Abstract
Bax is a pro-apoptotic cytosolic protein. In this work native (unphosphorylated) and JNK3 phosphorylated Bax proteins are studied on artificial bilayer membranes for pore formation. Phosphorylated Bax formed pore on the bilayer lipid membrane whereas native one does not. In cells undergoing apoptosis the pore formed by the phosphorylated Bax could be important in cytochrome c release from the mitochondrial intermembrane space to the cytosol. The low conductance (1.5 nS) of the open state of the phosphorylated Bax pore corresponds to pore diameter of 0.9 nm which is small to release cytochrome c (∼3.4 nm). We hypothesized that JNK3 phosphorylated Bax protein can form bigger pores after forming complexes with other mitochondrial proteins like VDAC, t-Bid etc. to release cytochrome c.
Keywords: Cytochrome c, Bax protein, JNK3, Phosphorylation, Ion channel
Abbreviations: JNK3, c-Jun N-terminal Kinase-3; Phospho-Bax, Phosphorylated Bax; MAPK, Mitogen Activated Protein Kinase; BLM, Bilayer Lipid Membrane; OMM, Outer Mitochondrial Membrane
Highlights
-
•
JNK3 phosphorylates Bax protein.
-
•
Monomeric full length native Bax protein do not form ion pore on BLM.
-
•
JNK3 phosphorylated full length Bax protein forms pore on BLM.
-
•
I–V plot of JNK3 phosphorylated Bax pore is linear.
-
•
Open probability of JNK3 phosphorylated Bax pore increases with increasing voltages.
1. Introduction
Bax is a pro-apoptotic cytosolic protein which is normally present in inactive state [1]. An apoptotic stimulus can cause activation of Bax by conformational rearrangement/s which is followed by its translocation towards mitochondria. At the mitochondrial outer membrane activated Bax promotes the release of cytochrome c (a mitochondrion intermembrane space protein) into the cytosol. Cytochrome c activates caspase proteases which degrade cytosolic proteins leading to apoptosis [1]. Thus, activation of Bax is an important step in apoptosis. Bax can be activated by different mechanisms [2], [3], [4], [5], [6]. One of the mechanism of Bax activation is by its phosphorylation [7], [8], [9], [10], [11], [12], [13], [14]. c-Jun N-terminal Kinases (JNKs) are cytosolic stress activated protein kinases. Bax phosphorylation by JNKs have been reported in a number of pathological states like lung cancers, nervous system disorders, liver disorders, heart diseases, muscular dystrophy, renal disorders and skin diseases [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. Furthermore, it is known that during apoptosis JNKs phosphorylated Bax promotes the release of cytochrome c into the cytosol but the mechanism how JNKs phosphorylated Bax interacts with the outer mitochondrial membrane and release it is not clear. One of the possibilities is that it forms mitochondrial membrane ion pores [25], [26], [27], [28]. In vitro studies on model membranes like artificial bilayer lipid membranes (BLMs) provide an effective means to understand the mechanism of action of membrane active molecules [29]. Keeping all this in view in the present work we have tested the possibility of ion pore formation by JNK3 phosphorylated Bax protein on artificial BLM using bilayer electrophysiology method.
2. Materials
GST protein (25 kDa) tagged monomeric full length human Bax protein (24 kDa) expressed in Escherichia coli was purchased from Signal Chem. Company, USA. Bax protein is difficult to express in E. coli cells and subsequently more difficult to purify as the protein is mostly in the inclusion bodies and is highly toxic to the host cells. Cells produce excess GST protein in order to protect themselves from the pro-apoptotic Bax protein and thus the purity of the supplied protein is only approximately 70%. Dually phosphorylated active JNK3 enzyme (JNK3 isoform is predominantly found in neurons) (human, recombinant) having more than 200 units/mg activity was purchased from Enzo Lifesciences, USA. Calf Intestinal Alkaline Phosphatase (CIAP) was purchased from New England Biolabs, Inc., USA. Peppermint stick phosphoprotein molecular weight standard, Pro-Q Diamond phosphoprotein gel stain and destain were obtained from Molecular probes, Inc. (Eugene, OR, USA). DPhPC lipid (1,2-DiPhytanoyl-sn-glycero-3-PhosphoCholine, neutral lipid) was purchased from Avanti Polar Lipids (Alabaster, AL, USA) and dissolved in chloroform at 25 mg/ml concentration.
3. Methods
3.1. Detection of phosphorylation of Bax by JNK3 enzyme using Pro-Q diamond dye
Phosphorylation of Bax protein by JNK3 enzyme was studied using the procedure standardized in our laboratory [30]. Pro-Q Diamond dye binds to the phosphate groups nonspecifically and thus identifies phosphorylated proteins distinctly from the unphosphorylated ones. Three microtubes were labeled as Negative Control (Bax protein + Mg2+ATP), Experimental Sample (Bax protein + JNK3 + Mg2+ATP) and Positive Control [Bax protein + JNK3 + Mg2+ATP + Calf Intestinal Alkaline Phosphatase (added later)]. Bax protein (8 μg), 0.5 μg of active JNK3 enzyme, ATP (Final concentration 100 μM) and MgCl2 (Final concentration 10 mM) were added to the respective tubes. Phosphorylation reaction was carried out by incubating the cocktail at 30 °C for 30 min. In the Positive Control 0.02 units of alkaline phosphatase was added and incubated for another 30 min at 30 °C. All the samples were precipitated by cold chloroform/methanol procedure. 30 μl of 1X sample buffer was added in all the tubes, pellets were dissolved and boiled for 5 min at 100 °C along with the phosphoprotein standard 2 μl in 12 μl of 1X sample buffer. Then resolved on 12.5% SDS-Polyacrylamide Tris-glycine gel (1.5 mm thickness). Staining of the gel was done using standard protocol available at the website of Molecular probes, Inc. (Eugene, OR, USA). Briefly, the gel was kept in a fixative solution (50% methanol, 10% acetic acid, 40% Milli Q water) for 30 min on a shaker. Then the fixative was replaced with fresh one and left overnight. Next day, gel was washed thrice with Milli Q water for 10 min each. It was stained using fluorescent Pro-Q Diamond phosphoprotein gel stain for approximately 1.5 h and then destained with Pro-Q Diamond phosphoprotein destain for 1.5 h thrice for 30 min each time in a dark room. The gel was washed thrice for 5 min each with Milli Q water and visualized on FLA-9000 phosphoimager (Fuji Fim Inc., Tokyo, Japan). After visualization, the gel was stained with coomassie blue dye and then destained to confirm the loading of equal amounts of Bax protein in all the lanes.
3.2. Bilayer electrophysiological studies of native (unphosphorylated) and JNK3 phosphorylated Bax proteins
Native and phosphorylated Bax proteins were tested for pore forming ability on the bilayer lipid membrane (BLM) as previously standardized in our laboratory [30]. Briefly, the apparatus consisted of a Perfusion BLM Cup (Warner Instruments Corp., Hamden, CT, USA) made up of polystyrene with a thin wall separating two aqueous compartments (cis and trans) of BLM chamber (Warner Instruments Corp.) containing BLM buffer [1 M KCl, 10 mM MgCl2, 10 mM HEPES (pH 7.4)]. The BLM Cup had a circular aperture with a diameter of 150 μm. Aqueous compartments were connected to Axopatch 200B amplifier (Axon Instruments, Sunnyvale, CA, USA) through a pair of matched Ag/AgCl electrodes. The voltage in the trans compartment was held at virtual ground (0 mV) by the amplifier and the cis compartment was connected to the headstage of the amplifier for applying the voltage. Chloroform from the DPhPC lipid solution was evaporated under nitrogen gas and the lipid was dissolved in n-decane, 8:20 (lipid: n-decane) v/v ratio. BLM was prepared by applying lipid solution to the BLM cup aperture. After formation of the leak free BLM, it was clamped at +10 mV holding potential and native or phosphorylated Bax protein (2 μg) was added to the cis side of BLM chamber and the solution was stirred slowly to promote protein insertion. Native (unphosphorylated) Bax protein did not show any current activity but phosphorylated Bax protein showed current activity indicating ion pore formation by it. Currents passing through phosphorylated Bax protein pore were filtered at the amplifier output by a 4-pole low pass Bessel filter and then by using an external low pass RC filter (Single pole) at 200 Hz frequency. Currents were acquired and digitized at 1 kHz sampling frequency using data acquisition pClamp 10.2 software (Clampex 10.2, Axon Instruments) through an analog to digital converter (Digidata 1440A, Axon Instruments).
4. Results
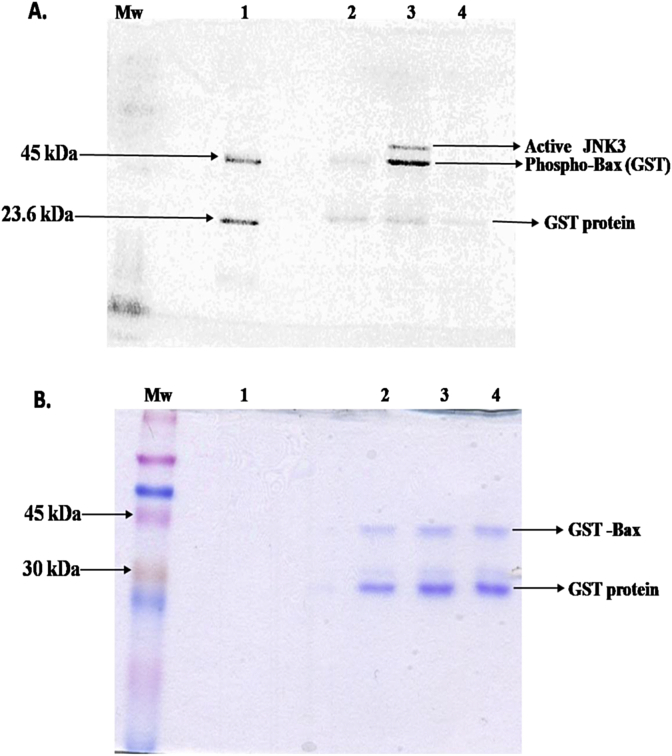
Fig. 1A shows gel electrophoresis data after Pro-Q Diamond dye staining and Fig. 1B shows coomassie blue dye stained image of the same gel. In Fig. 1A. Lane 1: Peppermint stick Phosphoprotein molecular weight standard. It contains a combination of six proteins, two are in the phosphorylated form (23.6 kDa, 45 kDa) and the rest four are in the unphosphorylated form (14.4 kDa, 18 kDa, 66.2 kDa, 116.25 kDa). Pro-Q Diamond dye binding was observed for only two phosphorylated proteins with molecular weights 23.6 kDa and 45 kDa in the phosphoprotein standard, which authenticates the activity of the dye. Lane 2: Negative Control, i.e. GST-Bax (49 kDa) + Mg2+ATP. We did not observe any dye binding to GST-Bax band in this lane which confirms Bax protein is neither pre-phosphorylated nor it undergoes self-phosphorylation in the presence of ATP without the JNK3 enzyme. Lane 3: Experimental Sample, i.e. GST-Bax + JNK3 enzyme + Mg2+ATP, dye binding was observed to the GST-Bax band. A faint band around 25 kDa was also observed in this lane indicating GST protein tag (as per the supplier's report) but as the intensity of the bound dye is the same in negative control and experimental sample, we concluded that dye binding to GST protein was because of non-specific interaction and not because of phosphorylation by JNK3 enzyme. As a whole, we conclude that Bax protein is phosphorylated by JNK3 enzyme. In this lane a band corresponding to active JNK3 was also seen that is because the purchased JNK3 enzyme is phosphorylated. We did not find any protein band with molecular weight higher than phosphorylated monomeric Bax in this lane which suggests neither JNK3 phosphorylated Bax formed oligomers nor it remained as Bax-JNK3 complex in the solution after the phosphorylation reaction was over. Lane 4: Positive Control, i.e. GST-Bax + JNK3 enzyme + Mg2+ATP + Alkaline Phosphatase. A decrease in the amount of the bound dye as compared to the experimental sample after alkaline phosphatase treatment shows that Bax protein phosphorylation by JNK3 is a reversible reaction. As demonstrated by our repeated sets of Pro-Q Diamond staining experiment (Fig. 1A) Bax protein undergoes reversible phosphorylation by JNK3 enzyme. Fig. 1B shows gel image after staining with coomassie blue dye. Equal intensity of dye binding to GST-Bax band in all the lanes confirms loading of equal amounts of Bax protein in all the lanes.
Fig. 1.
A. Representative Pro-Q Diamond phospho-protein stained SDS-Polyacrylamide (Tris-Glycine) gel image showing phosphorylation of Bax protein (GST tagged) by JNK3 enzyme. Lane 1: Phosphoprotein standard; Lane 2: Negative Control, i.e. Bax protein + Mg2+ATP; Lane 3: Experimental Sample, i.e. Bax protein + JNK3 + Mg2+ATP; Lane 4: Positive Control, i.e. Bax protein + JNK3 + Mg2+ATP + Calf Intestinal Alkaline Phosphatase. B. Coomassie blue dye stained image of the same gel, no bands could be seen in Lane 1 because of the low concentration of proteins in the phosphoprotein standard. Mw marker: Color Burst electrophoresis Marker C1992 (Mol. wt. 8000 ─ 220,000 Da, Sigma Chem. Company, USA). The size of the gel increases when stained with Pro-Q Diamond dye and then decreases to the original size during coomassie blue staining.
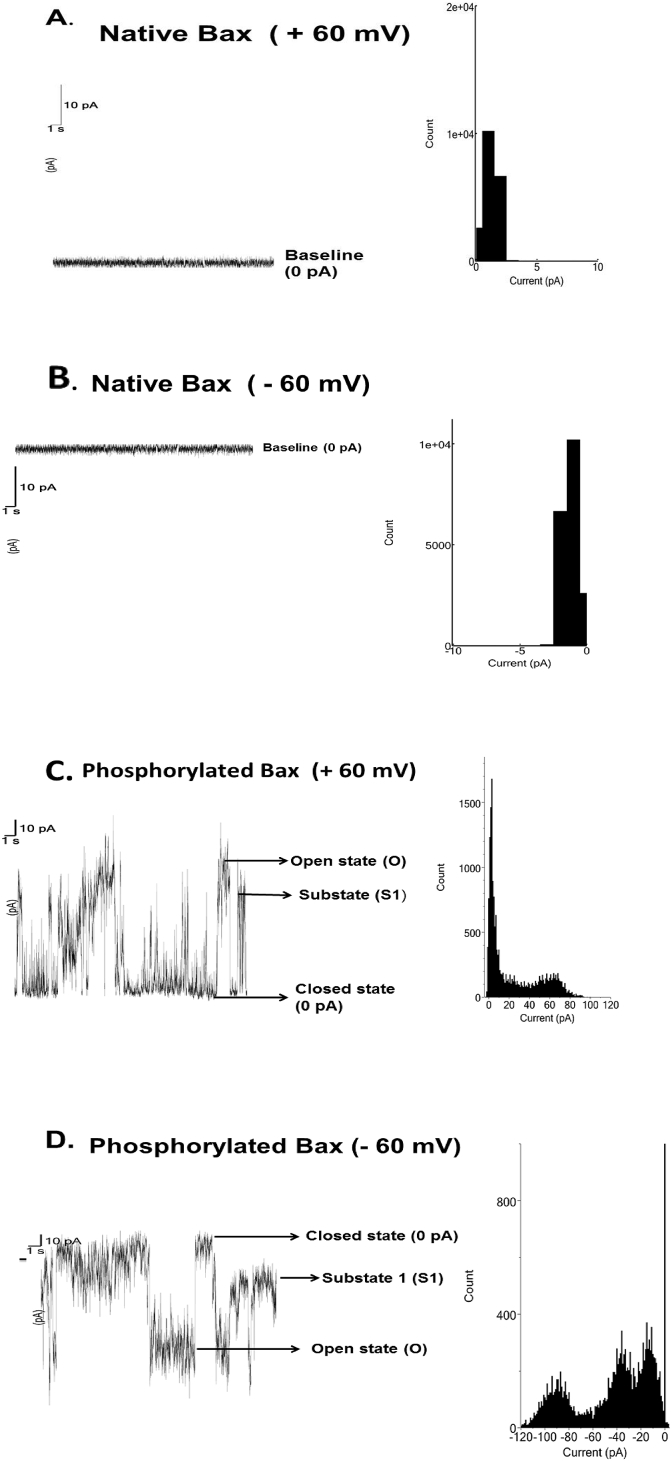
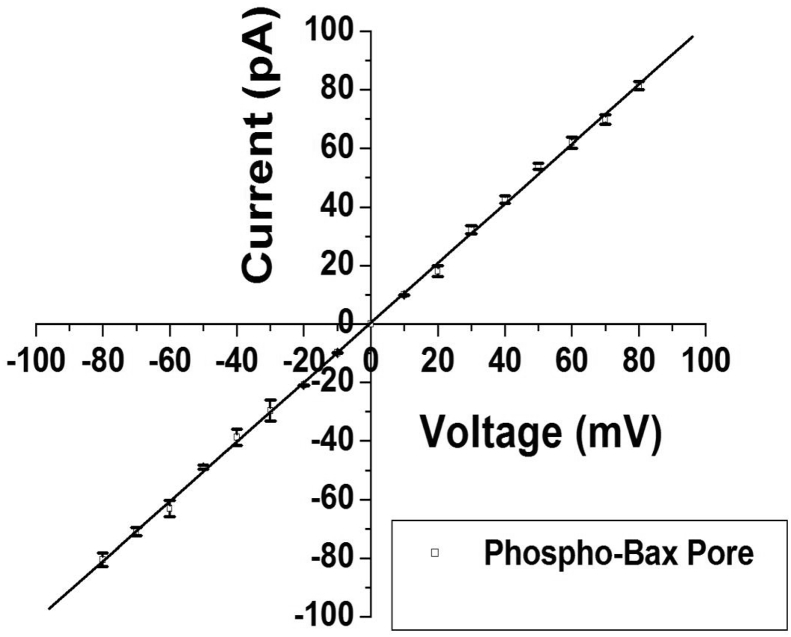
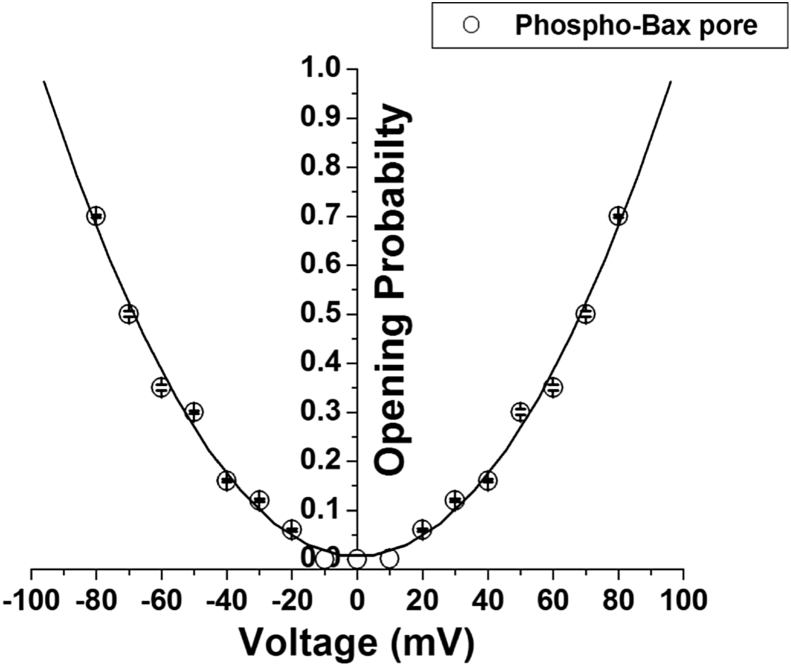
Fig. 2 shows representative current–time traces and their current amplitude histograms of the native (unphosphorylated) and phosphorylated (by JNK3) Bax protein. We first tested the native Bax protein for pore formation on BLM. We varied various parameters like Bax concentration (5–500 nM), KCl gradient (100 mM–1 M), addition of Bax to both sides of the bilayer chamber to promote its insertion into the BLM. Bax protein did not show any interaction with BLM under the present experimental condition (Fig. 2 A& B). We then tested JNK3 phosphorylated Bax protein for pore formation on BLM. Interestingly, phosphorylated Bax (Phospho-Bax) protein got inserted into the lipid bilayer and allowed ion passage through it indicating pore formation as shown in Fig. 2 (C & D). The conductance of the open state (O state) of the pore formed by Phospho-Bax protein was estimated to be approximately 1.5 nS; and a state 1 (S1) corresponding to approximately 1 nS was also identified. From the histograms we could make out that the conductance of S1 state is not half to that of open O state, thus S1 is a sub-state of the Phospho-Bax pore. Fig. 3 shows I–V plot of the full open state of the pore formed by the Phospho-Bax protein. Phospho-Bax pore shows linear I–V relation (ohmic behavior) at both the positive and the negative voltages. Fig. 4 shows the open probability of the full open state (O) of the phospho-Bax pore with respect to voltage. The open probability of the full open state (O) of the pore increases with the increase in voltage at both the positive and the negative voltages. At low voltages the open-close transitions or the gating events of the phospho-Bax pore are less frequent and the channel remains in closed state resulting in low open probability of the pore. As the voltage is increased the full open state of the pore is stabilized and the transitions from the open to the closed state becomes less frequent resulting in the increase in the open probability of the pore. This could be due to the fact that the electric field strength applied through the membrane potential is helping Phospho-Bax ion pore to go from the closed conformation to the open one and stabilize the latter state. The open probability curve of the phospho-Bax pore is U-shaped and it fits a second order polynomial function.
Fig. 2.
Representative current-time traces of JNK3 phosphorylated Bax protein recorded on a DPhPC membrane with a symmetrical bath solution of 1 M KCl, 10 mM MgCl2, 10 mM HEPES, pH 7.4 at 25 °C. A. Native Bax protein at +60 mV showing no deviations from the baseline current (0 pA); B. Native Bax protein at –60 mV showing no deviations from the baseline current; C. Phosphorylated Bax protein at +60 mV showing ion pore activity with a full open state (O), a sub-state 1 (S1) and a fully closed state (0 pA) which are represented by peaks in the histogram; D. Phosphorylated Bax protein at –60 mV showing pore activity with a full open state (O), a sub-state 1 (S1) and a closed state which are represented by the peaks in the histogram. Data shown is filtered at 200 Hz and sampled at 1 kHz frequency. Histogram analysis was done using AxoGraph X (Version 1.5.4, AxoGraph Inc., CA, USA).
Fig. 3.
Current–Voltage relation (I–V plot) of the full open state of the Phosphorylated (by JNK3) Bax protein pore. Pore state showing the maximum current value in the single-channel current–time traces was considered as full open state of the Phospho-Bax pore. Full open state current values were obtained from the current–time traces and their current amplitude histograms at different voltages. Finally, mean of the current values of the full open state obtained from three independent sets of experiments was shown by the I–V plot. I–V plot was obtained using pClamp 10.2 (Axon Instruments, Sunnyvale, CA, USA) and Origin 5.0 (Origin Lab Corp., MA, USA) software. I–V plot of the Phospho-Bax pore was fitted with a linear function. Best fit analysis of the experimental data was done using Origin 5.0. Values are Mean ± S.E. of three independent sets of experiments.
Fig. 4.
Dependence of open probability (Po) of the full open state of phosphorylated (by JNK3) Bax pore on applied membrane voltage (V). The open probability of the different states of the Phospho-Bax pore at a particular voltage was determined by selecting the different current levels showing the different states of the pore from single-channel traces. The scatter plot of the open probability of the full open state of the pore at different voltages was plotted using Origin 5.0 software and it fitted with a second order polynomial function. Values are Mean ± S.E. of three independent sets of experiments.
5. Discussion
Bax consists of nine α helices (α1–9), in inactive state, α5 helix constitutes the hydrophobic core of the protein and the other eight amphipathic α helices surround the core [31]. α9 helix is important for mitochondrial targeting of bax, in inactive state α9 helix is tethered to the α5 helix which prevents its mitochondrial targeting [32]. Upon induction of apoptosis, this α9–α5 helix interaction breaks leading to conformational rearrangements, which results in Bax activation and mitochondrial targeting [32]. The bulky hydrophobic side chains of amino acid residues of amphipathic α5–α6 helices interacts with the hydrophobic fatty acid chains of phospholipid bilayers resulting in integration of these helices into the BLM and formation of Bax pore by the α5–α6 helices [33], [34], [35]. In another study, Kim et al. replaced Threonine 167 with Aspartate (T167D) (phospho-mimetic mutant) by site directed mutagenesis and these mutants were found to translocate to mitochondria in these cells even without exposure to cell death stimulant [24]. Kim et al. hypothesized that hydrogen bonding between Thr167 and Trp170 might be important in keeping Bax in the cytoplasm by preventing the exposure of the α9 helix. It was proposed that the negatively charged nature of aspartate might be playing an important role in destabilising the interaction between α9–α5 helix and mitochondrial translocation.
In conclusion, we report that Bax can be phosphorylated by JNK3 in vitro. Moreover, native (unphosphorylated) Bax does not form ion pores on BLM and phosphorylation of Bax protein by JNK3 enzyme leads to induction of ion pore forming ability. JNKs are proline-directed Serine (S)/Threonine (T) kinases [24]. Phosphorylation of Bax by JNK3 would have transferred negatively charged phosphate groups on its proline directed Serine (Ser) and Threonine (Thr) residues which might be inducing ion pore formation. Although in our phosphorylation detection pro Q diamond dye experiment no oligomerization of Bax after phosphorylation by JNK3 was observed still with our current data it is difficult to conclude whether JNK3 phosphorylated Bax pore is formed by Bax monomer or oligomer due to several reasons (i) In solution organisation of Bax protein can be very different from its organisation in the bilayer lipid membrane environment. In a report it has been suggested that Bax might insert into the membrane as monomers and then undergoes oligomerization after inserting into the membrane [40]. This suggests that JNK3 phosphorylated Bax might also be undergoing oligomerization after inserting into the BLM in our experiments. (ii) The reported conductance of the oligomeric Bax pore by different groups varies from approximately 9 nS [41] and 1.5 nS [26] with the second one same as that JNK3 phosphorylated Bax pore conductance reported by us but unlike our results they observed oligomerization in pro Q diamond phosphorylation detection experiment. Thus, we think specific experiments are required to conclude whether JNK3 phosphorylated Bax pore is formed by a monomer or an oligomer.
The size of the JNK3 phosphorylated Bax pore can be calculated by assuming it a cylindrical structure and considering internal conductivity of the pore same as that in the bulk phase buffer. In one study Bax pore diameter has been calculated by considering these assumptions as 0.9 nm with reported single-channel conductance of the Bax pore as 1.5 ± 0.4 nS [26] which is same (conductance) as reported by us. This Bax pore diameter (0.9 nm) is smaller than the size of cytochrome c (∼3.4 nm). Thus, it is very likely that JNK3 phosphorylated Bax might also forms small pores, unable to leak cytochrome c but it can form larger channels by forming complexes with other outer mitochondrial proteins like Bak, Bif-1, truncated Bid, voltage dependent anion channel (VDAC) & ΔN BclxL etc. or after homo-oligomerization and promote leakage of cytochrome c [36], [37], [38], [39], [40]. It is proposed that during stress-induced apoptosis under in vivo conditions JNK3 Phosphorylated Bax pore interacts with the other outer mitochondrial membrane proteins forming large channels in the outer mitochondrial membrane, there by releasing cytochrome c and hence cell death. This issue is under investigation. Formation of pores by JNK3 Phosphorylated Bax protein during apoptosis will disturb the normal mitochondrial physiological processes like ion and metabolite transport between the cytosol and the mitochondria. Interaction of Bax with the outer mitochondrial membrane is an important check point in cellular stress-induced cell death. We believe these results would be helpful in designing new strategies to treat disorders involving cellular stress-mediated apoptotic death.
Conflict of interest
Both the authors Dr. Rajeev Gupta (Research Associate, All India Institute of Medical Sciences, India) and Prof. Subhendu Ghosh (Dept. of Biophysics, University of Delhi South Campus, India) declare no conflict of interest.
Acknowledgments
The authors acknowledge University of Delhi (RC/2015/9677 & DU DST PURSE Grant Phase II) for financial assistance. Dr. Rajeev Gupta gratefully acknowledges Department of Biotechnology (DBT), India for providing the Research Associate fellowship and All India Institute of Medical Sciences (AIIMS) (DRF-031/2015/RS) for maintaining the fellowship.
References
- 1.Basañez G., Soane L., Hardwick J.M. A new view of the lethal apoptotic pore. Plos Biol. 2012;10(9) doi: 10.1371/journal.pbio.1001399. e1001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terrones O., Etxebarria A., Landajuela A., Landeta O., Antonsson B., Basañez G. BIM and tBID are not mechanistically equivalent when assisting BAX to permeabilize bilayer membranes. J. Biol. Chem. 2008;283(12):7790–7803. doi: 10.1074/jbc.M708814200. [DOI] [PubMed] [Google Scholar]
- 3.Tafani M., Cohn J.A., Karpinich N.O., Rothman R.J., Russo M.A., Farber J.L. Regulation of intracellular pH mediates Bax activation in HeLa cells treated with staurosporine or tumor necrosis factor-α. J. Biol. Chem. 2002;277(51):49569–49576. doi: 10.1074/jbc.M208915200. [DOI] [PubMed] [Google Scholar]
- 4.Ausili A., Torrecillas A., Martínez-Senac M.M., Corbalán-García S., Gómez-Fernández J.C. The interaction of the bax C-terminal domain with negatively charged lipids modifies the secondary structure and changes its way of insertion into membranes. J. Struct. Biol. 2008;164:146–152. doi: 10.1016/j.jsb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Etxebarria A., Terrones O., Yamaguchi H., Landajuela A., Landeta O., Antonsson B., ..., Basañez G. Endophilin B1/Bif-1 stimulates bax activation independently from its capacity to produce large-scale membrane morphological rearrangements. J. Biol. Chem. 2009;284(7):4200–4212. doi: 10.1074/jbc.M808050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robin A.Y., Kumar K.K., Westphal D., Wardak A.Z., Thompson G.V., Dewson G., ..., Czabotar P.E. Crystal structure of Bax bound to the BH3 peptide of Bim identifies important contacts for interaction. Cell Death Dis. 2015;6(7) doi: 10.1038/cddis.2015.141. e1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonyan L., Renault T.T., Costa Novais M.J., Sousa M.J., Côrte-Real M., Camougrand N., ..., Manon S. Regulation of Bax/mitochondria interaction by AKT. FEBS Lett. 2015:13–21. doi: 10.1002/1873-3468.12030. [DOI] [PubMed] [Google Scholar]
- 8.Gardai S.J., Hildeman D.A., Frankel S.K., Whitlock B.B., Frasch S.C., Borregaard N., ..., Henson P.M. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J. Biol. Chem. 2004;279(20):21085–21095. doi: 10.1074/jbc.M400063200. [DOI] [PubMed] [Google Scholar]
- 9.Song J.Y., Ryu S.H., Cho Y.M., Kim Y.S., Lee B.M., Lee S.W., Choi J. Wip1 suppresses apoptotic cell death through direct dephosphorylation of BAX in response to γ-radiation. Cell Death Dis. 2013;4(8) doi: 10.1038/cddis.2013.252. e744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quast S., Berger A., Eberle J. ROS-dependent phosphorylation of Bax by wortmannin sensitizes melanoma cells for TRAIL-induced apoptosis. Cell Death Dis. 2013;4(10) doi: 10.1038/cddis.2013.344. e839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q., Sun S.Y., Khuri F., Curran W.J., Deng X. Mono- or double-site phosphorylation distinctly regulates the proapoptotic function of bax. Plos One. 2010;5(10) doi: 10.1371/journal.pone.0013393. e13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xin M., Gao F., May W.S., Flagg T., Deng X. Protein kinase Cζ abrogates the proapoptotic function of bax through phosphorylation. J. Biol. Chem. 2007;282(29):21268–21277. doi: 10.1074/jbc.M701613200. [DOI] [PubMed] [Google Scholar]
- 13.Min H., Ghatnekar G.S., Ghatnekar A.V., You X., Bu M., Guo X., ..., Huang Q. 2-Methoxyestradiol induced bax phosphorylation and apoptosis in human retinoblastoma cells via p38MAPK activation. Mol. Carcinog. 2012;51:576–585. doi: 10.1002/mc.20825. [DOI] [PubMed] [Google Scholar]
- 14.Arokium H., Ouerfelli H., Velours G., Camougrand N., Vallette F.M., Manon S. Substitutions of potentially phosphorylable serine residues of bax reveal how they may regulate its interaction with mitochondria. J. Biol. Chem. 2007;282(48):35104–35112. doi: 10.1074/jbc.M704891200. [DOI] [PubMed] [Google Scholar]
- 15.Wu C.H., Lin H.H., Yan F.P., Wu C.H., Wang C.J. Immunohistochemical detection of apoptotic proteins, p53/Bax and JNK/FasL cascade, in the lung of rats exposed to cigarette smoke. Arch. Toxicol. 2006;80(6):328–336. doi: 10.1007/s00204-005-0050-4. [DOI] [PubMed] [Google Scholar]
- 16.Kang C.D., Jang J.H., Kim K.W., Lee H.J., Jeong C.S., Kim C.M., ..., Chung B.S. Activation of c-jun N-terminal kinase/stress-activated protein kinase and the decreased ratio of Bcl-2 to Bax are associated with the auto-oxidized dopamine-induced apoptosis in PC12 cells. Neurosci. Lett. 1998;256(1):37–40. doi: 10.1016/s0304-3940(98)00751-4. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Ausman L.M., Russell R.M., Greenberg A.S., Wang X.D. Increased apoptosis in high-fat diet–induced nonalcoholic steatohepatitis in rats is associated with c-Jun NH2-terminal kinase activation and elevated proapoptotic Bax. J. Nutr. 2008;138(10):1866–1871. doi: 10.1093/jn/138.10.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhai C.L., Zhang M.Q., Zhang Y., Xu H.X., Wang J.M., An G.P., ..., Li L. Glycyrrhizin protects rat heart against ischemia-reperfusion injury through blockade of HMGB1-dependent phospho-JNK/Bax pathway. Acta Pharmacol. Sin. 2012;33(12):1477–1487. doi: 10.1038/aps.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wissing E.R., Boyer J.G., Kwong J.Q., Sargent M.A., Karch J., McNally E.M., ..., Molkentin J.D. P38α MAPK underlies muscular dystrophy and myofiber death through a Bax-dependent mechanism. Hum. Mol. Gen. 2014;23(20):5452–5463. doi: 10.1093/hmg/ddu270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y.Q., Liu Y.F., Ma X.M., Xiao Y.D., Wang Y.B., Zhang M.Z., ..., Xie F. Hydrogen-rich saline attenuates skin ischemia/reperfusion induced apoptosis via regulating Bax/Bcl-2 ratio and ASK-1/JNK pathway. J. Plast. Reconstr. Aesthet. Surg. 2015;68(7):e147–e156. doi: 10.1016/j.bjps.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y., Herdegen T. Cerebral ischemia provokes a profound exchange of activated JNK isoforms in brain mitochondria. Mol. Cell. Neurosci. 2009;41(2):186–195. doi: 10.1016/j.mcn.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Lei K., Nimnual A., Zong W.X., Kennedy N.J., Flavell R.A., Thompson C.B., ..., Davis R.J. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH2-terminal kinase. Mol. Cell. Biol. 2002;22(13):4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papadakis E.S., Finegan K.G., Wang X., Robinson A.C., Guo C., Kayahara M., Tournier C. The regulation of Bax by c-Jun N-terminal protein kinase (JNK) is a prerequisite to the mitochondrial-induced apoptotic pathway. FEBS Lett. 2006;580(5):1320–1326. doi: 10.1016/j.febslet.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 24.Kim B.J., Ryu S.W., Song B.J. JNK and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J. Biol. Chem. 2006;281(30):21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 25.Schlesinger P.H., Gross A., Yin X.M., Yamamoto K., Saito M., Waksman G., Korsmeyer S.J. Comparison of the ion channel characteristics of proapoptotic bax and antiapoptotic BCL-2. Proc. Natl. Acad. Sci. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin S.H., Perera M.N., Nguyen T., Datskovskiy D., Miles M., Colombini M. Bax forms two types of channels, one of which is voltage-gated. Biophys. J. 2011;101:2163–2169. doi: 10.1016/j.bpj.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin S.H., Cherian N., Wu B., Phee H., Cho C., Colombini M. Bax channel triplet: co-operativity and voltage gating. Biochem. J. 2014;459(2):397–404. doi: 10.1042/BJ20131441. [DOI] [PubMed] [Google Scholar]
- 28.Salvador-Gallego R., Mund M., Cosentino K., Schneider J., Unsay J., Schraermeyer U., ..., García-Sáez A.J. Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. EMBO J. 2016 doi: 10.15252/embj.201593384. e201593384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.N.D Weiner, 1973. The bimolecular lipid membrane: a system. By Mahendra K. Jain. Van Nostrand Reinhold, 450 W. 33rd St., New York, NY 10001, 1972.
- 30.Gupta R., Ghosh S. Phosphorylation of voltage-dependent anion channel by c-Jun N-terminal Kinase-3 leads to closure of the channel. Biochim. Biophys. Res. Commun. 2015;459(1):100–106. doi: 10.1016/j.bbrc.2015.02.077. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki M., Youle R.J., Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103(4):645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 32.Westphal D., Dewson G., Czabotar P.E., Kluck R.M. Molecular biology of Bax and Bak activation and action. BBA-Mol. Cell Res. 2011;1813(4):521–531. doi: 10.1016/j.bbamcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Cartron P.F., Arokium H., Oliver L., Meflah K., Manon S., Vallette F.M. Distinct domains control the addressing and the insertion of Bax into mitochondria. J. Biol. Chem. 2005;280(11):10587–10598. doi: 10.1074/jbc.M409714200. [DOI] [PubMed] [Google Scholar]
- 34.García-Sáez A.J., Coraiola M., Dalla Serra M., Mingarro I., Menestrina G., Salgado J. Peptides derived from apoptotic bax and Bid reproduce the poration activity of the parent full-length proteins. Biophys. J. 2005;88:3976–3990. doi: 10.1529/biophysj.104.058008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García-Sáez A.J., Mingarro I., Pérez-Payá E., Salgado J. Membrane-insertion fragments of Bcl-xL, Bax, and Bid. Biochemistry. 2004;43(34):10930–10943. doi: 10.1021/bi036044c. [DOI] [PubMed] [Google Scholar]
- 36.Parikh N., Koshy C., Dhayabaran V., Perumalsamy L.R., Sowdhamini R., Sarin A. The N-terminus and alpha-5, alpha-6 helices of the pro-apoptotic protein Bax, modulate functional interactions with the anti-apoptotic protein Bcl-xL. BMC Cell Biol. 2007;8(1):16. doi: 10.1186/1471-2121-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee J., Ghosh S. Bax increases the pore size of rat brain mitochondrial voltage-dependent anion channel in the presence of tBid. Biochem. Biophys. Res. Commun. 2004;323(1):310–314. doi: 10.1016/j.bbrc.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 38.Antonawich F.J., Krajewski S., Reed J.C., Davis J.N. Bcl-x1 Bax interaction after transient global ischemia. J. Cereb. Blood Flow. Metab. 1998;18(8):882–886. doi: 10.1097/00004647-199808000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Gupta R., Ghosh S. Bax and Bif-1 proteins interact on bilayer lipid membrane and form pore. Biochem. Biophys. Res. Commun. 2015;463(4):751–755. doi: 10.1016/j.bbrc.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Subburaj Y., Cosentino K., Axmann M., Pedrueza-Villalmanzo E., Hermann E., Bleicken S., ..., García-Sáez A.J. Bax monomers form dimer units in the membrane that further self-assemble into multiple oligomeric species. Nat. Commun. 2015;6(8042) doi: 10.1038/ncomms9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hetz C., Vitte P.A., Bombrun A., Rostovtseva T.K., Montessuit S., Hiver A., ..., Antonsson B. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J. Biol. Chem. 2005;280(52):42960–42970. doi: 10.1074/jbc.M505843200. [DOI] [PubMed] [Google Scholar]