Abstract
People with benign paroxysmal positional vertigo (BPPV) probably have otoconial particles displaced from the utricle into the posterior semicircular canal. This unilateral change in the inertial load distributions of the labyrinth may result in visual dependence and may affect balance control. The goal of this study was to explore the interaction between visual dependence and balance control. We compared 23 healthy controls to 17 people with unilateral BPPV on the Clinical Test of Sensory Interaction and Balance on compliant foam with feet together, the Rod-and-Frame Test and a Mental Rotation Test. In controls, but not BPPV subjects, subjects with poor balance scores had significantly greater visual dependence, indicating that reliance on visual cues can affect balance control. BPPV and control subjects did not differ on the mental rotation task overall but BPPV reaction time was greater at greater orietantions, suggesting that this cognitive function was affected by BPPV. The side of impairment was strongly related to the side of perceived bias in the Earth vertical determined by BPPV subjects, indicating the relationship between the effect of asymmetric otolith unloading with simultaneous canal loading on spatial orientation perception.
Keywords: BPPV, Rod and Frame Test, Mental Rotation, CTSIB, Balance testing, screening, Romberg, vestibular testing, visual dependence
1. Introduction
Accurate perception of vertical orientation is essential to achieving proper balance control. On Earth, the three types of vertical orientation are: the physical vertical, the behavioral/postural vertical, and the subjective perception of vertical [1]. This conscious perception of verticality was once thought to be just a bottom-up process, requiring integration of visual, vestibular, and somatosensory cues [5], but subsequent studies found that mental processes such as the awareness of body orientation also contribute to perception of verticality [2]. These representations of verticality are often affected by an individual's ability to reweight multi-modal sensory input dynamically. In other words, sometimes, when sensory inputs involved in spatial orientation conflict, one input is favored over the other [6].
Several studies have shown that humans have the ability for “sensory reweighting” for postural control. The term, sensory reweighting, refers to the ability to regulate and integrate sensory information dynamically to adapt to changing environments or sensory inputs [22, 23]. For example, an individual getting up in the middle of the night might leave the room lights off and might therefore rely more heavily on proprioception than vision in the minimal ambient light, but in daylight the same individual might rely more heavily on vision. Also, sensory reweighting occurs in astronauts who become more dependent on vision and less on proprioception and vestibular inputs when they adapt to the microgravity environment of spaceflight, [24, 25, 26]. Upon return to Earth they must then unlearn the sensory reweighting that was so useful in microgravity but which is maladaptive in normal gravitational conditions.
The concept of visual frame dependence incorporates the individual's ability to adapt sensorimotor control to situations by the selection of frames of reference. This characteristic may differ based on the individuals in question and their specific perceptual styles. Witkin et al. proposed a sliding scale from visual dependence to visual independence based on the individual's tendency to consider or ignore misleading visual inputs when making decisions regarding spatial orientation [11].
Increased visual dependence can affect an individual's locomotor performance and spatial orientation with greater perceptual response time in completing tasks [12], biased body orientation relative to the frame of reference [9, 13], diminished postural stability [13], and a decreased ability to adapt to sudden environmental changes [9, 14]. Visual dependence increases with advancing age [4, 8], putting older adults at increased risk for falls and decreased autonomy stemming from postural instability [16]. This rise in visual dependence with advancing age has is also related to reduced visual fixation stability, reduced visual attentional processing, and a decreased useful field of view, all of which lessen their abilities to perform daily living tasks [8].
The Rod-and-Frame Test [6] measures bias in an individual's subjective vertical and level of visual dependence. In this task, the subject sits in a darkened room with the head stabilized and attempts to adjust a rod to the vertical within the context of a tilted frame [6]. Initially involving a mechanical apparatus [7], the standard version of the test now is computerized [17, 18]. Persons who can accurately align the rod with the vertical, regardless of the orientation of the visual frame, are classified as “field independent”, while persons who significantly deviate from the vertical and align the rod with the direction of the tilt of the frame are classified as “field dependent”.
The Mental Rotation Test (MRT) is another measure of the integration of multiple systems for cognitive reasoning. Mental transformations of visuospatial images are crucial in both navigation and reasoning, requiring the use of multiple spatial frames of reference and the coordination of different neural processes, making the test one of both accuracy and reaction time [19]. For a matching task involving mental rotation of three-dimensional objects, reaction time increases and accuracy decreases linearly as the angular difference between the displayed images increases [20].
Spatial disorientation is characteristic of disorders of the vestibular system. A common vestibular disorder is benign paroxysmal positional vertigo (BPPV), in which the calcium carbonate crystals (otoconia) in the utricle of the vestibular labyrinth probably enter one of the semicircular canals, usually the posterior canal, and cause inertial changes in the semicircular canal and in the utricle, resulting in attacks of vertigo and abnormal nystagmus when the head changes position [3]. Agarwal et al. [3] reported that acutely ill, symptomatic BPPV patients and chronically ill, asymptomatic BPPV patients have increased visual dependence compared to controls. Moreover, in other studies [27, 28, 29] BPPV patients have shown reduced postural control abilities. In the current study, we investigated the interaction of balance abilities and visual field dependency characteristics in patients with symptomatic, unilateral BPPV of the posterior semicircular canal and control subjects. We tested performance on the Rod-and-Frame Test, on mental rotation ability in general with a Mental Rotation Test, and the Clinical Test of Sensory Interaction and Balance (CTSIB) [21] as a measure of standing balance control.
2. Methods
2.1 Subjects
Seventeen patient subjects had been diagnosed with unilateral BPPV of the posterior semicircular canal by board certified otolaryngologists, neurologists and internists. The diagnosis was verified with a positive response to the Dix-Hallpike maneuver. All patient subjects were recruited from the vestibular rehabilitation caseload of one of the authors (HSC). All patient subjects were tested in this study prior to being treated for BPPV. Twenty three healthy control subjects were also screened using the Dix-Hallpike maneuver and head impulse tests. See Table 1. All subjects gave informed consent prior to participating. This study was approved by the Institutional Review Board for Baylor College of Medicine and Affiliated Hospitals.
Table 1. Mean (SEM) of the number of subjects (N), age (years), gender distribution (male (M)/female (F)), and the number of subjects for each side affected by BPPV (right (R)/left (L)).
| Controls | Patients | |
|---|---|---|
| N | 23 | 17 |
| Age | 52 (±3.22) | 63 (±2.45) |
| Gender | 7 M, 16 F | 1 M, 16 F |
| BPPV Side | - | 8 R, 9 L |
2.2. Measurements
2.2.1 Clinical Test of Sensory Interaction and Balance (CTSIB)
All subjects performed the CTSIB with feet together while standing on 10 cm. medium density, compliant foam (Sunmate; Dynamic Systems, Leicester, NC), first with their eyes open, then closed, for a maximum of 30 seconds per trial. The time that the subject could stand on the foam until loss of balance was measured with a stopwatch. Loss of balance was defined as when the subject opened his eyes, moved his arms away from his body, or took a step [21]. The CTSIB in the eyes closed condition while standing on foam is considered a measure of the subject's ability to utilize vestibular inputs for postural control during standing, since the visual and proprioceptive inputs have been made nonexistent and unreliable, respectively.
2.2.2. Rod and Frame Test (RFT)
The RFT was performed in a darkened room with the subject seated erect and vertically aligned, with vision centered on the screen and the head stabilized. The computerized rod-and-frame program, developed using LabVIEW software (National Instruments, TX), was displayed on a high-resolution computer monitor that subjects viewed through a 0.6 m-long, 30 cm. × 30 cm. section tube. On this monitor, a single white line 1 cm.-wide and 20 cm.-long was displayed on a black background. Subjects were instructed to align the rod with the “perceived Earth vertical” using the keys on a gamepad. The rod could be rotated either clockwise or counterclockwise using the buttons on the gamepad, with a coarse adjustment step of 1.4° and a fine adjustment step of 0.1° for each button press.
Subjects pressed two buttons on the gamepad simultaneously to begin the next trial when they were satisfied with the rod's alignment. Subjects were instructed to close their eyes at the end of the trial for at least 5 seconds, and then open their eyes to view a blank white screen before the start of the next trial. Per the usual sequence used in previous studies, subjects were given eight trials, with both the rod and frame being rotated to either +18° (L) or -18° (R) with a frame tilt sequence of LLRRLLRR and a rod tilt sequence of LRRLLRRL [18]. The rod angle error relative to the actual vertical was measured for each of the trials. In 1974, Nyborg and Isaksen formulated a method to analyze the measurement obtained from the Rod-and-Frame Test [10], resulting in the measures of the frame effect and constant error. The constant error measures the subjects’ average bias in estimation of the true vertical across all trials and is characteristic of each subject. The frame effect is the main measure of visual dependence, which shows the magnitude of the error in the subjects’ estimate of the vertical caused by the tilted frame regardless of direction of frame or rod tilt, while also taking into account individual subjects’ bias [10].
2.2.3. Mental Rotation Test (MRT)
The MRT was given immediately following the RFT. The subject sat in the same position as during the RFT, but the 0.6 m viewing tube was removed. The initial presentation displayed a 3D figure composed of cubes for 3 seconds, followed by a blank screen for 2 seconds. Then, a pair of 3D figures appeared, one of which was congruent with the first figure shown, but had been rotated in the roll and/or pitch directions in either 0°, 20°, 40°, 80°, or 120°, while the other image shown was a different figure entirely. The subject had 10 seconds to determine which of the two figures matched the first figure, using the manual gamepad controller before the start of the next trial. The subject had to complete 26 trials as quickly and accurately as possible, making this a test of both accuracy and reaction time. Two trials were 0° pairs and six trials each were 20°, 40°, 80°, and 120° pairs. The main outcome measures were percentage accuracy and mean reaction time.
2.3 Statistical Analysis
Patients and controls were compared on normally distributed continuous variables by t-test, on non-normal data by Wilcoxon rank sum test, and on grouped data by chi-square/Fisher exact test. Using general linear mixed models, we compared healthy controls to patients on the mental rotation test. Least square mean for accuracy and reaction time was determined at baseline (0°) and for each degree of rotation (20°, 40°, 80°,120°) and was compared between the two groups . P values were adjusted for multiple comarision by tukey method. P<.05 was considered statistically significant. All analyses were performed in SAS Statistical Software (version 9.4, Carry, NC).
3. Results
3.1. CTSIB
BPPV subjects stood for significantly less time than controls during the eyes closed condition (Wilcoxon rank test p=0.0004). No difference was found between the two groups in the eyes open condition (p=0.23). Table 2 shows the average (SEM) time for which control and BPPV subjects were able to stand during the CTSIB test for the two visual conditions.
Table 2. CTSIB mean performance (SEM) in both eyes open and eyes closed conditions across subjects in the two groups.
| Eyes Open Time (sec.) |
Eyes Closed Time (sec.) |
|
|---|---|---|
| Controls | 29.09 (±.91) | 23.05(±2.49) |
| BPPV | 27.35 (±1.82) | 8.06 (±2.41) |
3.2. Rod-and-Frame Test (RFT)
In the RFT, the dependent variables were the magnitude of the subject's frame effect and the magnitude and direction of constant error. Frame effect gives a measure of a subject's level of field dependency, while constant error provides a measure of the subjects’ bias in their self-estimated vertical. The sign of the constant error indicates direction: a negative constant error indicates a rightward bias, while a positive constant error indicates a leftward bias. There were no significant differences between the two groups for these measures (p>0.05). Frame effect increased with age for the BPPV group (r=0.57, p=0.016), but not for the control group. The constant error was not significantly related to age for both the control and BPPV groups. See Table 3.
Table 3. Rod and Frame test mean (SEM) performance for Constant Error (self-perception error of vertical) and Frame Effect (visual dependence) across subjects in the two groups.
| Rod-and-Frame Test | ||
|---|---|---|
|
| ||
| Constant Error (°) | Frame Effect (°) | |
| Controls | .36 (±.36) | 10.62 (±.98) |
| BPPV | .91 (±.46) | 10.56 (±1.23) |
Approximately equal numbers of patient subjects had left side and right side BPPV. Patients with right BPPV were more likely to have a negative constant error and thus a rightward bias, and patients with left BPPV were more likely to have a positive constant error and a leftward bias, thus demonstrating a significant association (p=0.003) between the side of vestibular impairment and the direction of the bias of the subjects’ estimate of vertical away from the direction of gravity. See Figure 1.
Figure 1.
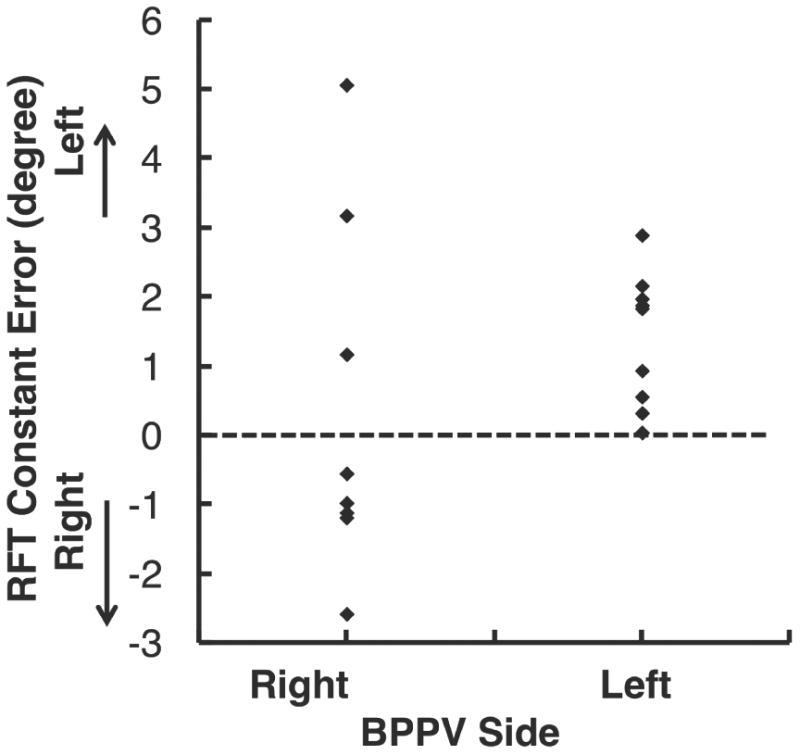
Relationship between the affected side of the BPPV subject and the constant error (degrees) as measured by the Rod and Frame Test for BPPV subjects. The constant error measures the subjects’ average bias in estimation of the true vertical across all trials. BPPV subjects showed a significant bias in the direction of perception of the true vertical to the affected BPPV side. The broken line indicates zero bias with respect to true Earth vertical.
As shown in Figure 2, performance on CTSIB in the eyes closed condition, most controls (N=16, 70%) did not fall and were able to perform CTSIB for 30 seconds. All controls who fell in less than 10 seconds (N=6) had higher frame effect scores. In fact the scores for early fallers (median: 14.8; interquatile range [IQR]: 12.2-15.8) was significantly higher than controls who did not fall and were able to perform CTSIB for 30 seconds (median= 8.0; IQR: 5.4-13.2) (p=.041), Wilcoxon rank test). This finding indicates that normal subjects who show a greater frame effect, and hence are relatively more visually dependent may have worse standing balance on the CTSIB eyes closed condition as they may be challenged by the compliant unstable support surface. In contrast, the majority of BPPV subjects (82%) fell early (≤10 min) and only 3 could perform the CTSIB task for nearly 30 seconds; these two groups did not differ significantly on frame effect magnitude (median, IQR: 12.3, 3.3-17.1 and 11.0; 5.4-14.8 respectively; p=.95) .
Figure 2.

CTSIB performance eyes closed time (seconds) vs the frame effect (degrees) as measured by the Rod and Frame Test for the control (A) and BPPV (B) subjects. In the control group (A), subjects who could perform the test for 10 seconds or less had a significantly higher average magnitude of the frame effect compared to the subjects who were able to complete the test at 30 seconds (p=0.02). The BPPV subjects (B) did not show this variation in relationship between their scores in the CTSIB with eyes closed and the frame effect.
3.3 Mental Rotation Test (MRT)
In this task, the dependent variables were reaction time and percentage correct, both overall and for each of the degrees of rotation in the task. Overall, the patient and control groups did not differ significantly on reaction time (p=.20) or accuracy (p=.13), as shown in Table 4. Tables 5 and 6 show the breakdown of the two measures across the five orientations. In general, for orientations from 0° to 40°, accuracy was significantly reduced for patients (p<.0001) and controls (<.0001). No significant change in accuracy was seen in orientations beyond 40° wihin either group (p>.05). This finding indicates that both controls and BPPV subjects performed the MRT with the same degree of accuracy across the different angular orientations.
Table 4.
Mental Rotation task mean (SEM) performance across subjects in the two groups.
| Mental Rotation Task | ||
|---|---|---|
|
| ||
| Reaction Time (sec.) | Percent Correct (%) | |
| Controls | 3.13 (±.20) | 43.30 (±2.00) |
| BPPV | 3.68 (±.23) | 39.95 (±2.33) |
Table 5.
Mental Rotation Task mean reaction time (SEM) broken down by degree of rotation across subjects in the two groups.
| Mental Rotation Task – Reaction Time (sec.) | |||||
|---|---|---|---|---|---|
|
| |||||
| 0° | 20° | 40° | 80° | 120° | |
| Controls | 2.93 (±.36) | 2.96 (±.23) | 3.06 (±.26) | 3.03 (±.23) | 3.44 (±.22) |
| BPPV | 2.73 (±.19) | 3.44 (±.21) | 3.70 (±.34) | 3.78 (±.26) | 3.82 (±.25) |
Table 6.
Mental Rotation Task mean (SEM) percentage correct broken down by degree of rotation across subjects in the two groups.
| Mental Rotation Task – Percentage Correct (%) | |||||
|---|---|---|---|---|---|
|
| |||||
| 0° | 20° | 40° | 80° | 120° | |
| Controls | 80.43 (±5.20) | 55.07 (±4.41) | 35.51 (±4.16) | 47.82 (±4.17) | 34.78 (±4.49) |
| BPPV | 70.59 (±7.50) | 59.80 (±4.30) | 33.33 (±4.04) | 35.29 (±4.49) | 31.37 (±3.16) |
The reaction time in BPPV patients increased significantly across orientations from 0° to 20° (p=.04). No further changes were found in BPPV subjects testest with oreintations greater than 20° (p>0.05). Among controls, no change in reaction time was found with increasing degrees of rotation (p>.05). Thus, control subjects performed MRT with no change in reaction time at all angular orientations, but BPPV subjects performed MRT more slowly at higher angular orientations compared to the 0° orientation condition..
Discussion
We examined the difference in performance on CTSIB, RFT and MRT between BPPV subjects and age-matched control subjects with no prior history of vertigo. Both subject groups performed MRT equally well overall in terms of accuracy and reaction time. Upon investigating the trends across increased angular rotations for both groups, however, we found that although the BPPV subjects were as accurate as the control subjects across the tested magnitude of rotations, they did so with slower reaction times at higher angular orientations compared to 0° rotations while the control subjects showed no change in reaction time compared to 0° rotations. Hence, BPPV seemed to decrease mental rotation capability by increasing the reaction times.
Most BPPV patients were unable to stand on foam with their eyes closed for 30 seconds, potentially indicating a decreased use of vestibular information even though the disruption caused by the abnormal transduction process is only on the affected side. This finding replicates our previous work [27, 28, 29]. The reason may be that the subjects used information bilaterally, and through vestibular callosal projections, so this asymmetrical input could have affected the vestibulospinal tracts output bilaterally. More importantly, patients with BPPV showed a tendency to bias their perception of true physical vertical, as measured by the constant error from the RFT, towards the side affected by BPPV. This finding indicates that these subjects had an imbalance in their perception of vertical between the intact and impaired sides. Hence they have a tendency to over-compensate the bias of their self-perception of vertical.
Control subjects who could not perform CTSIB with their eyes closed for 30 seconds showed significantly higher visual dependence scores than subjects who stood for the full 30 seconds. Exploration of this relationship in Figure 2 shows that all control subjects who were unable to complete the CTSIB test, because they stood for 10 seconds or less, showed a higher degree of visual dependence. Thus, these visually dependent control subjects, although possessing intact vestibular and somatosensory sensory systems, showed reduced postural stability control. This finding may indicate that either these subjects were more reliant on single sensory modality of vision while not making use of available vestibular or somatosensory inputs, even though the sensorimotor inputs may have been degraded by the compliant foam surface. By contrast, among the group of control subjects who were able to stand for 30 seconds during CTSIB some of the control subjects demonstrated greater visual dependence (> 10° frame effect) and performed equally well as subjects who showed a relatively smaller frame effect. Thus, control subjects who maintained postural stability on the CTSIB task with their eyes closed for the full 30 seconds should have been multi-sensory dependent, i.e. regardless of reliance on visual information they may have learnt to utilize information from multiple sensory systems including the vestibular and somatosensory systems to help maintain postural control.
We have shown that patients with unilateral BPPV of the posterior semicircular canal have varied magnitudes of visual dependency not significantly different from the controls as measured by the frame effect. Although the measure of visual dependence, i.e. the frame effect, in the RFT differed from that reportedly calculated in Agarwal et al., they found similar results in symptomatic BPPV subjects [3]. Agarwal et al., also measured responses to two additional dynamic visual dependence tests, the Rod and Disc test and Disc postural tests [3], which were not done in this study. They reported that both symptomatic and asymptomatic patient groups showed increased disc dependence and said that BPPV subjects were more visually dependent compared to controls, but only on the dynamic task. More importantly, their acutely ill BPPV patients who were tested pre-treatment did not differ significantly from controls on the static rod-and-frame test as was found in our study. That finding may be attributed to the high variability in static visual dependence measured in both the BPPV and control groups in both studies.
Agarwal et al [3] also reported that across the symptomatic and asymptomatic patient groups 85% of their subjects were able to complete the disc dependence test that required them to stand on a solid surface with feet placed at 30 degrees and heels 7 cm apart. BPPV patients in the present study, by contrast, performed the more challenging task of standing on foam with feet together and eyes closed. They were mostly unable to complete the CTSIB task, matching results from previous studies showing reduced postural control abilities in acute BPPV patients who were tested with feet placed shoulder width apart on a solid surface [27, 28, 29]. In this study we have shown that the reduced postural control in BPPV patients occurred regardless of the magnitude of the frame effect. However, as seen in Figure 2, three BPPV subjects were able to perform the CTSIB task in the eyes closed condition for 30 seconds -- the maximum length of the test. One was the BPPV subject with a relatively smaller frame effect (< 4 degree) showing that this BPPV subject was not visually dependent. The other two BPPV subjects showed a larger frame effect (>12 degree) indicating that they were highly visually dependent. Perhaps, in these three cases, the subjects could utilize information from either their vestibular receptors on the intact side or the somatosensory system that most patients and some controls mostly ignored or a combination of the two.
This co-variation of individuals’ sensory preferences in controls and BPPV subjects leads us to postulate that individuals who depend on a single sensory modality and reduced function within a given sensory modality will have a reduced ability to adapt their balance control abilities when exposed to a novel sensorimotor rearrangement compared to subjects who use multiple sensory modalities more equally. This idea has implications for balance training in elderly people, patients with vestibular disorders, and peripheral neuropathy, as well as in manned spaceflight. A few studies have established that when the vestibular signal is unreliable or not utilized appropriately, as in BPPV [3] or in vestibular neuritis [30] patients, subjects can reweight their sensory utilization during the integration process and rely excessively on visual input that can lead to visual vertigo. Canalith repositioning treatments reduce symptoms of vertigo in BPPV patients [28, 29]. However, BPPV patients take more time to recover fully their postural control abilities [28, 29] and thus the above mentioned rehabilitation strategies may not work for all subjects equally. Hence, balance training strategies that optimize the sensorimotor integration process by utilizing multiple sensory inputs will enable the development of personalized exercise prescriptions for these BPPV patients as well as in crewmembers to mitigate sensorimotor associated problems in exploration class missions after g-transitions.
Acknowledgments
Grant support: Supported by National Institutes of Health grant R01DC009031 (HSC), and by a grant from the National Space Biomedical Research Institute through NASA NCC 9-58 (APM, JJB).
References
- 1.Luyat M, Ohlmann T, Barraud P. Subjective vertical and postural activity. Acta Psychol. 1997;95:181–193. doi: 10.1016/s0001-6918(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 2.Barra J, Pérennou D, Thilo KV, Gresty M, Bronstein AM. The awareness of body orientation modulates the perception of visual vertical. Neuropsychologia. 2012;50:2492–2498. doi: 10.1016/j.neuropsychologia.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal K, Bronstein AM, Faldon ME, Mandalà M, Murray K, Silove Y. Visual dependence and BPPV. J Neurol. 2012;259:1117–1124. doi: 10.1007/s00415-011-6311-7. [DOI] [PubMed] [Google Scholar]
- 4.Slaboda JC, Keshner EA. Reorientation to vertical modulated by combined support surface tilt and virtual visual flow in healthy elders and adults with stroke. J Neurol. 2012;259:2664–2672. doi: 10.1007/s00415-012-6566-7. [DOI] [PubMed] [Google Scholar]
- 5.Mittelstaedt H. A new solution to the problem of the subjective vertical. Naturwissenschaften. 1983;70:272–281. doi: 10.1007/BF00404833. [DOI] [PubMed] [Google Scholar]
- 6.Witkin HA, Asch SE. Studies in space orientation; further experiments on perception of the upright with displaced visual fields. J Exp Psychol. 1984;38:762–82. doi: 10.1037/h0053671. [DOI] [PubMed] [Google Scholar]
- 7.Oltman PK. A portable rod-and-frame apparatus. Percept Motor Skill. 1968;26:503–506. doi: 10.2466/pms.1968.26.2.503. [DOI] [PubMed] [Google Scholar]
- 8.Agathos CP, Bernardin D, Huchet D, Scherlen A, Assaiante C, Isableu B. Sensorimotor and cognitive factors associated with the age-related increase of visual field dependence: a cross-sectional study. Age. 2015;37:67–85. doi: 10.1007/s11357-015-9805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isableu B, Ohlmann T, Cremieux J, Vuillerme N, Amblard B, Gresty MA. Individual differences in the ability to identify, select, and use appropriate frames of reference for perceptuo-motor control. Neurosci. 2010;169:1199–1215. doi: 10.1016/j.neuroscience.2010.05.072. [DOI] [PubMed] [Google Scholar]
- 10.Nyborg H. A method for analyzing performance in the rod-and-frame test I. Scand J Psychol. 1974;15:119–123. [Google Scholar]
- 11.Witkin HA, Lewis HB, Hertzman M, Machover K, Meissner PB, Wapner S. Personality through perception: an experimental and clinical study. Greenwood Press; Westport: 1954. [Google Scholar]
- 12.Yan JH. Cognitive styles affect choice response time and accuracy. Personal Individ Differ. 2010;48:747–751. [Google Scholar]
- 13.Isableu B, Ohlmann T, Cremieux J, Amblard B. Selection of spatial frame of reference and postural control variability. Exp Brain Res. 1997;114:584–589. doi: 10.1007/pl00005667. [DOI] [PubMed] [Google Scholar]
- 14.Brady RA, Peters BT, Batson CD, Ploutz-Snyder R, Mulavara AP, Bloomberg J/J/ Gait adaptability training is affected by visual dependency. Exp Brain Res. 2012;220:1–9. doi: 10.1007/s00221-012-3109-5. [DOI] [PubMed] [Google Scholar]
- 15.Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eikema DJA, Hatzitaki V, Tzovarad D, Papaxanthis C. Age-dependent modulation of sensory reweighting for controlling posture in a dynamic virtual environment. Age. 2012;34:1381–1392. doi: 10.1007/s11357-011-9310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagust J. Assessment of verticality perception by a rod-and-frame test: preliminary observations on the use of a computer monitor and video eye glasses. Arch Phys Med Rehabil. 2006;86:1062–1064. doi: 10.1016/j.apmr.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Isableu B, Gueguen M, Fourré B, Giraudet G, Amorim M. Assessment of visual field dependence: comparison between the mechanical 3D rod-and-frame test developed by Oltman in 1968 with a 2D computer-based version. J Vestib Res. 2008;18:239–247. [PubMed] [Google Scholar]
- 19.Zacks JM, Michelon P. Transformations of visuospatial images. Behav Cogn Neurosci Rev. 2005;4:96–118. doi: 10.1177/1534582305281085. [DOI] [PubMed] [Google Scholar]
- 20.Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171:701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- 21.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction on balance: suggestion from the field. Phys Ther. 1986;66:1548–1550. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 22.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 23.Mahboobin S, Loughlin P, Redfern MS, Sparto PJ. Sensory re-weighting in human postural control during moving-scene perturbations. Exp Brain Res. 2005;167:260–267. doi: 10.1007/s00221-005-0053-7. [DOI] [PubMed] [Google Scholar]
- 24.Reschke MF, Bloomberg JJ, Harm DL, Paloski WH, Layne C, McDonald V. Posture, locomotion, spatial orientation, and motion sickness as a function of space flight. Brain Res Brain Res Rev. 1998;28:102–117. doi: 10.1016/s0165-0173(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 25.Bloomberg JJ, Peters BT, Cohen HS, Mulavara AP. Enhancing astronaut performance using sensorimotor adaptability training. Front Syst Neurosci. 2015;9:129. doi: 10.3389/fnsys.2015.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seidler RD, Mulavara AP, Bloomberg JJ, Peters BT. Individual predictors of sensorimotor adaptability. Front Syst Neurosci. 2015;9:100. doi: 10.3389/fnsys.2015.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulavara AP, Cohen HS, Peters BT, Sangi-Haghpeykar H, Bloomberg JJ. New analyses of the Sensory Organization Test compared to the Clinical Test of Sensory Integration and Balance in patients with benign paroxysmal positional vertigo. Laryngoscope. 2013;123:2276–2280. doi: 10.1002/lary.24075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen HS, Kimball KT. Effectiveness of treatments for benign paroxysmal positional vertigo of the posterior canal. Otol Neurotol. 2005;26:1034–1040. doi: 10.1097/01.mao.0000185044.31276.59. [DOI] [PubMed] [Google Scholar]
- 29.Cohen HS, Sangi-Haghpeykar H. Canalith repositioning variations for benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2010;143:405–412. doi: 10.1016/j.otohns.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


