Abstract
Polycyclic Aromatic Hydrocarbons (PAHs) are potent carcinogens. Among these, dimethylbenz(a)anthracene (DMBA) is well known for its capacity to induce mammary carcinomas in female Sprague-Dawley (SD) rats. Ovariectomy suppresses the susceptibility of this model to DMBA, thus suggesting that the inducible action of the carcinogen depends on ovarian hormones. The promotion of DMBA-induced adenocarcinoma is accompanied by a series of neuroendocrine disruptions of both Hypothalamo-Pituitary-Gonadal (HPG) and Hypothalamo-Pituitary-Adrenal (HPA) axes and of the secretion of melatonin during the latency period of 2 months that precedes the occurrence of the first mammary tumor. The present review analyses the various neuroendocrine disruptions that occur along the HPG and the HPA axes, and the marked inhibitory effect of the carcinogen on melatonin secretion. The possible relationships between the neuroendocrine disruptions, which essentially consist in an increased pre-ovulatory secretion of 17β-estradiol and prolactin, associated with a marked reduction of melatonin secretion, and the decrease in gene expression of the receptors for aryl-hydrocarbons receptor (AhR) and 17β-estradiol (ERα; ERβ) are also discussed.
Keywords: Mammary cancer, Neuroendocrine disruption, Dimethylbenz(a)anthracene, Female rat
Abbreviation list: ACTH, Adrenocorticotropic hormone; AhR, Aryl hydrocarbon Receptor; ARNT, AhR nuclear translocator; CRH, Corticotropin releasing hormone; CYP, Cytochromes P450; DMBA, Dimethylbenz(a)anthracene; E2, 17β-estradiol; ERα and ERβ, Estrogen receptor; FSH, Folliculo-Stimulating Hormone; GnRH, Gonadotropin-Releasing Hormone; HPA, Hypothalamo-Pituitary-Adrenal; HPG, Hypothalamo-Pituitary-Gonadal; LH, Luteinizing hormone; PAHs, Polycyclic Aromatic Hydrocarbons; PRL, Prolactin; SD, Sprague-Dawley; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; XRE, Xenobiotic response elements
Highlights
-
•
Polycyclic Aromatic Hydrocarbons influence promotion of breast tumorigenesis.
-
•
Dimethylbenz(a)anthracene (DMBA) alters neuroendocrine axes and melatonin secretion.
-
•
DMBA modulates the activity of aryl hydrocarbon and 17β-estradiol receptors.
1. Introduction
A large number of polycyclic Aromatic Hydrocarbons (PAHs) have been identified as potent carcinogens and/or endocrine disruptors. PAHs are widely distributed in the environment as a result of incomplete combustion of fossil fuels and other organic molecules and are common contaminants of terrestrial and aquatic ecosystems [[1], [2] and therein]. Numerous compounds of the PAH family have been associated with the development of tumors in humans and experimental animals [3 and therein]. Among these, dimethylbenz(a)anthracene (DMBA) was extensively used as a model to study the molecular mechanisms of mammary carcinogenesis in Sprague-Dawley (SD) female rats [4]. Of particular interest is the observation that a single intra-gastric dose of DMBA is sufficient to induce mammary adenocarcinoma in contrast to the archetypal PAHs, benzo(a)pyrene or 3-methyl-cholanthrene for which the administration of several doses are needed [4].
The induction of mammary tumors by DMBA in rats is well documented. After metabolic activation in the mammary gland [5], the metabolites of the carcinogen interact with rapidly proliferating cells in the terminal end buds, forming DNA adducts and subsequent mutations, which in turn play a role in their transformation to malignant cells [6], [7], [8]. DMBA is a highly lipophilic molecule. The breast contains significant amount of adipose tissue, in which DMBA can concentrate at the epithelium contact before its metabolic activation and this property certainly correlates with the higher activity of DMBA at the mammary level. Other PAHs certainly act along the same line of events.
Sprague-Dawley (SD) rats are DMBA-sensitives, with a peak of susceptibility at 55–60 days of age. Ovariectomy suppresses this susceptibility, suggesting that the inducible action of the carcinogen is related to ovarian hormones and the ovarian cycle [9].
The promotion of DMBA-induced adenocarcinoma is accompanied by a series of neuroendocrine disruptions of the Hypothalamo-Pituitary-Gonadal (HPG) and Hypothalamo-Pituitary-Adrenal (HPA) axes and of the secretion of melatonin during the latency period of 2 months that precedes the occurrence of mammary tumors. The aim of this review is to report on the various neuroendocrine disruptions occurring along the closely interconnected HPG and HPA axes, and to analyze the marked effect of the carcinogen on melatonin secretion and their influence on the carcinogenic process [10]. The potential relationships between these neuroendocrine disruptions and the observed effects on the expression of the genes coding for the Aryl hydrocarbon Receptor (AhR) and for both estrogen receptors (ERα and ERβ), are also discussed.
2. The ovarian cycle of the female rat
The ovarian cycle of the female rat is much shorter (4-days) than that in the woman (28-days). However, the 2 major events, the follicular and the luteal phases, occurring during the 4-days cycle in the female rat are a simple reduction in time of the same events occurring during the 28-days cycle in the woman.
In the female rat, the duration of the follicular phase is of 2 days versus 14 days in the woman. The follicular phase is characterized by the secretion of the Folliculo-Stimulating Hormone (FSH) in the blood, which in turn, stimulates the secretion of 17β-estradiol (E2). At the end of that phase, a pre-ovulatory surge of E2 occurs which triggers pre-ovulatory surges of hypothalamic Gonadotropin-Releasing Hormone (GnRH), pituitary Luteinizing Hormone (LH), FSH and Prolactin(PRL). The surges of LH and FSH are provoked by the surge of the hypothalamic GnRH in the Hypothalamo-Pituitary portal blood system. The pre-ovulatory LH surge provokes the ovulation of the mature follicle. The luteal phase lasts 2 days in the female rat, versus 14 days in the woman and is characterized by the secretion of progesterone in response to the secretion of LH.
Another important difference between the ovarian cycles of the female rat and that of the woman is the absence of menstruation in the rodent. For that reason, the cycle of the female rat is named estrous and the cycle of the woman is named menstrual. In addition, there is no pre-ovulatory PRL surge during the menstrual cycle of the woman.
3. DMBA, the brain and Hypothalamo-Pituitary axes
After one single administration (15 mg/rat by gastric intubation), DMBA is able to cross the blood-brain barrier and to induce the expression of cytochromes P450 (CYP) in endothelial cells of blood brain interfaces [10]. Furthermore, labeled [H3]-DMBA and its metabolites have been identified in different area of the brain, including the hypothalamus, the pituitary gland, the pineal gland and the frontal cortex (Canonico M, Lenoir V and Kerdelhue B, unpublished results). In addition, DMBA disorganizes the neuronal plasma membranes in rat hypothalamus [11]. This pollutant is then able to disrupt the physiological processes regulated by those brain structures, including Hypothalamo-Pituitary axes.
3.1. Disruption of the Hypothalamo-Pituitary-Gonadal (HPG) axis
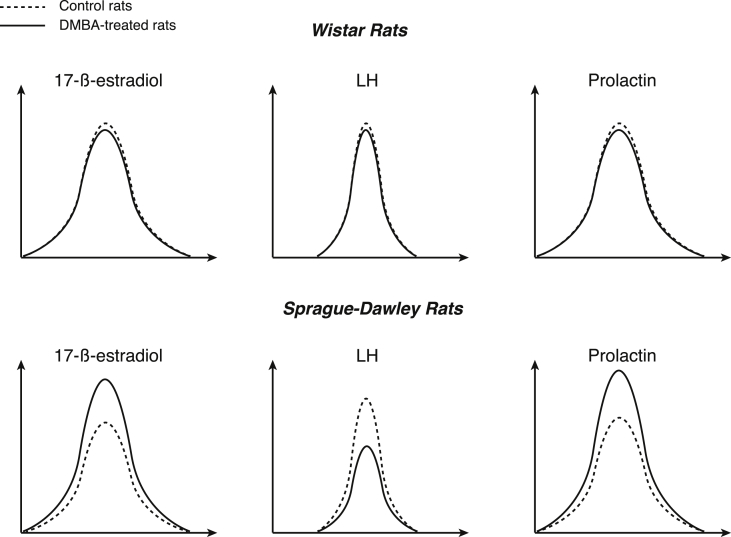
In female SD rats, a DMBA-sensitive strain, DMBA significantly inhibited LH and FSH surges and stimulated the PRL and the E2 surges in the afternoon of the proestrus at any estrous cycle after treatment [12], [13] (Fig. 1, Table 1). No difference was found for LH, FSH and PRL or E2 concentrations in the blood at other times of the estrous cycle. In addition, no difference was found for the level of any other anterior pituitary hormone at any time of the estrous cycle. In contrast, in female Wistar rats, a DMBA-resistant strain, the pre-ovulatory or basal LH, FSH, PRL and E2 secretions were not modified at any time of the estrous cycle after treatment with the carcinogen (Fig. 1). There was also no reported difference for any other pituitary hormone at any time of the estrous cycle [12], [13]. The pituitary gland is not the only area affected by DMBA exposure: ovariectomized SD rats that were pre-treated with DMBA exhibited a reduced GnRH release, as measured in vitro using synaptosomes from the mediobasal hypothalamus, together with a blunted in vivo LH release in response to E2 [14]. In addition, disturbances of the expression of the GnRH and its pituitary receptor genes occurred throughout the estrus cycle [15]. The early and persistent alterations in the hypothalamic centers that were involved in the neuroendocrine cyclicity of the HPG axis very likely resulted from a direct action of DMBA.
Fig. 1.
The 17β-estradiol, Luteinizing hormone (LH) and prolactin surges in female Wistar rats, a DMBA-insensitive strain (up), and in female Sprague-Dawley rats, a DMBA-sensitive strain (bottom) in the afternoon of the proestrus at any estrous cycle after treatment.
Table 1.
A summary of the DMBA effects on several neuroendocrine axes.
| Hypothalamus | ANT. pituitary | Pineal gland | Ovary | Adrenal gland | |
|---|---|---|---|---|---|
| AhR | −20% | −20% | |||
| ER-alpha | −15% | −20% | |||
| ER-beta | −25% | −20% | |||
| GnRH | +30% | ||||
| CRH | −20% | ||||
| LH | −30% | ||||
| FSH | −20% | ||||
| PRL | +30% | ||||
| Melatonin | −70% | ||||
| 17β-E2 | +20% | ||||
| Corticosterone | −60% |
In pre-menopausal women, the development of breast cancer is associated with reduced plasma GnRH and of FSH secretion in post menopausal women, thus suggesting the existence of neuroendocrine disruptions in the woman that could be involved in tumor promotion and that could be mimicked in a sensitive rat model with a single dose of a common PAH [16].
3.2. Disruption of the Hypothalamo-Pituitary-Adrenal (HPA) axis
The HPA axis includes the hypothalamus that secretes corticotropin releasing hormone (CRH) which stimulates the release of adrenocorticotropic hormone (ACTH) from the pituitary gland, thus allowing the secretion of glucocorticoids from the adrenal cortex. Glucocorticoids control stress reactions, the digestive and immune system, mood and emotions, sexuality, and energy storage and expenditure. Along the HPA axis, a long-term disregulation of the circadian and E2-induced corticosterone secretion was observed in DMBA-treated rats [17] (Table 1). The most important disregulation observed after administration of DMBA, is the almost complete abolition of the circadian rhythm of plasma corticosterone. This fact could be responsible for the change in the behavior of female rats in preparation for mammary cancers. In an open space system, DMBA-treated female rats exhibit a long-term reduction of anxiety level after treatment with the mammary carcinogen [18]. A relationship between breast cancer and stress response has been documented in the woman [Ref. in [19]].
3.3. Disruption of the secretion of melatonin from the pineal gland
Melatonin is a well-documented hormone secreted in a circadian fashion by the pineal gland and is implicated in many physiological interactions [20]. The circadian synthesis and release of melatonin is driven by the suprachiasmatic nucleus of the hypothalamus [21].
A single intragastric administration of DMBA to SD female rats induces a long-term marked decrease in the spontaneous and norepinephrine-induced melatonin secretion from the pineal gland, in an in vitro perfusion system (Table 1) [22]. Melatonin secretion was completely blunted when E2 was present in the perfusion medium, even at a low and physiological concentration (10−10 M) [22]. In contrast, the E2 antagonist tamoxifen, stimulated melatonin secretion [22]. Last but not least, melatonin had a preventive and curative effect on the mammary carcinogenesis induced by DMBA [23].
In the woman, it can be envisioned that the long-term inhibition of melatonin secretion by ovarian steroids might play a major role during the promotion phase of the mammary carcinogenesis. Whether or not a similar decrease in the secretion of melatonin from the pineal gland could be generated in the woman by non-identified carcinogenic environmental factors and therefore could constitute a promotional factor for breast cancer remains to be proven. Nevertheless, a disruption of melatonin secretion provoked by rotating night shifts increases the risk of breast cancer in the woman [24]. In addition, low levels of urinary melatonin have been associated with an increased risk of breast cancer in premenopausal women and increased melatonin levels have been related to a lower risk of invasive breast cancer in postmenopausal women [25]. In this context, any modification of melatonin secretion under the influence of breast cancer carcinogens should be placed under scrutiny.
The cellular and molecular actions of melatonin in relation with breast cancer have been well documented (ref in [26], [27]); melatonin oncostatic action is mainly due to its anti-estrogenic action involving the estrogen receptor alpha (ERα) (ref. in [28]).
4. The mechanisms of action triggered by DMBA
To summarize (Fig. 3), DMBA disrupts several key factors of both Hypothalamo-Pituitary axes in SD female rats: 1) along the HPG axis, we could hypothesize that the decreased expression of GnRH (and its secretion by the hypothalamus) and pituitary GnRH-R partly explains the blockade of LH and FSH surges and the maintenance of high E2 blood concentrations. It is noteworthy that PRL secretion by the pituitary gland is increased (which is consistent with the fact that PRL secretion does not depend on GnRH); 2) along the HPA axis, DMBA probably counteracts the effect of E2 on corticosterone secretion and abolishes almost completely the circadian secretion of the latter hormone. 3) In the melatoninergic system, DMBA and E2 act similarly for the blunting of the secretion of this hormone.
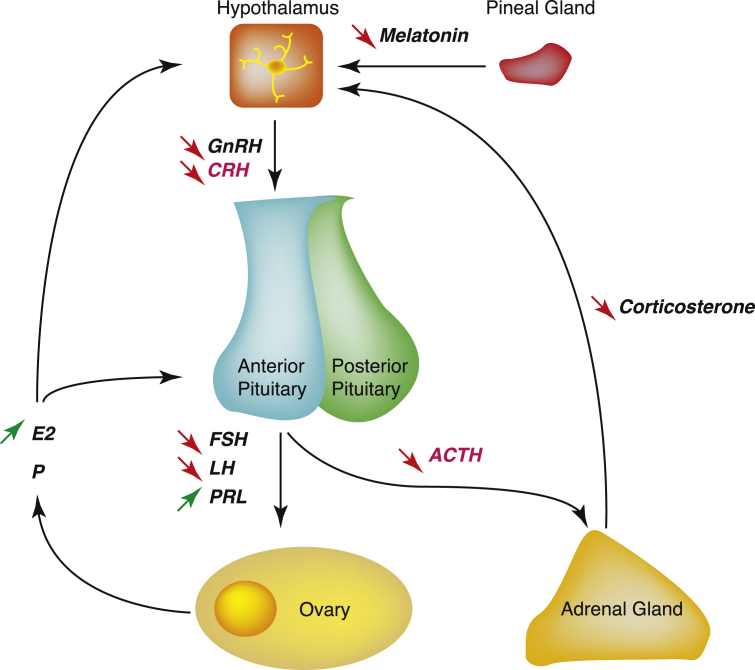
Fig. 3.
The neuro-endocrine mechanisms of action triggered by DMBA on both Hypothalamo-Pituitary-Adrenal and Hypothalamo-Pituitary-Gonadal axes in the SD female rat: DMBA alters melatonin secretion by the pineal gland, the secretion of GnRH and CRH by the hypothalamus which eventually impacts the pituitary (secretion of FSH, LH, PRL and ACTH), adrenal gland (secretion of corticosterone) and ovary functions (secretion of E2) and the feedback loops.
Overall, DMBA seems to exert pro- and anti-estrogenic effects on the neuroendocrine system, that can be partly explained by recent data and the direct/indirect effects of this carcinogen on several key cellular receptors, including the estrogen receptors (ERs) and the Aryl hydrocarbon Receptor (AhR), which have been shown to interact [29]. The AhR is a transcription factor, expressed in the central nervous system (including the hypothalamus, the hippocampus & the pituitary) [30]. Upon ligand binding (mostly xenobiotics), the AhR translocates to the nucleus where it heterodimerizes with the AhR nuclear translocator (ARNT) and the complex binds to xenobiotic response elements (XRE), regulating positively or negatively the transcription of target genes, including those encoding detoxification enzymes [31].
4.1. DMBA: an estrogen-mimetic in the short-term?
DMBA was previously shown to mimic neuroendocrine effects of E2 [11], [32]. These effects were very likely provoked by the binding of DMBA to ERs or to the AhR (or to both receptors since an intrinsic function of the AhR was documented as a possible key factor in female reproduction) [33]. Several studies showed that E2 down-regulated the expression of both GnRH and GnRH-R genes [15]. We could then hypothesize that DMBA acts similarly to E2 along the HPG axis, wherein it binds to ERs and subsequently delays the secretion of GnRH induced by the hypothalamus and the pituitary FSH/LH surges.
4.2. DMBA: acting negatively on receptor function in the long-term?
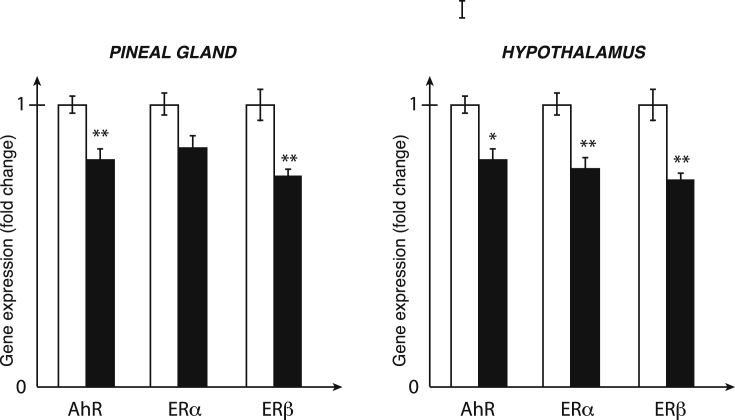
After one single administration of DMBA and although this xenobiotic is not a persistent organic pollutant, a long lasting and similar decrease in the expression of AhR, ERα and ERβ genes in the hypothalamus and the pineal gland was observed (Fig. 2) [34]. No change in the expression of the above-mentioned genes occurred in the hippocampus, a non-neuroendocrine structure [34]. In view of the well-documented action of estrogens on both HPG and HPA axes and of the strong interconnections between those two axes [35], the decrease in expression of the ERα and ERβ genes are very likely responsible for the constant disregulations of the HPG and HPA axes. We could then hypothesize that the E2-induced corticosterone secretion along the HPA axis, could be impacted on the long-term by the action of DMBA on the expression of ER [15].
Fig. 2.
mRNA expression of the AhR, ERα and ERβ genes in the hypothalamus and the pineal gland after one single administration of DMBA.
4.3. DMBA: a complex action on two receptors subsets?
Since DMBA is a polycyclic aromatic hydrocarbon and AhR ligand, we can hypothesize that, like most AhR ligands, it displays anti-estrogenic properties [36], [37]. For example, female SD rats that were treated with TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin), an AhR high affinity ligand, display delayed incidence of mammary tumors [38]. Only a limited number of studies were carried out on the nervous system of mammals with DMBA but alternative ligands, including TCDD, were sometimes used [39]. Experimental studies showed that animals exposed to AhR ligands displayed multiple symptoms including defects of motor coordination or spatial memory, two processes that can be regulated by estrogens and ERs [40], [41], [42], [43]. The interplay between the AhR and ERs is indeed quite complex: 1) AhR ligands decrease E2 blood concentration and stimulate the expression of both cytochromes P450 1A1 and 1B1 that use E2 as a substrate [30], [44], [45]. 2) AhR exerts a competitive action on ERs, using the same transcriptional co-activators (CBP/p300) [39]. 3) Inhibitory XREs have been characterized in the promoters of estrogen-responsive genes [46]. 4) AhR triggers the ubiquitination and degradation of ERα (forming a nuclear complex with CUL4B, an E3-ubiquitin ligase) [47]. However, pro-estrogenic properties could also be observed with unbound ER; AhR could then act as a co-activator on the promoter of estrogen-responsive genes [45]. In line with these observations, several studies showed a co-localization of the AhR and the ERs in the central nervous system; for example, a co-localization of the AhR with ERβ in the anterior pituitary [32] and the brain (Ref. in [38]) was described.
5. Conclusion
Besides its well-documented carcinogenic action at the mammary gland level, DMBA provokes long-term and constant neuroendocrine disruptions of the functioning of two major neuroendocrine axes, the HPG and the HPA. Several observations might be important to give a clue for the carcinogenesis process. First, the secretion of melatonin is reduced by DMBA (and E2) while the preventive and curative effects of melatonin on breast cancer are well known. Second, along the HPG axis, the increased DMBA-induced secretion of E2 and PRL favors the promotion of the mammary cancer while, along the HPA axis, the reduced secretion of corticosterone and the marked reduction of the circadian rhythm might account for the long-term reduction of anxiety level during the promotion phase of the mammary cancer.
Globally, several hypotheses could be enunciated regarding the consequences of ER and AhR down-regulation in both hypothalamus and pituitary structures. On a short-term mechanism of action, DMBA mimics E2 because it binds to E2R, but also stimulates a negative feedback loop through AhR activation, thus explaining the occurrence of pro- and anti-estrogenic effects. It is noteworthy that, following long-term exposure to DMBA, AhR mRNA is down regulated, thus suggesting that the global picture is far more complex than expected and requests additional investigations. Epigenetic mechanisms could also take place to explain the long-lasting effect of DMBA and needs to be explored because it could represent a novel type of cross-talks between AhR and ERs.
Conflict of interest:
The authors declare the absence of conflict of interest in relation to the present work.
Acknowledgements
This work was funded by the Université Paris Descartes PRES Sorbonne Paris Cité, the Institut National de la Santé et de la Recherche Médicale (INSERM), the Agence Nationale de la Recherche (ANR), ToxAhBrain, Programme CESA (ANR-13-CESA-0005-01), the Centre National de la Recherche Scientifique (CNRS).
References
- 1.Zhang Y., Dong S., Wang H., Tao S., Kiyama R. Biological impact of environmental aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ. Pollut. 2016;213:809–824. doi: 10.1016/j.envpol.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 2.Arfsten D.P., Schaeffer D.J., Mulveny D.C. The effects of near ultraviolet radiation on the toxic effects of polycyclic aromatic hydrocarbons in animals and plants. Ecotoxicol. Environ. Saf. 1996;33:1–24. doi: 10.1006/eesa.1996.0001. [DOI] [PubMed] [Google Scholar]
- 3.Santodonato J. Review of the estrogenic and antiestrogenic activity of polycyclic aromatic hydrocarbons: relationship to carcinogenicity. Chemosphere. 1997;34:835–848. doi: 10.1016/s0045-6535(97)00012-x. [DOI] [PubMed] [Google Scholar]
- 4.Huggins C., Grand L.C., Brillantes F.P. Mammary cancer induced by a single feeding of polynuclear hydrocarbons, and its suppression. Nature. 1961;189:204–207. doi: 10.1038/189204a0. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y., Yao Y., Liu S., Wang L., Moorthy B., Xiong D., Cheng T., Ding X., Gu J. Role of mammary epithelial and stromal P450 enzymes in the clearance and metabolic activation of 7,12-dimethylbenz(a)anthracene in mice. Toxicol. Lett. 2012;212:97–105. doi: 10.1016/j.toxlet.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniel F.B., Joyce N.J. DNA adduct formation by 7,12-dimethylbenz[a]anthracene and its noncarcinogenic 2-fluoro analogue in female Sprague-Dawley rats. J. Natl. Cancer Inst. 1983;70:111–118. [PubMed] [Google Scholar]
- 7.Russo J., Tait L., Russo I.H. Susceptibility of the mammary gland to carcinogenesis. III. The cell of origin of rat mammary carcinoma. Am. J. Pathol. 1983;113:50–66. [PMC free article] [PubMed] [Google Scholar]
- 8.Russo J., Tay L.K., Russo I.H. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res. Treat. 1982;2:5–73. doi: 10.1007/BF01805718. [DOI] [PubMed] [Google Scholar]
- 9.Dao T.L. The role of ovarian hormones in initiating the induction of mammary cancer in rats by polynuclear hydrocarbons. Cancer Res. 1962;22:973–981. [PubMed] [Google Scholar]
- 10.Granberg L., Ostergren A., Brandt I., Brittebo E.B. CYP1A1 and CYP1B1 in blood-brain interfaces: CYP1A1-dependent bioactivation of 7,12-dimethylbenz(a)anthracene in endothelial cells. Drug Metab. Dispos. 2003;31(:259–265. doi: 10.1124/dmd.31.3.259. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Segura L.M., Diolez-Bojda F., Lenoir V., Naftolin F., Kerdelhué B. Estrogen-like effects of the mammary carcinogen 7,12-dimethylbenz(alpha)anthracene on hypothalamic neuronal membranes. Brain Res. Bull. 1992;28:625–628. doi: 10.1016/0361-9230(92)90113-c. [DOI] [PubMed] [Google Scholar]
- 12.Kerdelhué B., El Abed A. Inhibition of preovulatory gonadotropin secretion and stimulation of prolactin secretion by 7,12-dimethylbenz(a)anthracene in Sprague-Dawley rats. Cancer Res. 1979;39:4700–4705. [PubMed] [Google Scholar]
- 13.El Abed A., Kerdelhué B., Castanier M., Scholler R. Stimulation of estradiol-17 beta secretion by 7,12-dimethylbenz (a) anthracene during mammary tumor induction in Sprague-Dawley rats. J. Steroid Biochem. 1987;26:733–738. doi: 10.1016/0022-4731(87)91047-8. [DOI] [PubMed] [Google Scholar]
- 14.Kerdelhué B., Peck E.J., Jr. In vitro LHRH release: correlation with the LH surge and alteration by a mammary carcinogen. Peptides. 1981;2:219–222. doi: 10.1016/s0196-9781(81)80037-x. [DOI] [PubMed] [Google Scholar]
- 15.Jakubowski M., Lenoir V., Jimenez-Linan M., Duval P., Israel L. Long-term effects of the mammary carcinogen 7,12-dimethylbenz(a) anthracene on hypothalamic gonadotropin-releasing hormone and its pituitary receptor gene expression, during the promotion stage, in female Sprague-Dawley rats. Breast Cancer Res. Treat. 2002;73:23–29. doi: 10.1023/a:1015282229388. [DOI] [PubMed] [Google Scholar]
- 16.Carrera-Gonzalez M.D.P., Ramirez-Exposito M.J., Duenas B., Martinez-Ferrol J., Mayas M.D., Martinez-Martos J.M. Putative relationship between hormonal status and serum pyrrolidone carboxypeptidase activity in pre- and post-menopausal women with breast cancer. Breast. 2012;21:751–754. doi: 10.1016/j.breast.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Yon de Jonage-Canonico M.B., Lenoir V., Scholler R., Kerdelhué B. Long-term dysregulation of circadian and 17-beta estradiol-induced LH, prolactin and corticosterone secretion after dimethylbenz(a) anthracene administration in the Sprague-Dawley female rat. Breast Cancer Res. Treat. 2005;92:47–50. doi: 10.1007/s10549-005-0270-6. [DOI] [PubMed] [Google Scholar]
- 18.De Jonage-Canonico M.B., Roubertoux P.L., Lenoir V., Carlier M., Kerdelhué B. Long term reduction in anxiety levels during the promotion phase of mammary adenocarcinoma induced by dimethylbenz (a) anthracene in female Sprague- Dawley rats. Open Neuroendocrinol. J. 2010;3:52–58. [Google Scholar]
- 19.Pyter Leah M. The influence of cancer on endocrine immune and behavioral stress responses. Physiol. Behav. Jun 2015 doi: 10.1016/j.physbeh.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 20.Reiter R.J. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- 21.Perreau-Lenz S., Kalsbeek A., Garidou M.L., Wortel J., van der Vliet J., van Heijningen C., Simonneaux V., Pévet P., Buijs R.M. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur. J. Neurosci. 2003;17:221–228. doi: 10.1046/j.1460-9568.2003.02442.x. [DOI] [PubMed] [Google Scholar]
- 22.De Jonage-Canonico M.B., Lenoir V., Martin A., Scholler R., Kerdelhué B. Long term inhibition by estradiol or progesterone of melatonin secretion after administration of a mammary carcinogen, the dimethyl benz(a)anthracene, in Sprague-Dawley female rat; inhibitory effect of melatonin on mammary carcinogenesis. Breast Cancer Res. Treat. 2003;79:365–377. doi: 10.1023/a:1024059824430. [DOI] [PubMed] [Google Scholar]
- 23.Lenoir V., De Jonage-Canonico M.B., Perrin M.H., Martin A., Scholler R., Kerdelhué B. Preventive and curative effect of melatonin on mammary carcinogenesis induced by dimethylbenz(a)anthracene in the female Sprague-Dawley rat. Breast Cancer Res. 2005;7:R470–R471. doi: 10.1186/bcr1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schernhammer E.S., Laden F., Speizer F.E., Willett W.C., Hunter D.J., Kawachi I., Coldit G.A. Rotating night shifts and risk of breast Cancer in women participating in the nurses' health study. J. Natl. Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 25.Brown S.B., Hankinson S.E., Eliassen A.H., Reeves K.W., Jing Q., Arcaro K.F., Wegrzyn L.R., Willett W.C., Schernhammer E.S. Urinary melatonin concentration and the risk of breast cancer in nurses' health study II. Am. J. Epidemiol. 2015;181:155–162. doi: 10.1093/aje/kwu261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant S.G., Melan M.A., Latimer J.J., Witt P.A. Melatonin and breast cancer: cellular mechanisms, clinical studies and future perspectives. Expert Rev. Mol. Med. 2009;11:e5. doi: 10.1017/S1462399409000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill S.M., Belancio V.P., Dauchy R.P., Brimer S., Mao L., Hauch A., Lundberg P.W., Summers W., Yuan L., Frash T., Blask D.E. Melatonin : an inhibitor of breast cancer. Endocr. Relat. Cancer. 2015;22:183–204. doi: 10.1530/ERC-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cos S., Gonzalez A., Martinez-Campa C., Mediavilla M.D., Alonso-Gonzalez C., Sanchez-Barcelo E.J. Estrogen-signaling pathway: a link between breast cancer and melatonin oncostatic actions. Cancer Detect. Prev. 2006;30:118–128. doi: 10.1016/j.cdp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Ruegg J., Swedenborg E., Wahlstrom D., Escande A., Balaguer P. The transcription factor aryl hydrocarbon receptor nuclear translocator functions as an estrogen receptor beta-selective coactivator, and its recruitment to alternative pathways mediates antiestrogenic effects of dioxin. Mol. Endocrinol. 2008;22:304–316. doi: 10.1210/me.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang P., Rannug A., Ahlbom E., Hakansson H., Ceccatelli S. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the expression of cytochrome P450 1A1, the aryl hydrocarbon receptor, and the aryl hydrocarbon receptor nuclear translocator in rat brain and pituitary. Toxicol. Appl. Pharmacol. 2000;169:159–167. doi: 10.1006/taap.2000.9064. [DOI] [PubMed] [Google Scholar]
- 31.Barouki R., Aggerbeck M., Aggerbeck L., Coumoul X. The aryl hydrocarbon receptor system. Drug Metabol. Drug Interact. 2012;27:3–8. doi: 10.1515/dmdi-2011-0035. [DOI] [PubMed] [Google Scholar]
- 32.Pasqualini C., Sarrieau A., Dussaillant M., Corbani M., Bojda-Diolez F. Estrogen-like effects of 7,12-dimethylbenz(a)anthracene on the female rat hypothalamo-pituitary axis. J. Steroid Biochem. 1990;36:485–491. doi: 10.1016/0022-4731(90)90092-7. [DOI] [PubMed] [Google Scholar]
- 33.Baba T., Mimura J., Nakamura N., Harada N., Yamamoto M. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol. Cell. Biol. 2005;25:10040–10051. doi: 10.1128/MCB.25.22.10040-10051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cadoudal T., Lenoir V., Penot G., Sathish I.S.H., Zhao Y., Coumoul X., Forest C., Kerdelhué B. Decrease in aryl hydrocarbon receptor and 17β-Estradiol receptor (A and B) gene expression in the hypothalamus and the pineal gland, after administration of dimethylbenz(A)Anthracene, a mammary carcinogen, to Sprague- Dawley female rats. J. Steroids Horm. Sci. 2006;5:127. [Google Scholar]
- 35.Kerdelhué B., Jones G.S., Gordon K., Seltman H., Lenoir V. Activation of the hypothalamo-anterior pituitary corticotropin-releasing hormone, adrenocorticotropin hormone and beta- endorphin systems during the estradiol 17 beta - induced plasma LH surge in the ovariectomized monkey. J. Neurosci. 1995;42:228–235. doi: 10.1002/jnr.490420210. [DOI] [PubMed] [Google Scholar]
- 36.Coumoul X. Dioxin and estradiol, a <<complex>> story. Med. Sci. Paris. 2007;23:701–702. doi: 10.1051/medsci/20072389701. [DOI] [PubMed] [Google Scholar]
- 37.Ohtake F., Takeyama K., Matsumoto T., Kitagawa H., Yamamoto Y. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 38.Holcomb M., Safe S. Inhibition of 7,12-dimethylbenzanthracene-induced rat mammary tumor growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Lett. 1994;82:43–47. doi: 10.1016/0304-3835(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 39.Kajta M., Wojtowicz A.K., Mackowiak M., Lason W. Aryl hydrocarbon receptor-mediated apoptosis of neuronal cells: a possible interaction with estrogen receptor signaling. Neuroscience. 2009;158:811–825. doi: 10.1016/j.neuroscience.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 40.Nishijo M., Kuriwaki J., Hori E., Tawara K., Nakagawa H. Effects of maternal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on fetal brain growth and motor and behavioral development in offspring rats. Toxicol. Lett. 2007;173:41–47. doi: 10.1016/j.toxlet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Seo B.W., Sparks A.J., Medora K., Amin S., Schantz S.L. Learning and memory in rats gestationally and lactationally exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Neurotoxicol. Teratol. 1999;21:231–239. doi: 10.1016/s0892-0362(98)00049-x. [DOI] [PubMed] [Google Scholar]
- 42.Thiel R., Koch E., Ulbrich B., Chahoud I. Peri- and postnatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin: effects on physiological development, reflexes, locomotor activity and learning behaviour in Wistar rats. Arch. Toxicol. 1994;69:79–86. doi: 10.1007/s002040050141. [DOI] [PubMed] [Google Scholar]
- 43.Carpenter K.D., Korach K.S. Potential biological functions emerging from the different estrogen receptors. Ann. N. Y. Acad. Sci. 2006;1092:361–373. doi: 10.1196/annals.1365.033. [DOI] [PubMed] [Google Scholar]
- 44.Coumoul X., Diry M., Robillot C., Barouki R. Differential regulation of cytochrome P450 1A1 and 1B1 by a combination of dioxin and pesticides in the breast tumor cell line MCF-7. Cancer Res. 2001;61:3942–3948. [PubMed] [Google Scholar]
- 45.Tsuchiya Y., Nakajima M., Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227:115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Safe S., Wormke M., Samudio I. Mechanisms of inhibitory aryl hydrocarbon receptor-estrogen receptor crosstalk in human breast cancer cells. J. Mammary Gland. Biol. Neoplasia. 2000;5:295–306. doi: 10.1023/a:1009550912337. [DOI] [PubMed] [Google Scholar]
- 47.Ohtake F., Baba A., Takada I., Okada M., Iwasaki K. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature. 2007;446:562–566. doi: 10.1038/nature05683. 8. [DOI] [PubMed] [Google Scholar]