Abstract
Background
A blunted reward positivity (RewP), an event-related potential (ERP) elicited by feedback indicating monetary gain relative to loss, was recently shown to prospectively predict the development of adolescent-onset depression. Time-frequency based representations of this activity (e.g., reward-related delta) have also been associated with depression. The present study is a reanalysis of the time-domain RewP investigation to examine the incremental value of time-frequency indices in the prediction of adolescent-onset depression.
Methods
The sample included 444 13 to 15 year-old girls with no lifetime history of a depressive disorder. At baseline, adolescents completed a monetary guessing task, and both time-domain and time-frequency analyses were conducted on the ERP response to gain and loss feedback. Lifetime psychiatric history in the adolescent and a biological parent were evaluated with diagnostic interviews, and the adolescents’ current depressive symptoms were assessed using a self-report questionnaire. Adolescents were interviewed again approximately 18-months later to identify first-onset depressive disorder.
Results
Blunted reward-related delta predicted first-onset depressive disorder 18-months later, independent of the time-domain RewP and psychosocial risk factors (i.e., adolescent baseline depressive symptoms, adolescent and parental psychiatric history). In contrast, loss-related theta did not predict the development of depression. Reward-related delta increased sensitivity (73.8% to 82.8%) and positive predictive value (45.0% to 70.9%) for first-onset depressive disorder when applied in parallel and in series, respectively, with baseline depressive symptoms and the time-domain RewP.
Conclusions
The present study provides evidence that frequency-based representations of ERPs provide incremental value in the prediction of psychiatric disorders.
Keywords: adolescence, depression, event-related potentials, prospective, reward, time-frequency
Introduction
Adolescence is period of increased vulnerability for depression. The point prevalence of depression escalates markedly after age 14 (1), with the lifetime prevalence of depression increasing nearly twofold across adolescence from 8.4% to 15.4% (2). Sex differences in depression also arise during adolescence, with girls relative to boys being twice as likely to experience depression by the end of adolescence (3). Adolescent-onset depression is associated with a host of negative consequences, including academic difficulties, physical illness, and poor social functioning (4–9); moreover, the disorder often persists into adulthood (10, 11) where the economic burden amounts to billions of dollars (12–14). To address this important public health priority, there has been a growing interest in biomarkers of depression that could aid in early identification and prevention efforts (15).
Abnormalities in the brain’s reward circuitry have been implicated in the development of depression (16, 17), and multiple neural measures of reward system activation have been identified as potential biomarkers of depression. For example, functional MRI (fMRI) studies have indicated that blunted striatal activation to rewards is cross-sectionally associated with childhood depression (18) and family history (i.e., risk) of depression (19, 20), and prospectively predicts the emergence of depressive symptoms (21, 22) and diagnoses in adolescents (23). However, there are important limitations regarding the application of fMRI in clinical psychiatry practice and research (e.g., high cost of imaging) and youth populations (e.g., excluding adolescents with braces) (24).
Event-related potentials (ERP) have also been employed to examine reward system activation in children and adolescents. ERPs provide several advantages over other neurobiological measures of reward sensitivity, including low cost, minimal invasiveness, and the ability to be used in children and adolescents who have contraindications for fMRI (e.g., braces, claustrophobia). The reward positivity (RewP) is a positive-going deflection in the ERP signal that occurs approximately 250 to 350 ms following feedback indicating monetary gains that is absent or reduced following losses (25, 26). The reward positivity is an ideal biomarker given its excellent reliability (27, 28) and validity, including associations with self-report and behavioral measures of reward sensitivity (29) and fMRI-based activation in the medial prefrontal cortex and striatum (30–32). In children and adolescents, a blunted reward positivity has been cross-sectionally associated with increased depressive symptoms (33), depressive disorders (34), and family history (i.e., risk) of depression (35, 36), and prospectively predicts major depressive episodes (37).
In a recent investigation of 444 13 to 15 year-old girls with no lifetime history of a depressive disorder (38), a blunted RewP predicted an increased likelihood of developing a first-onset depressive disorder 18-months later, independent of other prominent psychosocial risk factors (e.g., baseline adolescent depressive symptoms, adolescent and parental lifetime psychiatric history). In addition, the investigation examined the sensitivity, specificity, and positive and negative predictive value of the RewP, which are important characteristics of screening tools or disease tests that require binary decisions (e.g., does a patient have a disease, should a patient receive treatment, etc.), in the prediction of first-onset depressive disorder. Sensitivity and specificity reflect the probability a test result will be positive when the disease is present and negative when the disease is absent, respectively. Positive and negative predictive value take into account the prevalence rate of the disease and indicate the proportion of positive and negative results that are true positives and true negatives, respectively. The RewP improved sensitivity and positive predictive value of first-onset depressive disorder when used in conjunction with baseline depressive symptoms. Specifically, baseline depressive symptoms and the RewP each had relatively low sensitivity (50.0% and 47.5%, respectively) and positive predictive value (26.1%, and 18.6% respectively). However, when the measures were applied in parallel (i.e., if the individual tests positive on either test, the disease is present) sensitivity increased to 73.8%. Moreover, when the measures were applied in series (i.e., the individual must test positive on both measures to have the disease) positive predictive value increased to 45.0%. These results provide promising evidence that biomarkers like the RewP provide incremental value in the prediction of psychiatric disorders.
The RewP is typically scored as the average activity in a window of the ERP; more complex factor analytic approaches (e.g., principal components analysis; PCA) differentially weight time-points to quantify the RewP. All of these time-domain representations of the RewP fail to consider the fact that ERPs have distinct frequency characteristics. Indeed, many common ERPs reflect multiple frequency components. For instance, time-frequency analysis of feedback to monetary gains and losses indicates two distinct effects in the same time range as the RewP (39): increased activity in the delta frequency band (< 3 Hz) following feedback indicating monetary gain, and increased activity in the theta frequency band (4 to 7 Hz) following feedback indicating monetary loss (39–43). Scoring the RewP as the average activity in a time window conflates these distinct effects. In fact, reward-related delta and loss-related theta are only moderately correlated with the time-domain RewP (43, 44), suggesting that frequency-based representations provide unique information that is not apparent with the time-domain analysis. Source localization has suggested the striatum for reward-related delta and the anterior cingulate cortex for loss-related theta (43). Although the exact functions of these frequency-based representations remain unknown, some research suggests that reward-related delta may index motivational relevance and salience of feedback (45) whereas loss-related theta represents the processing of error feedback (42).
A small number of studies that have examined time-frequency representations of neural activity to monetary feedback in relation to depression. In one investigation of undergraduate students, more blunted reward-related delta was associated with greater depressive symptoms, but loss-related theta was not associated with depression (43). In a second investigation, depressed adolescent girls, relative to those with no lifetime history of depression, exhibited greater loss-related theta, but there were no group differences in reward-related delta (44). These initial studies suggest that time-frequency activity to monetary feedback is associated with depression. However, time-frequency representations of neural activity to monetary feedback have not been examined in relation to the development of depression. In addition, it is unclear whether reward-related delta or loss-related theta constitute unique biomarkers of depression, independent of the time-domain RewP and other prominent psychosocial risk factors.
The present study leveraged data from the aforementioned sample of 444 13 to 15 year-old girls with no lifetime history of depression and examined whether time-frequency indices of neural activity to monetary feedback prospectively predicted first-onset depressive disorder. We hypothesized that smaller reward-related delta and greater loss-related theta would predict a greater likelihood of first-onset depressive disorder, and these relationships would be independent of the time-domain RewP and psychosocial risk factors (i.e., baseline adolescent depression symptoms, adolescent and parental psychiatric history). The present study also examined whether the time-frequency indices had incremental predictive value relative to the time-domain RewP and baseline depressive symptoms by determining whether it provided increased sensitivity, specificity, and positive and negative predictive value for first-onset depressive disorder. We hypothesized that the time-frequency indices would provide increased sensitivity and positive predictive value when applied in parallel and series, respectively, with baseline depressive symptoms and the time-domain RewP.
Methods and Materials
Participants
The sample consisted of 550 girls between ages 13.5 and 15.5 years (M = 14.4 years, SD = 0.63) and a biological parent (93.9% mothers) who participated in the Adolescent Development of Emotions and Personality Traits (ADEPT) project, a longitudinal study of risk for adolescent depression. See (38) for more details regarding the sample characteristics.
Measures
The adolescents’ psychiatric history was ascertained with the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL) (46). The K-SADS-PL was administered to the adolescent at the baseline assessment by trained interviewers who were closely supervised by clinical psychologists (R.K., D.N.K., and G.P.). Participants were allowed to meet criteria for non-depressive psychiatric disorders (e.g., anxiety disorders). At the 18-month follow-up assessment, the K-SADS-PL was administered again to the adolescent to assess change in diagnostic status across the interval. The present study focused on first-onset depressive disorder (major depressive disorder, dysthymia, or depressive disorder not otherwise specified).
Parental psychiatric history was assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (47). The SCID was administered at the baseline assessment to the biological parent accompanying the participant by trained interviewers who were closely supervised by clinical psychologists (R.K., D.N.K., and G.P.).
Adolescent depressive symptoms at the baseline assessment were measured with the expanded version of the Inventory of Depression and Anxiety Symptoms (48), which is a 99-item factor-analytically derived self-report inventory of empirically distinct dimensions of depression and anxiety symptoms. Symptoms are rated for the past two weeks on a Likert-type scale ranging from 1 (not at all) to 5 (extremely). The present study focused on the 10-item dysphoria subscale, which is the core symptom dimension of depression (48–50).
Procedure
Doors task
The doors task (25) was administered using Presentation, version 17.2 (Neurobehavioral Systems, Berkeley, CA, USA) and consisted of three blocks of 20 trials. Each trial began by presenting two identical doors. Participants were instructed to select the left or right door by clicking the left or right mouse button, respectively. Participants were told that they could either win $0.50 or lose $0.25 on each trial. The image of the doors was presented until participants made a selection. Next, a fixation cross was presented for 1000 ms, and feedback was subsequently presented for 2000 ms. A monetary gain was indicated by a green arrow pointing upward and a monetary loss by a red arrow pointing downward. The feedback stimulus was followed by a fixation cross presented for 1500 ms, immediately followed by the message “Click for next round.” This prompt remained on the screen until participants responded with a button press to initiate the next trial, ensuring that participants remained active and engaged during the task. There were 30 gain and 30 loss trials.
EEG recording and processing
EEG recording and processing parameters were consistent with previous investigations using the doors task (37). Continuous EEG was recorded using an elastic cap with 34 electrode sites placed according to the 10/20 system. Electrooculography (EOG) was recorded using four additional facial electrodes: two placed approximately 1 cm outside of the right and left eyes and two placed approximately 1 cm above and below the right eye. Sintered Ag/AgCl electrodes were used. EEG and EOG were recorded using the ActiveTwo system (BioSemi, Amsterdam) and digitized with a sampling rate of 1024 Hz using a low-pass fifth-order sinc filter with a half-power cutoff of 204.8 Hz. For the EEG electrodes, a common mode sense active electrode producing a monopolar (non-differential) channel was used as a recording reference. The EOG electrodes produced two bipolar channels that measured horizontal and vertical eye movements.
Offline time-domain data processing was identical to the previous investigation using the same sample and measures (38). Feedback-locked ERPs were averaged separately for monetary gains and losses and scored as the mean amplitude from 250 to 350 ms following feedback at FCz, where the difference between gains and losses was maximal. Offline time-frequency data processing was conducted using a combination of EEGLAB toolbox, version 13.6.5b (51) and customized Matlab scripts, version R2016a (MathWorks, Natick, MA, USA). Data were re-referenced to the average of the left and right mastoids, band-pass filtered (.01 to 30 Hz), and eye blinks were removed using independent component analyses. Feedback-locked epochs were extracted with a duration of 6000 ms, beginning 3000 ms before feedback presentation. Epochs containing artifacts were identified and rejected using FASTER (52).
Feedback-locked ERPs at FCz were averaged separately for gains and losses and decomposed into their time-frequency representation by multiplying the power spectrum of the ERP by the power spectrum of a set of complex Morlet wavelets that increased by 50 logarithmic steps from 1 to 50 Hz. An estimate of frequency band-specific power at each time point was computed by squaring the absolute value of the complex signal. Power was normalized using a decibel (dB) transformation where the baseline activity was the average power at each frequency condition from −200 to 0 ms prior to feedback onset.
In order to reduce the time-frequency data into distinguishable components whose timing and frequency range were associated with monetary gain and loss feedback, a principle components analysis (PCA) was conducting according to published guidelines (53). Time-frequency surfaces were rearranged into vectors and a PCA was performed on the data using the ERP PCA Toolkit, version 2.52 (54). The PCA used the time-frequency vectors as variables and participants and trial types as observations. Varimax rotation was used and forty-three factors that accounted for 94.51% of the variance were extracted based on the resulting Scree plot (55). To assess the timing and frequency distribution of the components, the factors were translated back into normalized power and rearranged into surfaces. Twenty factors accounted for at least 1% of the variance, and two of those factors resembled the time and frequency distribution of delta activity (accounting for 2.98% of the variance) and theta activity (accounting for 13.99% of the variance) and were consistent with previous findings (39, 41, 56).
Data Analysis
Participants were excluded from the analyses if they had a lifetime history of depressive disorder not otherwise specified at baseline (n = 34), were missing diagnostic interview or self-report data at baseline or the 18-month follow-up assessment (n = 38), did not complete the doors task (n = 30), or had outlier RewP values that were more than three standard deviations from the mean (n = 4), resulting in a final sample of 444 participants. The ERP response to monetary gain and loss feedback has often been examined using a difference score. However, recent evidence has indicated that residuals provide a more reliable ERP measure (27, 57, 58). Residuals are calculated using linear regression as the residual response to the condition of interest adjusting for the comparison condition. In the present study, three residuals were calculated: time-domain ERP response to monetary gains adjusting for the ERP response to monetary losses, time-frequency delta activity to monetary gains adjusting for delta activity to monetary losses, and time-frequency theta activity to monetary losses adjusting for theta activity to monetary gains. Logistic regression was conducted to determine whether the reward-related delta and loss-related theta residuals predicted first-onset depressive disorder. Multiple logistic regression was conducted to determine whether these associations were independent of the time-domain RewP residual and the other psychosocial risk factors.
Receiver operating characteristic curve analyses were conducted to determine area under the curve, sensitivity, and specificity for significant time-frequency predictors of first-onset depressive disorder. These values were used in combination with the prevalence of first-onset depressive disorder in this sample (9.0%) to calculate positive and negative predictive value. The time-frequency factors were continuous measures; therefore, multiple sensitivity, specificity, and positive and negative predictive values were calculated based on a range of cutoffs (0.5, 1.0, 1.5, and 2.0 standard deviations from the mean). Combined sensitivity and specificity for the three tests (A, B, and C) using parallel testing was calculated using the formulas (A)SEN + (B)SEN + (C)SEN − [(A)SEN × (B)SEN] − [(A)SEN × (C)SEN] − [(B)SEN × (C)SEN] +[(A)SEN × (B)SEN × (C)SEN] and (A)SPEC × (B)SPEC × (C)SPEC, respectively, and for series testing was calculated using the formulas (A)SEN × (B)SEN × (C)SEN and (A)SPEC + (B)SPEC + (C)SPEC − [(A)SPEC × (B)SPEC] − [(A)SPEC × (C)SPEC] − [(B)SPEC × (C)SPEC] +[(A)SPEC × (B)SPEC × (C)SPEC], respectively. The combined sensitivity and specificity values were then used to calculate the combined positive and negative predictive values. Analyses were conducted in IBM SPSS Statistics, version 22.0 (IBM, Armonk, N.Y.).
Results
Figures 1 and 2 display the time-domain and time-frequency, respectively, representation of the ERP response to monetary gain and loss feedback. Figure 3 displays the PCA-derived time-frequency factors for delta activity to monetary gains (top) and theta activity to monetary losses (bottom). As expected, delta activity was greater in response to monetary gains (M = 3.26 dB, SD = 5.26) than the response to losses (M = 1.49 dB, SD = 5.60), t(444) = 4.89, p < .001, while theta activity was greater in response to monetary losses (M = 10.42 dB, SD = 6.04) than the response to gains (M = 7.23, dB, SD = 6.32), t(444) = 8.63, p < .001. The time-domain RewP residual was positively associated with the reward-related delta residual, r(444) = .12, p = .015, and negatively associated with the loss-related theta residual, r(444) = −.13, p = .006. The reward-related delta and loss-related theta residuals were not correlated with each other, r(444) = −.01, ns.
Figure 1.
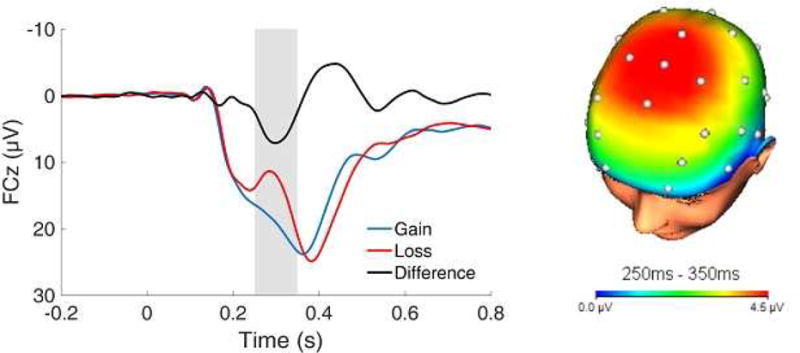
Time-domain event-related potential (ERP) waveforms at FCz and three-dimensional scalp topography for the gain minus loss difference (i.e., the reward positivity; RewP). The shaded region of the waveform shows the segment (250 to 350 ms) where the RewP was scored.
Figure 2.
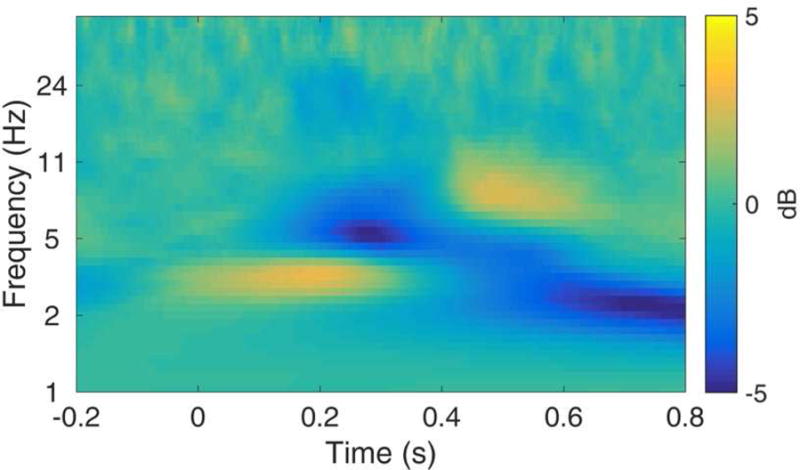
Time-frequency plot for the gain minus loss difference at FCz, with more yellow indicating greater activity to gain relative to loss and more blue indicating greater activity to loss relative to gain.
Figure 3.
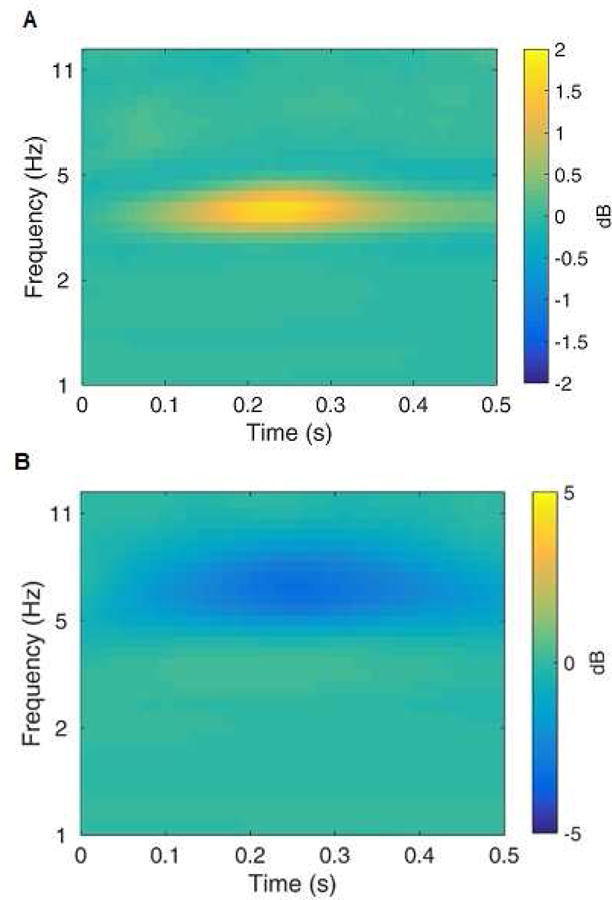
Time-frequency principal components analysis (PCA) factors for delta (panel A) and theta (panel B) activity. The time-frequency plots represent the gain minus loss difference, with more yellow indicating greater activity to gain relative to loss and more blue indicating greater activity to loss relative to gain.
In the logistic regression analyses, the reward-related delta residual was multiplied by −1 to produce the inverse relationship, such that more positive values indicated a reduced neural response to rewards. Results indicated that a smaller reward-related delta residual predicted a greater likelihood of developing first-onset depressive disorder (OR = 1.46, 95% CI = 1.07–1.99, p = .018). However, the loss-related theta residual did not predict first-onset depressive disorder (OR = 1.08, 95% CI = 0.78–1.49, ns). In addition, as shown in Table 1, a multiple logistic regression analysis indicated that a smaller reward-related delta residual predicted first-onset depressive disorder independent of the time-domain RewP residual, loss-related theta residual, and the other measured psychosocial risk factors.1,2
Table 1.
Multiple Regression with Baseline Assessment Risk Measures Predicting First-Onset Depressive Disorder at the 18-Month Follow-Up Assessment
| R2 | χ2 | Adjusted OR | 95% CI | |
|---|---|---|---|---|
| .15 | 31.23*** | |||
| Adolescent Dysphoria Symptoms | 1.57 | 1.17–2.10** | ||
| Adolescent Reward Positivity (RewP) | 1.50 | 1.03–2.18* | ||
| Adolescent Reward-Related Delta | 1.50 | 1.06–2.11* | ||
| Adolescent Loss-Related Theta | 1.07 | 0.76–1.51 | ||
| Adolescent Lifetime Anxiety Disorder | 1.96 | 0.92–4.17 | ||
| Adolescent Lifetime Behavioral Disorder | 1.18 | 0.30–4.60 | ||
| Parental Lifetime Depressive Disorder | 1.49 | 0.70–3.17 | ||
| Parental Lifetime Anxiety Disorder | 1.10 | 0.54–2.24 | ||
| Parental Lifetime Substance Use Disorder | 1.00 | 0.47–2.15 |
Note. Age was included as a covariate in the analyses. Adolescent RewP and reward-related delta demonstrated an inverse relationship with first-onset depressive disorder. Therefore, the RewP and reward-related delta were multiplied by −1 to produce the inverse relationship, such that more positive values indicated a reduced neural response to rewards, in order to better compare the reward measures with the other risk measures. The RewP, reward-related delta, loss-related theta, and dysphoria symptom measures were z-transformed to allow for direct comparison of the adjusted odds ratios. Adolescent and parental lifetime psychiatric disorder measures were dichotomous independent variables (0 = absent, 1 = present) and first-onset depressive disorder was the dichotomous dependent variable (0 = absent, 1 = present). OR = odds ratio; CI = confidence interval.
p < .05,
p < .01,
p < .001
Table 2 lists sensitivity, specificity, and positive and negative predictive values for the reward-related delta residual predicting first-onset depressive disorder. Similar to the time-domain RewP (38), reward-related delta provided relatively high specificity and negative predictive value that approached 100%, but relatively low sensitivity and positive predictive value that were ≤ 42.5%. As shown in Table 3, parallel testing produced increased sensitivity but decreased specificity using a low cutoff for baseline depressive symptoms, the RewP, and reward-related delta. A 0.5 standard deviation cut-off for all three measures for parallel testing produced a maximum sensitivity of 82.8%. In contrast, series testing produced increased specificity but decreased sensitivity when using a high cut-off for all three measures. A 1.5 standard deviation cutoff for the RewP and a 2.0 standard deviation cut-off for baseline dysphoria symptoms and reward-related delta for series testing produced a maximum positive predictive value of 70.9%. Negative predictive value remained high irrespective of cutoff or testing approach (≥ 91.0%).
Table 2.
Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value for Adolescent Reward-Related Delta Predicting First-Onset Depressive Disorder at the 18-Month Follow-Up Assessment
| Area Under the Curve | Cutoff | SEN | SPEC | PPV | NPV |
|---|---|---|---|---|---|
| .60 | +2.0 SD | 12.5 | 96.0 | 23.6 | 91.7 |
| +1.5 SD | 17.5 | 91.8 | 17.4 | 91.8 | |
| +1.0 SD | 32.5 | 84.4 | 17.1 | 92.7 | |
| +0.5 SD | 42.5 | 75.2 | 14.5 | 93.0 |
Note. The reward-related delta was multiplied by −1 to produce the inverse relationship, such that more positive values indicated a reduced neural response to rewards. The table includes sensitivity, specificity, and positive and negative predictive values for a range of cut-offs for adolescent reward-related delta. PPV = positive predictive value; NPV = negative predictive value; SD = standard deviation; SEN = sensitivity, SPEC = specificity.
Table 3.
Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value for Adolescent Baseline Dysphoria Symptoms, Reward Positivity (RewP), and Reward-Related Delta Predicting First-Onset Depressive Disorder at the 18-Month Follow-Up Assessment when Applied in Parallel and in Series
| Adolescent Baseline Assessment
|
Applied in Parallel
|
Applied in Series
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysphoria Symptoms | Reward Positivity (RewP) | Reward-Related Delta | SEN | SPEC | PPV | NPV | SEN | SPEC | PPV | NPV |
| +2.0 SD | +1.5 SD | +2.0 SD | 34.9 | 86.8 | 20.8 | 93.1 | 0.2 | 100.0 | 70.9 | 91.0 |
| +2.0 SD | +1.5 SD | +1.5 SD | 38.6 | 83.0 | 18.4 | 93.2 | 0.3 | 100.0 | 63.0 | 91.0 |
| +2.0 SD | +1.5 SD | +1.0 SD | 49.8 | 76.5 | 17.3 | 93.9 | 0.6 | 100.0 | 63.0 | 91.0 |
| +2.0 SD | +1.5 SD | +0.5 SD | 57.2 | 68.2 | 15.1 | 94.2 | 0.8 | 99.9 | 58.4 | 91.1 |
| +2.0 SD | +1.0 SD | +2.0 SD | 40.5 | 77.5 | 15.1 | 92.9 | 0.4 | 100.0 | 57.4 | 91.0 |
| +2.0 SD | +1.0 SD | +1.5 SD | 43.9 | 74.1 | 14.3 | 93.0 | 0.5 | 99.9 | 48.5 | 91.0 |
| +2.0 SD | +1.0 SD | +1.0 SD | 54.1 | 68.2 | 14.4 | 93.8 | 1.0 | 99.9 | 48.5 | 91.1 |
| +2.0 SD | +1.0 SD | +0.5 SD | 60.9 | 60.8 | 13.3 | 94.0 | 1.3 | 99.8 | 43.7 | 91.1 |
| +2.0 SD | +0.5 SD | +2.0 SD | 55.4 | 64.5 | 13.4 | 93.6 | 0.8 | 99.9 | 58.6 | 91.1 |
| +2.0 SD | +0.5 SD | +1.5 SD | 57.9 | 61.7 | 13.0 | 93.7 | 1.1 | 99.9 | 49.8 | 91.1 |
| +2.0 SD | +0.5 SD | +1.0 SD | 65.6 | 56.8 | 13.1 | 94.3 | 2.0 | 99.8 | 49.8 | 91.1 |
| +2.0 SD | +0.5 SD | +0.5 SD | 70.7 | 50.6 | 12.4 | 94.6 | 2.6 | 99.7 | 44.9 | 91.2 |
| +1.5 SD | +1.5 SD | +2.0 SD | 42.6 | 83.5 | 20.3 | 93.6 | 0.4 | 100.0 | 68.3 | 91.0 |
| +1.5 SD | +1.5 SD | +1.5 SD | 45.9 | 79.8 | 18.3 | 93.7 | 0.5 | 100.0 | 60.1 | 91.0 |
| +1.5 SD | +1.5 SD | +1.0 SD | 55.7 | 73.5 | 17.2 | 94.4 | 1.0 | 99.9 | 60.1 | 91.1 |
| +1.5 SD | +1.5 SD | +0.5 SD | 62.3 | 65.5 | 15.2 | 94.6 | 1.3 | 99.9 | 55.4 | 91.1 |
| +1.5 SD | +1.0 SD | +2.0 SD | 47.5 | 74.5 | 15.5 | 93.5 | 0.6 | 99.9 | 54.4 | 91.0 |
| +1.5 SD | +1.0 SD | +1.5 SD | 50.5 | 71.2 | 14.8 | 93.6 | 0.9 | 99.9 | 45.5 | 91.1 |
| +1.5 SD | +1.0 SD | +1.0 SD | 59.5 | 65.6 | 14.6 | 94.2 | 1.6 | 99.8 | 45.5 | 91.1 |
| +1.5 SD | +1.0 SD | +0.5 SD | 65.5 | 58.5 | 13.5 | 94.5 | 2.1 | 99.7 | 40.7 | 91.1 |
| +1.5 SD | +0.5 SD | +2.0 SD | 60.6 | 62.0 | 13.6 | 94.1 | 1.3 | 99.9 | 55.6 | 91.1 |
| +1.5 SD | +0.5 SD | +1.5 SD | 62.9 | 59.3 | 13.3 | 94.2 | 1.8 | 99.8 | 46.8 | 91.1 |
| +1.5 SD | +0.5 SD | +1.0 SD | 69.6 | 54.6 | 13.2 | 94.8 | 3.3 | 99.6 | 46.8 | 91.2 |
| +1.5 SD | +0.5 SD | +0.5 SD | 74.1 | 48.7 | 12.5 | 95.0 | 4.3 | 99.4 | 41.9 | 91.3 |
| +1.0 SD | +1.5 SD | +2.0 SD | 50.2 | 78.5 | 18.8 | 94.1 | 0.5 | 100.0 | 64.0 | 91.0 |
| +1.0 SD | +1.5 SD | +1.5 SD | 53.1 | 75.0 | 17.4 | 94.2 | 0.8 | 99.9 | 55.5 | 91.1 |
| +1.0 SD | +1.5 SD | +1.0 SD | 61.6 | 69.1 | 16.5 | 94.8 | 1.4 | 99.9 | 55.5 | 91.1 |
| +1.0 SD | +1.5 SD | +0.5 SD | 67.3 | 61.6 | 14.8 | 95.0 | 1.9 | 99.8 | 50.6 | 91.1 |
| +1.0 SD | +1.0 SD | +2.0 SD | 54.5 | 70.0 | 15.2 | 94.0 | 0.9 | 99.9 | 49.6 | 91.1 |
| +1.0 SD | +1.0 SD | +1.5 SD | 57.1 | 67.0 | 14.6 | 94.0 | 1.2 | 99.8 | 40.8 | 91.1 |
| +1.0 SD | +1.0 SD | +1.0 SD | 64.9 | 61.7 | 14.3 | 94.7 | 2.3 | 99.7 | 40.8 | 91.2 |
| +1.0 SD | +1.0 SD | +0.5 SD | 70.1 | 55.0 | 13.3 | 94.9 | 3.0 | 99.5 | 36.2 | 91.2 |
| +1.0 SD | +0.5 SD | +2.0 SD | 65.9 | 58.3 | 13.5 | 94.5 | 1.8 | 99.8 | 50.9 | 91.1 |
| +1.0 SD | +0.5 SD | +1.5 SD | 67.8 | 55.8 | 13.2 | 94.6 | 2.5 | 99.7 | 42.0 | 91.2 |
| +1.0 SD | +0.5 SD | +1.0 SD | 73.7 | 51.4 | 13.0 | 95.2 | 4.6 | 99.4 | 42.0 | 91.3 |
| +1.0 SD | +0.5 SD | +0.5 SD | 77.6 | 45.8 | 12.4 | 95.4 | 6.0 | 99.0 | 37.4 | 91.4 |
| +0.5 SD | +1.5 SD | +2.0 SD | 61.7 | 70.2 | 17.0 | 94.9 | 0.8 | 99.9 | 60.2 | 91.1 |
| +0.5 SD | +1.5 SD | +1.5 SD | 63.9 | 67.2 | 16.1 | 95.0 | 1.1 | 99.9 | 51.5 | 91.1 |
| +0.5 SD | +1.5 SD | +1.0 SD | 70.5 | 61.9 | 15.5 | 95.5 | 2.0 | 99.8 | 51.5 | 91.2 |
| +0.5 SD | +1.5 SD | +0.5 SD | 74.8 | 55.1 | 14.2 | 95.7 | 2.7 | 99.7 | 46.6 | 91.2 |
| +0.5 SD | +1.0 SD | +2.0 SD | 65.0 | 62.7 | 14.7 | 94.8 | 1.3 | 99.9 | 45.6 | 91.1 |
| +0.5 SD | +1.0 SD | +1.5 SD | 67.0 | 59.9 | 14.2 | 94.8 | 1.8 | 99.7 | 37.0 | 91.1 |
| +0.5 SD | +1.0 SD | +1.0 SD | 73.0 | 55.2 | 13.9 | 95.4 | 3.3 | 99.5 | 37.0 | 91.2 |
| +0.5 SD | +1.0 SD | +0.5 SD | 77.0 | 49.2 | 13.0 | 95.6 | 4.3 | 99.1 | 32.6 | 91.3 |
| +0.5 SD | +0.5 SD | +2.0 SD | 73.8 | 52.2 | 13.2 | 95.3 | 2.5 | 99.7 | 46.8 | 91.2 |
| +0.5 SD | +0.5 SD | +1.5 SD | 75.3 | 49.9 | 12.9 | 95.3 | 3.5 | 99.4 | 38.1 | 91.2 |
| +0.5 SD | +0.5 SD | +1.0 SD | 79.8 | 46.0 | 12.7 | 95.8 | 6.5 | 99.0 | 38.1 | 91.5 |
| +0.5 SD | +0.5 SD | +0.5 SD | 82.8 | 41.0 | 12.2 | 96.0 | 8.5 | 98.3 | 33.7 | 91.6 |
Note. The RewP and reward-related delta were multiplied by −1 to produce the inverse relationship, such that more positive values indicated a reduced neural response to rewards. The table includes sensitivity, specificity, and positive and negative predictive values for a range of cut-offs for adolescent dysphoria symptoms, the RewP residual, and reward-related delta residual. In parallel testing if either test is positive (i.e., a blunted RewP, blunted reward-related delta, or greater dysphoria symptoms) the condition (i.e., depression risk) is present, and in series testing if all tests are positive the condition is present. The maximum sensitivity and positive predictive value are bolded. PPV = positive predictive value; NPV = negative predictive value; SD = standard deviation; SEN = sensitivity, SPEC = specificity.
Discussion
In a sample of 444 13 to 15 year-old girls with no lifetime history of a depressive disorder, the present study examined whether frequency-domain representations of ERPs prospectively predicted first-onset depressive disorder. Time-frequency analyses indicated that the neural activity in the time range of the RewP was characterized by increased delta activity to monetary gains and increased theta activity to monetary losses. Prospective analyses indicated that blunted reward-related delta predicted first-onset depressive disorder 18-months later, independent of the time-domain RewP and other prominent psychosocial risk factors (adolescent depressive symptoms, adolescent and parental psychiatric history). There was no relationship between loss-related theta and the development of depression. Finally, delta activity to monetary gains provided increased sensitivity and positive predictive value for first-onset depressive disorder when applied in parallel and in series, respectively, with baseline depressive symptoms and the time-domain RewP. Overall, the present study is the first to demonstrate a prospective relationship between delta-band activity to rewards and the development of adolescent-onset depression—and to show that this effect is independent of the time-domain RewP.
Time-domain representations of ERPs (i.e., average activity in a window) do not parse frequency-related characteristics of a time-varying neural response. Thus, time-domain ERP scores potentially confound different types of activity. Indeed, in the current study, time-frequency representations were only moderately correlated with the time-domain RewP score, and only gain-related delta prospectively predicted depression.
The present results are consistent with a previous investigation indicating that reward-related delta is associated with depression (43, cf.44). The time-domain RewP has been linked to activation in the striatum (30, 56), a region involved in decision-making and reward processing (59). Reward-related delta has also been associated with activation in the striatum, while loss-related theta has been associated with activation in the anterior cingulate cortex (43). The time-domain and time-frequency evidence suggests that abnormalities in reward-related striatal activity play an important role in the development of depression. Indeed, fMRI-based striatal activation to rewards has also been shown to prospectively predict the development of depression (23), and deep brain stimulation of the striatum has been shown to treat refractory depression (60–62). These results provide converging evidence across multiple modalities that a blunted neural response to rewards is a potential biomarker for the development of depression.
Delta band representations of the RewP yielded complementary information that improved the ability to predict adolescent-onset depression over and above time-domain RewP. In a previous investigation involving the same sample, the sensitivity of baseline depressive symptoms increased (50.0% to 73.8%) when it was applied in parallel with the RewP, and the positive predictive value of baseline depressive symptoms increased nearly twofold (26.1% to 45.0%) when it was applied in series with the RewP. In the present study, the addition of time-frequency reward-related delta further increased the sensitivity (73.8% to 82.8%) and positive predictive value (45.0% to 70.9%) when applied in parallel and in series, respectively, with baseline depressive symptoms and the time-domain RewP. Frequency-based representations of ERPs might be a simple measure to examine in conjunction with more traditional time-domain measures in studies of individual differences and psychiatric disorders.
These results provide intriguing insights regarding the potential role of neural biomarkers in the prediction of depression. Adolescence is a period of increased risk for depression, but most adolescents will not experience a depressive disorder. Therefore, the negative predictive value of a screening tool for adolescent-onset depression will always be high as there is a relatively small chance of obtaining a false “true” negative. However, the addition of screening tools with high specificity, such as the RewP and reward-related delta, to parallel testing has the potential to significantly improve sensitivity, while the addition of these screening tools to series testing has the potential to significantly improve positive predictive value. The parallel versus series testing approach using neural biomarkers may be relevant for different clinical purposes. For example, parallel testing might be used in an effort to identify as many at risk individuals as possible and administer an economical, low-intensity preventative measure (e.g., psychoeducational groups). In contrast, series testing might be used to identify individuals who are more likely to actually develop psychopathology and are administered a more costly, time-consuming preventative measure (e.g., individual or family-based psychotherapy). In a clinical setting, series testing could be utilized in a cost-effective manner, such as a self-report symptom measure being administered as a first-line screening tool, and in individuals who test positive (i.e., report elevated symptoms) the neural biomarker would be assessed to further assess risk status.
The present study results should be interpreted in the context of several limitations. First, the sample consisted of 13 to 15 year-old girls, and the findings may not generalize to other populations (e.g., boys, adult-onset depression). Second, the guessing task focused on monetary reward, and it is possible that social reward might be more salient in adolescents and provide stronger relationships with depression (20, 63, 64). Finally, this study focused on adolescent-onset depression given the overarching aims of the project, but time-frequency indices of neural activity to feedback have also been associated with other psychiatric disorders (e.g., externalizing disorders) (65). Future studies should examine whether time-frequency indices can parse risk for different classes of psychiatric disorders.
Acknowledgments
This project was funded by National Institute of Mental Health grant R01 MH093479 awarded to Dr. Kotov. We thank the adolescents and parents who contributed their time and energy to the ADEPT project. We also appreciate the countless contributions of the research assistants and staff to this study. A special thank you to Molly Gromatsky for her leadership and hard work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
See (38) for rates of adolescent psychiatric disorders and parental psychiatric disorders.
High neuroticism is also a prominent risk factor for the development of depression (66). As part of the larger ADEPT project, self-reported personality traits were also measured using the Big Five Inventory (67, 68) in the adolescent girls, and a supplementary logistic regression analysis was conducted to determine whether the RewP and reward-related delta residuals predicted first-onset depressive disorder independent of the aforementioned psychosocial risk factors and self-reported neuroticism. Results indicated that both the RewP residual (adjusted OR = 1.52, 95% CI = 1.05–2.21, p = .028) and reward-related delta (adjusted OR = 1.50, 95% CI = 1.07–2.11, p = .019) predicted the development of first-onset depressive disorder.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Lewinsohn PM, Rohde P, Seeley JR. Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clin Psychol Rev. 1998;18:765–794. doi: 10.1016/s0272-7358(98)00010-5. [DOI] [PubMed] [Google Scholar]
- 2.Merikangas KR, He J, Burstein M, Swendsen J, Avenevoli S, Case B, et al. Service utilization for lifetime mental disorders in U.S. adolescents: results of the National Comorbidity Survey-Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2011;50:32–45. doi: 10.1016/j.jaac.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- 4.Birmaher B, Ryan ND, Williamson DE, Brent Da, Kaufman J, Dahl RE, et al. Childhood and adolescent depression: a review of the past 10 years. Part I. J Am Acad Child Adolesc Psychiatry. 1996;35:1427–1439. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Hammen C, Burge D, Burney E, Adrian C. Longitudinal study of diagnoses in children of women with unipolar and bipolar affective disorder. Arch Gen Psychiatry. 1990;47:1112–1117. doi: 10.1001/archpsyc.1990.01810240032006. [DOI] [PubMed] [Google Scholar]
- 6.Kandel DB, Davies M. Adult sequelae of adolescent depressive symptoms. Arch Gen Psychiatry. 1986;43:255–262. doi: 10.1001/archpsyc.1986.01800030073007. [DOI] [PubMed] [Google Scholar]
- 7.Kovacs M. Presentation and course of major depressive disorder during childhood and later years of the life span. J Am Acad Child Adolesc Psychiatry. 1996;35:705–15. doi: 10.1097/00004583-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Rao U, Hammen C, Daley SE. Continuity of depression during the transition to adulthood: a 5-year longitudinal study of young women. J Am Acad Child Adolesc Psychiatry. 1999;38:908–15. doi: 10.1097/00004583-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Ryan ND, Puig-Antich J, Ambrosini P, Rabinovich H, Robinson D, Nelson B, et al. The clinical picture of major depression in children and adolescents. Arch Gen Psychiatry. 1987;44:854–61. doi: 10.1001/archpsyc.1987.01800220016003. [DOI] [PubMed] [Google Scholar]
- 10.Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, et al. Depressed Adolescents Grown Up. JAMA. 1999;281:1707–1713. doi: 10.1001/jama.281.18.1707. [DOI] [PubMed] [Google Scholar]
- 11.Weissman MM, Wolk S, Wickramaratne P, Goldstein RB, Adams P, Greenwald S, et al. Children with prepubertal-onset major depressive disorder and anxiety grown up. Arch Gen Psychiatry. 1999;56:794–801. doi: 10.1001/archpsyc.56.9.794. [DOI] [PubMed] [Google Scholar]
- 12.Luppa M, Heinrich S, Angermeyer MC, König H-H, Riedel-Heller SG. Cost-of-illness studies of depression: a systematic review. J Affect Disord. 2007;98:29–43. doi: 10.1016/j.jad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. J Am Med Assoc. 2003;289:3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States. J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 15.Singh I, Rose N. Biomarkers in psychiatry. Nature. 2009;460:202–207. doi: 10.1038/460202a. [DOI] [PubMed] [Google Scholar]
- 16.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–25. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, et al. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47:1031–40. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67:380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olino TM, Silk JS, Osterritter C, Forbes EE. Social reward in youth at risk for depression: A preliminary investigation of subjective and neural differences. J Child Adolesc Psychopharmacol. 2015;25:711–721. doi: 10.1089/cap.2014.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiol Dis. 2013;52:66–74. doi: 10.1016/j.nbd.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanson JL, Hariri AR, Williamson DE. Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol Psychiatry. 2015;78:598–605. doi: 10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stringaris A, Vidal-Ribas Belil P, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, et al. The brain’s response to reward anticipation and depression in adolescence: Dimensionality, specificity, and longitudinal predictions in a community-based sample. Am J Psychiatry. 2015;172:1215–1223. doi: 10.1176/appi.ajp.2015.14101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stringaris A. Editorial : Neuroimaging in clinical psychiatry – when will the pay off begin ? J Child Psychol Psychiatry. 2015;12:1263–1265. doi: 10.1111/jcpp.12490. [DOI] [PubMed] [Google Scholar]
- 25.Proudfit GH. The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology. 2015;52:449–459. doi: 10.1111/psyp.12370. [DOI] [PubMed] [Google Scholar]
- 26.Holroyd CB, Pakzad-Vaezi KL, Krigolson OE. The feedback correct-related positivity: Sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology. 2008;45:688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- 27.Levinson A, Speed B, Hajcak G. Reliability of the electrocortical response to gains and losses in the Doors task. Psychophysiology. 2017;54:601–607. doi: 10.1111/psyp.12813. [DOI] [PubMed] [Google Scholar]
- 28.Luking KR, Nelson BD, Infantolino ZP, Sauder CL, Hajcak G. Internal consistency of fMRI and EEG measures of reward in late childhood and early adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:289–297. doi: 10.1016/j.bpsc.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bress JN, Hajcak G. Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology. 2013;50:610–616. doi: 10.1111/psyp.12053. [DOI] [PubMed] [Google Scholar]
- 30.Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: A combined ERP and fMRI study. Neuroimage. 2011;57:1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 31.Foti D, Weinberg A, Dien J, Proudfit GH. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Hum Brain Mapp. 2011;32:2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker MPI, Nitsch AM, Miltner WHR, Straube T. A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. J Neurosci. 2014;34:3005–12. doi: 10.1523/JNEUROSCI.3684-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bress JN, Smith E, Foti D, Klein DN, Hajcak G. Neural response to reward and depressive symptoms in late childhood to early adolescence. Biol Psychol. 2012;89:156–162. doi: 10.1016/j.biopsycho.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belden AC, Irvin K, Hajcak G, Kappenman ES, Kelly D, Karlow S, et al. Neural correlates of reward processing in depressed and healthy preschool-age children. J Am Acad Child Adolesc Psychiatry. 2016;55:1081–1089. doi: 10.1016/j.jaac.2016.09.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foti D, Kotov R, Klein DN, Hajcak G. Abnormal neural sensitivity to monetary gains versus losses among adolescents at risk for depression. J Abnorm Child Psychol. 2011;39:913–924. doi: 10.1007/s10802-011-9503-9. [DOI] [PubMed] [Google Scholar]
- 36.Kujawa A, Proudfit GH, Klein DN. Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. J Abnorm Psychol. 2014;123:287–97. doi: 10.1037/a0036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50:74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- 38.Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G. Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. Am J Psychiatry. 2016;173:1223–1230. doi: 10.1176/appi.ajp.2016.15121524. [DOI] [PubMed] [Google Scholar]
- 39.Bernat EM, Nelson LD, Baskin-Sommers AR. Time-frequency theta and delta measures index separable components of feedback processing in a gambling task. Psychophysiology. 2015;52:626–637. doi: 10.1111/psyp.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernat EM, Nelson LD, Holroyd CB, Gehring WJ, Patrick CJ. Separating cognitive processes with principal components analysis of EEG time-frequency distributions. Proc SPIE. 2008;7074:70740S–70740S–10. [Google Scholar]
- 41.Nelson LD, Patrick CJ, Collins P, Lang AR, Bernat EM. Alcohol impairs brain reactivity to explicit loss feedback. Psychopharmacology. 2011;218:419–428. doi: 10.1007/s00213-011-2323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35:968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foti D, Weinberg A, Bernat EM, Proudfit GH. Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clin Neurophysiol. 2015;126:1338–1347. doi: 10.1016/j.clinph.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb CA, Auerbach RP, Bondy E, Stanton CH, Foti D, Diego A, et al. Abnormal neural responses to feedback in depressed adolescents. J Abnorm Psychol. 2017;126:19–31. doi: 10.1037/abn0000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knyazev GG. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci Biobehav Rev. 2007;31:377–395. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 47.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) 1997 [Google Scholar]
- 48.Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, et al. Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II) Assessment. 2012;19:399–420. doi: 10.1177/1073191112449857. [DOI] [PubMed] [Google Scholar]
- 49.Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, et al. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS) Psychol Assess. 2007;19:253–268. doi: 10.1037/1040-3590.19.3.253. [DOI] [PubMed] [Google Scholar]
- 50.Watson D, O’Hara MW, Chmielewski M, McDade-Montez EA, Koffel E, Naragon K, Stuart S. Further validation of the IDAS: evidence of convergent, discriminant, criterion, and incremental validity. Psychol Assess. 2008;20:248–259. doi: 10.1037/a0012570. [DOI] [PubMed] [Google Scholar]
- 51.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Nolan H, Whelan R, Reilly RB. FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. J Neurosci Methods. 2010;192:152–162. doi: 10.1016/j.jneumeth.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 53.Bernat EM, Williams WJ, Gehring WJ. Decomposing ERP time-frequency energy using PCA. Clin Neurophysiol. 2005;116:1314–1334. doi: 10.1016/j.clinph.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 54.Dien J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. J Neurosci Methods. 2010;187:138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Cattell R. The Scree test for the number of factors. Multivariate Behav Res. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- 56.Foti D, Weinberg A, Bernat EM, Proudfit GH. Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clin Neurophysiol. 2015;126:1338–1347. doi: 10.1016/j.clinph.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer A, Lerner M, Reyes A, Laird R, Hajcak G. Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology. 2017;54:114–122. doi: 10.1111/psyp.12664. [DOI] [PubMed] [Google Scholar]
- 58.Weinberg A, Venables NC, Proudfit GH, Patrick CJ. Heritability of the neural response to emotional pictures: evidence from ERPs in an adult twin sample. Soc Cogn Affect Neurosci. 2015;10:424–434. doi: 10.1093/scan/nsu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–77. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 61.Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 62.Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Soc Cogn Affect Neurosci. 2014;9:1798–1807. doi: 10.1093/scan/nst175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forbes EE. Where’s the fun in that? Broadening the focus on reward function in depression. Biol Psychiatry. 2009;66:199–200. doi: 10.1016/j.biopsych.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bernat EM, Nelson LD, Steele VR, Gehring WJ, Patrick CJ. Externalizing psychopathology and gain-loss feedback in a simulated gambling task: dissociable components of brain response revealed by time-frequency analysis. J Abnorm Psychol. 2011;120:352–364. doi: 10.1037/a0022124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klein DN, Kotov R, Bufferd SJ. Personality and depression: explanatory models and review of the evidence. Annu Rev Clin Psychol. 2011;7:269–295. doi: 10.1146/annurev-clinpsy-032210-104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.John OP, Donahue EM, Kentle RL. The Big Five Inventory–Versions 4a and 54. Berkley, CA: University of California, Berkele, Institute of Personality and Social Research; 1991. [Google Scholar]
- 68.John OP, Naumann LP, Soto CJ. Paradigm shift to the integrative big-five trait taxonomy: History, measurement, and conceptual issues. Handb Personal Theory Res. 2008:114–158. [Google Scholar]


