Abstract
Voltage-Dependent Anion Channel (VDAC) phosphorylated by c-Jun N-terminal Kinase-3 (JNK3) was incorporated into the bilayer lipid membrane. Single-channel electrophysiological properties of the native and the phosphorylated VDAC were compared. The open probability versus voltage curve of the native VDAC displayed symmetry around the voltage axis, whereas that of the phosphorylated VDAC showed asymmetry. This result indicates that phosphorylation by JNK3 modifies voltage-dependence of VDAC.
Keywords: Phosphorylation, Voltage-Dependent Anion Channel, c-Jun N-terminal Kinase-3, Bilayer electrophysiology
Abbreviations: Phospho-VDAC, Phosphorylated Voltage-Dependent Anion Channel; JNK3, c-Jun N-terminal Kinase-3; MAPK, mitogen activated protein kinase; BLM, bilayer lipid membrane
Highlights
-
•
Phosphorylation of purified VDAC by JNK3 has been carried out in vitro.
-
•
Single-channel currents from native and phosphorylated VDAC have been recorded.
-
•
Open probability vs. voltage curve of native VDAC is symmetric.
-
•
Phospho-VDAC open probability is asymmetric i.e. modified at negative voltages.
-
•
This result indicates phosphorylation by JNK3 modifies voltage-dependence of VDAC.
1. Introduction
Post-translational modifications like phosphorylation, nitrosylation etc. regulate the activity of ion channels in cells [1]. The effect of phosphorylation on the gating of ion channels purified from tissues can be checked in vitro by incorporating them on the artificially prepared bilayer lipid membranes (BLM) by two different ways. One way is to carry out the in vitro phosphorylation reaction and then study the electrophysiological properties of the phosphorylated channel by incorporating it into the BLM [2], [3]. The other is to first incorporate the ion channel into the BLM and then carry out the phosphorylation reaction [4], [5], [6], [7]. The latter has been studied by us where we have reported that phosphorylation of rat brain purified outer mitochondrial membrane Voltage-Dependent Anion Channel (VDAC) by c-Jun N-terminal Kinase-3 (JNK3) enzyme leads to closure of the channel [7]. However, the former, we believe, is important as it would help understanding the ion channel structure–function relationship at the molecular level [2], [3].
VDAC is a porin which is present at the nuclear envelope, endosomes, plasma membrane, at the sarcoplasmic/endoplasmic reticulum membrane and at the outer mitochondrial membrane in cells [8]. The structure of VDAC consists of an N-terminal α-helix and a β-barrel cylinder which forms its lumen or pore [8]. At the outer mitochondrial membrane, it controls the transport of ions, adenine nucleotides like ATP and energy related metabolites between the mitochondria and the cytosol by voltage-dependent gating. At voltages ≤ ±20 mV VDAC remains in open-state and favors anion transport, whereas at voltages >20 mV it preferably displays steady lower-conductance closed state(s) (VDAC closed state(s) are not fully closed and are also known as sub-states) which favor cation transport. Also, VDAC has been shown to be important in promoting cytochrome c release during mitochondrion-mediated apoptosis by different proposed mechanisms [9]. JNKs are cytosolic mitogen activated protein kinases (MAPKs) which regulate normal physiological functions like immune responses, cell and tissue morphogenesis and also they are involved in pathological processes [10], [11]. JNKs are activated by phosphorylation and after activation translocate towards mitochondrion in cells. There are indirect evidences which suggest that activation of JNKs by phosphorylation during mitochondrial apoptosis can lead to phosphorylation of VDAC. A parallel increase in the levels of phosphorylated VDAC, phosphorylated JNKs and cytosolic cytochrome c has been reported during mitochondrion-mediated apoptosis in renal ischemia-reperfusion injury [10]. Furthermore, in cervical cancer cells, arsenic oxide treatment has been shown to result in induction of mitochondrion-mediated apoptosis with JNK1/2 activation and homo-dimerization of VDAC [11]. JNK3 isoform of JNKs has been shown to get activated in brain cells during neurodegeneration [12]. After activation JNK3 translocates toward mitochondrion and results in biochemical modulation of outer mitochondrial membrane proteins leading to apoptosis [12]. In the present work JNK3 phosphorylated purified mitochondrial VDAC has been incorporated into the BLM and its single-channel electrophysiological properties have been studied.
2. Materials and methods
2.1. Purification of VDAC
VDAC was purified from rat brain mitochondria by standard method [7], [13]. Purified mitochondria were allowed to swell by resuspending them in a chilled hypotonic solution containing 10 mM Tris–HCl (pH 7.4) and 1 mM K+-EDTA for 15 min. Meanwhile, mitochondrial concentration (mg/ml) was determined. Mitochondrial suspension was centrifuged at 27,000g for 10 min. The swollen mitochondrial pellet was gently resuspended in a solution containing 10 mM Tris–HCl, 1 mM K+-EDTA and 3% (v/v) Triton X-100 detergent (pH 7.4) at a final concentration of 5 mg/ml. Triton X-100 gently solubilizes outer mitochondrial membrane proteins and VDAC only forms Triton X-100-Lipid-VDAC micelles at 5 mg/ml concentration. The suspension was centrifuged at 44,000g for 30 min. Supernatant was loaded on a hydroxyapatite (0.1 g/mg mitochondria):Celite (2:1 w/w) dry column and pure VDAC was eluted from the column in the initial 1 ml fractions. Permission for this experiment was obtained from the Committee for the Purpose of Control and Supervision of Experiments on Animals, India.
2.2. Phosphorylation of VDAC
Phosphorylation of purified VDAC by JNK3 was carried out and checked using Pro-Q Diamond dye method as standardized in our laboratory [7], [14]. Pro-Q Diamond dye binds to the phosphate groups non-specifically and thus identifies phosphorylated proteins distinctly from the unphosphorylated ones. Initial fractions obtained from the hydroxyapatite:celite column containing high amounts of purified VDAC were chosen for the phosphorylation reaction. Triton X-100 shields VDAC in purified preparations which would reduce VDAC phosphorylation by JNK3 but it is necessary to maintain the solubility of VDAC. VDAC (600 μl) was equally divided and added into three eppendorf tubes corresponding to Negative Control (VDAC + Mg2+ATP), Experimental Sample (VDAC + JNK3 + Mg2+ATP) and Positive Control [VDAC + JNK3 + Mg2+ATP + Alkaline Phosphatase (added later)]. 0.32 μl (0.5 mg) dually phosphorylated His-JNK3 enzyme (Enzo Life Sciences, USA), ATP (Final concentration 100 mM) and MgCl2 (Final concentration 10 mM) were added to the respective tubes. Phosphorylation reaction was carried out by incubating the cocktail at 30 °C for 30 min. In the positive control, 0.5 μl (0.1 mg) of calf intestinal alkaline phosphatase prepared in 10 mM HEPES–KOH buffer (pH 7.4) was added and incubated for another 30 min at 30 °C. The samples were precipitated using chloroform:methanol mixture. Protein pellets were air dried and dissolved in 1× sample buffer. All the tubes were boiled for 5 min at 100 °C along with the peppermint stick phosphoprotein molecular weight standards (Molecular probes, Inc., Eugene, OR, USA), 2 μl in 12 μl of 1× sample buffer. Samples were resolved on 12.5% SDS-PAGE at a constant 100 V and the gel was fixed in 50% Methanol and 10% Acetic acid for 30 min on a shaker. Fixative was replaced with fresh one and left overnight. Next day, gel was washed thrice with Milli-Q water for 15 min each. It was stained using fluorescent Pro-Q Diamond phosphoprotein gel stain (Molecular probes, Inc., Eugene, OR, USA) for 2 h and then de-stained with Pro-Q Diamond phosphoprotein destaining solution (Molecular probes, Inc., Eugene, OR, USA) for 1.5 h thrice for 30 min each time in a dark room on the shaker. The gel was washed thrice for 5 min each with Milli-Q water and visualized on FLA-9000 phosphoimager (Fuji Fim Inc., Tokyo, Japan). After visualization, the gel was silver stained to show VDAC was loaded equally in all the wells.
2.3. Single-channel bilayer electrophysiological recordings of native and phosphorylated VDAC
Native and Phosphorylated VDAC were incorporated into the BLM as standardized in our laboratory [5], [6], [7], [14]. The apparatus consisted of a perfusion BLM cup (Warner Instruments Corp., Hamden, CT) made up of polystyrene with a thin wall separating two aqueous compartments (cis and trans) of BLM chamber (Warner Instruments) containing BLM buffer [1 M KCl, 10 mM MgCl2, 10 mM HEPES (pH 7.4)]. The BLM cup had a circular aperture with a diameter of 150 μm. Aqueous compartments were connected to an amplifier (Axopatch 200B, Molecular devices, CA, USA) through a pair of matched Ag/AgCl electrodes. The voltage in the transcompartment was held at virtual ground by the amplifier and the cis compartment was connected to the headstage for applying the desired voltage. Lipid mixture containing DPhPE (1,2-DiPhytanoyl-sn-glycero-3-PhosphoEthanolamine) and DPhPC (1,2-DiPhytanoyl-sn-glycero-3-PhosphatidylCholine) (Avanti Polar Lipids, Alabaster, AL) (8:2 v/v ratio) in chloroform was dried under nitrogen gas and then dissolved in double volume of n-decane. BLM was prepared by applying lipid mixture to the BLM cup aperture. After formation of the BLM, 10 μl of the native or the phosphorylated VDAC solution (in 3% Triton X-100) was added to the cis chamber buffer (1 ml) such that the concentration of Triton X-100 remained only 0.03% during the incorporation step and the solution was stirred slowly to promote VDAC insertion at +10 mV holding potential. As soon as insertion of the first molecule on BLM was detected from the current activity, buffer in the cis chamber was perfused with fresh one. Single-channel currents were filtered at the amplifier output by a low pass Bessel filter at 1 kHz frequency. Currents were secondarily filtered by an external low pass filter at 200 Hz frequency and then digitized at 1 kHz sampling frequency using data acquisition software pClamp 10.2 (Molecular devices) through an analog to digital converter (Digidata 1440A, Molecular devices).
2.4. Analysis of bilayer electrophysiological data
-
(i)
Full records of single-channel current–time traces of native and phosphorylated VDAC (recorded on stable & non leaky BLM) at different voltages were analyzed. Native VDAC showed its characteristic open-state (O) and a sub-state (S1) whereas in case of phosphorylated VDAC we did not observe the characteristic VDAC open-state (∼4 nS), thus we have labeled its maximum current open-state as P-state (∼3.2 nS). At each voltage, current value (I) of the native VDAC steady-state and the phospho-VDAC open P-state was noted for drawing the current–voltage (I–V) plot of the native and the phosphorylated VDAC. Finally, Mean ± S.E. of the current values at different voltages from three independent sets of experiments were shown by the I–V plot. I–V plot was obtained using data analysis pClamp 10.2 (Clampfit 10.2, Molecular devices) and Origin 5.0 software (Origin Lab Corp., MA). Best fit analysis of the experimental data was done using Origin 5.0. Differences between the native and the phosphorylated VDAC I–V data was statistically tested using Students's paired t-test and p-value was calculated using GraphPad software. Statistical significance of the results was defined at the 95% confidence level.
-
(ii)
For representation, best single-channel current–time traces (10–35 s duration) of native and phosphorylated VDAC from three independent sets of experiments were selected from the full records (shown in Fig. 2, Fig. 3) and their current amplitude histograms (bin size = 1) at different voltages were drawn and each peak of the histogram representing the different states of VDAC was fitted with a distribution function using pClamp 10.2 software (Clampfit 10.2). The different states of the native and the phosphorylated VDAC were identified from the current values of the peaks of the histograms.
-
(iii)
The open probability at a particular voltage was determined using pClamp 10.2 software by selecting the different current levels from the full records of single-channel traces and open probability vs. voltage (V) curve was drawn. The open probability of VDAC at a voltage V is defined as the fraction of time for which the channel was in open-state divided by the total time of recording it spent in partially closed sub-states. In this case we have compared the opening probability of native VDAC open-state (O) and the phosphorylated VDAC open P-state (P). Paired t-test of the mean of open probability (at the positive and the negative voltages) vs. Voltage data of the native and the phosphorylated VDAC was done to statistically test the differences between the native and the phosphorylated VDAC using GraphPad software. Statistical significance of the results was defined at the 95% confidence level.
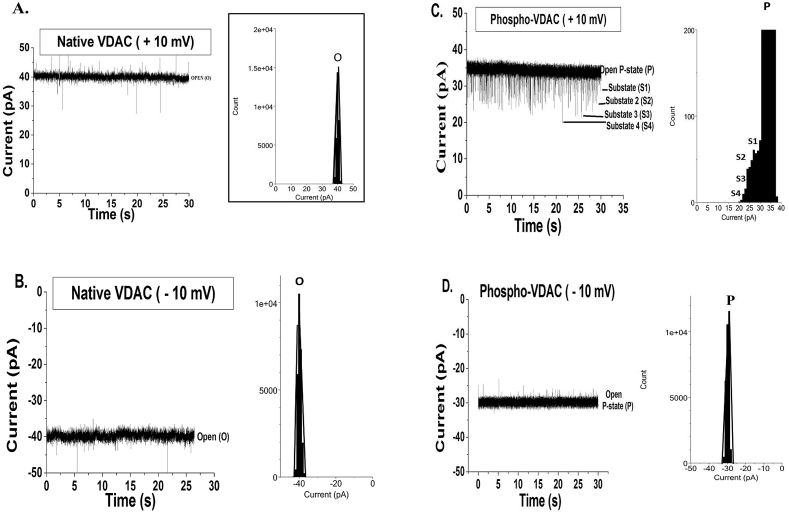
Fig. 2.
Best representative single-channel current–time traces chosen from three different sets of experiments (and their histograms) recorded with respect to the ground on a DPhPE/DPhPC membrane with a symmetrical bath solution of 1 M KCl, 10 mM MgCl2, 10 mM HEPES, pH 7.4 at 25 °C. A. Native VDAC at +10 mV showing its characteristic open-state (O) (O = 40 pA) which is represented by one peak fitted with Gaussian function in the histogram; B. Native VDAC at −10 mV showing the characteristic open-state (O) (O = −40 pA) which is represented by one peak fitted with Gaussian function in the histogram; C. Phosphorylated (by JNK3) VDAC at +10 mV showing the open P-state (P current = 32 pA) and frequent flickering to four substates (S1, S2, S3 and S4) which are seen in the histogram; D. Phosphorylated (by JNK3) VDAC at −10 mV showing the open P-state (P current = −32 pA) which is represented by one peak fitted with Gaussian function in the histogram. In all the traces the baseline is at 0 pA and it represents the fully closed state of the channel.
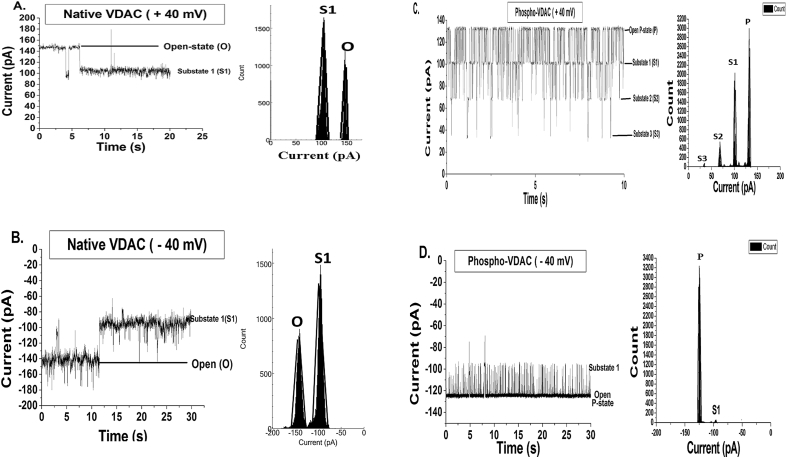
Fig. 3.
Best representative single-channel current–time traces chosen from three different sets of experiments (and their histograms) recorded with respect to the ground on a DPhPE/DPhPC membrane with a symmetrical bath solution of 1 M KCl, 10 mM MgCl2, 10 mM HEPES, pH 7.4 at 25 °C. A. Native VDAC at +40 mV showed a fluctuating open-state (O) and a steady substate S1 (O current = 150 pA & S1 current = 100 pA) which are represented by two peaks fitted with Gaussian functions in the histogram; B. Native VDAC at −40 mV showing a fluctuating open-state (O) and a steady substate S1 (O current = −150 pA & S1 = −100 pA) which are represented by two peaks fitted with Gaussian functions in the histogram; C. Phosphorylated (by JNK3) VDAC at +40 mV showing the open P-state (P current = 128 pA) and three substates (S1, S2 & S3) (S1 current = 96.4 pA; S2 = 62 pA & S3 = 29 pA) which are represented by four peaks fitted with Gaussian functions in the histogram; D. Phosphorylated (by JNK3) VDAC at −40 mV showing the open P-state (P = −128 pA) and a substate (S1 = −100 pA) which are represented by two peaks fitted with Gaussian functions in the histogram. In all the traces the baseline is at 0 pA and it represents the fully closed state of the channel.
3. Results and discussion
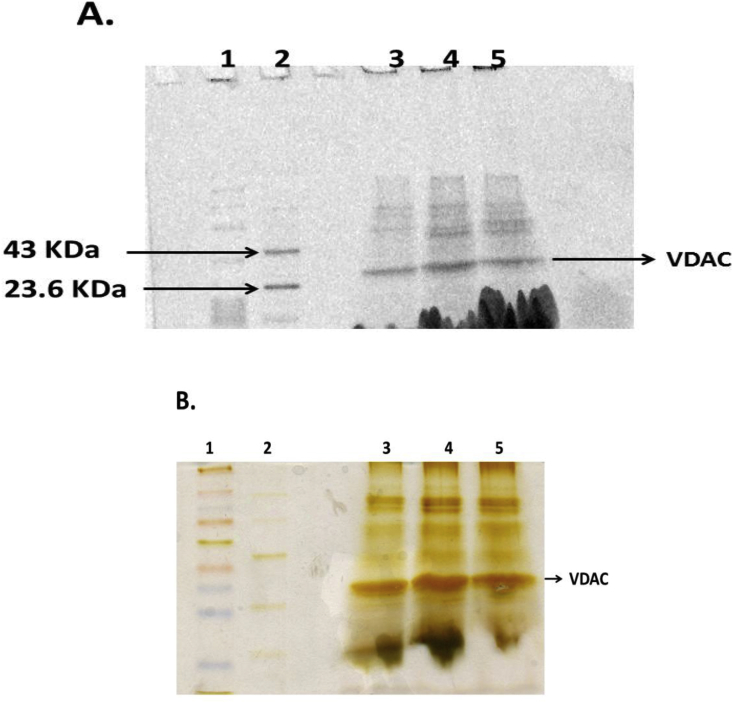
In the present work we have demonstrated that JNK3 phosphorylates rat brain purified mitochondrial VDAC in vitro and in vitro phosphorylation leads to changes in its single-channel electrophysiological properties. Fig. 1A shows the image of the Pro-Q Diamond stained gel and Fig. 1B shows the silver-stained image of the same gel. Lane 1: Color burst electrophoresis molecular weight marker C1992 (Mol. wt. 8000–220,000 Da, Sigma Chem. Company, USA). Lane 2: Peppermintstick phosphoprotein molecular weight standard. It contains a combination of six proteins, two are in the phosphorylated form (23.6 kDa, 45 kDa) and the rest four are in the unphosphorylated form (14.4 kDa, 18 kDa, 66.2 kDa, 116.25 kDa). Pro-Q Diamond dye binding was observed for only two phosphorylated proteins with molecular weight 23.6 kDa and 43 kDa in the standard sample, which authenticates the activity of the dye. Lane 3: Negative Control, i.e. VDAC (35 kDa) + Mg2+ATP. We did detect some dye binding to VDAC in this lane which shows that VDAC is either pre-phosphorylated or it has undergone self-phosphorylation in the presence of ATP without the enzyme. Lane 4: Experimental Sample, i.e. VDAC + JNK3 enzyme + Mg2+ATP, in this case more Pro-Q Diamond dye binding was observed to the VDAC band as compared to the negative control which suggests VDAC is phosphorylated by JNK3. Lane 5: Positive Control, i.e. VDAC + JNK3 enzyme + Mg2+ATP + alkaline phosphatase. A decrease in the amount of dye bound to the VDAC band as compared to the experimental sample after alkaline phosphatase treatment in this lane shows VDAC phosphorylation by JNK3 is reversible. As demonstrated by our repeated sets of Pro-Q Diamond Staining Experiment (Fig. 1A) rat brain mitochondrial VDAC is phosphorylated by JNK3. In Fig. 1B equal intensity of the VDAC monomer band in the positive and negative controls as well as in the experimental sample confirms equal loading of VDAC in these lanes. After confirming that JNK3 phosphorylates VDAC in vitro, we studied whether in vitro phosphorylation affects channel gating.
Fig. 1.
Representative Pro-Q Diamond stained SDS-PAGE gel image showing phosphorylation of rat brain purified mitochondrial VDAC by JNK3. Lane 1: Color burst electrophoresis molecular weight marker C1992 (Mol. wt. 8000–220,000 Da, Sigma Chem. Company, USA). Lane 2: Phosphoprotein standard. Lane 3: Negative Control, i.e. VDAC + Mg2+ATP. Lane 4: Experimental Sample i.e. VDAC + JNK3 + Mg2+ATP. Lane 5: Positive Control i.e. VDAC + JNK3 + Mg2+ 565 ATP + Alkaline Phosphatase. B. Gel image after silver staining. The size of the gel increases when stained with Pro-Q Diamond dye and decreases to the original size during silver staining.
There are three isoforms of VDAC, VDAC-1, VDAC-2 and VDAC-3 present in all the tissues with VDAC-1 being most abundant in most of the tissues [15]. The electrophysiological properties of the three VDAC isoforms are different. VDAC-1 and VDAC-2 isoforms have an open single-channel conductance in the range of 3.5–4 nS (in 1 M KCl) at ≤ ±20 mV, sigmoidal steady-state current I–V plot and bell shaped, symmetrical open probability curve whereas human VDAC-3 has a conductance of ≈90 pS with high open probability than other isoforms at voltages higher than ±40 mV [15], [16], [17]. VDAC-2 and VDAC-3 have a high prevalence in the mammalian testis [16], [17]. Bilayer electrophysiological recordings obtained from the purified native VDAC sample (which would contain all the three VDAC isoforms) and the JNK3 phosphorylated VDAC were observed. We found JNK3 phosphorylated VDAC electrophysiological behavior to be different than all the native VDAC isoforms. Furthermore, we have also done control experiments in which only VDAC (without JNK3) was incubated with the phosphorylation buffer at 30 °C for 30 min and then reconstituted on BLM. We found that the electrophysiological properties of 30 °C incubated VDAC was not different from the native VDAC recordings (data not shown). This suggests that different electrophysiological behavior of JNK3 phosphorylated VDAC is not obtained as a result of incubation at 30 °C. Fig. 2(A–D) shows best representative current–time traces and their histograms (chosen from three independent sets of experiments) obtained from the native and the JNK3 phosphorylated VDAC at 10 mV. At ±10 mV native VDAC remained in open-state (O) (O state current = ±40 pA) for most of the time of recording, but phosphorylated VDAC showed frequent flickering between the lower-conductance substates (S1, S2, S3 and S4) and the open P-state (P) (P current = ±32 pA). The calculated P-state conductance at ±10 mV was 3.2 nS (in 1 M KCl buffer) which is different from the open or the sub-states conductance of the three native VDAC isoforms. Fig. 3(A–D) shows best representative current–time traces and their histograms from three independent sets of experiments of the native and the phosphorylated VDAC at ±40 mV. We confirmed that there is single incorporated phosphorylated VDAC in the BLM as follows. In Fig. 3D, it can be noted that the phosphorylated channel remains mainly in open P-state at −40 mV (−128 pA). As current steps corresponding to the gating of more than one channel is not observed during long recordings at −40 mV, we infer that there must be only one incorporated channel in the BLM. Furthermore, in Fig. 3C it is observed that the phosphorylated VDAC at +40 mV displays continuous fluctuations between the different states (P, S1, S2 & S3). However, it does not show direct transitions from the open P-state to the lowest current substate (S3) (P ↔ S3) or from current substate S1 to substate 3 (S1 ↔ S3). Had there been multiple channels present the aforesaid transitions (P ↔ S3 & S1 ↔ S3) would have been observed at some point of time during the long recording. All these observations suggest that there is only one inserted phosphorylated channel.
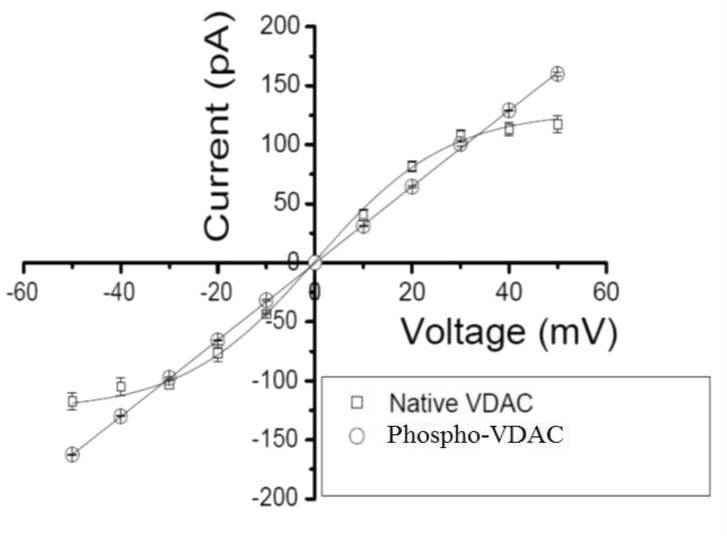
At +40 mV (Fig. 3A) native VDAC showed a fluctuating open-state (O) and a predominant steady sub-state S1 (O = ∼150 pA, Po ≈ 0.4; S1 = ∼100 pA, Po ≈ 0.6), whereas JNK3 phosphorylated VDAC (Fig. 3C) showed an open P-state (P) (P = 128 pA, Po ≈ 0.52) and three sub-states (S1 = 96.4 pA, Po ≈ 0.37; S2 = 62 pA, Po ≈ 0.1 and S3 = 29 pA, Po ≈ 0.01). At −40 mV (Fig. 3B) native VDAC showed a fluctuating open-state (O) and a sub-state S1 (O = −150 pA, Po ≈ 0.4; S1 = −100 pA, Po ≈ 0.6), whereas the phosphorylated channel (Fig. 3D) showed high open probability of open P-state (P = −128 pA, Po ≈ 0.96) and a substate 1 (S1). The high open probability of the phosphorylated VDAC at−40 mV suggests stabilization (locking) of the VDAC pore in the open P-state as a result of phosphorylation. Fig. 4 shows the current–voltage relation (I–V) of the native VDAC steady substate current (S1) and the phosphorylated VDAC open P-state current. I–V plot of the native and the phosphorylated VDAC suggests that there are differences in single-channel current values of the native and the phosphorylated VDAC. The results of student's paired t-test of the I–V data at the positive and the negative voltages show that the two tailed p-values (0.6794 and 0.4983) are greater than 0.05 at both the positive and the negative voltages. These results suggest that the differences in single-channel current values of the native and the phosphorylated VDAC are statistically insignificant.
Fig. 4.
Single-channel current–voltage relation (I–V plot) of the native VDAC steady-state current and the P-state current of the phosphorylated VDAC. The scatter plots of current (I) versus voltage (mV) are made using Origin 5.0 software. Native VDAC plot is fitted with a sigmoidal function whereas that of the phosphorylated VDAC is fitted with a linear function. Values are Mean ± S.E. of three independent sets of best experiments.
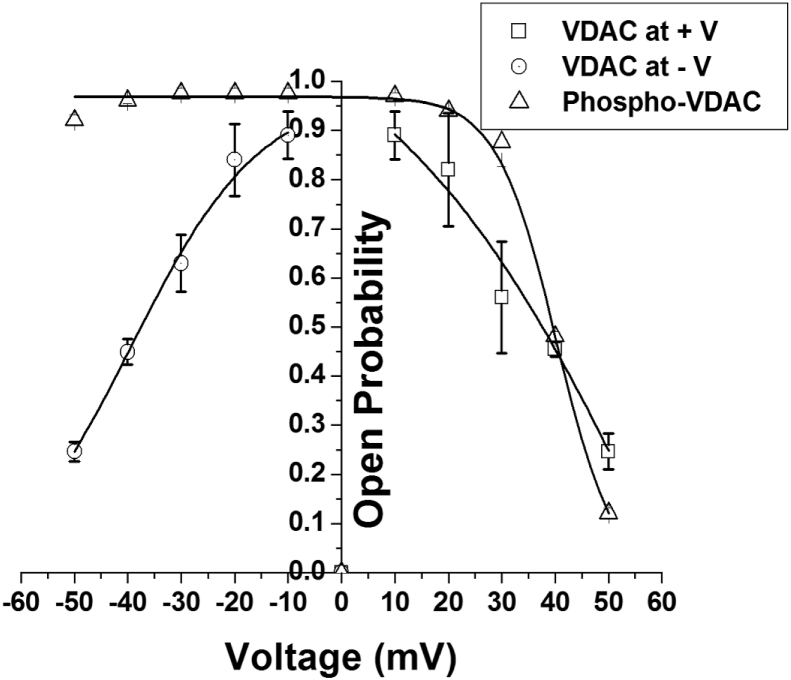
Fig. 5 shows the open-state probability vs. applied membrane potential plot of the native and the phosphorylated VDAC. The open probability distribution curve of native VDAC is symmetric at the positive and at the negative voltages and is fitted with two Boltzmann fits because native VDAC is reported to have two different, independent gating processes operative at the positive and the negative potentials, whereas phosphorylated VDAC P-state open-probability curve is found to be asymmetric and it fitted well with a single Boltzmann function. The data points of the open probability of the native and the phosphorylated VDAC coincided at the positive voltages, but at the negative voltages the open probability of phosphorylated VDAC was higher than that of the native VDAC at each voltage. From the paired t-test of the mean open probability vs. voltage data it is found that at the negative voltages the two tailed p-value (0.0343 < 0.05) is statistically significant but at the positive voltages the p-value (0.3081 > 0.05) is insignificant. This establishes that open probability of the phosphorylated VDAC P-state is different from that of the native VDAC open-state at the negative voltages but the same at the positive voltages.
Fig. 5.
Dependence of Open Probability (Po) of the native and the phosphorylated (by JNK3) VDAC on applied membrane voltage (mV). The open probability of VDAC at a voltage V is defined as the fraction of time for which the channel was in open-state divided by the total time of recording it spent in partially closed sub-states. The open probability at a particular voltage is determined using pClamp 10.2 software by selecting the different current levels from the single-channel traces. At voltages ≤ ±20 mV native VDAC remains mainly in open-state, so its open-state probability was plotted against voltage (V) whereas at voltages >20 mV it also shows sub-state (s) so the open-probability of open-state (O) is calculated relative to the sub-states. In case of phosphorylated VDAC we did not observe characteristic VDAC open-state (∼4 nS) thus we have labeled its maximum current open-state as open P-state. We have compared the opening probability of native VDAC open-state (O) and the phosphorylated VDAC P-state (P). The scatter plots of the open probability versus voltage are made using Origin 5.0 software. Native VDAC plot is fitted with two Boltzmann functions one at positive and the other at the negative voltages, whereas of the phosphorylated VDAC is fitted with a single Boltzmann function. Values are Mean ± S.E. of three independent sets of best experiments.
For molecular interpretation of the different electrophysiological behavior of the JNK3 phosphorylated VDAC we referred to the structure of rat VDAC-1. Structure of VDAC consists of a flexible N-terminal α-helix attached to the 19 β-strands which forms its cylindrical lumen [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]. VDAC is believed to possess a single voltage sensor with net positive charge and its lumen is also overall positively charged. Asymmetric voltage-dependence of the open probability curve of the phosphorylated VDAC at the positive and the negative voltages suggests that phosphorylation is affecting the voltage-sensing domains of VDAC. It also supports the existence of two separate closure (gating) mechanisms working independently at the positive and the negative voltages. Several experiments have been done in the past to identify the voltage-sensing domains in VDAC like multiple negative charges were introduced by selectively modifying VDAC amino-acids amino groups with succinic anhydride and this has been shown to result in a decrease of VDAC voltage-sensitivity [29], [30]. This suggested that voltage-dependence of VDAC depends on the charged state of certain positively charged amino acid residues like lysines which constitute its voltage sensor and is also sensitive to ion flow [31], [32], [33], [34]. Furthermore, it was shown that increasing the positive charge of VDAC at certain amino acid sites increases the voltage-dependence of VDAC at both the positive and negative voltages and vice-versa [35]. Deletion of N-terminal region enriched with positively charged amino-acid residues resulted in altered voltage-dependent gating of VDAC [36]. These experiments suggested that flexible N-terminal α-helix acts as voltage sensor in VDAC. A proposed model for VDAC gating suggested that N-terminal α-helix resides in the pore lumen in the open-state and interacts with some defined β-barrel strands which forms the inside pore wall [37], [38]. Movement of the N-terminal voltage sensor from inside the pore lumen to the membrane surface in two opposite directions leads to voltage-dependent closure of VDAC at the higher positive and the negative potentials [37], [38]. Thus, VDAC closed state (s) (substates) at positive voltages are structurally different from that at negative voltages. Furthermore, in bilayer reconstitution experiments it was shown that changing the pH of the cis and trans side buffer surrounding VDAC affects the voltage-dependence of VDAC asymmetrically [39]. All these experiments suggest that VDAC can behave asymmetrically at the positive and negative potentials. Recently it has been shown that voltage-dependent gating in VDAC is possible even when N-terminal helix is fixed with the pore wall β-strands and thus the model involving movement of voltage sensor from pore inside to the membrane surface is being debated [40], [41], [42]. But still this model is used to explain voltage-dependent gating in VDAC. JNKs are proline directed serine–threonine kinases (Ser/Thr–Pro), in our case phosphorylation by JNK3 would have added negatively charged phosphate groups to serine/threonine residues of the VDAC voltage-sensor and also the amino acid residues on the β-strands with which voltage sensor interacts during gating. Phosphorylation of voltage sensor by JNK3 would have decreased its overall positive charge and lead to decrease in the movement of voltage-sensor in the direction which leads to decreased voltage-dependent closure at the negative potentials. As VDAC-1 is the most abundant isoform on the outer mitochondrial membrane in rat brain, we predicted the plausible sites for JNK3 phosphorylation in VDAC using rat VDAC-1 structure. First 26 amino acids at the N-terminus of VDAC-1 most likely form the voltage sensor. According to the rat VDAC-1 primary amino acid sequence, threonine at 6th position is a proline directed site and thus could be a putative JNK3 target. Furthermore, in a report phosphorylation of VDAC-1 at Ser-104 site has been shown to oppose the anion transport through the pore and hence decrease the VDAC conductance at low voltages as seen in our results [43]. Also, Ser-104 is a proline directed site in VDAC-1. Ser-104 is located on the 6th β-strand in the transmembrane (TM) region representing VDAC-1 pore. Similarly, Serine 137 Glutamate (S137E) phospho-mimetic mutant of native VDAC-1 has been reported to result in more frequent transitions to lower-conductance states from the open-state at lower negative voltages (−10 mV) increasing the open probability of lower-conducting state like our after phosphorylation results [44]. Ser-137 is a proline directed site in VDAC-1 and it is located on the 9th β-strand in the TM region of VDAC-1. Deletion of 9th and 10th β-strands has been shown to lead to asymmetry in conductance-voltage (G-V) plot of VDAC-1 [41]. Thus, Ser-137 could be a plausible JNK3 phosphorylation site in VDAC-1. Both Ser-104 and Ser-137 are present in the transmembrane region and might be exposed to JNK3 when phosphorylation is carried out in tube and may not be exposed in bilayer lipid membrane incorporated VDAC.
As mentioned in the introduction in one of our previous work we have reported that ‘on bilayer phosphorylation’ of rat brain mitochondrial VDAC by JNK3 leads to significant reduction in the open-probability and conductance of the channel at both the positive and the negative voltages [7]. During in vitro phosphorylation in tube, purified VDAC is present in the micellar form, shielded by the Triton X-100 detergent molecules. Thus, both hydrophilic faces (which in in vivo condition faces the cytosol and the inner mitochondrial membrane) of VDAC would only be partially exposed to the kinase during the reaction. Moreover, while carrying out phosphorylation in tube in contrast to ‘on bilayer phosphorylation’ the membrane environment required for proper folding and voltage-dependent gating of VDAC is absent [45], [46]. These could be the reasons why phosphorylation in tube in contrast to on bilayer phosphorylation lead to phosphorylation only at domains controlling the VDAC voltage-dependence at the negative voltages.
This study solves and raises several important questions on VDAC gating mechanisms. First, VDAC voltage-dependent gating can be selectively modified at the negative potentials but what conformational rearrangements leads to such a change needs to be worked out. Second question is, what would be the ionic selectivity (anionic/cationic) of the phosphorylated VDAC open-state and the sub-states? We believe given the established role of JNK3 in neurodegeneration and VDAC in mitochondrial apoptosis JNK3 phosphorylation site localization in VDAC would be of great biomedical importance in future.
Conflict of interest
Both the authors declare no conflict of interest.
Acknowledgments
Both the authors acknowledge University of Delhi for the R & D Project and the DU-DST PURSE Grant. Dr. Rajeev Gupta gratefully acknowledges Prof. H.N. Mallick (Dept. of Physiology, AIIMS, New Delhi) for help and support. Dr. Rajeev Gupta also acknowledges Department of Biotechnology, India for providing the Research Associate fellowship and All India Institute of Medical Sciences, New Delhi for maintaining the fellowship.
References
- 1.Schulz D.J., Temporal S., Barry D.M., Garcia M.L. Mechanisms of voltage-gated ion channel regulation: from gene expression to localization. Cell Mol. Life Sci. 2008;65:2215–2231. doi: 10.1007/s00018-008-8060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheldon K.L., Maldonado E.N., Lemasters J.J., Rostovtseva T.K., Bezrukov S.M. Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin. PLoS One. 2011;6:e25539. doi: 10.1371/journal.pone.0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckford P.D., Li C., Ramjeesingh M., Bear C.E. Cystic fibrosis transmembrane conductance regulator (CFTR) potentiator VX-770 (ivacaftor) opens the defective channel gate of mutant CFTR in a phosphorylation-dependent but ATP-independent manner. J. Biol. Chem. 2012;287(44):36639–36649. doi: 10.1074/jbc.M112.393637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ismailov I.I., Benos D.J. Effects of phosphorylation on ion channel function. Kidney Int. 1995;48:1167–1179. doi: 10.1038/ki.1995.400. [DOI] [PubMed] [Google Scholar]
- 5.Bera A.K., Ghosh S. Dual mode of gating of voltage-dependent anion channel as revealed by phosphorylation. J. Struct. Biol. 2001;135:67–72. doi: 10.1006/jsbi.2001.4399. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee J., Ghosh S. Phosphorylation of rat brain mitochondrial voltage-dependent anion as a potential tool to control leakage of cytochrome c. J. Neurochem. 2006;98:670–676. doi: 10.1111/j.1471-4159.2006.03853.x. [DOI] [PubMed] [Google Scholar]
- 7.Gupta R., Ghosh S. Phosphorylation of voltage-dependent anion channel by c-Jun N-terminal Kinase-3 leads to closure of the channel. Biochem. Biophys. Res. Commun. 2015;459:100–106. doi: 10.1016/j.bbrc.2015.02.077. [DOI] [PubMed] [Google Scholar]
- 8.Colombini M. The VDAC channel: molecular basis for selectivity. BBA Mol. Cell Res. 2016;1863(10):2498–2502. doi: 10.1016/j.bbamcr.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 9.McCommis K.S., Baines C.P. The role of VDAC in cell death: friend or foe? BBA Biomembranes. 2012;1818(6):1444–1450. doi: 10.1016/j.bbamem.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahfoudh-Boussaid A., Zaouali M.A., Hauet T., Hadj-Ayed K., Miled A.H., Ghoul-Mazgar S., Saidane-Mosbahi D., Rosello-Catafau J., Abdennebi H.B. Attenuation of endoplasmic reticulum stress and mitochondrial injury in kidney with ischemic postconditioning application and trimetazidine treatment. J. Biomed. Sci. 2012;19:1–14. doi: 10.1186/1423-0127-19-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J., Qian H., Li Y., Wang Y., Zhang X., Liang X., Fu M., Lin C. Therapeutic effect of arsenic trioxide (As2O3) on cervical cancer in vitro and in vivo through apoptosis induction. Cancer Biol. Ther. 2007;6:580–586. doi: 10.4161/cbt.6.4.3887. [DOI] [PubMed] [Google Scholar]
- 12.Barrientos S.A., Martinez N.W., Yoo S., Jara J.S., Zamorano S., Hetz C., Twiss J.L., Alvarez J. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J. Neurosci. 2011;31:966–978. doi: 10.1523/JNEUROSCI.4065-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Pinto V., Prezioso G., Palmieri F. A simple and rapid method for the purification of the mitochondrial porin from mammalian tissues. Biochim. Biophys. Acta. 1987;905:499–502. doi: 10.1016/0005-2736(87)90480-9. [DOI] [PubMed] [Google Scholar]
- 14.Gupta R., Ghosh S. JNK3 phosphorylates Bax protein and induces ability to form pore on bilayer lipid membrane. Biochimie Open. 2017;4:41–46. doi: 10.1016/j.biopen.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messina A., Reina S., Guarino F., De Pinto V. VDAC isoforms in mammals. BBA Biomembranes. 2012;1818(6):1466–1476. doi: 10.1016/j.bbamem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Menzel V.A., Cassará M.C., Benz R., De Pinto V., Messina A., Cunsolo V., Salleti R., Hinsch K.D., Hinsch E. Molecular and functional characterization of VDAC2 purified from mammal spermatozoa. Biosci. Rep. 2009;29(6):351–362. doi: 10.1042/BSR20080123. [DOI] [PubMed] [Google Scholar]
- 17.Checchetto V., Reina S., Magri A., Szabo I., De Pinto V. Recombinant human voltage dependent anion selective channel isoform 3 (hVDAC3) forms pores with a very small conductance. Cell Physiol. Biochem. 2014;34(3):842–853. doi: 10.1159/000363047. [DOI] [PubMed] [Google Scholar]
- 18.Malia T.J., Wagner G. NMR structural investigation of the mitochondrial outer membrane protein VDAC and its interaction with antiapoptotic Bcl-xL. Biochemistry. 2007;46(2):514–525. doi: 10.1021/bi061577h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayrhuber M., Meins T., Habeck M., Becker S., Giller K., Villinger S., Vonrhein C., Griesinger C., Zweckstetter M., Zeth K. Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15370–15375. doi: 10.1073/pnas.0808115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiller S., Garces R.G., Malia T.J., Orekhov V.Y., Colombini M., Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ujwal R., Cascio D., Colletier J.P., Faham S., Zhang J., Toro L., Ping P., Abramson J. The crystal structure of mouse VDAC1 at 2.3 Å resolution reveals mechanistic insights into metabolite gating. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colombini M. The published 3D structure of the VDAC channel: native or not? Trends Biochem. Sci. 2009;34:382–389. doi: 10.1016/j.tibs.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Hiller S., Abramson J., Mannella C., Wagner G., Zeth K. The 3D structures of VDAC represent a native conformation. Trends Biochem. Sci. 2010;35:514–521. doi: 10.1016/j.tibs.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villinger S., Briones R., Giller K., Zachariae U., Lange A., de Groot B.L., Griesinger C., Becker S., Zweckstetter M. Functional dynamics in the voltage-dependent anion channel. Proc. Natl. Acad. Sci. U. S. A. 2010;107(52):22546–22551. doi: 10.1073/pnas.1012310108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eddy M.T., Andreas L., Teijido O., Su Y., Clark L., Noskov S.Y., Wagner G., Rostovtseva T.K., Griffin R.G. Magic angle spinning nuclear magnetic resonance characterization of voltage-dependent anion channel gating in two-dimensional lipid crystalline bilayers. Biochemistry. 2015;54(4):994–1005. doi: 10.1021/bi501260r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge L., Villinger S., Mari S.A., Giller K., Griesinger C., Becker S., Müller D.J., Zweckstetter M. Molecular plasticity of the human voltage-dependent anion channel embedded into a membrane. Structure. 2016;24(4):585–594. doi: 10.1016/j.str.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shuvo S.R., Ferens F.G., Court D.A. The N-terminus of VDAC: structure, mutational analysis, and a potential role in regulating barrel shape. BBA Biomembranes. 2016;1858(6):1350–1361. doi: 10.1016/j.bbamem.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Briones R., Weichbrodt C., Paltrinieri L., Mey I., Villinger S., Giller K., Lange A., Zweckstetter M., Griesinger C., Becker S., Steinem C., de Groot B.L. Voltage dependence of conformational dynamics and subconducting states of VDAC-1. Biophys. J. 2016;111(6):1223–1234. doi: 10.1016/j.bpj.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doring C., Colombini M. Voltage dependence and ion selectivity of the mitochondrial channel, VDAC, are modified by succinic anhydride. J. Membr. Biol. 1985;83:81–86. doi: 10.1007/BF01868740. [DOI] [PubMed] [Google Scholar]
- 30.Doring C., Colombini M. The mitochondrial voltage-dependent channel, VDAC, is modified asymmetrically by succinic anhydride. J. Membr. Biol. 1985;83:87–94. doi: 10.1007/BF01868741. [DOI] [PubMed] [Google Scholar]
- 31.Bowen K.A., Tam K., Colombini M. Evidence for titratable gating charges controlling the voltage dependence of the outer mitochondrial membrane channel, VDAC. J. Membr. Biol. 1985;86:51–59. doi: 10.1007/BF01871610. [DOI] [PubMed] [Google Scholar]
- 32.Zizi M., Byrd C., Boxus R., Colombini M. The voltage-gating process of the voltage-dependent anion channel is sensitive to ion flow. Biophys. J. 1998;75(2):704–713. doi: 10.1016/S0006-3495(98)77560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirzabekov T.A., Ermishkin L.N. The gate of mitochondrial porin channel is controlled by a number of negative and positive charges. FEBS Lett. 1989;249:375–378. doi: 10.1016/0014-5793(89)80662-3. [DOI] [PubMed] [Google Scholar]
- 34.De Pinto V., Al Jamal J.A., Benz R., Palmieri F. Positive residues involved in the voltage-gating of the mitochondrial porin-channel are localized in the external moiety of the pore. FEBS Lett. 1990;274(1–2):122–126. doi: 10.1016/0014-5793(90)81345-o. [DOI] [PubMed] [Google Scholar]
- 35.Thomas L., Blachly-Dyson E., Colombini M., Forte M. Mapping of residues forming the voltage sensor of the voltage-dependent anion-selective channel. Proc. Natl. Acad. Sci. U. S. A. 1993;90:5446–5449. doi: 10.1073/pnas.90.12.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popp B., Benz R., Neupert W., Lill R. The role of the N and C termini of recombinant Neurospora mitochondrial porin in channel formation and voltage-dependent gating. J. Biol. Chem. 1996;271(23):13593–13599. doi: 10.1074/jbc.271.23.13593. [DOI] [PubMed] [Google Scholar]
- 37.Song J., Midson C., Blachly-Dyson E., Forte M., Colombini M. The sensor regions of VDAC are translocated from within the membrane to the surface during the gating processes. Biophys. J. 1998;74:2926–2944. doi: 10.1016/S0006-3495(98)78000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mertins B., Psakis G., Grosse W., Back K.C., Salisowski A., Reiss P., Koert U., Essen L.O. Flexibility of the N-terminal mVDAC1 segment controls the channel's gating behavior. PLoS One. 2012;7(10):e47938. doi: 10.1371/journal.pone.0047938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teijido O., Rappaport S.M., Chamberlin A., Noskov S.Y., Aguilella V.M., Rostovtseva T.K., Bezrukov S.M. Acidification affects voltage-dependent anion channel functioning asymmetrically: role of salt bridges. J. Biol. Chem. August 22, 2014;289(34):23670–23682. doi: 10.1074/jbc.M114.576314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teijido O., Ujwal R., Hillerdal C.O., Kullman L., Rostovtseva T.K., Abramson J. Affixing the N-terminal alpha helix of the voltage dependent anion channel to the channel's wall does not prevent its voltage gating. J. Biol. Chem. 2012;287(14):11437–11445. doi: 10.1074/jbc.M111.314229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reina S., Magrì A., Lolicato M., Guarino F., Impellizzeri A., Maier E., Benz R., Ceccarelli M., De Pinto V., Messina A. Deletion of β-strands 9 and 10 converts VDAC1 voltage-dependence in an asymmetrical process. BBA Bioenergetics. 2013;1827(6):793–805. doi: 10.1016/j.bbabio.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Zachariae U., Schneider R., Briones R., Gattin Z., Demers J.P., Giller K., Maier E., Zweckstetter M., Griesinger C., Becker S., Benz R., de Groot B.L. β-Barrel mobility underlies closure of the voltage-dependent anion channel. Structure. 2012;20:1540–1549. doi: 10.1016/j.str.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villinger S., Giller K., Bayrhuber M., Lange A., Griesinger C., Becker S., Zweckstetter M. Nucleotide interactions of the human voltage-dependent anion channel. J. Biol. Chem. 2014;289(19):13397–13406. doi: 10.1074/jbc.M113.524173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tewari S.G., Zhou Y., Otto B.J., Dash R.K., Kwok W.M., Beard D.A. Markov chain Monte Carlo based analysis of post-translationally modified VDAC gating kinetics. Front. Physiol. 2015;5:513. doi: 10.3389/fphys.2014.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rostovtseva T.K., Kazemi N., Weinrich M., Bezrukov S.M. Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes. J. Biol. Chem. 2006;281(49):37496–37506. doi: 10.1074/jbc.M602548200. [DOI] [PubMed] [Google Scholar]
- 46.Shanmugavadivu B., Apell H.J., Meins T., Zeth K., Kleinschmidt J.H. Correct folding of the β-barrel of the human membrane protein VDAC requires a lipid bilayer. J. Mol. Biol. 2007;368(1):66–78. doi: 10.1016/j.jmb.2007.01.066. [DOI] [PubMed] [Google Scholar]