Abstract
CRISPR/Cas9 system confers molecular immunity in archeal and bacterial species against invading foreign nucleic acids. CRISPR/Cas9 system is used for genome engineering applications across diverse eukaryotic species. In this study, we demonstrate the utility of the CRISPR/Cas9 genome engineering system for drug target validation in human cells. Pladienolide B is a natural macrolide with antitumor activities mediated through the inhibition of pre-mRNA splicing. To validate the spliceosomal target of Pladienolide B, we employed the CRSIPR/Cas9 system to introduce targeted mutations in the subunits of the SF3B complex in the HEK293T cells. Our data reveal that targeted mutagenesis of the SF3b1 subunit exhibited higher levels of resistance to Pladienolide B. Therefore, our data validate the spliceosomal target of Pladienolide B and provide a proof of concept on using the CRISPR/Cas9 system for drug target identification and validation.
Keywords: CRIPSR/Cas9, Pladienolide B, Spliceosome, Pre-mRNA splicing, Drug target validation, Drug discovery
Abbreviations: SSNs, Site Specific Nucleases; ZFN, Zing Finger Nucleases; TALENs, Transcription Like Effector Nucleases; CRISPR, Clustered Regulatory Interspaced Short Palindromic Repeats; Cas9, CRISPR associated protein; sgRNA, Guide RNA; NHJE, Non-Homologous End-Joining; HR, Homologous Recombination; DSB, double strand break; PB, Pladienolide B; AB, Alamar Blue; T7EI, T7 Endonuclease I
Highlights
-
•
CRISPR/Cas9 system serves as an excellent tool for drug target validation.
-
•
Pladienolide B an antitumor macrolide known to bind to the SF3b complex and inhibit pre-mRNA splicing.
-
•
Use of CRISPR/Cas9 system to confirm and validate the Pladienolide B spliceosomal target.
-
•
Our data confirm that the SF3b1 is a specific target for Pladienolide B.
Targeted modification of genomes holds much promise in functional analysis studies in basic biology and applied biotechnology [1], [2]. Site-specific nucleases (SSNs) that can be customized to specifically bind and cleave a user-defined sequence have been developed including zinc finger nucleases (ZFNs), transcription activator like nucleases (TALENs) and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated (Cas9) systems [3], [4], [5], [6]. SSNs generate double strand breaks (DSBs) that are repaired by either the error-prone non-homologous end joining (NHEJ) or the precise homology directed repair (HDR), where a template is provided to copy the information across the break [7], [8]. Due to its facile engineering and higher efficiencies, CRISPR/Cas9 system is the platform of choice for targeted editing and mutagenesis of eukaryotic genomes [9], [10]. Therefore, CRISPR/Cas9 has been used across diverse eukaryotic species for targeted genome editing and regulation and for functional analysis studies [3], [4], [5].
Studies of drug target discoveries were hindered by the lack of efficient and reproducible-targeted modification of mammalian genomes. The efficiency of the CRISPR/Cas9 system, and the ability of modifying several targets simultaneously (multiplexing), and amenability of sgRNA library construction and screening are poised to expedite the drug discovery and development efforts for different applications including the treatment of human diseases [5], [11], [12], [13], [14], [15]. Pladienolide B (PB), a natural product with antitumor activities isolated from Streptomyces platensis and shown to bind the SAP130 subunit of the SF3B complex [16], [17]. The SF3B complex is part of the U2 snRNP complex [18], [19], [20]. Binding of PB to the SF3B complex blocks splicing and prompts nuclear export of intron containing transcripts [17], [21], [22]. Here, we attempted to validate the molecular target of the PB in the SF3B complex. Therefore, we generated sgRNAs with binding specificities to all SF3B subunits. We performed transfections into HEK293T cells using Cas9 endonuclease driven by the β-chicken promoter and sgRNA driven by the U6 promoter (Fig. S1A). We designed several sgRNAs to target some subunits of the spliceosome complex that is including some of SF3B complex such as SF3B1, SF3B2, SF3B3 [23] and SF3A complex such as SF3A3 (Fig. S1B and Supporting information and Table S2). Catalytically inactive Cas9 (dCas9) was used as a negative control (Fig. S1A). Three days post-transfection, cells were collected and genomic DNA purified and subjected to T7 Endonuclease I (T7EI) mutation detection assays. Our data reveal high levels of targeted mutagenesis of the SF3B subunits as demonstrated by T7EI mutation detection assays (Fig. 1A–D). To validate the targeted mutagenesis efficiencies and precisely determine the nature of indels, we PCR-amplified fragments encompassing the target sites, SF3B1, SF3B2, SF3B3, and SF3A3, and cloned the amplicons. Subsequently, we subjected these clones to Sanger sequencing and analyzed the presence of indels and the efficiency of targeted mutagenesis (Fig. 1A–D). We achieved 23% of targeted mutagenesis of the SF3B1 subunit, 37.5% for the SF3B2, 35.7% for the SF3B3 subunit and 20% for the SF3A3 subunit (Fig. 1E).
Fig. 1.
Detection of the targeted modification of each spliceosome subunit by T7EI assay and Sanger sequencing. (A–D) T7EI assays and alignment of Sanger sequencing reads of PCR amplicons encompassing the targets of SF3b1, SF3b2, SF3b3 and SF3a3 genes respectively. SgRNAs targets are highlighted in red, the PAM sequence is underlined, dashes indicate nucleotide deletions, nucleotides highlighted in blue indicate insertions, and nucleotides highlighted in green indicate substitutions. Arrow indicates the expected size of the DNA bands of corresponding amplicons cleaved by T7EI. (E) Genome editing efficiency in each subunit estimated by the number of mutant clones divided by the total number of sequenced clones.
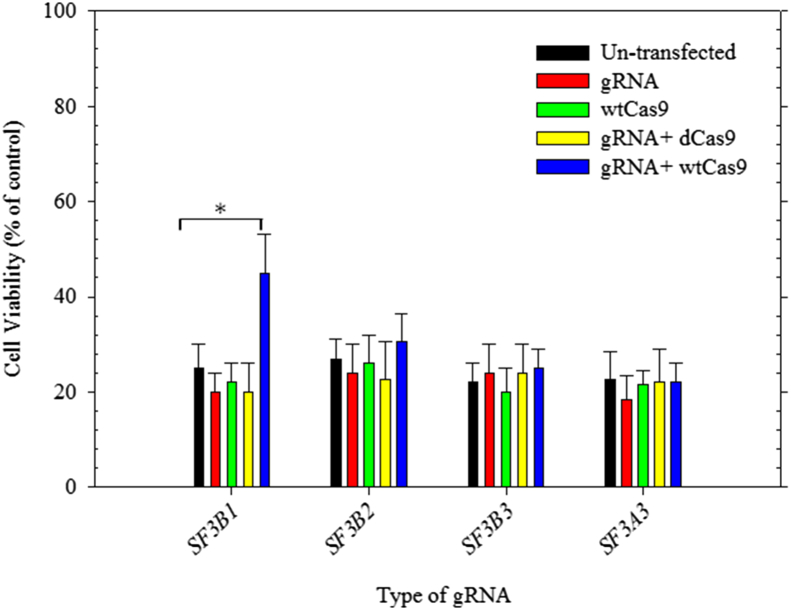
Having achieved higher levels of targeted mutagenesis in all subunits of the SF3B complex, we proceeded to investigate the effects of the targeted mutagenesis of each subunit to the sensitivity to PB. Three days post transfection, cells were collected and seeded again in a 24 well culture plate and cultured for 24 h prior treatment with increasing concentrations of PB (1–100 nM) and incubated for 24 h at 37 °C humidified incubator with 5% CO2. Subsequently, at the end of incubation time the media was removed and we performed cell viability assays using Almar Blue (AB) [24], [25], [26]. Using this assay, it is possible to spectrophotometrically measure the cellular proliferation. Resazurin (oxidized form) is blue and non-fluorescent, whereas resorufin (reduced form) is red and highly fluorescent. Therefore, AB reduction is a suitable indicator of the cellular viability [27]. The AB assays were carried out according to manufacturer's instructions. In each experiment, wells containing only the AB solution without cells were also prepared and incubated for 5 h (Fig. 2). HEK293T cells edited in SF3B1 subunit of the spliceosome complex and treated with 100 nM concentration of PB showed a red color when compared to the cells un-transfected or transfected with only sgRNA or wtCas9 only indicating more resorufin accumulation and high level of resistance to the PB (Fig. 2). Higher cell viability levels (50% ± 8) were detected in population of cells co-transfected with wtCas9 and a sgRNA targeting SF3B1 compared to lower cell viability levels (23% ± 5) for cells co-transfected with dCas9 and sgRNA targeting SF3B1 (Fig. 2). However, no significant difference has been observed in HEK293T cells co-transfected with SF3B2, SF3B3 or SF3A3 indicating that SF3b1 is the bona fide target of the PB antitumor drug. Our data confirm the conclusions of previous work from Yokoi et al., where a mutation in the gene for SF3B1 was shown to confer resistance to the inhibitory action of PB [17]. Therefore, CRISPR/Cas9 system could be used to identify a target of known drugs by using a specific subset of sgRNAs and screening their potential targets. Furthermore, sgRNA libraries with genome-wide targets could be employed to generate functional knock outs for the purpose of identifying drug targets (Fig. S2). Our data validate that the SF3B1 is the bona fide molecular target the PB and indicate the power of this technology in identifying existing and novel drug targets. Such applications would revolutionize the drug discovery and development efforts, and lead to the next generation of smart and personalized drugs.
Fig. 2.
Effect of the specific gene editing on the toxicity of the antitumor macrolide Pladienolide B on the HEK293T cells. (A) Cell viability was assessed by colorimetric assay using Alamar Blue Cell Viability Reagent (Life Technologies, UK). HEK293T cells edited in various genes (SF3A3, SF3B1, SF3B2, and SF3B3) and treated with 100 nM of Pladienolide B for 24 h at 37 °C humidified incubator with 5% CO2. Specific deletion of SF3B1 displayed high level of resistance to the PB when compared to the un-transfected cells or cells transfected only with wtCas9, or sgRNA or dCas9. Result is a representative of three independent experiments and the bars represents the mean ± SD and *P < 0.01.
Our study extends the utility of the CRISPR/Cas9 system for drug discovery and development applications. Molecular target validation of PB would unlock the possibilities of developing new synthetic analogues that are cheaper and more stable with superb antitumor activities. Furthermore, target knockouts of genes of key cellular machineries, could be used to discover novel drugs from synthetic and natural chemical libraries. Different platforms for genome-wide interrogation of gene function have been recently developed [28]. Such platforms could be utilized to identify the molecular targets of existing drugs, and for novel drug discovery (Fig. S2). Furthermore, such a platform would be helpful in identifying interacting genes and pathways with the primary drug target. By manipulating those interacting genes and pathways, we could increase the efficacy of the current drugs and develop much more powerful drugs. In conclusion, CRISPR/Cas9 system serves as a key tool in the molecular toolbox for drug discovery and development.
Competing interests
Authors declare no competing interests in regards to this project.
Author contribution statement
M.M., M.A., and A.E., designed research, M.A., and A.E., performed research, M.M., and M.A., analyzed data, M.A., prepared the figures, and M.M., and M.A., wrote the manuscript. All authors reviewed the manuscript.
Acknowledgments
We thank the Bioscience Core Facility at King Abdullah University of Science and Technology (KAUST) for technical assistance. We also thank the members of the genome engineering group at KAUST for their helpful discussions and technical assistance throughout the preparation of the manuscript. This research was funded King Abdullah University of Science and Technology (KAUST).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.biopen.2016.02.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Maggio I., Goncalves M.A. Genome editing at the crossroads of delivery, specificity, and fidelity. Trends Biotechnol. 2015;33:280–291. doi: 10.1016/j.tibtech.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Yang X. Applications of CRISPR-Cas9 mediated genome engineering. Mil. Med. Res. 2015;2:11. doi: 10.1186/s40779-015-0038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cathomen T., Joung J.K. Zinc-finger nucleases: the next generation emerges. Mol. Ther. 2008;16:1200–1207. doi: 10.1038/mt.2008.114. [DOI] [PubMed] [Google Scholar]
- 4.Chandrasegaran S., Carroll D. Origins of programmable nucleases for genome engineering. J. Mol. Biol. 2015 doi: 10.1016/j.jmb.2015.10.014. http://dx.doi.org/10.1016/j.jmb.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang W., Bikard D., Cox D., Zhang F., Marraffini L.A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 2013;31:233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukacsovich T., Yang D., Waldman A.S. Repair of a specific double-strand break generated within a mammalian chromosome by yeast endonuclease I-SceI. Nucleic Acids Res. 1994;22:5649–5657. doi: 10.1093/nar/22.25.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouet P., Smih F., Jasin M. Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 1994;91:6064–6068. doi: 10.1073/pnas.91.13.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sander J.D., Joung J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore J.D. The impact of CRISPR-Cas9 on target identification and validation. Drug Discov. Today. 2015;20:450–457. doi: 10.1016/j.drudis.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Neggers J.E., Vercruysse T., Jacquemyn M., Vanstreels E., Baloglu E., Shacham S., Crochiere M., Landesman Y., Daelemans D. Identifying drug-target selectivity of small-molecule CRM1/XPO1 inhibitors by CRISPR/Cas9 genome editing. Chem. Biol. 2015;22:107–116. doi: 10.1016/j.chembiol.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Shi J., Wang E., Milazzo J.P., Wang Z., Kinney J.B., Vakoc C.R. Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nat. Biotechnol. 2015;33:661–667. doi: 10.1038/nbt.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shuvalov O., Barlev N.A. Current genome editing tools in gene therapy: new approachesto treat cancer. Curr. Gene Ther. 2015;15:511–529. doi: 10.2174/1566523215666150818110241. [DOI] [PubMed] [Google Scholar]
- 15.Smurnyy Y., Cai M., Wu H., McWhinnie E., Tallarico J.A., Yang Y., Feng Y. DNA sequencing and CRISPR-Cas9 gene editing for target validation in mammalian cells. Nat. Chem. Biol. 2014;10:623–625. doi: 10.1038/nchembio.1550. [DOI] [PubMed] [Google Scholar]
- 16.Kotake Y., Sagane K., Owa T., Mimori-Kiyosue Y., Shimizu H., Uesugi M., Ishihama Y., Iwata M., Mizui Y. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat. Chem. Biol. 2007;3:570–575. doi: 10.1038/nchembio.2007.16. [DOI] [PubMed] [Google Scholar]
- 17.Yokoi A., Kotake Y., Takahashi K., Kadowaki T., Matsumoto Y., Minoshima Y., Sugi N.H., Sagane K., Hamaguchi M., Iwata M., Mizui Y. Biological validation that SF3b is a target of the antitumor macrolide pladienolide. FEBS J. 2011;278:4870–4880. doi: 10.1111/j.1742-4658.2011.08387.x. [DOI] [PubMed] [Google Scholar]
- 18.Bonnal S., Vigevani L., Valcarcel J. The spliceosome as a target of novel antitumour drugs. Nat. Rev. Drug Discov. 2012;11:847–859. doi: 10.1038/nrd3823. [DOI] [PubMed] [Google Scholar]
- 19.Singh R.K., Cooper T.A. Pre-mRNA splicing in disease and therapeutics. Trends Mol. Med. 2012;18:472–482. doi: 10.1016/j.molmed.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb T.R., Joyner A.S., Potter P.M. The development and application of small molecule modulators of SF3b as therapeutic agents for cancer. Drug Discov. Today. 2013;18:43–49. doi: 10.1016/j.drudis.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Effenberger K.A., Anderson D.D., Bray W.M., Prichard B.E., Ma N., Adams M.S., Ghosh A.K., Jurica M.S. Coherence between cellular responses and in vitro splicing inhibition for the anti-tumor drug pladienolide B and its analogs. J. Biol. Chem. 2014;289:1938–1947. doi: 10.1074/jbc.M113.515536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato M., Muguruma N., Nakagawa T., Okamoto K., Kimura T., Kitamura S., Yano H., Sannomiya K., Goji T., Miyamoto H., Okahisa T., Mikasa H., Wada S., Iwata M., Takayama T. High antitumor activity of pladienolide B and its derivative in gastric cancer. Cancer Sci. 2014;105:110–116. doi: 10.1111/cas.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golas M.M., Sander B., Will C.L., Luhrmann R., Stark H. Molecular architecture of the multiprotein splicing factor SF3b. Science. 2003;300:980–984. doi: 10.1126/science.1084155. [DOI] [PubMed] [Google Scholar]
- 24.Rampersad S.N. Multiple applications of Alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Basel) 2012;12:12347–12360. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vega-Avila E., Pugsley M.K. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc. West Pharmacol. Soc. 2011;54:10–14. [PubMed] [Google Scholar]
- 26.White M.J., DiCaprio M.J., Greenberg D.A. Assessment of neuronal viability with Alamar blue in cortical and granule cell cultures. J. Neurosci. Methods. 1996;70:195–200. doi: 10.1016/s0165-0270(96)00118-5. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed S.A., Gogal R.M., Jr., Walsh J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 28.Xu Q.W., Zhao W., Wang Y., Sartor M.A., Han D.M., Deng J., Ponnala R., Yang J.Y., Zhang Q.Y., Liao G.Q., Qu Y.M., Li L., Liu F.F., Zhao H.M., Yin Y.H., Chen W.F., Zhang Y., Wang X.S. An integrated genome-wide approach to discover tumor-specific antigens as potential immunologic and clinical targets in cancer. Cancer Res. 2012;72:6351–6361. doi: 10.1158/0008-5472.CAN-12-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.