Abstract
Never in mitosis gene-A (NIMA)-related expressed kinase 2 (NEK2) has been recently reported to play a role in tumor progression, drug resistance and tumorigenesis. However, little is known about the effects of NEK2 in hepatocellular carcinoma (HCC) metastasis and the underlying mechanism. NEK2 expression levels were examined by immunochemistry, qRT-PCR and western blot analyses in HCC cell lines and HCC tissues. A Transwell assay was used to determine the migration and invasion capacity of NEK2-silenced or NEK2-overexpressing HCC cells. Cell proliferation was investigated by MTT [(3-(4,5)-dimethylthiazol(-z-y1)-3,5-di-phenytetrazolium bromide] assay. The expression levels of epithelial-mesenchymal transition (EMT) markers in NEK2-silenced or NEK2-overexpressing HCC cells were examined by western blot analyses and qRT-PCR. The correlations between NEK2 expression and clinicopathological characteristics were further analyzed. Gene microarray was further used to analyze the effect of NEK2 expression on downstream cell signals. Our study showed that NEK2 was overexpressed in human HCC (37.84%; 98/259). NEK2 overexpression was significantly associated with liver non-capsulation and predicted poor survival outcomes in HCC patients after hepatectomy. In addition, NEK2 significantly enhanced HCC cell invasive ability. Mechanistically, we found that the epithelial-mesenchymal transition (EMT) plays a pivotal role in the NEK2-mediated promotion of HCC cell invasion. Furthermore, we provided evidence that signaling through the Wnt, NF-κB, focal adhesion, VEGF, Hippo and p53 pathways may be downstream of NEK2. Our findings highlight the importance of NEK2 in HCC metastasis and suggest that NEK2 is a reliable prognostic marker for HCC patients after hepatectomy.
Keywords: never in mitosis gene-A (NIMA)-related expressed kinase 2 (NEK2), hepatocellular carcinoma, epithelial-mesenchymal transition
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant cancers and major health problem worldwide (1). Although HCC treatments have improved within recent years, patients with HCC often face an unfavorable prognosis, mainly due to tumor recurrence and metastasis after liver resection. Metastasis, the main risk to the long-term survival of HCC patients, involves a complicated process of invasion-metastasis cascades (2). Therefore, a better understanding of the molecular mechanisms underlying HCC metastasis is necessary for its prevention, diagnosis and treatment.
Never in mitosis gene-A (NIMA)-related expressed kinase 2 (NEK2), a serine/threonine centrosomal kinase, is highly expressed and activated during the S and G2 phases of the cell cycle and plays a pivotal role in regulating centrosome separation and mitotic progression (3). Aberrant NEK2 expression can cause chromosome instability (CIN) as well as abnormal chromosome content. Aberrant NEK2 expression has been reported in cancer cells (4), and NEK2 has recently been identified as a potential biomarker of several cancers, such as non-small lung cancer (5) and pancreatic ductal adenocarcinoma (6). Most studies have focused on the function of NEK2 in centrosome regulation as well as spindle formation (7), whereas little is known about the other functions of NEK2 in cancer. Previous reports with small patient populations have identified NEK2 as a biomarker of HCC (8,9), but few have provided evidence that NEK2 can promote HCC migration and invasion as well as the mechanisms underlying this process.
In the present study, we demonstrated that NEK2 overexpression predicted poor prognoses in HCC patients after hepatectomy. Moreover, NEK2 can promote HCC metastasis through induction of the epithelial-mesenchymal transition (EMT). We further analyzed the downstream signaling targets of NEK2 that were related to HCC metastasis.
Materials and methods
Cell lines
The human liver cancer cell lines MHCC97H, MHCC97L, SMMC-7721, HepG2, Huh7, BEL7402, and HCCLM3 and the normal human liver cell line LO2 were purchased from the Typical Culture Preservation Committee Cell Bank of the Chinese Academy of Science, Shanghai, China. The liver cancer cell lines were cultured in DMEM with 10% FBS (Thermo Fisher Scientific, MA, USA) at 37°C in a humidified incubator with 5% CO2.
NEK2 overexpression and knockdown cell lines
The NEK2 overexpression, knockdown lentivirus and negative control lentivirus vectors were purchased from GeneChem (GeneChem, Shanghai, China). The transfection was performed according to the manufacturer's instructions. Briefly, the full-length NEK2 overexpression lentivirus was transfected into MHCC97L cells, and the knockdown virus was transfected into HCCLM3 cells. In the meantime, the negative control virus was transfected into MHCC97L or HCCLM3 cells as controls. Puromycin (3 µg/ml) was then used to select the stable clones. The cDNA clone and shRNA sequences are listed in Table I.
Table I.
The sequence of cDNA clone and shRNA of NEK2.
| Name | Sequence |
|---|---|
| NEK2-RNAi-1 | TGTATTGAGTGAGCTGAAA |
| NEK2-RNAi-2 | AGACGAGCAAAGAAGAAAT |
| NEK2-RNAi-3 | TTACTCTGATGAATTGAAT |
| ORF nucleotide sequence of NEK2 (transcript variant 3) | TTTTTTGTTAGACGAAGCTTGGGCTGCAGGTCGACTCTA |
| GAGGATCCCCGGGTACCGGTCGCCACCATGCCTTCCCG | |
| GGCTGAGGACTATGAAGTGTTGTACACCATTGGCACAG | |
| GCTCCTACGGCCGCTGCCAGAAGATCCGGAGGAAGAGT | |
| GATGGCAAGATATTAGTTTGGAAAGAACTTGACTATGG | |
| CTCCATGACAGAAGCTGAGAAACAGATGCTTGTTTCTG | |
| AAGTGAATTTGCTTCGTGAACTGAAACATCCAAACATC | |
| GTTCGTTACTATGATCGGATTATTGACCGGACCAATACA | |
| ACACTGTACATTGTAATGGAATATTGTGAAGGAGGGGA | |
| TCTGGCTAGTGTAATTACAAAGGGAACCAAGGAAAGGC | |
| AATACTTAGATGAAGAGTTTGTTCTTCGAGTGATGACTC | |
| AGTTGACTCTGGCCCTGAAGGAATGCCACAGACGAAGT | |
| GATGGTGGTCATACCGTATTGCATCGGGATCTGAAACC | |
| AGCCAATGTTTTCCTGGATGGCAAGCAAAACGTCAAGC | |
| TTGGAGACTTTGGGCTAGCTAGAATATTAAACCACGAC | |
| ACGAGTTTTGCAAAAACATTTGTTGGCACACCTTATTAC | |
| ATGTCTCCTGAACAAATGAATCGCATGTCCTACAATGA | |
| GAAATCAGATATCTGGTCATTGGGCTGCTTGCTGTATGA | |
| GTTATGTGCATTAATGCCTCCATTTACAGCTTTTAGCCA | |
| GAAAGAACTCGCTGGGAAAATCAGAGAAGGCAAATTC | |
| AGGCGAATTCCATACCGTTACTCTGATGAATTGAATGA | |
| AATTATTACGAGGATGTTAAACTTAAAGGATTACCATC | |
| GACCTTCTGTTGAAGAAATTCTTGAGAACCCTTTAATAG | |
| CAGATTTGGTTGCAGACGAGCAAAGAAGAAATCTTGAG | |
| AGAAGAGGGCGACAATTAGGAGAGCCAGAAAAATCGC | |
| AGGATTCCAGCCCTGTATTGAGTGAGCTGAAACTGAAG | |
| GAAATTCAGTTACAGGAGCGAGAGCGAGCTCTCAAAGC | |
| AAGAGAAGAAAGATTGGAGCAGAAAGAACAGGAGCTT | |
| TGTGTTCGTGAGAGACTAGCAGAGGACAAACTGGCTAG | |
| AGCAGAAAATCTGTTGAAGAACTACAGCTTGCTAAAGG | |
| AACGGAAGTTCCTGTCTCTGGCAAGTAATCCAGAGTCT | |
| CACTTTGTTGCCCAGGCTGGAATGCAGTGGTGTGATCAC | |
| AGCTCAATGTAGCTAGCCTGTGGAATGTGTGTCAGTTA | |
| GGGTGTGGAAAGTCCCCAGGCTCCCCAGCAGGC |
MTT [3-(4,5)-dimethylthiazol(-z-y1)-3,5-di-phenyltetrazolium bromide] assay
Cell growth was examined using MTT assays. MHCC97L-control, MHCC97L-NEK2, HCCLM3-control, HCCLM3-shNEK2 cells were added to 96-well plates at concentration of 1×103 cells/well and placed at 37°C with 5% CO2 incubator. Then MTT reagent (0.5 mg/ml) (Sigma-Aldrich, St. Louis, MO, USA) was added to each well and further incubated for 4 h after indicated hours. Dimethyl sulfoxide (150 µl) was added. The plates were read at wavelength of 490 nm.
Migration and invasion assay
Cell migration and invasion were tested using a Transwell assay (Corning, NY, USA) as we have reported previously (10). The cells were placed into the upper chamber of the insert using Matrigel (Corning). After a 48-h incubation at 37°C, the cells adhering to the lower membrane of the insert were counted after staining with 0.1% crystal violet for 10 min. The number of the cells was observed using a Leica microscope (Leica, Wetzlar, Germany).
Quantitative real-time PCR
Total RNA was isolated using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) from the frozen tissue samples or HCC cell lines according to the manufacturer's protocol. Generation of cDNA from RNA was carried out using a cDNA conversion kit (Takara, Shiga, Japan) at 37°C for 15 min. The resultant products were then amplified using the SYBR Green PCR kit (Toyobo, Tokyo, Japan) for qRT-PCR analysis. The Ct values were measured during the amplification phase while the amplification plots were analyzed using Bio-Rad IQ5 software (Bio-Rad Laboratories Inc., CA, USA). All quantifications were normalized to the level of endogenous GAPDH as a control, and the procedure was performed as the previously reported (11). The primers were all purchased from Genecopia (Guangzhou, China).
Gene microarray
An Agilent Gene Expression array (Kangchen Biotech Inc.), containing >41,000 transcripts (http://www.Kangchen.com.cn), was used to investigate the transcriptional profiles of the MHCC97L-control and MHCC97L-NEK2 cells. The microarray datasets were normalized in GeneSpring GX using the Agilent FE one-color scenario. Differentially expressed genes were identified via fold-change screening.
Western blot analysis
The proteins of interest were obtained from lysed cells, fractioned by SDS-PAGE, and subsequently transferred to PVDF membranes (Roche Life Sciences, Switzerland). The membranes were then blocked with 5% skim milk in TBST for 1 h at room temperature and then incubated with the specific primary antibodies overnight at 4°C. Tubulin was used as a loading control. After incubation, the membranes were washed with TBST and incubated with HRP-conjugated secondary antibody. The antigen-antibody complex was detected with enhanced chemiluminescence regents (Merck Millipore, MA, USA). The antibodies are listed in Table II.
Table II.
Primary antibodies.
| Antibody name | Source |
|---|---|
| NEK2 | Abcam (55550) |
| β-tubulin | Abcam (6046) |
| E-cadherin | Cell Signaling Technology (9782) |
| N-cadherin | Cell Signaling Technology (9782) |
| α-catenin | Abcam (ab51032) |
| Vimentin | Cell Signaling Technology (5741) |
Immunofluorescence
The cells were seeded on coverslips to analyze cellular immunofluorescence. When the cells reached a confluence of 60%, they were fixed with 4% paraformaldehyde in PBS for 15 min, washed twice with PBS, and then incubated with primary antibodies against E-cadherin (Proteintech Group, Chicago, IL, USA) or N-cadherin (Cell Signaling Technology) overnight at 4°C. The cells were then incubated with FITC-conjugated goat anti-mouse or anti-rabbit IgG according to the source of the primary antibody (Genecopia) and counterstained with DAPI (Genecopia) for nuclear identification. A Leica DMRA fluorescence microscope (Leica) was used to obtain the images.
Patients and follow-up
In total, 259 patients diagnosed with HCC after hepatectomy were enrolled from the First Affiliated Hospital, Sun Yat-Sen University, Guangdong, China, between 2006 and 2009. All surgical specimens were histologically determined as HCC. Patients under 18 years of age and those with incomplete clinical, laboratory, or follow-up data were excluded. The last follow-up was conducted in December 2012. The disease-free survival (DFS) and overall survival (OS) were calculated from date of surgery to the date of recurrence or HCC-associated death, respectively. Informed consent was received from each enrolled patient, and the research was carried out with approval from the Ethics Committee of the First Affiliated Hospital of Sun Yat-Sen University (Guangdong, China). All patients in this study were classified according to the American Joint Committee on Cancer (AJCC) and tumor node metastasis (TNM) classification system.
Immunochemistry analysis for HCC patients
A tissue microarray containing tumor samples as well as the matched adjacent non-cancerous tissue from HCC patients enrolled in the study was constructed as previously described (12). A Dako Real Envision kit (K5007; Dako Denmark A/S, Denmark) was used for IHC staining. For antigen retrieval, the slides were boiled in a pressure cooker at maximum heat for 2 min, which contained 0.01 mol/l sodium citrate (pH 6.0), and then cooled to room temperature. Primary antibodies against NEK2 (1:100 dilution; Abcam, Cambridge, UK), E-cadherin (1:200 dilution; Cell Signaling Technology, Inc., MA, USA), and N-cadherin (1:200 dilution; Cell Signaling Technology, Inc.) were used for this study. A five-point scoring system as follows was used to assess staining: 0, no positive cells; 1, >0-25% positive cells; 2, >25-50% positive cells; 3, >50-75% positive cells; and 4, >75% positive cells. To maintain objectivity, we also applied a four-point scoring system as follows to describe the intensity of staining: 0, negative staining; 1, weak staining/light yellow; 2, moderate staining/yellow-brown; and 3, strong staining/brown. The NEK2, E-cadherin and N-cadherin immunoreactivity scores (IRSs) were calculated by adding the staining score to the intensity score. Cases with an IRS >4 were defined as high expression and cases with an IRS ≤4 were defined as low expression. Three independent pathologists without access to the clinicopathological data scored the staining. The IRS was determined only when all of the examining pathologists assigned a consistent score to the sample. When different scores were obtained, a consensus score was reached by discussion.
Statistical analysis
Statistical analyses were performed using SPSS 17.0. Data were expressed as the mean ± standard error of the mean (SEM) from at least three independent experiments. Quantitative data were compared between groups using Student's t-test. Categorical data were analyzed using the χ2 test or Fisher's exact test. Spearman's rank analysis was used to analyze the correlations between different protein expression levels. The Kaplan-Meier method and log-rank test were used to analyze the overall survival and the disease-free survival curve and differences. The independent factors that influenced survival and recurrence based on the variables selected from the univariate analysis were determined using the Cox proportional hazards model. Values of p<0.05 were considered as statistically significant.
Results
NEK2 expression is elevated in liver cancer cell lines and tissues
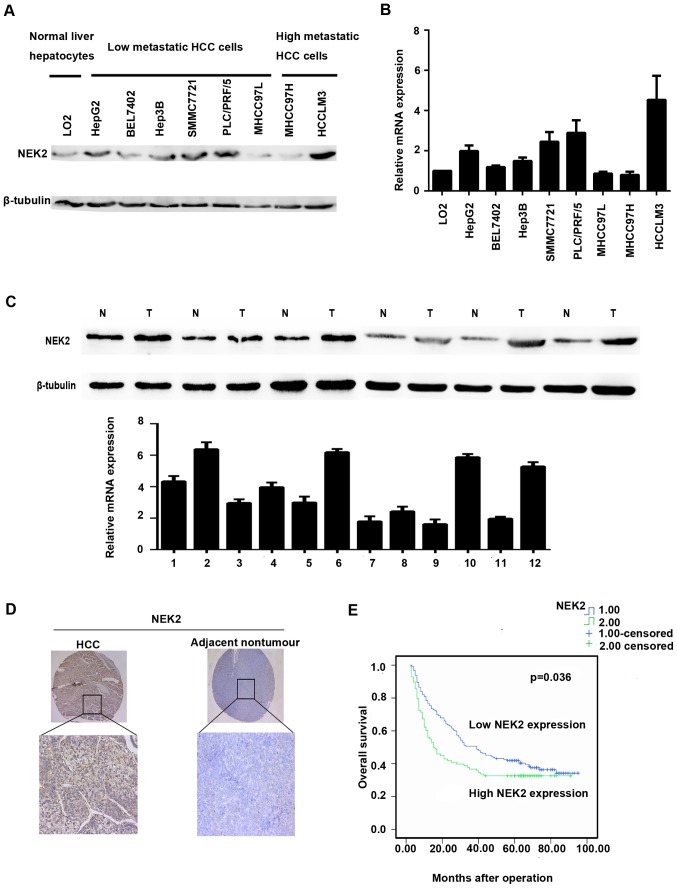
Western blot and qRT-PCR analyses were used to investigate the expression levels of NEK2 in liver cancer cell lines and tissues. Compared with the LO2 cell line, NEK2 expression was elevated in the low metastasis potential liver cancer cell lines HepG2, BEL7402, Hep3B, SMMC7721, and PLC/PRF/5 and the high metastasis potential HCC cell line HCCLM3 by qRT-PCR. However, NEK2 expression was downregulated in MHCC97L and MHCC97H cells (Fig. 1B). Western blot analysis additionally confirmed such results (Fig. 1A).
Figure 1.
Expression of NEK2 in HCC. (A) Western blot analysis of NEK2 in different HCC cell lines. (B) qRT-PCR of NEK2 in different HCC cell lines. (C) NEK2 is upregulated in human HCC tissues by western blot analysis and qRT-PCR. (D) IHC staining of NEK2 expression in adjacent non-cancerous tissues and HCC tissues. (E) Kaplan-Meier analysis of the correlation between NEK2 expression and overall survival.
NEK2 expression was further identified in 8 paired fresh HCC tissues and adjacent non-cancerous liver tissues. qRT-PCR analysis showed that mRNA levels of NEK2 were markedly elevated in 6 HCC tissues when compared with the adjacent non-cancerous liver tissues (Fig. 1B). In accordance with the qRT-PCR results, NEK2 protein expression was significantly higher in HCC tissues than in adjacent non-cancerous tissues in 6/8 cases (Fig. 1C).
NEK2 expression is correlated with poor HCC prognosis
To further evaluate the significance of NEK2 expression in HCC prognosis, we employed another HCC tissue microarray with follow-up data from 259 pairs of HCC and adjacent non-cancerous tissues. The IHC staining demonstrated that NEK2 expression was dramatically enhanced in 37.84% of the HCC samples (98/259) when compared with the adjacent non-tumorous specimens (Table III); HCC patients with higher NEK2 expression exhibited shorter median OS time (15.5 months) compared with patients who had lower levels of NEK2 expression (39.0 months) (Fig. 1E). In addition, HCC patients with higher NEK2 expression levels had shorter overall survival than did those with lower NEK2 expression levels (1-, 3- and 5-year OS: 76.5, 50.6 and 42.0% vs. 56.1, 36.7 and 32.7%, respectively). The 1-, 3- and 5-year disease-free survival (DFS) rates for HCC patients with higher or lower NEK2 expression levels were 46.9, 30.6 and 26.5% and 50.3, 34.2 and 29.1% respectively, with no significant difference between the two groups (p=0.171). Furthermore, univariate analysis revealed that NEK2 overexpression was significantly correlated with tumor size (p=0.031) and non-capsulation (p=0.019). However, no statistical connections were found between NEK2 expression and other clinicopathological parameters, such as age, sex, hepatitis B surface antigen level, α-fetoprotein (AFP) level, tumor number, liver cirrhosis, Edmondson grading, or vascular invasion (Table III). Notably, HCC patients with NEK2-positive tumor exihibited shorter overall survival (p=0.036) than did patients with NEK2-negative tumors. Moreover, multivariate analysis revealed that higher expression of NEK2, bigger tumor size, multiple tumors, non-capsulation, and vascular invasion were independent risk factors associated with decreased survival (Table IV). Collectively, the clinical data indicated that NEK2 is correlated with poor prognosis in HCC patients.
Table III.
Correlation between NEK2 expression and clinicopathological characteristics of HCC patients.
| NEK2 | ||||
|---|---|---|---|---|
| Category | n | Low expression | High expression | p-value |
| Sex | ||||
| Female | 29 | 20 | 9 | 0.543 |
| Male | 230 | 141 | 89 | |
| Age | ||||
| <60 | 198 | 127 | 71 | 0.291 |
| ≥60 | 61 | 34 | 27 | |
| HBsAg | ||||
| Negative | 31 | 18 | 13 | 0.695 |
| Positive | 228 | 139 | 84 | |
| AFP | ||||
| ≥200 | 148 | 94 | 54 | 0.608 |
| <200 | 111 | 67 | 44 | |
| Size | ||||
| ≥5 | 188 | 109 | 79 | 0.031 |
| <5 | 71 | 52 | 19 | |
| Tumor nos. | ||||
| >1 | 84 | 49 | 35 | 0.413 |
| 1 | 175 | 112 | 63 | |
| Liver cirrhosis | ||||
| Present | 206 | 125 | 81 | 0.347 |
| Absent | 53 | 36 | 17 | |
| Capsulation | ||||
| Capsulated | 96 | 55 | 41 | 0.019 |
| Non-capsulated | 163 | 106 | 57 | |
| Vascular invasion | ||||
| Absent | 208 | 132 | 76 | 0.422 |
| Present | 51 | 29 | 22 | |
| Edmondson grade | ||||
| I–II | 201 | 126 | 75 | 0.760 |
| III–IV | 58 | 35 | 23 | |
p<0.05 is highlighted as bold text.
Table IV.
Univariate and multivariate analysis of risk factors associated with overall survival of HCC patients.
| Overall survival | |||||
|---|---|---|---|---|---|
| Category | Subcategory | Univariate analysis | HR (95%) | Multivariate analysis | HR (95%) |
| Sex | Male | 0.037 | 1.037–3.221 | NA | NA |
| Female | |||||
| Age | <60 | 0.927 | NA | NA | NA |
| ≥60 | |||||
| HBs-Ag | Negative | 0.252 | NA | NA | NA |
| Positive | |||||
| AFP (ng/ml) | <200 | 0.008 | 1.122–2.118 | NA | NA |
| ≥200 | |||||
| Tumor size (cm) | <5 | <0.001 | 1.703–3.446 | <0.001 | 1.324–2.941 |
| ≥5 | |||||
| Tumor no. | Single | <0.001 | 0.332–0.622 | 0.009 | 0.462–0.896 |
| Multiple | |||||
| Liver cirrhosis | Absent | 0.375 | NA | NA | NA |
| Present | |||||
| Capsulation | Capsulated | <0.001 | 0.367–0.678 | 0.034 | 0.479–0.971 |
| Non-capsulated | |||||
| Vascular invasion | Absent | <0.001 | 2.160–4.315 | 0.002 | 1.095–2.076 |
| Present | |||||
| Edmondson grade | I–II | 0.004 | 1.174–2.310 | NA | NA |
| III–IV | |||||
| ELMO1 | Lower expression | 0.039 | 1.017–1.897 | 0.012 | 0.457–0.835 |
| Higher expression | |||||
p<0.05 is highlighted as bold text.
NEK2 can promote cell migration and invasion
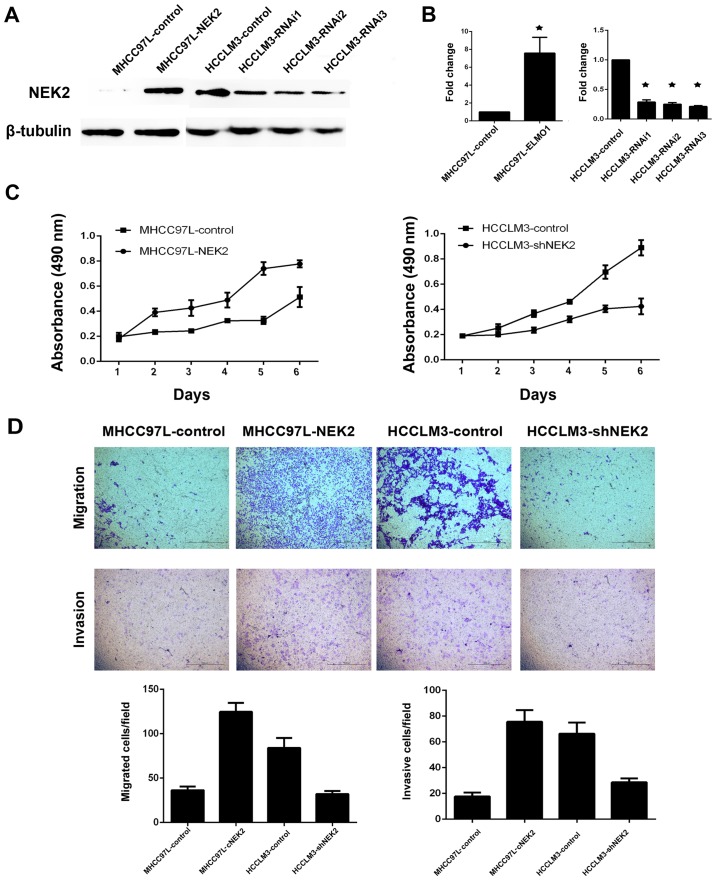
As tumor non-capsulation is a significant risk factor for tumor metastasis, higher NEK2 expression was correlated with tumor non-capsulation in our study. Therefore, we determined whether NEK2 plays a role in HCC cell proliferation or invasion. As NEK2 expression levels were upregulated in HCCLM3 cells (high metastasis liver cancer cell line) and downregulated in MHCC97L cells (low metastasis liver cancer cell line), we established two stable cell lines (MHCC97L-NEK2 and HCCLM3-shNEK2) using lentivirus. MTT assay showed that overexpression of NEK2 in MHCC 97L cells (MHCC97L-NEK2) can promote cell proliferation, whereas knockdown of NEK2 in highly metastatic HCCLM3 cells (HCCLM3-shNEK2) resulted in a remarkable suppression of cell proliferation (Fig. 2C). Furthermore, Transwell migration and Matrigel invasion assays revealed that overexpression of NEK2 dramatically inhibited cell migration and invasion when compared with control cells (Fig. 2D). Our results demonstrated that NEK2 can promote cell proliferation, migration and invasion.
Figure 2.
NEK2 can promote HCC cell proliferation and metastasis. Western blot analysis (A) and qRT-PCR (B) showed successful overexpression and knockdown of NEK2 in HCC cells. (C) Proliferation of indicated cells was examined by MTT assays. (D) Transwell cell migration and invasion assays of indicated cells.
NEK2 induces epithelial mesenchymal transition (EMT)
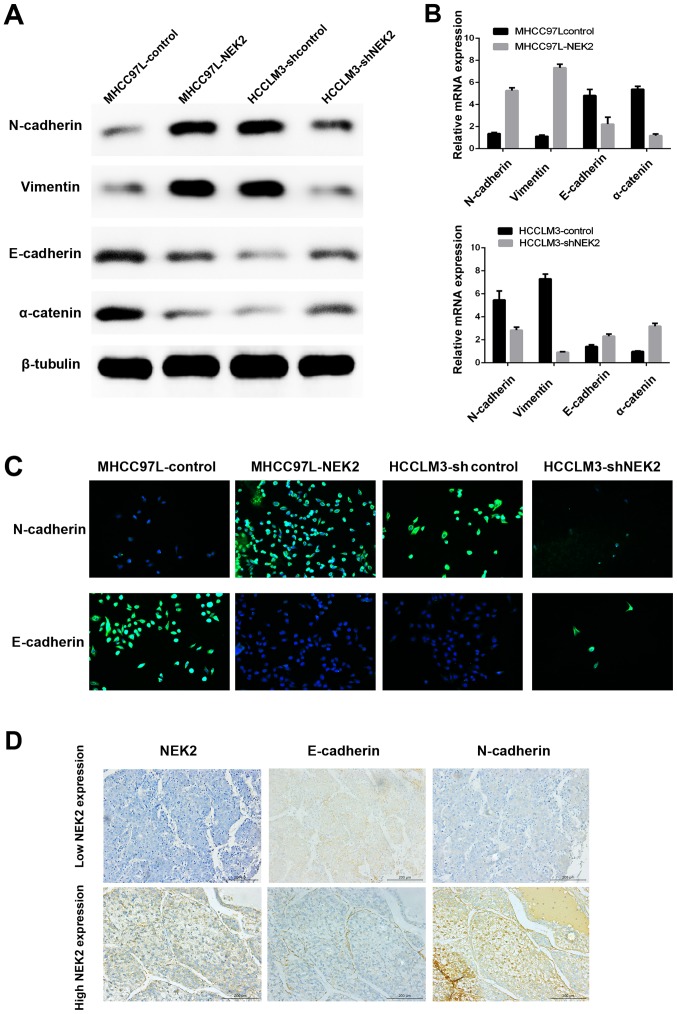
A previous study indicated that NEK2 contributed to altered β-catenin localization from the intercellular adherens junction to the cytoplasm and nucleus, which is a key process during EMT and an invasive phenotype typical of HCC. Here, we confirmed that NEK2 can induce EMT by immunofluorescence (IF), qRT-PCR, western blot analysis and IHC of EMT molecular marker expression. We showed that NEK2 overexpression increased the levels of mesenchymal marker (N-cadherin and vimentin) expression and decreased epithelial marker (E-cadherin and α-catenin) expression by qRT-PCR and western blot analyses. Conversely, knocking down NEK2 had the opposite effect (Fig. 3A and B). Moreover, IF showed that ectopic expression of NEK2 suppressed E-cadherin expression, while inducing N-cadherin expression in MHCC97L-NEK2 cells. In contrast, E-cadherin expression was enhanced, whereas N-cadherin expression was inhibited, in HCCLM3-shNEK2 cells compared with the parental HCCLM3 cells (Fig. 3C). Furthermore, IHC of tissue microarray data showed that the levels of E-cadherin and N-cadherin were strikingly altered in HCC tumor samples expressing high levels of NEK2 (Fig. 3D). N-cadherin was upregulated, whereas E-cadherin was downregulated (Fig. 3D). These results suggested that NEK2 can affect the expression of epithelial and mesenchymal markers and may induce EMT in HCC cells.
Figure 3.
NEK2 induces EMT of HCC cells. Western blot analysis (A) and qRT-PCR (B) of EMT markers in MHCC97L-control, MHCC97L-NEK2, HCCLM3-control and HCCLM3-shNEK2. (C) Representative IF images of cell E-cadherin and N-cadherin expression in MHCC97L-NEK2, HCCLM3-shNEK2 and their respective parental cells. (D) Representative IHC images of HCC tissue microarrays for E-cadherin and N-cadherin in the HCC tissue microarray.
NEK2 can regulate metastasis-related pathways
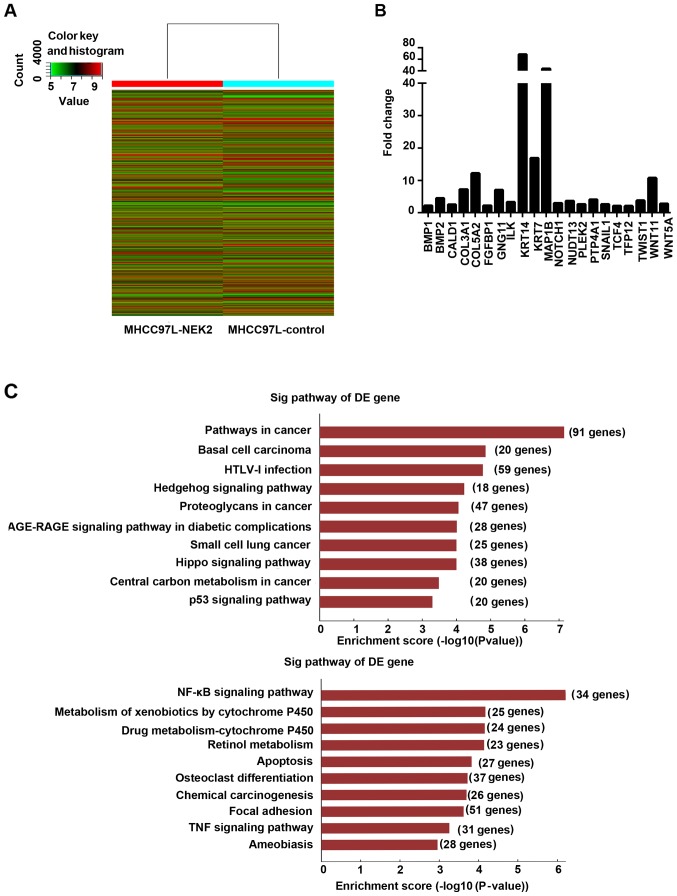
To study the genes and signaling pathways regulated by NEK2 in HCC, we subjected MHCC97L-control and MHCC97L-NEK2 cells to gene expression microarray analysis. EMT-related gene analysis revealed that NOTCH1, SNAIL1, TWIST1, WNT11, and WNT5A were upregulated in MHCC97L-NEK2 cells compared with the MHCC97L-control (Fig. 4B). Then, signaling pathways influenced by NEK2 were analyzed. Notably, the Wnt, NF-κB, focal adhesion, and VEGF signaling pathways were activated by NEK2 overexpression. Interestingly, tumor suppression pathways, such as the Hippo and p53 pathways, were downregulated when NEK2 was overexpressed (Fig. 4C). Our data provided novel insights into NEK2 regulation of HCC cells and suggested that NEK2 regulates gene expression and signaling involved in EMT.
Figure 4.
Downstreams of NEK2 in HCC cells. (A) Heat map showing the differences in gene expression between parental MHCC97L and MHCC97L-NEK2 cells. (B) EMT-related genes regulated by NEK2. (C) Cell signals suppressed (upper panel) and activated (lower panel) by NEK2 overexpression.
Discussion
In this study, we identified that overexpression of NEK2 predicted poor prognosis for HCC patients after hepatectomy. We then demonstrated that overexpression of NEK2 led to EMT in HCC cells and promoted HCC cell migration and invasion. Moreover, we discovered that Wnt or NF-κB signaling may be involved in the NEK2-induced EMT process in HCC using gene expression microarray.
Aberrant NEK2 activities resulted in failed regulation of centrosome duplication, causing aneuploidy and, therefore, oncogenic effects (13). Recently, accumulating evidence has demonstrated that NEK2 is overexpressed in several neoplastic diseases, such asbreast carcinoma, testicular seminomas, diffuse large B cell lymphomas and prostate cancer (14–17). Further studies should focus on the expression levels of NEK2 in HCC. Zhang et al (18) reported that NEK2 expression was higher in HepG2 cells than in Huh7, SMMC-7721 and BEL7402 cells. However, these authors did not compare the expression levels of NEK2 between cancer cell lines and normal liver cell lines. Li et al (9) found that the NEK2 expression level is higher in SMMC-7721 cells than in the normal liver cell line HL-7702. Neither study could identify differences in NEK2 expression levels in high or low potential metastasis HCC cell lines. In our study, we found that the NEK2 expression level was elevated in cancer cell lines relative to the normal liver cell line LO2, and we also found NEK2 expression was higher in the high metastasis potential HCC cell line (HCCLM3) than in the low metastasis potential HCC cell line (MHCC97L). Furthermore, we revealed that NEK2 expression levels were much higher in HCC tissues when compared with the adjacent non-cancerous tissues. Collectively, such data suggest that NEK2 is overexpressed in HCC.
Emerging evidence has shown that NEK2 is an independent risk for cancer patients prognosis and identified NEK2 as a putative oncogene. NEK2 overexpression is a frequent event in cancer cells. For instance, NEK2 was found to be upregulated in breast tissue when compared with adjacent non-cancerous tissue, and its upregulation was closely linked to poor prognosis and high recurrence in patients (14). Zhou et al (19) investigated the levels of 56 genes related to drug resistance using clinical data, and found that NEK2 was the gene most strongly associated with overall survival in myeloma. Further study also identified NEK2 as an effective tumor proliferation marker of poor prognosis for non-small cell lung cancer (5). Recently, several studies have highlighted the potential role of NEK2 in predicting HCC patient prognosis. One report indicated that NEK2 was a promising biomarker for HCC recurrence by analyzing 50 HCC patients who underwent hepatectomy. They showed that NEK2 had a statistically significant association with DFS although not with OS, which was completely contradictory to our results. The study population was relatively small and the background hepatitis C virus infection was present in most cases, which might explain the big difference compared with our results.
Another report revealed that NEK2 was related with diolame complete, tumor nodule number and recurrence (9). However, like the above-mentioned study, this study concerned only 63 patients, which might not provide strong evidence to determine the relationship between NEK2 status and HCC characteristics. These authors then verified the relationship of NEK2 expression with p-AKT and MMP-2 by IHC, but they did not explore the underlying mechanism as to how NEK2 promotes cell migration and invasion. Consistent with previous studies, our study confirmed the clinical significance of NEK2 as an independent prognostic marker for HCC patients after hepatectomy in more specimens, and confirmed that NEK2 expression level was related to tumor size and tumor non-capsulation. Taken together, our data revealed that NEK2 may serve as an independent risk factor for HCC. Unlike previous reports concerning NEK2 expression level in HCC, we enrolled more HCC patients (259 cases), which could provide more exact evidence for the function of NEK2 in HCC and further performed experiments in vitro to explore the role of NEK2 expression in cell migration and invasion as well as its underlying mechanism.
Notably, as we discovered that the NEK2 expression level is related to tumor non-capsulation in clinical specimens, of interest, we employed functional experiments to confirm our suspicion. Interestingly, upregulated NEK2 expression fosters the migration and invasion ability in HCC cells, whereas downregulated NEK2 expression results in decreasing migration and invasion ability. This upregulation pattern and its invasion and metastasis activator function as characterized here in HCC cells were similar to those functions as previously demonstrated in pancreatic cancer (20). Nevertheless, the underlying biological mechanism by which overexpression of NEK2 promotes cancer cell invasion remains poorly understood. Currently, little information underlying the molecular mechanism of NEK2 regulation of cancer cell invasion is available. A previous report indicated that NEK2 may contribute to HCC metastasis, which revealed that NEK2 may mediate liver cancer cell migration via pAKT signaling and matrix metalloproteinase (MMP) activation (21), however, such signaling might not be the unique mechanism by which NEK2 promotes HCC metastasis. One recent report demonstrated that NEK2 contributed to altered β-catenin localization from the intercellular adherens junction to the cytoplasm and nucleus (22), a key process of EMT and an invasive phenotype typical of HCC (23). Therefore, we speculated that NEK2 is involved mainly in the events that trigger EMT. Notably, the present study provides further evidence that overexpressed NEK2 result in upregulated N-cadherin and downregulated E-cadherin in HCC cells; conversely, silenced NEK2 expression showed contrary results. Furthermore, these EMT process-related markers all showed similarly correlated staining patterns for NEK2 in HCC clinical tissue microarray. Based on our results, we speculated that NEK2 can induce EMT in HCC.
Our findings provided evidence that NEK2 is a critical mediator of HCC cell invasion and that activation of EMT is its novel regulating mechanism. However, how NEK2 induces EMT remains unclear. One recent report indicated that overexpression of NEK2 can activate AKT and Jnk pathways and upregulate Wnt/Wingless signaling and alter the expression of Rho1, Rac1 and E-cadherin (24). Consistently, our study identified that upregulation of NEK2 can alter Wnt, NF-κB, focal adhesion and VEGF signaling. All of the above signaling pathways are classical signaling pathways that are involved in cancer cell invasion and metastasis (25–28). Interestingly, overexpression of NEK2 could suppress the Hippo signaling pathway and p53 signaling pathway, which were reported as cancer progression suppressors by numerous studies (29,30). Collectively, our data highlight the potential role of NEK2 in regulating several cancer-related signaling pathways. Nevertheless, future studies to assess how NEK2 regulation of such pathways promotes HCC cell invasion will be warranted, and whether these pathways take part in NEK2 induction of EMT is required.
In conclusion, our results revealed an oncogenic role for NEK2 in HCC and as an independent prognosis indicator for HCC patients. Forced expression of NEK2 in HCC cells promoted cell proliferation, migration and invasion, at least partly via EMT activation, and provided evidence that Wnt, NF-κB, focal adhesion, VEGF, Hippo and p53 signaling pathways may be downstream of NEK2. Therefore, NEK2 may be a novel target for the treatment of HCC.
Acknowledgements
This study was supported by National Natural Science Foundation of China (81302142; 81172039), the Natural Science Foundation of Guangdong Province (S2011010005864; 2014A030313108), and the Young Teacher Training Program of Sun Yat-Sen University (15ykpy15).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Hu CM, Zhu J, Guo XE, Chen W, Qiu XL, Ngo B, Chien R, Wang YV, Tsai CY, Wu G, et al. Novel small molecules disrupting Hec1/Nek2 interaction ablate tumor progression by triggering Nek2 degradation through a death-trap mechanism. Oncogene. 2015;34:1220–1230. doi: 10.1038/onc.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang Y, Zhang X. Targeting NEK2 as a promising therapeutic approach for cancer treatment. Cell Cycle. 2016;15:895–907. doi: 10.1080/15384101.2016.1152430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong X, Guan X, Liu W, Zhang L. Aberrant expression of NEK2 and its clinical significance in non-small cell lung cancer. Oncol Lett. 2014;8:1470–1476. doi: 10.3892/ol.2014.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ning Z, Wang A, Liang J, Liu J, Zhou T, Yan Q, Wang Z. Abnormal expression of Nek2 in pancreatic ductal adenocarcinoma: A novel marker for prognosis. Int J Clin Exp Pathol. 2014;7:2462–2469. [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong AL, Lee S, Park JS, Han S, Jang CY, Lim JS, Lee MS, Yang Y. Cancerous inhibitor of protein phosphatase 2A (CIP2A) protein is involved in centrosome separation through the regulation of NIMA (never in mitosis gene A)-related kinase 2 (NEK2) protein activity. J Biol Chem. 2014;289:28–40. doi: 10.1074/jbc.M113.507954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wubetu GY, Morine Y, Teraoku H, Yoshikawa M, Ishikawa D, Yamada S, Ikemoto T, Saito YU, Imura S, Shimada M. High NEK2 expression is a predictor of tumor recurrence in hepatocellular carcinoma patients after hepatectomy. Anticancer Res. 2016;36:757–762. [PubMed] [Google Scholar]
- 9.Li G, Zhong Y, Shen Q, Zhou Y, Deng X, Li C, Chen J, Zhou Y, He M. NEK2 serves as a prognostic biomarker for hepatocellular carcinoma. Int J Oncol. 2017;50:405–413. doi: 10.3892/ijo.2017.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Y, Wang J, Zhang L, Shen S, Guo R, Yang Y, Chen W, Wang Y, Chen G, Shuai X. Theranostical nanosystem-mediated identification of an oncogene and highly effective therapy in hepatocellular carcinoma. Hepatology. 2016;63:1240–1255. doi: 10.1002/hep.28409. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Zhang JX, Huang LL, He LJ, Liao YJ, Lai YR, Deng HX, Tian XP, Kung HF, Xie D, et al. Low expression of BARX2 in human primary hepatocellular carcinoma correlates with metastasis and predicts poor prognosis. Hepatol Res. 2015;45:228–237. doi: 10.1111/hepr.12340. [DOI] [PubMed] [Google Scholar]
- 12.Huang LL, Zhang Y, Zhang JX, He LJ, Lai YR, Liao YJ, Tian XP, Deng HX, Liang YJ, Kung HF, et al. Overexpression of NKX6.1 is closely associated with progressive features and predicts unfavorable prognosis in human primary hepatocellular carcinoma. Tumour Biol. 2015;36:4405–4415. doi: 10.1007/s13277-015-3080-4. [DOI] [PubMed] [Google Scholar]
- 13.Cappello P, Blaser H, Gorrini C, Lin DC, Elia AJ, Wakeham A, Haider S, Boutros PC, Mason JM, Miller NA, et al. Role of Nek2 on centrosome duplication and aneuploidy in breast cancer cells. Oncogene. 2014;33:2375–2384. doi: 10.1038/onc.2013.183. [DOI] [PubMed] [Google Scholar]
- 14.Marina M, Saavedra HI. Nek2 and Plk4: Prognostic markers, drivers of breast tumorigenesis and drug resistance. Front Biosci (Landmark Ed) 2014;19:352–365. doi: 10.2741/4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbagallo F, Paronetto MP, Franco R, Chieffi P, Dolci S, Fry AM, Geremia R, Sette C. Increased expression and nuclear localization of the centrosomal kinase Nek2 in human testicular seminomas. J Pathol. 2009;217:431–441. doi: 10.1002/path.2471. [DOI] [PubMed] [Google Scholar]
- 16.de Vos S, Hofmann WK, Grogan TM, Krug U, Schrage M, Miller TP, Braun JG, Wachsman W, Koeffler HP, Said JW. Gene expression profile of serial samples of transformed B-cell lymphomas. Lab Invest. 2003;83:271–285. doi: 10.1097/01.LAB.0000053913.85892.E9. [DOI] [PubMed] [Google Scholar]
- 17.Zeng YR, Han ZD, Wang C, Cai C, Huang YQ, Luo HW, Liu ZZ, Zhuo YJ, Dai QS, Zhao HB, et al. Overexpression of NIMA-related kinase 2 is associated with progression and poor prognosis of prostate cancer. BMC Urol. 2015;15:90. doi: 10.1186/s12894-015-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang MX, Xu XM, Zhang P, Han NN, Deng JJ, Yu TT, Gan YY, He XQ, Long ZX. Effect of silencing NEK2 on biological behaviors of HepG2 in human hepatoma cells and MAPK signal pathway. Tumour Biol. 2016;37:2023–2035. doi: 10.1007/s13277-015-3993-y. [DOI] [PubMed] [Google Scholar]
- 19.Zhou W, Yang Y, Xia J, Wang H, Salama ME, Xiong W, Xu H, Shetty S, Chen T, Zeng Z, et al. NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancers. Cancer Cell. 2013;23:48–62. doi: 10.1016/j.ccr.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokuryo T, Hibino S, Suzuki K, Watanabe K, Yokoyama Y, Nagino M, Senga T, Hamaguchi M. Nek2 siRNA therapy using a portal venous port-catheter system for liver metastasis in pancreatic cancer. Cancer Sci. 2016;107:1315–1320. doi: 10.1111/cas.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu SM, Lin SL, Lee KY, Chuang HC, Feng PH, Cheng WL, Liao CJ, Chi HC, Lin YH, Tsai CY, et al. Hepatoma cell functions modulated by NEK2 are associated with liver cancer progression. Int J Cancer. 2017;140:1581–1596. doi: 10.1002/ijc.30559. [DOI] [PubMed] [Google Scholar]
- 22.Neal CP, Fry AM, Moreman C, McGregor A, Garcea G, Berry DP, Manson MM. Overexpression of the Nek2 kinase in colorectal cancer correlates with beta-catenin relocalization and shortened cancer-specific survival. J Surg Oncol. 2014;110:828–838. doi: 10.1002/jso.23717. [DOI] [PubMed] [Google Scholar]
- 23.Wang ZC, Gao Q, Shi JY, Guo WJ, Yang LX, Liu XY, Liu LZ, Ma LJ, Duan M, Zhao YJ, et al. Protein tyrosine phosphatase receptor S acts as a metastatic suppressor in hepatocellular carcinoma by control of epithermal growth factor receptor-induced epithelial-mesenchymal transition. Hepatology. 2015;62:1201–1214. doi: 10.1002/hep.27911. [DOI] [PubMed] [Google Scholar]
- 24.Das TK, Dana D, Paroly SS, Perumal SK, Singh S, Jhun H, Pendse J, Cagan RL, Talele TT, Kumar S. Centrosomal kinase Nek2 cooperates with oncogenic pathways to promote metastasis. Oncogenesis. 2013;2:e69. doi: 10.1038/oncsis.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen EA, Menon R, Bailey KM, Thomas DG, Van Noord RA, Tran J, Wang H, Qu PP, Hoering A, Fearon ER, et al. Activation of Wnt/β-catenin in Ewing sarcoma cells antagonizes EWS/ETS function and promotes phenotypic transition to more metastatic cell states. Cancer Res. 2016;76:5040–5053. doi: 10.1158/0008-5472.CAN-15-3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi W, Ye Z, Zhuang L, Li Y, Shuai W, Zuo Z, Mao X, Liu R, Wu J, Chen S, et al. Olfactomedin 1 negatively regulates NF-κB signalling and suppresses the growth and metastasis of colorectal cancer cells. J Pathol. 2016;240:352–365. doi: 10.1002/path.4784. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Li Y, Xing Y, Cao B, Yang F, Yang T, Ai Z, Wei Y, Jiang J. The interaction between cancer stem cell marker CD133 and Src protein promotes focal sdhesion kinase (FAK) phosphorylation and cell migration. J Biol Chem. 2016;291:15540–15550. doi: 10.1074/jbc.M115.712976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin X, Li HR, Lin XF, Yu ME, Tu XW, Hua ZD, Lin M, Xu NL, Han LL, Chen YS. Silencing of Livin inhibits tumorigenesis and metastasis via VEGF and MMPs pathway in lung cancer. Int J Oncol. 2015;47:657–667. doi: 10.3892/ijo.2015.3058. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Liu X, Luo J, Xiao W, Ye X, Chen M, Li Y, Zhang GJ. Notch3 inhibits epithelial-mesenchymal transition by activating Kibra-mediated Hippo/YAP signaling in breast cancer epithelial cells. Oncogenesis. 2016;5:e269. doi: 10.1038/oncsis.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adriaens C, Standaert L, Barra J, Latil M, Verfaillie A, Kalev P, Boeckx B, Wijnhoven PW, Radaelli E, Vermi W, et al. p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nat Med. 2016;22:861–868. doi: 10.1038/nm.4135. [DOI] [PubMed] [Google Scholar]