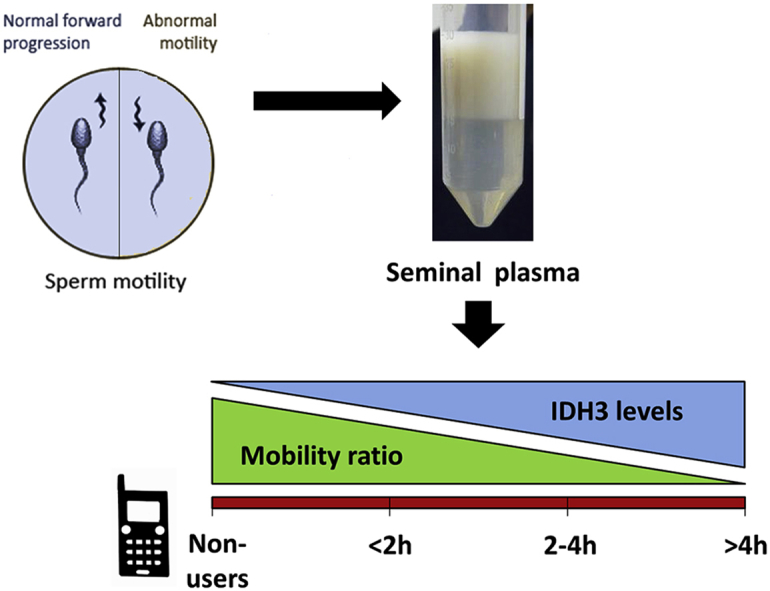
Abstract
NAD+-dependent Isocitrate Dehydrogenase (NAD+-IDH) could be one of the cell phone radiation targets. Enzyme activity alteration may lead to decline in sperm motility during radio-frequency electromagnetic waves (RF-EMW) exposure. The current case control study aimed to investigate the possible relationship between mitochondrial NAD+-IDH activity in human seminal plasma and sperm motility among asthenozoospermic cellular phone users. A total number of ninety idiopathic infertile males referred from the Department of Dermatology and Andrology, were enrolled in this study. NAD+-IDH activity was measured in human seminal plasma by spectrophotometer. Computer-aided sperm analysis (CASA) following WHO criteria has been used for semen analyses. The results showed that IDH activity was increased in patients with prolonged cell phone daily use ≥4 h/day. Its level, correlated negatively with either the motility ratio percentages (r = −0.46, p < 0.001) or the progressive motility percentages (r = −0.50, p < 0.001) in the study groups. The current study suggests that NAD+-IDH in human seminal plasma could be one of seminal plasma biomarkers reflecting the mitochondrial function of spermatozoa. Alteration of its level could reflect the defective motility of sperms among some cases of cellular phone users.
Keywords: Mobile phone electromagnetic radiations, Isocitrate dehydrogenase, Human, Sperm parameters
Graphical abstract
Highlights
-
•
Increased seminal NAD+-IDH activity with prolonged mobile daily use ≥4 h/day.
-
•
Semen NAD+-IDH levels correlated negatively with the sperm motility parameters.
-
•
Semen NAD+-IDH level could be one of biomarkers reflecting the mitochondrial function.
1. Introduction
The growing popularity of mobile usage is associated with increased concern regarding its radiation (RF-EMW; radiofrequency electromagnetic waves) harmful effects on human health, brain and fertility among others [1], [2], [3].
RF-EMW exposure effects on male fertility has been studied and evaluated in many animal and human studies but the results are inconsistent. The main semen parameter that the majority of studies have shown to be significantly affected is the motility [1], [4], [5], [6], [7], [8], [9], [10], [11].
Spermatozoa are highly specialized cells, offering utilities for studying several basic aspects of metabolic control such as the role of ATP (adenosine triphosphate) homeostasis for cell function [12]. As the energy metabolism of mitochondria is a main factor supporting multiple functions of the sperm, they harbor significant metabolic pathways during germ cell development and fertilization [13]. However, the most important aspect of mitochondria function in all cell types is ATP production, which can be used in the case of spermatozoa for maintaining motility of the sperms that represents one of the main determinants of male fertility. Consequently, the presence of structural and functional alterations in mitochondria from asthenozoospermic (i.e. percentage of progressively motile spermatozoa <32%; fifth percentile relative to the average fertile male) [14] subjects supports the vital role played by these organelles in sperm motility energy maintenance [15].
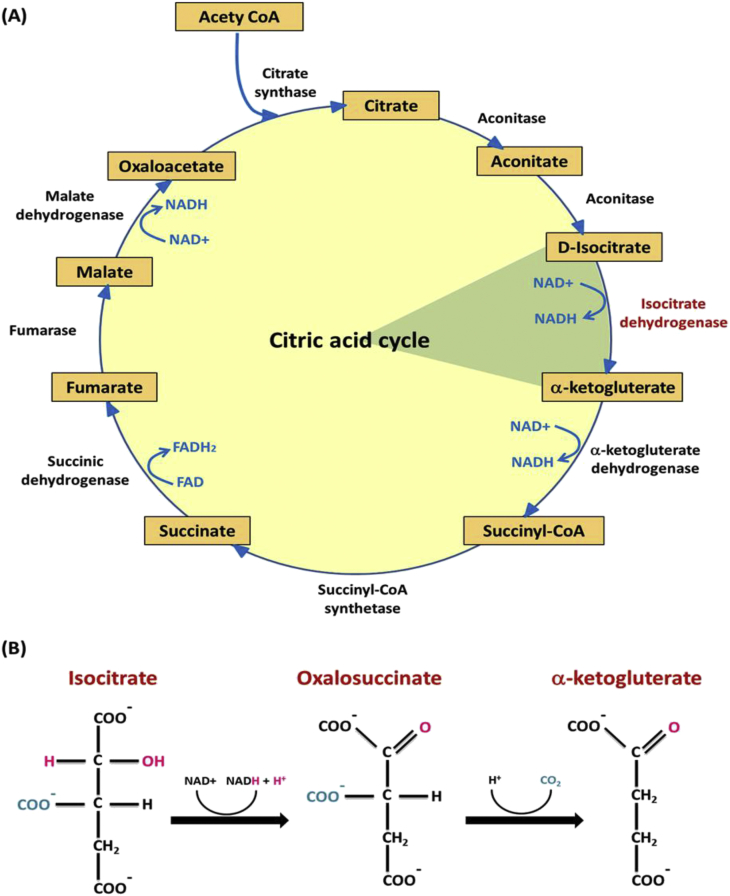
Krebs cycle (tricarboxylic acid cycle; TCA, citric acid cycle), is a group of enzyme-catalyzed chemical reactions (Fig. 1A) of key importance in all living cells that use oxygen as part of cellular respiration. In eukaryotes, it occurs in the mitochondrial matrix as a part of a metabolic pathway involved in the chemical conversion of carbohydrates, lipids and proteins into carbon dioxide and water generating a form of usable energy. The metabolic step in this cycle catalyzed by isocitrate dehydrogenase, occupies a central position in the intermediary metabolism, links multiple synthetic and catabolic pathways and could be rate limiting [16].
Fig. 1.
Citric acid cycle and NAD+-dependent IDH action. (A) The rate-limiting step of the citric acid cycle catalyzed by isocitrate dehydrogenase, is one of the irreversible reactions in the citric acid cycle due to its large –ΔG (negative free energy change) (B) The two-step process of isocitrate dehydrogenase action.
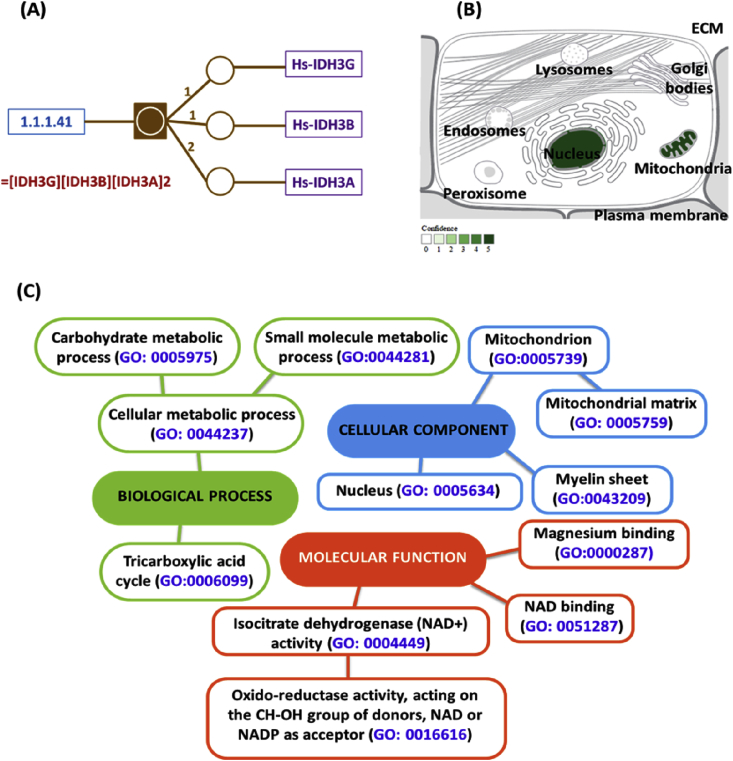
Isocitrate dehydrogenase (IDH) is a large, ubiquitous, and very ancient protein sub-family member playing key roles in energy metabolism. IDHs can be divided into two main groups according to coenzyme specificity: NAD+-specific IDHs (EC 1.1.1.41, NAD+-IDH) and NADP+-specific IDHs (EC 1.1.1.42, NADP-IDH) [17]. Eukaryotic NAD+-IDHs are heterotetramer (Fig. 2A); localized in the mitochondria (Fig. 2B), regulating the metabolic fluxes and the generation of ATP in the TCA cycle. This later isoenzyme catalyzes allosterically the oxidative NAD+-dependent decarboxylation of isocitrate to α-ketoglutarate (α-KG) and CO2 while converts NAD+ to NADH in the presence of Mn2+ or Mg2+. This is a two-step process involves oxidation of isocitrate to oxalosuccinate, followed by the decarboxylation of the β-carboxyl group to form the α-ketoglutarate (Fig. 1B).
Fig. 2.
Structure subunits, subcellular localization and gene ontology analysis of NAD+-dependent IDH enzyme (EC 1.1.1.41). (A) It is a heterotetramer that is composed of two alpha subunits, one beta subunit, and one gamma subunit. All the subunits have similar molecular masses and amino acid sequences. The IDH α subunit appears to possess the enzymatic catalytic activity [Data source: Biocyc14 databases: http://biocyc.org/META]. (B) The evidence confidence is color coded, ranging from low confidence (light green) to higher confidence (dark green). Absence of localization evidence is indicated by white color (Data source: Compartment web server: http://compartments.jensenlab.org/). (C) Gene ontology analysis of IDH enzyme [Data from: NextProt beta (http://www.nextprot.org) and GeneCards human gene database (http://www.genecards.org/)].
Many previous studies reported that RF-EMW emitted from commercially available cell phones have no thermal effect [18]. However, several views were proposed to elucidate the disruption of metabolic pathways by this type of waves. Some of these views are based on experimental evidences and some on hypothetical models. NAD+-specific IDH could be one of the targets of cell phone radiation and alternation in its activity leads to decrease production of ATP in mammalian cells [19]. Since sperm motility depends on the ATP active generation, such a mechanism might cause a decline in sperm motility during RF exposure. Hence, the current study aimed to investigate the relationship between sperm motility and the mitochondrial NAD+-dependent IDH activity in the seminal plasma of asthenozoospermic cellular phone users.
2. Materials and methods
2.1. Participants
The current case control study was carried out on ninety non-smoker males [median age; 36 years (range 25–50)] with idiopathic infertility and using mobile phone model GSM1800/1900 MHz for at least one year (except group A). We tried to select patients with minimum exposure to other environmental sources of electromagnetic waves as much as possible. Their habits or work were away from base station (EM tower) and don't expose directly to another source of EMW as computer, microwaves nor have no time for watching television. Patients with systemic disease, orchitis or varicocele had also excluded. The participants referred from the Department of Dermatology and Andrology (FOM-SCU), Ismailia, Egypt. Detailed history (general, medical, surgical, sexual, social and history related to the female partner) and inquires related to mobile use habits; possession, daily standby position and daily transmission times had been taken from all participants. They were subjected to physical examination and divided into 4 sub-groups according to their mobile usage per day: group A: no use (n = 15); group B: <2 h/day (n = 25); group C: 2–4 h/day (n = 25); and group D: >4 h/day (n = 25). The study was carried out in accordance with the Helsinki Declaration guidelines. An informed consent has been taken from all study participants.
2.2. EMW doses calculation in the exposed populations
Each participant accepted to attend the infertility clinic weekly for one month with his own mobile (s) (provided that there was no any limitations of its habitual use over research period). Minute number of all mobile calls and each call period per week from the phone document, has been calculated by the physicians (all followed a single systematic recording methodology). Individuals whose statements do not agree with their usage of cellular phone (s) as presented in their cell phone documents have been excluded.
2.3. Semen collection and analysis
Semen samples were collected after 3–5 days of abstinence, liquefied for 30 min at 37 °C and analyzed within 60 min after ejaculation according to WHO guidelines; 2010 [14]. The detailed procedure has been described previously [3]. A computer-assisted semen analyzer (Hamilton Thorne, HTM-IVOS, Version 12, Baumann Medical AG, Wetzikon, Switzerland), was used for sperm concentration and kinetic measurements. Sperm motility was classified into: class a and b = fast and weak forward motility, respectively; class c = non-progressive motility; class d = immobile spermatozoa. Sperm viability has been evaluated by eosin exclusion test [14]. Seminal plasmas were obtained by centrifugation at 1500–2000 rpm for 10 min at room temperature. The supernatant was kept at −20 °C until the day of enzyme assay.
2.4. NAD+-dependent IDH activity assay
IDH activity in seminal plasma was estimated in the department of Medical Biochemistry, Faculty of Medicine Suze Canal University, using IDH Assay kit (Cat. No. K756-100, BioVision Inc., USA) (www.biovision.com), according to the manufacturer's instructions. The test based on utilization of the samples IDH to isocitrate (provided by the manufacturer) as a substrate leading to NADH generation and development of the color proportionally that can be quantified colorimetrically at 450 nm. The test detection sensitivity was 0.01 mU (i.e. samples below this limit have been considered negative).
Briefly, the reaction was started by adding 50 μl of the reaction mix (containing 38 μl IDH assay buffer, 2 μl IDH substrate, 8 μl developer and 2 μl NAD+) to each 96-well plate containing either the test samples (50 μl seminal plasma), the positive control (2 μl adjusted to 50 μl with assay buffer) or standards (with final concentration of 0, 2, 4, 6, 8 and 10 nmol/well as described by the manufacturer). After 3 min at 37 °C incubation, OD at 450 nm (A0) was measured in a micro-plate reader (Ceres UV900 HDI, Bio-Tek Instrument Inc., USA). Another measurement was taken at the same wavelength after 2 h at 37 °C incubation [20].
Calculation of the results: The zero standard values were subtracted from all standards and test samples readings. The standard curve of the NADH was plotted and the activity of IDH in the test samples was calculated by application of the ΔOD (A1 -A0) of each sample to the NADH standard curve to get a B (nmol) of NADH generated by IDH during the time of the reaction (ΔT = T2 − T1; in min). Then the following equation was applied to each sample: IDH activity nmol/min/ml (mU/ml) = B (in nmol)/ΔT (in min) × V (volume of the sample in ml added into the reaction well). The unit of enzyme activity was defined as the amount of enzyme which brings about the formation of 1 μmole of NADH in one minute under the test condition as defined above [20]. The intra-assay precision of the same samples gave coefficient of variations less than 5%.
2.5. Statistical analysis
Results are shown as mean ± SEM. Since semen parameters and IDH activities in seminal plasma were not normally distributed, data comparisons were performed using Kruskal–Wallis tests followed by Tukey's multiple comparison test. Comparison of the frequencies between groups was carried out using Chi-square and Fisher's exact tests when appropriate. Correlations were calculated by Pearson's correlation and simple regression tests using Texasoft WINKS, 4.651 software (Texas, USA). Statistical significance was considered for p < 0.05.
3. Results
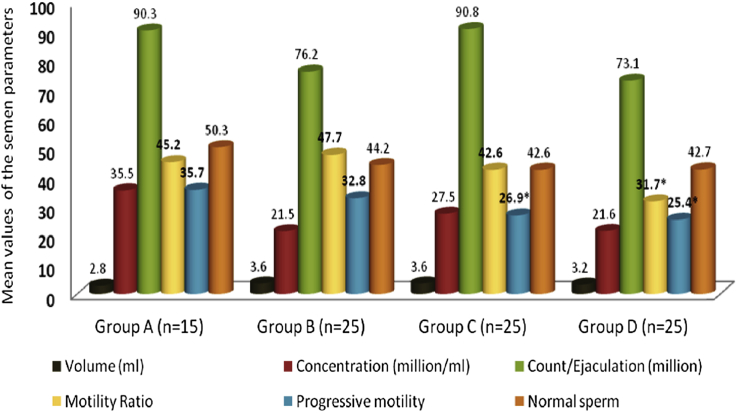
The results for various semen parameters including the kinetic one that have been assessed by CASA for the semen samples provided by the enrolled 90 participants, were analyzed and presented in Fig. 3. The motility ratio was significantly decreased in group D in comparison to controls (31.7 ± 3.8 vs. 45.2 ± 5.6; mean ± SEM, respectively). The progressive motility percentage, in addition, was significantly lower in group C and D (26.9 ± 1.9 and 25.4 ± 3.7; mean ± SEM, respectively) when compared to control group (35.7 ± 5.5; mean ± SEM, P < 0.05). Although group D showed a lower mean count/ejaculate (million) and an increase in dead sperm and atypical forms in comparison to other groups, these values did not reach statistical significance (P > 0.05; Fig. 3).
Fig. 3.
Basic parameters of semen samples in the study groups. *P < 0.05.
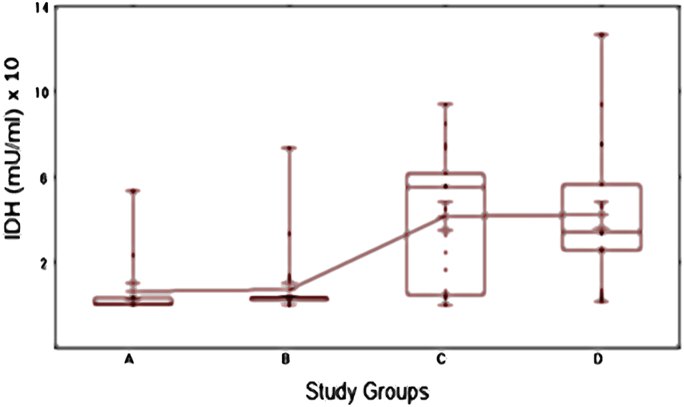
Fig. 4 showed the mean isocitrate dehydrogenase levels (mU/ml) in the studied groups C and D were 41.6 ± 6.5 and 42.2 ± 6.3 (mean ± SEM), respectively. These patients had statistically significant higher mean values compared to patients in group A and B (6.5 ± 3.7 and 7.4 ± 3.0, respectively).
Fig. 4.
Box and Whiskers plot of seminal plasma NAD+-IDH levels (mU/ml) among the studied groups. The median and interquartile ranges (25th −75th percentile) are shown as solid horizontal lines and the means of all groups are connected. Participants exposed more to radiofrequency waves (groups C and D: 2–4 h/day and >4 h/day, respectively) had a significantly higher mean isocitrate dehydrogenase level; IDH (mU/ml) compared with the other groups (overall P < 0.001).
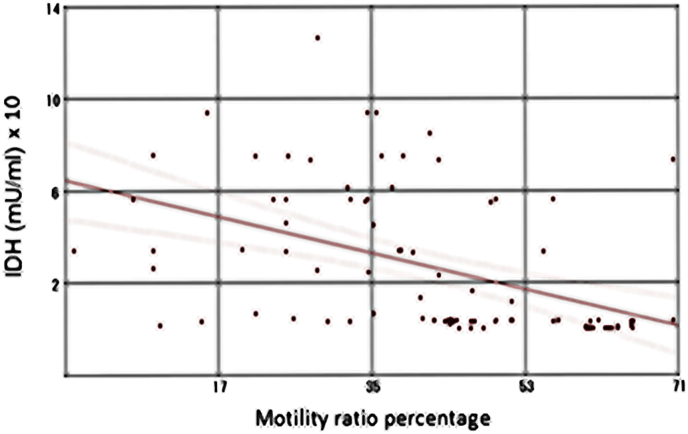
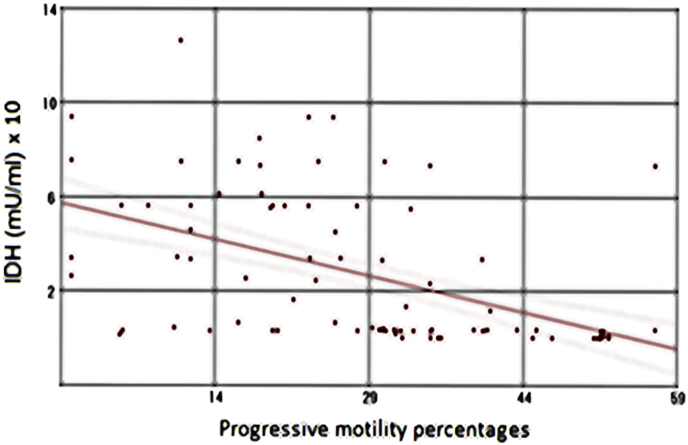
Notably, a moderate statistically significant reverse correlation between isocitrate dehydrogenase levels (mU/ml) and either the motility ratio percentages (Fig. 5; r = −0.46, P < 0.001) or the progressive motility percentages (Fig. 6; r = −0.50, P < 0.001) in the study groups were found. Otherwise, no any significant correlation between IDH level and other semen parameters has been detected.
Fig. 5.
Correlation between seminal plasma IDH levels (mU/ml) and the motility ratio percentages in the study participants (n = 90) (r = −0.46, P < 0.001).
Fig. 6.
Correlation between seminal plasma IDH levels (mU/ml) and the progressive motility percentages in the study participants (n = 90) (r = −0.50, P < 0.001).
4. Discussion
Given the ubiquity of mobile phone use, the possible hazards of RF-EMR from these devices that could potentially affect semen quality and sperm parameters have raised public concern in recent years [21].
Several studies reported significant correlations between mobile radiation and sperm health [22], [23], [24], and many revealed that the adverse changes, increased with the radiation exposure amount [2], [25]. The possible mechanisms by which RF-EMR might produce these changes are controversial [24], [26].
The current study revealed a decrease in motility ratio and the progressive motility percentage in patients with prolonged cell phone daily use ≥4 h/day, while the decline in sperm mean count/ejaculate in those patients, did not reach statistical significance when compared to controls. These results are in line with previous studies that found a significant negative effect of cell phone exposure on human sperm motility [1], [2], [7], [10], [27], [28], [29], [30]. Adams et al. [24] in their meta-analysis of ten pooled experimental (in vitro) and observational (in vivo) human studies (n = 1492), concluded that RF-EMR may have both thermal and non-thermal effects on biological tissue. The latter effect is postulated to increase the production of the ROS (reactive oxygen species) and this may lead to DNA damage. These outcomes we identified in the same study group with longer time of EMR exposure in our previous work [3]. Regarding the thermal effects “may be largely due to the heat generated by the handsets rather than the RF-EMR, since the EMR frequencies released from cell phones are thought to have negligible heating effects” [11], [31]. Adams and coworkers explained that “if the impact of mobile phones was mainly due to heating rather than radiation, an effect on sperm concentration rather than parameters such as viability and motility, which are linked with DNA integrity, would be expected” [24].
We correlated for the first time, in the current work, NAD+-dependent IDH activity in seminal plasma and semen parameters including sperm motility among asthenozoospermic cellular phone users. Unexpectedly, we found that NAD+-dependent IDH values were significantly increased in patients with prolonged cell phone daily use ≥4 h/day in comparison to control group. We believe that at least two pathways may be responsible for NAD+-dependent IDH presence in semen of the study cases. The first source might be membrane leakage under the effects of EMR and the second one might be a secretion product that contains IDH. Therefore, one or both of these sources may be the source of IDH in seminal plasma of the prolonged cell phone users of the current study. In line with our results, Leventerler et al. [32] evaluated malate dehydrogenase activity (another citric acid cycle enzyme) in their samples of the human seminal plasma among normozoospermic and infertile males and proposed that a problem on metabolic pathways might lead to increase in enzyme activity as a defense mechanism. Noguera Velasco et al. [33] reported the lactate dehydrogenase-C4 isoenzyme could be determined in seminal plasma due to the enzyme outward diffusion from the spermatozoa or to their destruction. However, the current study isoenzyme (i.e. NAD+-dependent IDH) occurs only in mitochondria matrix, hence our first assumption as membrane leakage or damage under EMR effects more powerful than secretion or passive diffusion mechanism. It is noted that NADP+-dependent IDH activity that has mitochondrial and cytosolic isozymes, is especially high in cardiac tissue and is often monitored in the blood of patients with myocardial infarction. IDH activity detection in the arterial blood suggests severe tissue damage with soluble (cytosolic) IDH leakage into the blood [20]. We recommend future detailed studies on IDH activity of seminal plasma and sperm homogenates especially in a group of azoospermia to infer the source of seminal IDH, either from the ejaculated sperms or other tissues contributing to semen secretion (e.g. prostate gland and the seminal vesicles). Such studies will be useful to understand the mechanisms underlying the changes of seminal IDH values in response to EMR exposure.
In the current study, NAD+-dependent IDH values were significantly correlated negatively with either the motility ratio percentages or the progressive motility percentages in the study groups. Muratori and coworkers [34], found that proteins related to sperm motility and differentiation could be categorized into (a) energy related enzymes in mitochondrial and glycolytic pathways, (b) structural proteins, and (c) activating signal transducers. It is well known that much ATP is consumed to support sperm movement. The two main pathways for this ATP production in mammalian sperm are glycolysis and mitochondrial oxidative phosphorylation [35], [36], [37]. As NAD+-dependent IDH is a key enzyme in the TCA cycle, abnormal IDH expression might disrupt sperm motility as a result of TCA cycle dysfunction. At the proteome level, Zhao et al. [38], have identified lower expression of IDH subunit-α in asthenozoospermic patients by matrix-assisted laser desorption ionization time of flight (MALDI-TOF) analysis. For the first time, this seems contradictory to our results, but these investigators run their analysis only on mature sperms homogenate separated from the seminal plasma and their patients not exposed to EMR.
Study of RF-EMR exposure outcomes on enzymes could contribute to state mechanisms by which this radiation affects sperms. It has been shown that enzyme activity could be augmented, reduced or did not alter depending on the exposure parameters (frequency, modulation, exposure duration, SAR), enzyme type and localization [39], [40].
In conclusion, our biochemical analysis revealed that seminal plasma NAD+-IDH could be a target of or a response to RF-EMW exposure that might compromise TCA cycle, contributing to the defective sperm motility among some cases of male infertility characterized by asthenozoospermia. Further larger scale multi-centered studies are highly recommended to confirm our results.
Conflicts of interest
The authors declare no competing financial interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgment
The authors thank all study participants and our colleagues in the Department of Dermatology and Andrology, Suez Canal University Hospital, for their referral of cases for laboratory assessment.
References
- 1.Agarwal A., Desai N.R., Makker K. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: an in vitro pilot study. Fertil. Steril. 2009;92:1318–1325. doi: 10.1016/j.fertnstert.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 2.De Iuliis G.N., Newey R.J., King B.V. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS One. 2009;4:e6446. doi: 10.1371/journal.pone.0006446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mostafa Rashad M., Elmoemen Eman A., Fawzy Manal S. Possible impact(s) of cell phone electromagnetic radiation on human sperm parameters. Hum. Androl. 2012;2:49–55. [Google Scholar]
- 4.Davoudi M., Brossner C., Kuber W. The influence of electromagnetic waves on sperm motility. in German, “Der Einfluß elektromagnetischer Wellen auf die Spermienmotilität”J. für Urologie und Urogynäkologie. 2002;9:18–22. [Google Scholar]
- 5.Fejes I., Zavaczki Z., Szollosi J. Is there a relationship between cell phone use and semen quality? Arch. Androl. 2005;51:385–393. doi: 10.1080/014850190924520. [DOI] [PubMed] [Google Scholar]
- 6.Kilgallon S.J., Simmons L.W. Image content influences men's semen quality. Biol. Lett. 2005;1:253–255. doi: 10.1098/rsbl.2005.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erogul O., Oztas E., Yildirim I. Effects of electromagnetic radiation from a cellular phone on human sperm motility: an in vitro study. Arch. Med. Res. 2006;37:840–843. doi: 10.1016/j.arcmed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Wdowiak A., Wdowiak L., Wiktor H. Evaluation of the effect of using mobile phones on male fertility. Ann. Agric. Environ. Med. 2007;14:169–172. [PubMed] [Google Scholar]
- 9.Deepinder F., Makker K., Agarwal A. Cell phone and male infertility: dissecting the relationship. Reprod. Biomed. Online. 2007;15:266–270. doi: 10.1016/s1472-6483(10)60338-0. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal A., Deepinder F., Sharma R.K. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil. Steril. 2008;89:124–128. doi: 10.1016/j.fertnstert.2007.01.166. [DOI] [PubMed] [Google Scholar]
- 11.La Vignera S., Condorelli R.A., Vicari E. Effects of the exposure to mobile phones on male reproduction: a review of the literature. J. Androl. 2012;33:350–356. doi: 10.2164/jandrol.111.014373. [DOI] [PubMed] [Google Scholar]
- 12.Kamp G., Büsselmann G., Lauterwein J. Spermatozoa: models for studying regulatory aspects of energy metabolism. Experientia. 1996;52:487–494. doi: 10.1007/BF01919321. [DOI] [PubMed] [Google Scholar]
- 13.Piomboni P., Focarelli R., Stendardi A. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012;35:109–124. doi: 10.1111/j.1365-2605.2011.01218.x. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO) fifth ed. 2010. WHO Laboratory Manual for the Examination and Processing of Human Semen. WHO, Geneva:World Health Organization. [Google Scholar]
- 15.Stendardi A., Focarelli R., Piomboni P. Evaluation of mitochondrial respiratory efficiency during in vitro capacitation of human spermatozoa. Int. J. Androl. 2011;34:247–255. doi: 10.1111/j.1365-2605.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- 16.Yadav R.N.S., Singh S.N. Regulation of rat liver NADP+ -isocitrate dehydrogenase during aging. J. Biosci. 1980;2:15–22. [Google Scholar]
- 17.Wu M.C., Tian C.Q., Cheng H.M. A novel type II NAD+-Specific Isocitrate Dehydrogenase from the Marine Bacterium Congregibacter litoralis KT71. PLoS One. 2015;10:e0125229. doi: 10.1371/journal.pone.0125229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan J.G., Agresti M., Bruce T. Effect of cellular phone emission on sperm motility in rats. Fertil. Steril. 2007;88:957–964. doi: 10.1016/j.fertnstert.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Nylund R., Leszczynski D. Mobile phone radiation causes changes in gene and protein expression in human endothelial cell lines and the response seems to be genome- and proteome dependent. Proteomics. 2006;6:4769–4780. doi: 10.1002/pmic.200600076. [DOI] [PubMed] [Google Scholar]
- 20.J. Williamson, A.M. Campbell, Isocitrate dehydrogenase parameters of enzyme activity, in: S.J. Karcher (Ed.), Tested studies for laboratory teaching, vol. 20. Proceedings of the 20th Workshop/Conference of the Association for Biology Laboratory Education (ABLE), pp. 137–163. http://www.zoo.utoronto.ca/able.
- 21.SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks) 2015. Potential Health Effects of Exposure to Electromagnetic Fields (EMF) [Google Scholar]
- 22.Falzone N., Huyser C., Fourie F. In vitro effect of pulsed 900 MHz GSM radiation on mitochondrial membrane potential and motility of human spermatozoa. Bioelectromagnetics. 2008;29:268–276. doi: 10.1002/bem.20390. [DOI] [PubMed] [Google Scholar]
- 23.Falzone N., Huyser C., Becker P. The effect of pulsed 900-MHz GSM mobile phone radiation on the acrosome reaction, head morphometry and zona binding of human spermatozoa. Int. J. Androl. 2010;34:20–26. doi: 10.1111/j.1365-2605.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- 24.Adams J.A., Galloway T.S., Mondal D. Effect of mobile telephones on sperm quality: a systematic review and meta-analysis. Environ. Int. 2014;70:106–112. doi: 10.1016/j.envint.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Freour T., Barriere P. Wi-Fi decreases human sperm motility and increases sperm DNA fragmentation. Fertil. Steril. 2012;97:e14. doi: 10.1016/j.fertnstert.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Falzone N., Huyser C., Franken D.R. Mobile phone radiation does not induce pro-apoptosis effects in human spermatozoa. Radiat. Res. 2010;174:169–176. doi: 10.1667/RR2091.1. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed L., Baig N.M. Mobile phone RF-EMW exposure to human spermatozoa: an in vitro study. Pak J. Zool. 2011;43:1147–1154. [Google Scholar]
- 28.Sajeda S., Al-Watter Y. Effect of mobile phone usage on semen analysis in infertile men. Tikrit J. Pharm. Sci. 2011;7:77–82. [Google Scholar]
- 29.Liu K., Li Y., Zhang G. Association between mobile phone use and semen quality: a systemic review and meta-analysis. Andrology. 2014;2:491–501. doi: 10.1111/j.2047-2927.2014.00205.x. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal A., Durairajanayagam D. Are men taking their reproductive health away? Asian J. Androl. 2015;17:433–434. doi: 10.4103/1008-682X.140963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal A., Singh A., Hamada A. Cell phones and male infertility: a review of recent innovations in technology and consequences. Int. Braz J. Urol. 2011;37:432–454. doi: 10.1590/s1677-55382011000400002. [DOI] [PubMed] [Google Scholar]
- 32.Leventerler H., Taga S., Urunsak İ.F. Malate dehydrogenase activity in human seminal plasma and spermatozoa homogenates. Cukurova Med. J. 2013;38:648–658. [Google Scholar]
- 33.Noguera Velasco J.A., Tovar Zapata I., Martinez Hernandez P. Lactic dehydrogenase-C4 activity in seminal plasma and male infertility. Fertil. Steril. 1993;60:331–335. doi: 10.1016/s0015-0282(16)56107-x. [DOI] [PubMed] [Google Scholar]
- 34.Muratori M., Luconi M., Marchiani S. Molecular markers of human sperm functions. Int. J. Androl. 2009;32:25–45. doi: 10.1111/j.1365-2605.2008.00875.x. [DOI] [PubMed] [Google Scholar]
- 35.Mukai C., Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm. Biol. Reprod. 2004;4:540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- 36.Miki K., Qu W., Goulding E.H. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz-Pesini E., Díez-Sánchez C., López-Pérez M.J. The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr. Top. Dev. Biol. 2007;77:3–19. doi: 10.1016/S0070-2153(06)77001-6. [DOI] [PubMed] [Google Scholar]
- 38.Zhao C., Huo R., Wang F.Q. Identification of several proteins involved in regulation of sperm motility by proteomic analysis. Fertil. Steril. 2007;87:436–438. doi: 10.1016/j.fertnstert.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 39.Eraslan G., Bilgili A., Akdogan M. Studies on antioxidant enzymes in mice exposed to pulsed electromagnetic fields. Ecotoxicol. Environ. Saf. 2007;66:287–289. doi: 10.1016/j.ecoenv.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Ammari M., Jacquet A., Lecomte A. Effect of head-only sub-chronic and chronic exposure to 900-MHz GSM electromagnetic fields on spatial memory in rats. Brain Inj. 2008;22:1021–1029. doi: 10.1080/02699050802530599. [DOI] [PubMed] [Google Scholar]