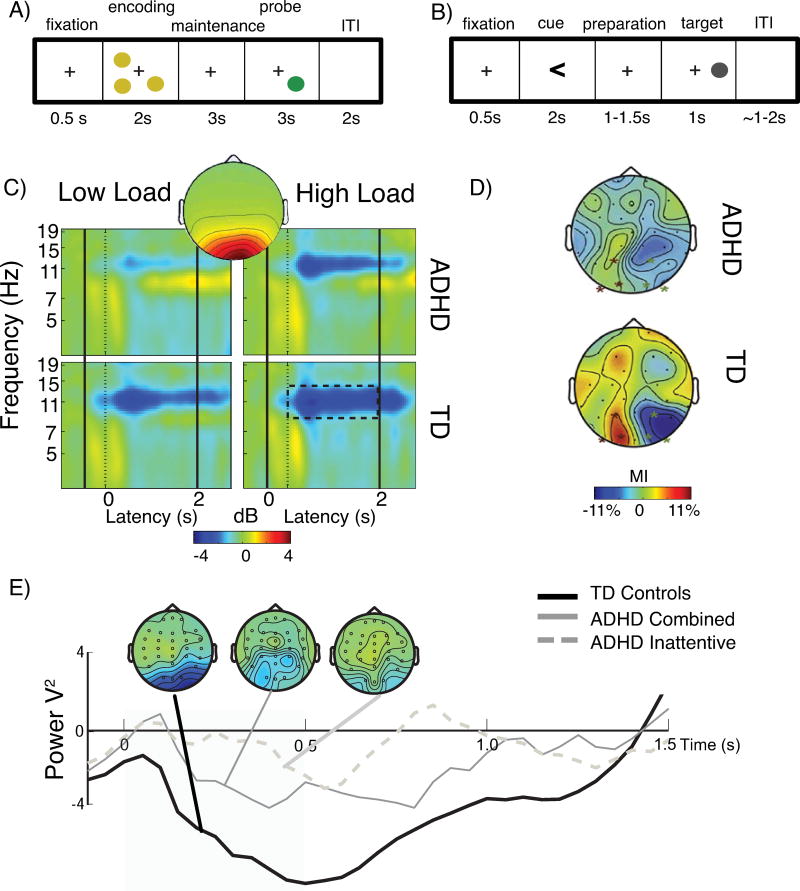
Across several types of attention and working memory type tasks (Fig. 2ab), differences between children with ADHD and controls have been observed in modulation of alpha band oscillatory power. For example, within a spatial working memory (SWM) delayed match-to-sample task (Fig. 2a), robust ADHD (54) diagnostic group effects were observed during the encoding phase of the task when compared to typically developing (TD) controls (Fig. 2c). During this encoding phase, control children showed an event-related decrease (ERD) in alpha band power, consistent with increased attention to and processing of the visual inputs. In children with ADHD, however, the alpha ERD during encoding was attenuated (Cohen’s d>0.79), which occurred primarily at low load rather than high load, was more prominent among younger children (7–10 years) versus older children (11–14 years) with ADHD, and was predictive of task performance. This finding is broadly consistent with reports by Mazaheri et al. (58, 59), who, using cross-modal attention and flanker tasks, also found attenuated alpha ERD in ADHD (Fig 2e). The alpha ERD finding was significant after an informative (‘response preparation’) cue but not after a null cue (suggesting a tight coupling to attentional processes) and was associated with reaction time benefit among TD children but not those with ADHD (59). In visuospatial attention paradigms (60) (Fig. 2b), alpha ERD arises as a lateralization effect, where alpha power decreases over the hemisphere contralateral to the attended visual hemifield relative to alpha power increases over the ipsilateral hemisphere. Using this paradigm, Vollebregt et al (61) observed that boys with ADHD were unable to modulate lateralized alpha in posterior regions when compared to typically developing peers (Fig. 2d), however, alpha lateralization was not associated to performance in either group. In a study of lateralized activity in the motor cortex, Yordanova et al (62) reported exaggerated suppression of alpha activity over sensorimotor cortex (i.e., mu wave) in response to non-attended (distractor) stimuli, potentially an indicator of enhanced processing of distractors and deficient inhibition of motor cortical networks. Attenuated lateralization of alpha may be indicative of inappropriate allocation of attention between attended and ignored streams of inputs. Finally, Heinrich (63) reported higher alpha power (which likely represents attenuated alpha ERD) during attention network task segments without stimulus processing or overt behavior among children with ADHD compared to controls, consistent with poor attentional allocation during the task.
Figure 2. Alpha ERD is attenuated in ADHD during visual attention.
In the spatial working memory task (A) participants encode the spatial location of 1 or 3 (low load) or 5 or 7 (high load) dots. Following a maintenance interval, they indicate if the probe dot occurs in the same or different location than any of the stimuli in the encoding stimulus. Attenuation of alpha event-related decrease (ERD) in ADHD was apparent during the 2-sec encoding period (C) (relative to pre-stimulus baseline). This effect was most pronounced at low load among children with ADHD (top left). Alpha ERD plots are calculated from the time-courses of a single occipitally-distributed (inset) independent component. Figure reproduced with permission from (54). (E) A similar result was reported by Mazaheri et al (59), in a cued spatial attention task. Attenuation of alpha ERD at electrode Oz in response to cues (cue duration is 1 s) was more pronounced in ADHD Inattentive Type than ADHD Combined Type (left panel), relative to TD controls. Figure reproduced with permission from (59). In the prototypical cued spatial attention task (B), a cue indicates the most likely location of the upcoming target stimulus (e.g., left). Following a preparation interval, the target appears either on right or left, requiring participants to indicate on which side the target appeared. In this paradigm, alpha ERD is lateralized, greater in the hemisphere contralateral to the hemifield indicated by the cue (attended, e.g., right) than in the hemisphere ipsilateral to the hemifield indicated by the cue (ignored, e.g., left). The normalized difference can be quantified as a modulation index (MI), the difference in alpha power for left minus right attention cues. The expected topography of the MI during the preparation interval is evident in panel (D) for typically developing (TD) boys, a relative decrease in alpha power for contralateral cues (attended) and increase for ipsilateral cues (ignored). This effect was significantly attenuated in boys with ADHD. Figure reproduced with permission from (61). In both (A) and (B), ITI is intertrial interval.
We note that alpha ERD group differences seem to be associated primarily with ADHD inattentive symptoms. Lenartowicz et al (54) reported a correlation between alpha ERD and inattentive symptoms (p=0.008), but less so for hyperactive symptoms (p=0.08). In the Mazaheri et al (59) study, alpha ERD was attenuated among adolescents with Inattentive Type but not with Combined Type ADHD (see Fig. 2e). Similarly, alpha ERD deficits were not observed by Gomarus et al (64) during a visual selective memory task where the ADHD sample was characterized primarily by hyperactive-impulsive behaviors. Overall, alpha ERD is attenuated primarily in ADHD inattentive type, consistent with ineffective selective attention to visual inputs, and is often associated with poorer task performance (accuracy, reaction time or reaction time variability).
Aberrant alpha modulation has also been consistently observed in studies examining adults with ADHD during attentional tasks. While performing a flanker task, posterior alpha ERD was significantly attenuated among adults with ADHD during visuospatial orienting (65). During stimulus processing in a N-back working memory task, adults with ADHD exhibited reduced alpha ERD in frontal channels relative to controls. Attenuated alpha ERD was particularly pronounced during the low versus high load condition (66), an interaction that was also present in the SWM study with children (c.f., Fig. 2c) (54). This suggests that aberrant alpha modulation may interact with sluggish recruitment of attention or maintenance of vigilance, which is more difficult in easier task conditions. Finally, MEG studies have found that adults with ADHD also have difficulty sustaining posterior hemispheric alpha lateralization during visuospatial attention (c.f., Fig 2b) in the period between the cue and target, particularly when attending to the left visual hemifield (67). A follow up study revealed a similar deficit in alpha power observed over sensorimotor cortex (i.e., mu wave) (68). Coupled with behavioral performance results, the authors suggested that adults with ADHD have an attention bias to the right visual field, which has been linked not only to ADHD severity but also other ADHD risk factors such as gender, handedness, and genetic factors (69). Collectively, adult ADHD studies are consistent with effects observed in children and support the notion of continued deficits in the ability to modulate alpha power across development, with a potential rightward bias in alpha power.
Neural mechanisms underlying alpha oscillatory activity
A mechanistic understanding of alpha oscillations has clear implications for the neural circuitry underlying deficient attention control in ADHD. Seminal in vivo (70, 71) and in vitro (72–74) experiments of thalamic alpha, and studies of occipital alpha (75–77) in the dog have identified a circuit between excitatory thalamocortical cells and inhibitory reticular neurons that generates alpha oscillations in thalamocortical neurons via a feedback loop between excitation and inhibition (78, 79). These studies were initially interpreted as supporting the hypothesis that alpha oscillations were indicative of the brain in an idling state (80). This is because the thalamic generator of alpha is dependent on decreasing arousal (79, 81, 82), whereby ascending cholinergic projections “deinactivate” (i.e., inactivation gate reopens and activation gate closes) low-threshold-Ca2+ channels, which reduces the reactivity of cortex to inputs (83–85). And while alpha oscillations are typically strongest over occipital cortex, they are also detectable in sensorimotor (the “mu” wave) and temporal cortices (the “tau” wave) (86–89), supporting a general mechanism by which sensory processing is gated by the thalamus. Hence, core thalamo-cortical interactions may play an important role in the aberrant alpha patterns observed in ADHD at rest. It is noteworthy that the dependence of thalamic generators of alpha on decreasing arousal is reminiscent of and consistent with energetic (low-arousal) models of ADHD etiology (90). However, given a lack of consistency in group differences in alpha during rest, further research is warranted to establish if links exist between alpha-generating thalamo-cortical interactions, alpha at rest and ADHD diagnosis.
In addition to the thalamo-cortical mechanisms, the modulation of alpha during task is thought to represent fronto-parietal interactions biasing activity in occipital cortex in line with attentional goals. This idea is supported by: (a) recordings in (primarily) primate occipital cortex of alpha generators in deep layers (which receive inputs from cortical regions other than thalamus) (91–93); (b) intracranial and MEG recordings, and Granger causality modeling showing that alpha (and beta) range oscillations carry feedback information from higher-order association areas (in contrast to >30Hz gamma oscillations, most prominent in superficial layers and carrying feedforward information) (94–99); and (c) disruption of frontal/parietal activities by transcranial magnetic stimulation that compromises performance and alpha modulation during visual attention (83, 100–102). Attenuated alpha ERD in ADHD is therefore a likely indicator of weakened attention control and, given prior association of fronto-parietal circuitry with alpha power (103–106), it predicts weakened interactions between the fronto-parietal network and occipital cortex during tasks. Consistent with this prediction, alpha ERD impairments do not appear to indicate an impairment with basic sensory processing as alpha ERD is independent of perceptual processing (53); it can occur before (101, 107, 108) or after (109) the stimulus, and can be absent during a stimulus when no post-perceptual processing is required (110).
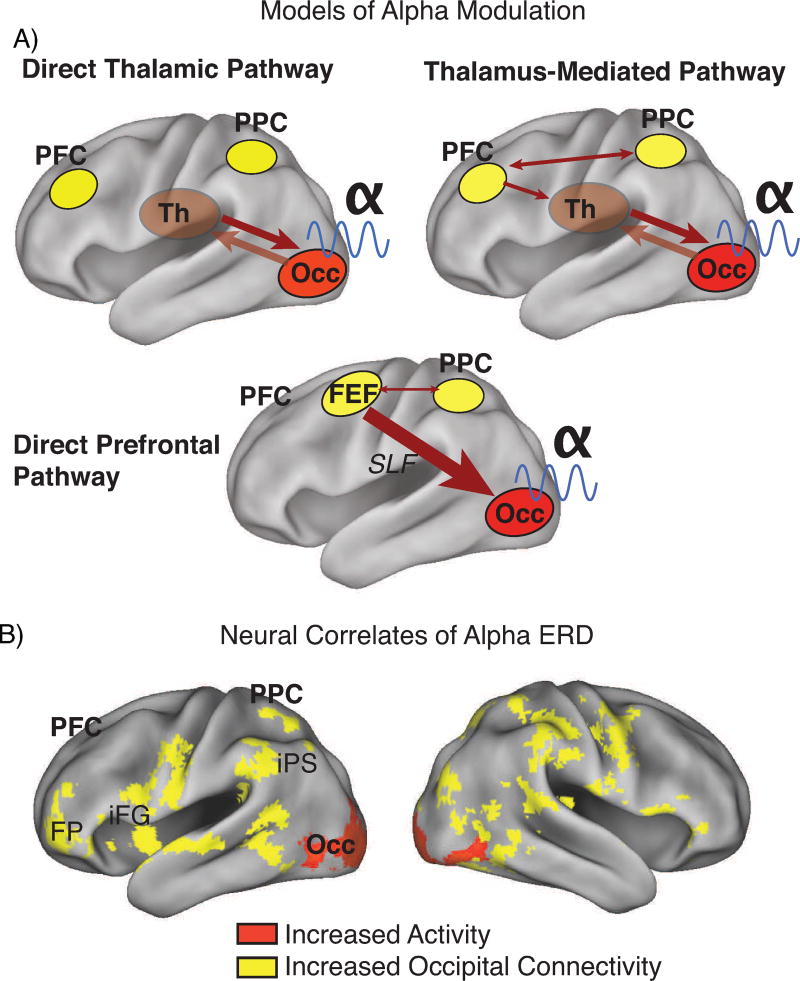
It is an outstanding question whether thalamus (111) or fronto-parietal interactions via either the thalamus and/or the superior longitudinal fasciculus (112) (Fig. 3a), are critical in generating the aberrant alpha patterns in ADHD. A thalamic impairment can certainly account for ineffective fronto-parietal activities (e.g., contributing to poor alpha ERD) because the thalamus (in particular the pulvinar nuclei) displays attentional modulation signals and has been shown to drive alpha synchrony in primate occipital cortex during attentional selection (113, 114). It may thus be a mediating structure for fronto-parietal top-down control. In turn, the relationship between thalamic generators of alpha and ascending cholinergic projections (79, 81, 82) implies that faulty arousal regulation could impact both thalamic and fronto-parietal activities. It is noteworthy that these alternatives are analogous with (i.e., capture the same circuits as) existing multi-pathway models of ADHD (e.g., 115). Further research into the mechanisms of alpha generation versus modulation will be imperative in distinguishing the critical pathways behind both alpha (and related behavioral) deficits in ADHD, and thereby informing existing models. Increasingly promising are multimodal approaches such as concurrent EEG-fMRI, which has been fruitful in non-invasively confirming the associations between alpha power and thalamic, occipital and fronto-parietal activities (103–106, 116–122). Extensions of such approaches to map the functional connectivity of alpha in ADHD (123, 124) may prove particularly revealing. Indeed, a recent study used concurrent EEG-fMRI recordings during SWM (Fig. 2a) in a small sample of adolescent boys with and without ADHD (N=30, 15 ADHD; 121). Overall, alpha ERD during SWM encoding was associated with occipital activation and fronto-parieto-occipital functional connectivity (Fig. 3b), with the latter predicting ADHD symptoms and response variability. The degree to which these two substrates were recruited differed by diagnosis, with greater occipital activation in controls and greater fronto-parieto-occipital connectivity in ADHD. The finding is consistent with the pattern of results in the larger EEG-only sample (54), namely that ADHD participants had to work harder (through recruitment of executive function fronto-parietal mechanisms) to compensate for a poor visual attention response.
Figure 3. Candidate neural mechanisms of alpha modulation include thalamo-occipital and fronto-parietal interactions.
(A) Modulation of alpha in occipital cortex is likely the result of one of three pathways: bidirectional interactions between occipital cortex and thalamus (Direct Thalamic Pathways), or fronto-parietal interactions exerting top-down influence over occipital activities either via thalamus (Thalamus-Mediated Pathway) or directly (Direct Prefrontal Pathway) via the superior longitudinal fasiculus. (B) Results from a small (n=21) concurrent EEG-fMRI study (121) indicates that alpha ERD in the encoding phase of a spatial working memory trial (c.f., Fig. 2ac) is correlated with both increases in occipital cortex activation and strengthening of functional connectivity between occipital cortex and fronto-parietal regions that include frontal pole, inferior frontal gyrus, post-central sulcus, and, in posterior cortex, intraparietal sulcus and lateral/superior occipital regions. The connectivity also included thalamus (not shown). The data thus support the thalamus-mediated and direct frontal models. Overlays in this image are regression parameters, with threshold at z>2.0, p<0.05 (whole-brain corrected for multiple comparisons using Gaussian random field theory) and mapped to the PALS atlas of human cortex, PFC=prefrontal cortex, PPC=posterior parietal cortex, Th=thalamus, Occ=occipital cortex, iPS=intraparietal sulcus, iFG=inferior frontal gyrus, FP=frontal pole, FEF=frontal eye fields, SLF=superior longitudinal fasiculus.
Oscillations as biomarkers of ADHD
Can measures of alpha oscillations serve as a biomarker of ADHD? Given the large effect sizes of group differences in alpha modulation, and clearly defined mechanistic targets, it seems the answer ought to be yes. However, large effect sizes are not sufficient to define a biomarker, which additionally needs to show reliability as well as both sensitivity (ability to detect the disorder) and specificity (ability to discriminate between disorders). Less commonly reported alpha measures such as lateralization, coherence, and mean/peak frequency have not been well studied with respect to reliability, however, several previous studies indicate high within-subject reliability of alpha ERD. Neuper et al (125) (n=29, 18–45 years) reported a Cronbach’s alpha>0.85 and r(27)>0.7 test-retest reliability of alpha ERD (up to 107 days apart) during numerical processing (125). Similar results were reported for resting state alpha power by Tenke et al (126), in 39 adults (18–65 years), test-retest reliability of 0.84 recorded 5–16 days apart, and by McEvoy et al (127) (n=20, 18–29 years), test-retest correlation >0.8 in psychomotor vigilance task and >0.9 in a Sternberg working memory task, recorded 7 days apart. Impressively, Näpflin, Wildi and Sarnthein evaluated both resting state alpha (128) and alpha ERD in a modified Sternberg working-memory task (129) in test-retest sessions 12–40 months apart (n=55, 19–79 years). They were able to predict if the oscillatory metrics came from within the same subject or from different subjects with a sensitivity over 87% and specificity over 99%. Thus, we cautiously conclude that alpha ERD is a reliable signature within individual, an important property for a biomarker, though it is notable that all of these studies were performed in adults and may not generalize to children.
However, the sensitivity and specificity of alpha ERD are questionable, and we suggest that alpha ERD, like its theta-beta ratio predecessor, is not likely to provide a reliable biomarker of ADHD diagnosis. The reason for this conclusion lies in the clinical (130), mechanistic (131, 132), and etiologic (133) heterogeneity of the disorder, which likely degrades the reliability of putative biomarkers of ADHD. For example, the ADHD 200 competition, which challenged scientists to develop diagnostic group classifiers for ADHD based on over 700 MRI datasets, had accuracy rates ranging from 43% to 62% (mean 56%), with the highest prediction accuracy of 62.5% coming from a prediction model that did not include any imaging data at all (134).
Several EEG/ERP studies have had more success using multivariate EEG profiles, (~90%, e.g., 135, 136, 137) but the high accuracy results require further validation because of potential statistical model overfitting. This is because of either small sample size precluding ability to split the data into independent training and testing sets (N < 22 per group; 135, 136, 138, 139), or the common practice of selecting classification features from the same dataset that is subsequently used for the classification (i.e., artificially inflating diagnostic classification accuracy) (140). For instance, in two large-sample EEG studies, Mueller et al (141) reported diagnostic classification accuracy of 92% (n=150), and Tenev et al (142) reported an accuracy of 82% (n=112), but in both cases the features used for the classification were those that were most discriminant in the sample, thus creating circularity in the analysis (critique also applies to the findings of Hammer et al (143) who cited 92.5% classification based on fMRI data). Notably, in an independent validation sample of 17 adults, Mueller et al (137) reported an impressive accuracy of 94%, yet because the validation sample was comprised of only individuals with ADHD, it is impossible to assess whether the classifier was inaccurately labeling all new data as ADHD (i.e., specificity). Moreover, across the studies, there is a lack of consistency in the features that are most effective in diagnostic classification (i.e., in EEG studies: TBR, absolute or relative power within various frequency bands, fractal measures, and event-related potential components (22)). We may therefore conclude that past classification efforts, including those using theta-beta ratio, have not yielded reliable diagnostic classification results, a finding that is not surprising if we consider the distribution overlap in EEG features across groups (e.g., Fig. 1b for alpha ERD).
It may be a more useful exercise to consider the prognostic utility of alpha oscillatory effects as a biomarker of a cognitive process (and associated neural circuits), developmental outcomes, or treatment response rather than diagnosis. As noted previously, attenuated alpha ERD was associated with inattentive symptoms (54) and subtype of ADHD (58, 144) and much less so with the ADHD combined subtype (64, 144). Moreover, stronger alpha ERD is predictive of better task performance both in studies of ADHD (54, 144) and otherwise, with alpha power predicting success of visual discrimination (108), errors on no-go trials (145) and successful inhibition of distractor items during working memory (146). Alpha oscillations may therefore be considered a putative predictor of visual attention processes and related behavioral outcomes. In the context of ADHD, this may translate into prediction of inattentive symptoms and how they may change with development or in response to treatment. The practical significance lies in the strong relationship between attention processes and real-life outcomes. We know that working memory deficits can have significant effects on academic achievement, educational attainment (repeating a grade, special education classes, learning disabilities) and IQ (147), which contribute significantly to occupational, academic, and social functioning in adulthood. Furthermore, the demonstrated population-level heterogeneity in alpha band activity (40, 41) may be framed as a potential advantage of EEG based-measures, if it reveals neurophysiologically distinct clusters. If so, alpha suppression may potentially be used not only as a measure of treatment response but also a predictor of which treatment may be effective for a given individual.
Conclusions, challenges and future directions
EEG and MEG oscillatory activity have long been used to quantify neural mechanisms and network interactions underlying cognitive processes such as attention. Alpha ERD appears to be a robust, yet relatively unexplored (in ADHD) putative biomarker of attentional impairment that subsequently impacts performance on WM and other executive function tasks. Despite its potential utility, there remain a number of challenges in the interpretation of alpha that need to be addressed. First, the group differences in alpha ERD that we have described require replication in larger samples, under identical task conditions. For instance, while attenuation of alpha suppression during SWM encoding and attenuation of alpha lateralization in ADHD are hypothesized to stem from similar mechanisms, a study comparing the paradigms (and alpha measures) within the same population would be instructive. Similarly, while most group differences have been reported over occipital electrodes/cortex (Table 1), some group effects have also been reported over frontal electrodes and/or over sensorimotor cortex. It is not currently known if these alpha measures in various regions represent different or overlapping mechanisms. Moreover, effects of pre-stimulus alpha on group differences in alpha modulation have not been systematically considered and likely introduce another source of variability (e.g., pre-stimulus alpha differences were present in (63) but not (54). Finally, it is not clear if alpha suppression deficit reflects a fundamental dysfunction in associated circuitry or if this is a downstream effect (e.g., a problem with arousal).
In addition, more work is needed to address clinical correlates associated with alpha ERD. In terms of inattention symptoms, it would be important to understand whether alpha ERD indexes specific types of inattention, such as distractibility, a lack of vigilance, or daydreaming. More specificity with respect to which inattention symptoms are represented by alpha ERD may support its use as a biomarker of treatment response or developmental outcomes. Such specificity would also be instructive in interpreting deficits in alpha modulation in other disorders (e.g., alpha suppression impairment during working memory in patients with schizophrenia (148) and, in a visual attention task among those with autism (149). Finally, further research is critical to ascertain whether alpha ERD is indeed predictive of clinical features typically associated with working memory deficits. If so, the association could potentially reveal shared neural mechanisms underlying inattention and academic achievement, or, identify risk for highly co-morbid diagnoses such as learning disability among children with ADHD. Remaining challenges notwithstanding, the promising research findings described herein suggest that alpha ERD is a strong prospect for future studies aimed at examining underlying neural mechanisms and putative biomarkers of ADHD.
Acknowledgments
This work was supported by National Institutes of Health grants MH101282 and NS97484 (to SKL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2(4):229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 2.Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13(2):121–34. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- 4.Mangun GR, Hillyard SA. Allocation of visual attention to spatial locations: tradeoff functions for event-related brain potentials and detection performance. Percept Psychophys. 1990;47(6):532–50. doi: 10.3758/bf03203106. [DOI] [PubMed] [Google Scholar]
- 5.Luck SJ, Heinze HJ, Mangun GR, Hillyard SA. Visual event-related potentials index focused attention within bilateral stimulus arrays. II. Functional dissociation of P1 and N1 components. Electroencephalogr Clin Neurophysiol. 1990;75(6):528–42. doi: 10.1016/0013-4694(90)90139-b. [DOI] [PubMed] [Google Scholar]
- 6.Hopf JM, Luck SJ, Boelmans K, Schoenfeld MA, et al. The neural site of attention matches the spatial scale of perception. J Neurosci. 2006;26(13):3532–40. doi: 10.1523/JNEUROSCI.4510-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hopf JM, Boelmans K, Schoenfeld MA, Luck SJ, Heinze HJ. Attention to features precedes attention to locations in visual search: evidence from electromagnetic brain responses in humans. J Neurosci. 2004;24(8):1822–32. doi: 10.1523/JNEUROSCI.3564-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Association AP. Diagnostic and statistical manual of mental disorders (5th ed.) Washington, DC: Author; 2013. [Google Scholar]
- 9.Sibley MH, Swanson JM, Arnold LE, Hechtman LT, et al. Defining ADHD symptom persistence in adulthood: optimizing sensitivity and specificity. J Child Psychol Psychiatry. 2017;58(6):655–662. doi: 10.1111/jcpp.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doshi JA, Hodgkins P, Kahle J, Sikirica V, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry. 2012;51(10):990–1002 e2. doi: 10.1016/j.jaac.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Jasper H, Solomon P, Bradley C. Electroencephalographic analyses of behavior problem children. Am J Psychiatry. 1938;95(3):641. [Google Scholar]
- 12.Satterfield JH, Cantwell DP, Satterfield BT. Pathophysiology of the hyperactive child syndrome. Archives of General Psychiatry. 1974;31:839–844. doi: 10.1001/archpsyc.1974.01760180079010. [DOI] [PubMed] [Google Scholar]
- 13.Snyder SM, Hall JR. A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. J Clin Neurophysiol. 2006;23(5):440–55. doi: 10.1097/01.wnp.0000221363.12503.78. [DOI] [PubMed] [Google Scholar]
- 14.Loo SK, Makeig S. Clinical utility of EEG in attention-deficit/hyperactivity disorder: a research update. Neurotherapeutics. 2012;9(3):569–87. doi: 10.1007/s13311-012-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monastra VJ, Lubar JF, Linden M. The development of a quantitative electroencephalographic scanning process for attention deficit-hyperactivity disorder: reliability and validity studies. Neuropsychology. 2001;15(1):136–44. doi: 10.1037//0894-4105.15.1.136. [DOI] [PubMed] [Google Scholar]
- 16.Snyder SM, Quintana H, Sexson SB, Knott P, et al. Blinded, multi-center validation of EEG and rating scales in identifying ADHD within a clinical sample. Psychiatry Res. 2008;159(3):346–58. doi: 10.1016/j.psychres.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Buyck I, Wiersema JR. Resting electroencephalogram in attention deficit hyperactivity disorder: Developmental course and diagnostic value. Psychiatry Res. 2014;216(3):391–7. doi: 10.1016/j.psychres.2013.12.055. [DOI] [PubMed] [Google Scholar]
- 18.Liechti MD, Valko L, Muller UC, Dohnert M, et al. Diagnostic value of resting electroencephalogram in attention-deficit/hyperactivity disorder across the lifespan. Brain Topogr. 2013;26(1):135–51. doi: 10.1007/s10548-012-0258-6. [DOI] [PubMed] [Google Scholar]
- 19.Loo SK, Cho A, Hale TS, McGough J, et al. Characterization of the theta to beta ratio in ADHD: identifying potential sources of heterogeneity. J Atten Disord. 2013;17(5):384–92. doi: 10.1177/1087054712468050. [DOI] [PubMed] [Google Scholar]
- 20.Ogrim G, Kropotov J, Hestad K. The quantitative EEG theta/beta ratio in attention deficit/hyperactivity disorder and normal controls: Sensitivity, specificity, and behavioral correlates. Psychiatry Research. 2012;198(3):482–488. doi: 10.1016/j.psychres.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 21.Poil SS, Bollmann S, Ghisleni C, O'Gorman RL, et al. Age dependent electroencephalographic changes in attention-deficit/hyperactivity disorder (ADHD) Clin Neurophysiol. 2014;125(8):1626–38. doi: 10.1016/j.clinph.2013.12.118. [DOI] [PubMed] [Google Scholar]
- 22.Lenartowicz A, Loo SK. Use of EEG to diagnose ADHD. Curr Psychiatry Rep. 2014;16(11):498. doi: 10.1007/s11920-014-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arns M, Conners CK, Kraemer HC. A decade of EEG Theta/Beta Ratio Research in ADHD: a meta-analysis. J Atten Disord. 2013;17(5):374–83. doi: 10.1177/1087054712460087. [DOI] [PubMed] [Google Scholar]
- 24.Bresnahan SM, Barry RJ. Specificity of quantitative EEG analysis in adults with attention deficit hyperactivity disorder. Psychiatry Res. 2002;112(2):133–44. doi: 10.1016/s0165-1781(02)00190-7. [DOI] [PubMed] [Google Scholar]
- 25.Chabot RJ, Serfontein G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biol Psychiatry. 1996;40(10):951–63. doi: 10.1016/0006-3223(95)00576-5. [DOI] [PubMed] [Google Scholar]
- 26.Koehler S, Lauer P, Schreppel T, Jacob C, et al. Increased EEG power density in alpha and theta bands in adult ADHD patients. J Neural Transm (Vienna) 2009;116(1):97–104. doi: 10.1007/s00702-008-0157-x. [DOI] [PubMed] [Google Scholar]
- 27.Bresnahan SM, Anderson JW, Barry RJ. Age-related changes in quantitative EEG in attention-deficit/hyperactivity disorder. Biol Psychiatry. 1999;46(12):1690–7. doi: 10.1016/s0006-3223(99)00042-6. [DOI] [PubMed] [Google Scholar]
- 28.Bresnahan SM, Barry RJ, Clarke AR, Johnstone SJ. Quantitative EEG analysis in dexamphetamine-responsive adults with attention-deficit/hyperactivity disorder. Psychiatry Res. 2006;141(2):151–9. doi: 10.1016/j.psychres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Hermens DF, Williams LM, Lazzaro I, Whitmont S, et al. Sex differences in adult ADHD: a double dissociation in brain activity and autonomic arousal. Biol Psychol. 2004;66(3):221–33. doi: 10.1016/j.biopsycho.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Loo SK, Hale TS, Hanada G, Macion J, et al. Familial clustering and DRD4 effects on electroencephalogram measures in multiplex families with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;29(4):368–77. [PMC free article] [PubMed] [Google Scholar]
- 31.van Dongen-Boomsma M, Lansbergen MM, Bekker EM, Kooij JJ, et al. Relation between resting EEG to cognitive performance and clinical symptoms in adults with attention-deficit/hyperactivity disorder. Neurosci Lett. 2010;469(1):102–6. doi: 10.1016/j.neulet.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 32.Barry RJ, Clarke AR, Johnstone SJ, Brown CR. EEG differences in children between eyes-closed and eyes-open resting conditions. Clin Neurophysiol. 2009;120(10):1806–11. doi: 10.1016/j.clinph.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: differences in two subtypes of attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2001;112(5):815–26. doi: 10.1016/s1388-2457(01)00487-4. [DOI] [PubMed] [Google Scholar]
- 34.El-Sayed E, Larsson JO, Persson HE, Rydelius PA. Altered cortical activity in children with attention-deficit/hyperactivity disorder during attentional load task. J Am Acad Child Adolesc Psychiatry. 2002;41(7):811–9. doi: 10.1097/00004583-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Loo SK, Hale TS, Macion J, Hanada G, et al. Cortical activation patterns in ADHD during arousal, activation, and sustained attention. Neuropsychologia. 2009;47(10):2114–2119. doi: 10.1016/j.neuropsychologia.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magee CA, Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Examining the diagnostic utility of EEG power measures in children with attention deficit/hyperactivity disorder. Clin Neurophysiol. 2005;116(5):1033–40. doi: 10.1016/j.clinph.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Nazari MA, Wallois F, Aarabi A, Berquin P. Dynamic changes in quantitative electroencephalogram during continuous performance test in children with attention-deficit/hyperactivity disorder. Int J Psychophysiol. 2011;81(3):230–6. doi: 10.1016/j.ijpsycho.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Woltering S, Jung J, Liu Z, Tannock R. Resting state EEG oscillatory power differences in ADHD college students and their peers. Behav Brain Funct. 2012;8:60. doi: 10.1186/1744-9081-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pomerov V, Mueller A, Candrian G, Grin-Yatsenko V, Kropotov J. Group independent component analysis (gICA) and current source density (CSD) in the study of EEG in ADHD adults. Clinical Neurophysiology. 2014;125:83–97. doi: 10.1016/j.clinph.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Clarke AR, Barry RJ, Dupuy FE, Heckel LD, et al. Behavioural differences between EEG-defined subgroups of children with Attention-Deficit/Hyperactivity Disorder. Clinical Neurophysiology. 2011;122(7):1333–1341. doi: 10.1016/j.clinph.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 41.Loo SK, McGough J, McCracken J, Smalley SL. Parsing heterogeneity in attention-deficit hyperactivity disorder using EEG-based subgroups. Journal of Child Psychology and Psychiatry. doi: 10.1111/jcpp.12814. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berger H. Über das Elektroenzephalogramm des Menschen. Archiv für Psychiatrie und Nervenkrankheiten. 1929;87:527–570. [Google Scholar]
- 43.Adrian ED, Matthews BH. The interpretation of potential waves in the cortex. J Physiol. 1934;81(4):440–71. doi: 10.1113/jphysiol.1934.sp003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfurtscheller G, Stancak A, Jr, Neuper C. Event-related synchronization (ERS) in the alpha band--an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24(1–2):39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- 45.Thut G, Miniussi C. New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn Sci. 2009;13(4):182–9. doi: 10.1016/j.tics.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Foxe JJ, Snyder AC. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front Psychol. 2011;2:154. doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Research Reviews. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Payne L, Guillory S, Sekuler R. Attention-modulated alpha-band oscillations protect against intrusion of irrelevant information. J Cogn Neurosci. 2013;25(9):1463–76. doi: 10.1162/jocn_a_00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Diepen RM, Miller LM, Mazaheri A, Geng JJ. The Role of Alpha Activity in Spatial and Feature-Based Attention. eNeuro. 2016;3(5) doi: 10.1523/ENEURO.0204-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Diepen RM, Mazaheri A. Cross-sensory modulation of alpha oscillatory activity: suppression, idling, and default resource allocation. Eur J Neurosci. 2017;45(11):1431–1438. doi: 10.1111/ejn.13570. [DOI] [PubMed] [Google Scholar]
- 51.Handel BF, Haarmeier T, Jensen O. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J Cogn Neurosci. 2011;23(9):2494–502. doi: 10.1162/jocn.2010.21557. [DOI] [PubMed] [Google Scholar]
- 52.Klimesch W. EEG-alpha rhythms and memory processes. International Journal of Psychophysiology. 1997;26(1–3):319–340. doi: 10.1016/s0167-8760(97)00773-3. [DOI] [PubMed] [Google Scholar]
- 53.Klimesch W, Fellinger R, Freunberger R. Alpha oscillations and early stages of visual encoding. Front Psychol. 2011;2:118. doi: 10.3389/fpsyg.2011.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lenartowicz A, Delorme A, Walshaw PD, Cho AL, et al. Electroencephalography correlates of spatial working memory deficits in attention-deficit/hyperactivity disorder: vigilance, encoding, and maintenance. J Neurosci. 2014;34(4):1171–82. doi: 10.1523/JNEUROSCI.1765-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12(8):877–82. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- 56.Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012;16(12):606–17. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathewson KE, Lleras A, Beck DM, Fabiani M, et al. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front Psychol. 2011;2:99. doi: 10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazaheri A, Coffey-Corina S, Mangun GR, Bekker EM, et al. Functional Disconnection of Frontal Cortex and Visual Cortex in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2010;67(7):617–623. doi: 10.1016/j.biopsych.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 59.Mazaheri A, Fassbender C, Coffey-Corina S, Hartanto TA, et al. Differential Oscillatory Electroencephalogram Between Attention-Deficit/Hyperactivity Disorder Subtypes and Typically Developing Adolescents. Biol Psychiatry. 2014;76(422–429) doi: 10.1016/j.biopsych.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Posner MI. Orienting of Attention. Quarterly Journal of Experimental Psychology. 1980 Feb;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 61.Vollebregt MA, Zumer JM, Ter Huurne N, Buitelaar JK, Jensen O. Posterior alpha oscillations reflect attentional problems in boys with Attention Deficit Hyperactivity Disorder. Clin Neurophysiol. 2016;127(5):2182–91. doi: 10.1016/j.clinph.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 62.Yordanova J, Kolev V, Rothenberger A. Event-related oscillations reflect functional asymmetry in children with attention deficit/hyperactivity disorder. Suppl Clin Neurophysiol. 2013;62:289–301. doi: 10.1016/b978-0-7020-5307-8.00018-1. [DOI] [PubMed] [Google Scholar]
- 63.Heinrich H, Busch K, Studer P, Erbe K, et al. EEG spectral analysis of attention in ADHD: implications for neurofeedback training? Front Hum Neurosci. 2014;8:611. doi: 10.3389/fnhum.2014.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomarus HK, Wijers AA, Minderaa RB, Althaus M. Do children with ADHD and/or PDD-NOS differ in reactivity of alpha/theta ERD/ERS to manipulations of cognitive load and stimulus relevance? Clin Neurophysiol. 2009;120(1):73–9. doi: 10.1016/j.clinph.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 65.Hasler R, Perroud N, Meziane HB, Herrmann F, et al. Attention-related EEG markers in adult ADHD. Neuropsychologia. 2016;87:120–33. doi: 10.1016/j.neuropsychologia.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 66.Missonnier P, Hasler R, Perroud N, Herrmann FR, et al. EEG anomalies in adult ADHD subjects performing a working memory task. Neuroscience. 2013;241:135–46. doi: 10.1016/j.neuroscience.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 67.ter Huurne N, Onnink M, Kan C, Franke B, et al. Behavioral consequences of aberrant alpha lateralization in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74(3):227–33. doi: 10.1016/j.biopsych.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 68.Ter Huurne N, Lozano-Soldevilla D, Onnink M, Kan C, et al. Diminished modulation of preparatory sensorimotor mu rhythm predicts attention-deficit/hyperactivity disorder severity. Psychol Med. 2017;47(11):1947–1956. doi: 10.1017/S0033291717000332. [DOI] [PubMed] [Google Scholar]
- 69.Hale TS, Smalley SL, Hanada G, Macion J, et al. Atypical alpha asymmetry in adults with ADHD. Neuropsychologia. 2009;47(10):2082–8. doi: 10.1016/j.neuropsychologia.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deschenes M, Paradis M, Roy JP, Steriade M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. J Neurophysiol. 1984;51(6):1196–219. doi: 10.1152/jn.1984.51.6.1196. [DOI] [PubMed] [Google Scholar]
- 71.Roy JP, Clercq M, Steriade M, Deschenes M. Electrophysiology of neurons of lateral thalamic nuclei in cat: mechanisms of long-lasting hyperpolarizations. J Neurophysiol. 1984;51(6):1220–35. doi: 10.1152/jn.1984.51.6.1220. [DOI] [PubMed] [Google Scholar]
- 72.Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297(5865):406–8. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- 73.Jahnsen H, Llinas R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984;349:227–47. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jahnsen H, Llinas R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984;349:205–26. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lopes da Silva FH, Vanliero Th, Schrijer CF, Vanleeuw Ws. Organization of Thalamic and Cortical Alpha Rhythms - Spectra and Coherences. Electroencephalography and Clinical Neurophysiology. 1973;35(6):627–639. doi: 10.1016/0013-4694(73)90216-2. [DOI] [PubMed] [Google Scholar]
- 76.Lopes Da Silva FH, Storm Van Leeuwen W. The cortical source of the alpha rhythm. Neurosci Lett. 1977;6(2–3):237–41. doi: 10.1016/0304-3940(77)90024-6. [DOI] [PubMed] [Google Scholar]
- 77.Lopes da Silva FH, Vos JE, Mooibroek J, Van Rotterdam A. Relative contributions of intracortical and thalamo-cortical processes in the generation of alpha rhythms, revealed by partial coherence analysis. Electroencephalogr Clin Neurophysiol. 1980;50(5–6):449–56. doi: 10.1016/0013-4694(80)90011-5. [DOI] [PubMed] [Google Scholar]
- 78.Steriade M, Deschenes M. The thalamus as a neuronal oscillator. Brain Res. 1984;320(1):1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- 79.Jones EG. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann N Y Acad Sci. 2009;1157:10–23. doi: 10.1111/j.1749-6632.2009.04534.x. [DOI] [PubMed] [Google Scholar]
- 80.Pfurtscheller G. Event-related synchronization (ERS): an electrophysiological correlate of cortical areas at rest. Electroencephalogr Clin Neurophysiol. 1992;83(1):62–9. doi: 10.1016/0013-4694(92)90133-3. [DOI] [PubMed] [Google Scholar]
- 81.Steriade M, Gloor P, Llinas RR, Lopes de Silva FH, Mesulam MM. Report of IFCN Committee on Basic Mechanisms. Basic mechanisms of cerebral rhythmic activities. Electroencephalogr Clin Neurophysiol. 1990;76(6):481–508. doi: 10.1016/0013-4694(90)90001-z. [DOI] [PubMed] [Google Scholar]
- 82.Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. Journal of Neurophysiology. 2006;95(6):3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 83.Romei V, Brodbeck V, Michel C, Amedi A, et al. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cerebral Cortex. 2008;18(9):2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chaumon M, Busch NA. Prestimulus neural oscillations inhibit visual perception via modulation of response gain. J Cogn Neurosci. 2014;26(11):2514–29. doi: 10.1162/jocn_a_00653. [DOI] [PubMed] [Google Scholar]
- 85.Lange J, Oostenveld R, Fries P. Reduced occipital alpha power indexes enhanced excitability rather than improved visual perception. J Neurosci. 2013;33(7):3212–20. doi: 10.1523/JNEUROSCI.3755-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pfurtscheller G, Zalaudek K, Neuper C. Event-related beta synchronization after wrist, finger and thumb movement. Electroencephalogr Clin Neurophysiol. 1998;109(2):154–60. doi: 10.1016/s0924-980x(97)00070-2. [DOI] [PubMed] [Google Scholar]
- 87.Pfurtscheller G, Neuper C. Event-related synchronization of mu rhythm in the EEG over the cortical hand area in man. Neurosci Lett. 1994;174(1):93–6. doi: 10.1016/0304-3940(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 88.Anderson KL, Ding M. Attentional modulation of the somatosensory mu rhythm. Neuroscience. 2011;180:165–80. doi: 10.1016/j.neuroscience.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 89.Pfurtscheller G. Induced oscillations in the alpha band: functional meaning. Epilepsia. 2003;44(Suppl 12):2–8. doi: 10.1111/j.0013-9580.2003.12001.x. [DOI] [PubMed] [Google Scholar]
- 90.Sergeant JA. A cognitive energetic model of hyperactivity. International Journal of Psychology. 1996;31(3–4):1722–1722. [Google Scholar]
- 91.Bollimunta A, Chen Y, Schroeder CE, Ding M. Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. J Neurosci. 2008;28(40):9976–88. doi: 10.1523/JNEUROSCI.2699-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bollimunta A, Mo J, Schroeder CE, Ding M. Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J Neurosci. 2011;31(13):4935–43. doi: 10.1523/JNEUROSCI.5580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scheeringa R, Koopmans PJ, van Mourik T, Jensen O, Norris DG. The relationship between oscillatory EEG activity and the laminar-specific BOLD signal. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(24):6761–6766. doi: 10.1073/pnas.1522577113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, et al. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron. 2015;85(2):390–401. doi: 10.1016/j.neuron.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 95.Michalareas G, Vezoli J, van Pelt S, Schoffelen JM, et al. Alpha-Beta and Gamma Rhythms Subserve Feedback and Feedforward Influences among Human Visual Cortical Areas. Neuron. 2016;89(2):384–97. doi: 10.1016/j.neuron.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fontolan L, Morillon B, Liegeois-Chauvel C, Giraud AL. The contribution of frequency-specific activity to hierarchical information processing in the human auditory cortex. Nature Communications. 2014;5 doi: 10.1038/ncomms5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fries P. Rhythms for Cognition: Communication through Coherence. Neuron. 2015;88(1):220–235. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Popov T, Kastner S, Jensen O. FEF-Controlled Alpha Delay Activity Precedes Stimulus-Induced Gamma-Band Activity in Visual Cortex. J Neurosci. 2017;37(15):4117–4127. doi: 10.1523/JNEUROSCI.3015-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis MA, et al. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc Natl Acad Sci U S A. 2014;111(40):14332–41. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci. 2009;29(18):5863–72. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Romei V, Gross J, Thut G. On the role of prestimulus alpha rhythms over occipitoparietal areas in visual input regulation: correlation or causation? J Neurosci. 2010;30(25):8692–7. doi: 10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marshall TR, O'Shea J, Jensen O, Bergmann TO. Frontal Eye Fields Control Attentional Modulation of Alpha and Gamma Oscillations in Contralateral Occipitoparietal Cortex. Journal of Neuroscience. 2015;35(4):1638–1647. doi: 10.1523/JNEUROSCI.3116-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Munck JC, Goncalves SI, Huijboom L, Kuijer JP, et al. The hemodynamic response of the alpha rhythm: an EEG/fMRI study. Neuroimage. 2007;35(3):1142–51. doi: 10.1016/j.neuroimage.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 104.Laufs H, Kleinschmidt A, Beyerle A, Eger E, et al. EEG-correlated fMRI of human alpha activity. NeuroImage. 2003;19(4):1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y, Bengson J, Huang H, Mangun GR, Ding M. Top-down Modulation of Neural Activity in Anticipatory Visual Attention: Control Mechanisms Revealed by Simultaneous EEG-fMRI. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scheeringa R, Petersson KM, Oostenveld R, Norris DG, et al. Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage. 2009;44(3):1224–38. doi: 10.1016/j.neuroimage.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 107.Hanslmayr S, Aslan A, Staudigl T, Klimesch W, et al. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37(4):1465–73. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 108.Ergenoglu T, Demiralp T, Bayraktaroglu Z, Ergen M, et al. Alpha rhythm of the EEG modulates visual detection performance in humans. Brain Res Cogn Brain Res. 2004;20(3):376–83. doi: 10.1016/j.cogbrainres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 109.Freunberger R, Klimesch W, Griesmayr B, Sauseng P, Gruber W. Alpha phase coupling reflects object recognition. Neuroimage. 2008;42(2):928–35. doi: 10.1016/j.neuroimage.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 110.Hanslmayr S, Klimesch W, Sauseng P, Gruber W, et al. Visual discrimination performance is related to decreased alpha amplitude but increased phase locking. Neurosci Lett. 2005;375(1):64–8. doi: 10.1016/j.neulet.2004.10.092. [DOI] [PubMed] [Google Scholar]
- 111.Saalmann YB, Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;71(2):209–23. doi: 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marshall TR, Bergmann TO, Jensen O. Frontoparietal Structural Connectivity Mediates the Top-Down Control of Neuronal Synchronization Associated with Selective Attention. PLoS Biol. 2015;13(10):e1002272. doi: 10.1371/journal.pbio.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The Pulvinar Regulates Information Transmission Between Cortical Areas Based on Attention Demands. Science. 2012;337(6095):753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suffczynski P, Kalitzin S, Pfurtscheller G, da Silva FHL. Computational model of thalamo-cortical networks: dynamical control of alpha rhythms in relation to focal attention. International Journal of Psychophysiology. 2001;43(1):25–40. doi: 10.1016/s0167-8760(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 115.Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51(4):356–67. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 116.Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, et al. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. J Neurosci. 2010;30(30):10243–50. doi: 10.1523/JNEUROSCI.1004-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luchinger R, Michels L, Martin E, Brandeis D. EEG-BOLD correlations during (post-)adolescent brain maturation. Neuroimage. 2011;56(3):1493–505. doi: 10.1016/j.neuroimage.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 118.Goncalves SI, de Munck JC, Pouwels PJ, Schoonhoven R, et al. Correlating the alpha rhythm to BOLD using simultaneous EEG/fMRI: inter-subject variability. Neuroimage. 2006;30(1):203–13. doi: 10.1016/j.neuroimage.2005.09.062. [DOI] [PubMed] [Google Scholar]
- 119.Goldman RI, Stern JM, Engel J, Jr, Cohen MS. Acquiring simultaneous EEG and functional MRI. Clin Neurophysiol. 2000;111(11):1974–80. doi: 10.1016/s1388-2457(00)00456-9. [DOI] [PubMed] [Google Scholar]
- 120.Goldman RI, Stern JM, Engel J, Jr, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13(18):2487–92. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lenartowicz A, Lu S, Rodriguez C, Lau E, et al. Alpha desynchronization and frontoparietal connectivity during spatial working memory encoding deficits in ADHD: A simultaneous EEG-fMRI study. Neuroimage: Clinical. 2016;11:210–23. doi: 10.1016/j.nicl.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zumer JM, Scheeringa R, Schoffelen JM, Norris DG, Jensen O. Occipital alpha activity during stimulus processing gates the information flow to object-selective cortex. PLoS Biol. 2014;12(10):e1001965. doi: 10.1371/journal.pbio.1001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lenartowicz A, Lu S, Rodriguez C, Lau EP, et al. Alpha desynchronization and frontoparietal connectivity during spatial working memory encoding deficits in ADHD: A simultaneous EEG-fMRI study. Neuroimage Clin. 2016;11:210–23. doi: 10.1016/j.nicl.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scheeringa R, Petersson KM, Kleinschmidt A, Jensen O, Bastiaansen MC. EEG alpha power modulation of fMRI resting-state connectivity. Brain Connect. 2012;2(5):254–64. doi: 10.1089/brain.2012.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Neuper C, Grabner RH, Fink A, Neubauer AC. Long-term stability and consistency of EEG event-related (de-)synchronization across different cognitive tasks. Clin Neurophysiol. 2005;116(7):1681–94. doi: 10.1016/j.clinph.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 126.Tenke CE, Kayser J, Pechtel P, Webb CA, et al. Demonstrating test-retest reliability of electrophysiological measures for healthy adults in a multisite study of biomarkers of antidepressant treatment response. Psychophysiology. 2017;54(1):34–50. doi: 10.1111/psyp.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McEvoy LK, Smith ME, Gevins A. Test-retest reliability of cognitive EEG. Clin Neurophysiol. 2000;111(3):457–63. doi: 10.1016/s1388-2457(99)00258-8. [DOI] [PubMed] [Google Scholar]
- 128.Napflin M, Wildi M, Sarnthein J. Test-retest reliability of resting EEG spectra validates a statistical signature of persons. Clin Neurophysiol. 2007;118(11):2519–24. doi: 10.1016/j.clinph.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 129.Napflin M, Wildi M, Sarnthein J. Test-retest reliability of EEG spectra during a working memory task. Neuroimage. 2008;43(4):687–93. doi: 10.1016/j.neuroimage.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 130.Willcutt EG, Nigg JT, Pennington BF, Solanto MV, et al. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. 2012;121(4):991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57(11):1424–35. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 132.Sonuga-Barke EJS, Castellanos FX. A common core dysfunction in attention-deficit/hyperactivity disorder: A scientific red herring? Behavioral and Brain Sciences. 2005;28(3):443-+. [Google Scholar]
- 133.Hawi Z, Cummins TD, Tong J, Johnson B, et al. The molecular genetic architecture of attention deficit hyperactivity disorder. Mol Psychiatry. 2015;20(3):289–97. doi: 10.1038/mp.2014.183. [DOI] [PubMed] [Google Scholar]
- 134.ADHD.Consortium. The ADHD-200 Consortium: A Model to Advance the Translational Potential of Neuroimaging in Clinical Neuroscience. Front Syst Neurosci. 2012;6:62. doi: 10.3389/fnsys.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Abibullaev B, An J. Decision Support Algorithm for Diagnosis of ADHD Using Electroencephalograms. Journal of Medical Systems. 2012;36(4):2675–2688. doi: 10.1007/s10916-011-9742-x. [DOI] [PubMed] [Google Scholar]
- 136.Nazhvani AD, Boostani R, Afrasiabi S, Sadatnezhad K. Classification of ADHD and BMD patients using visual evoked potential. Clin Neurol Neurosurg. 2013;115(11):2329–35. doi: 10.1016/j.clineuro.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 137.Mueller A, Candrian G, Grane VA, Kropotov JD, et al. Discriminating between ADHD adults and controls using independent ERP components and a support vector machine: a validation study. Nonlinear Biomed Phys. 2011;5:5. doi: 10.1186/1753-4631-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ahmadlou M, Adeli H. Wavelet-Synchronization Methodology: A New Approach for EEG-Based Diagnosis of ADHD. Clinical Eeg and Neuroscience. 2010;41(1):1–10. doi: 10.1177/155005941004100103. [DOI] [PubMed] [Google Scholar]
- 139.Sadatnezhad K, Boostani R, Ghanizadeh A. Classification of BMD and ADHD patients using their EEG signals. Expert Systems with Applications. 2011;38(3):1956–1963. [Google Scholar]
- 140.Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mueller A, Candrian G, Kropotov JD, Ponomarev VA, Baschera GM. Classification of ADHD patients on the basis of independent ERP components using a machine learning system. Nonlinear Biomed Phys. 2010;4(Suppl 1):S1. doi: 10.1186/1753-4631-4-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tenev A, Markovska-Simoska S, Kocarev L, Pop-Jordanov J, et al. Machine learning approach for classification of ADHD adults. Int J Psychophysiol. 2014;93(1):162–6. doi: 10.1016/j.ijpsycho.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 143.Hammer R, Cooke GE, Stein MA, Booth JR. Functional neuroimaging of visuospatial working memory tasks enables accurate detection of attention deficit and hyperactivity disorder. Neuroimage Clin. 2015;9:244–52. doi: 10.1016/j.nicl.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mazaheri A, van Schouwenburg MR, Dimitrijevic A, Denys D, et al. Region-specific modulations in oscillatory alpha activity serve to facilitate processing in the visual and auditory modalities. Neuroimage. 2014;87:356–62. doi: 10.1016/j.neuroimage.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 145.Mazaheri A, Nieuwenhuis ILC, van Dijk H, Jensen O. Prestimulus Alpha and Mu Activity Predicts Failure to Inhibit Motor Responses. Human Brain Mapping. 2009;30(6):1791–1800. doi: 10.1002/hbm.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hamidi M, Slagter HA, Tononi G, Postle BR. Repetitive Transcranial Magnetic Stimulation Affects behavior by Biasing Endogenous Cortical Oscillations. Front Integr Neurosci. 2009;3:14. doi: 10.3389/neuro.07.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, et al. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J Consult Clin Psychol. 2004;72(5):757–66. doi: 10.1037/0022-006X.72.5.757. [DOI] [PubMed] [Google Scholar]
- 148.Erickson MA, Albrecht MA, Robinson B, Luck SJ, Gold JM. Impaired suppression of delay-period alpha and beta is associated with impaired working memory in schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(3):272–279. doi: 10.1016/j.bpsc.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Keehn B, Westerfield M, Muller RA, Townend J. Autism, attention, and alpha oscillations: An electrophysiological study of attention capture. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. doi: 10.1016/j.bpsc.2017.06.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Baving L, Laucht M, Schmidt M. Atypical frontal brain activation in ADHD: Preschool and elementary school boys and girls. J Am Acad Child Adolesc Psychiatry. 1999;38(11):1363–1371. doi: 10.1097/00004583-199911000-00010. [DOI] [PubMed] [Google Scholar]