Abstract
Surface pre-reacted glass-ionomer (S-PRG)-containing dental materials, including composite and coating resins have been used for the restoration and/or prevention of dental cavities. S-PRG is known to have the ability to release aluminum, boron, fluorine, silicon, and strontium ions. Aluminum ions are known to be inhibitors whereas boron, fluorine, silicon, and strontium ions are known to be promoters of mineralization, via osteoblasts. However, it remains to be clarified how an aqueous eluate obtained from S-PRG containing these ions affects the ability of mesenchymal stem cells (MSCs), which are known to be present in dental pulp and bone marrow, to differentiate into osteogenic cell types. The present study demonstrated that 200- to 1,000-fold-diluted aqueous eluates obtained from S-PRG significantly upregulated the mRNA expression level of the osteogenic differentiation marker alkaline phosphatase in human MSCs (hMSCs) without exhibiting the cytotoxic effect. In addition, the 500- to 1,000-fold-diluted aqueous eluates obtained from S-PRG significantly and clearly promoted mineralization of the extracellular matrix of hMSCs. It was additionally demonstrated that hMSCs cultured on the cured resin composites containing S-PRG fillers exhibited osteogenic differentiation in direct correlation with the weight percent of S-PRG fillers. These results strongly suggested that aqueous eluates of S-PRG fillers promoted hard tissue formation by hMSCs, implicating that resins containing S-PRG may act as a useful biomaterial to cover accidental exposure of dental pulp.
Keywords: surface pre-reacted glass-ionomer, water-soluble factor, osteoblastic differentiation, mesenchymal stem cell
Introduction
Dental restoration is frequently performed using resin composites to repair missing parts of teeth caused by dental trauma or traumatic damage. Resin composites consist of three different materials: An organic phase (matrix), a dispersed phase (inorganic filler), and an interfacial phase (coupling agent to bind the filler to the organic resin) (1). The inorganic filler in the resin composite determines mainly its physical and mechanical characteristics. The filler is known to suppress thermal expansion and polymerization shrinkage of the resin composites (2) as well as to give it fracture resistance (3).
Surface pre-reacted glass-ionomer (S-PRG) fillers are prepared by an acid-based reaction between fluoro-boro-aluminosilicate glass and polyacrylic acid solution (4). Intriguingly, S-PRG fillers are known to release ions of aluminum, boron, fluorine, sodium, silicon, and strontium (5). Among these, fluorine ion strengthen the resistance of teeth against acid exposure by producing fluoride-substituted hydroxyapatite (HA) (6). Siiya et al previously reported that temporary resin-based filling materials containing over 10% S-PRG filler content have anti-demineralization effects on adjacent dentin (7). In addition, ions of boron, silicon, and strontium have the ability to promote bone formation by inducing osteoblastic differentiation of undifferentiated mesenchymal cells as follows: i) Boron ions promoted extracellular matrix (ECM) mineralization by inducing osteoblastic differentiation of rat bone marrow-derived mesenchymal stem cells (MSCs) (8). Boron ions also induced odontogenic and osteogenic differentiation of human tooth germ stem cells (9). In addition, Doğan et al demonstrated that boron-containing poly-(lactide-co-glycolide-acid) (PLGA) scaffolds promoted the in vivo healing of a bone defect in a femur. Moreover, they showed that a transplantation of rat adipose-derived stem cells embedded in the boron-containing PLGA scaffolds further promoted the bone healing of the femur (10). ii) Orthosilicic acid induced osteoblastic differentiation of human osteoblast-like cells and expression of collagen type I in the cells (11). Tetraethyl orthosilicate-containing poly(ε-caprolactone) nanofiber scaffolds promoted the proliferative activity and osteoblastic differentiation of human osteogenic sarcoma cells SaOS-2 (12). In addition, Wang et al demonstrated that a 1:1 mixture of silica- and silicatein-containing bead-like scaffolds exhibited strong bone regenerative activity in rabbit (13). iii) Strontium-substituted HA more potently induced alkaline phosphatase (ALP) activity, osteoblast differentiation marker RUNX2 expression in rat primary osteoblasts, and ECM mineralization in the cells compared with non-substituted HA (14). Loading of strontium onto chitosan-based hydrogel scaffolds significantly improved the inducibility of stem cells derived from human exfoliated deciduous teeth to differentiate into osteoblasts (15). In addition, the substitution of calcium by strontium in a calcium zinc silicate mineral coated on the surface of a biomedical implant made of Ti clearly improved the rate of osseointegration on the surface of the implant (16). In contrast, aluminum ions are known to inhibit osteoblastic differentiation of undifferentiated mesenchymal cells and induce toxic effects on bone formation. Aluminum trichloride suppressed the differentiation of rat osteoblastic lineage cells through the inactivation of the Wnt/β-catenin signal transduction pathway (17). Aluminum trichloride also suppressed transforming growth factor (TGF)-β-induced ECM mineralization by rat osteoblasts through interruption of Smad-dependent signal pathways (18). In addition, aluminum trichloride induced the apoptosis of osteoblasts by disrupting calcium ion homeostasis and activating calmodulin-dependent kinase II-mediated signal pathway (19). Moreover, an intake of drinking water containing aluminum trichloride decreased the bone mineral density in rats (20). However, it remains to be clarified how aqueous eluates obtained from S-PRG containing ions of aluminum, boron, silicon, and strontium can affect the ability of MSCs, which are known to be present in dental pulp and bone marrow, to proliferate and differentiate into osteogenic lineage cells.
Here, we examined how aqueous eluates obtained from S-PRG affect the viability and osteoblastic differentiation of MSCs. In addition, we evaluated the status of osteogenic differentiation of human MSCs (hMSCs) cultured on S-PRG-containing composite resins.
Materials and methods
Cell culture
Human bone marrow-derived MSCs, UE7T-13 cells immortalized by overexpression of human papillomavirus E7, and human telomerase reverse transcriptase (hTERT) (21,22) were obtained from the Health Science Research Resources Bank (JCRB no. 1154; Japan Health Sciences Foundation). UE7T-13 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified incubator with an atmosphere of 5% CO2.
Preparation of aqueous eluate of S-PRG fillers
A total of 20 g of powdered S-PRG fillers was thoroughly mixed with 20 ml of DMEM in a 50-ml plastic centrifuge tube using a tube rotator (rgos. Technologies, Inc., Elgin, IL, USA) at 40 rpm for 24 h at room temperature. Then, the suspension of the S-PRG fillers in DMEM was aliquoted into 2-ml centrifuge tubes, and the filler particles in the suspension were centrifuged at 10,000 × g for 15 min at 4°C. The supernatant was sterilized by filtration using a nylon filter membrane with a pore size of 0.22 µm (33 mm diameter; Sigma-Aldrich).
Cell viability assay
The cell viability was evaluated using an alamarBlue assay (AbDSerotec, Oxon, UK) according to the manufacturer's instruction. The assay includes an indicator that fluoresces and undergoes colorimetric change when reduced by mitochondrial respiration, which is proportional to the number of living cells. The cells were cultured with DMEM containing 10% alamarBlue solution to evaluate the viability of the cells, and the cells were cultured for an additional 4.5 h. The absorbance in each well was measured using a micro plate reader (Tosoh Corp., Tokyo, Japan). The data are shown as values of Abs570-Abs600. Each experiment was repeated three times with six wells dedicated to each time point.
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA from UE7T-13 cells was isolated with ISOGENII reagent (Nippon Gene, Toyama, Japan) according to the manufacturer's instructions. First-strand cDNA was synthesized from total RNA using the PrimeScript RT reagent kit (Takara Bio, Inc., Otsu, Japan). PCR was subsequently performed on a Thermal Cycler Dice Real Time System (Takara Bio, Inc.) using SYBR Premix Ex Taq II (Takara-Bio) with specific oligonucleotide primers (human ALP, 5′-GGACCATTCCCACGTCTTCAC-3′ (forward) and 5′-CCTTGTAGCCAGGCCCATTG-3′ (reverse); human GAPDH, 5′-GCACCGTCAAGGCTGAGAAC-3′ (forward) and 5′-ATGGTGGTGAAGACGCCAGT-3′ (reverse)). The mRNA expression of ALP was normalized to that of GAPDH, and the relative expression levels are shown as fold increase or decrease relative to the control.
Detection of ECM mineralized by the cells
ECM mineralization was evaluated by Alizarin red S (A5533; Sigma-Aldrich) staining after the fixation with 4% paraformaldehyde. Then, the dye bound to the calcified nodules was extracted by 5% formic acid. The absorbance of the extracted solution was measured using a micro plate reader. The data are shown as values of Abs405-Abs600. Each experiment was repeated three times with five wells dedicated to each time point.
Elemental analysis of ions released from S-PRG filler
Elemental analysis of ions (Al, B, F, Na, Si, and Sr, which were presumed to be contained in the glass core composition) released from the S-PRG filler was performed using an inductively coupled plasma (ICP) atomic emission spectrometer (ICP-AES: ICPS-8000; Shimadzu Corporation, Kyoto, Japan) or fluoride electrode method (fluoride electron, model 9609BN; pH/ion meter, model 720A, Orion Research).
Analysis using ICP was conducted after preparing calibration curves corresponding to each element (standard solution concentrations; Si: 0, 0.5, 1, 5 ppm; Sr: 0, 5, 20, 50 ppm; B: 0, 10, 50, 100 ppm; Al: 0, 0.5, 5, 10 ppm; Na: 0, 0.5, 20, 50 ppm). Fluoride was analyzed using a fluoride ion electrode method after preparing calibration curves (standard solution concentrations: 0.1, 1, 5, 10 ppm). An ionic strength adjuster (TISAB III, Orion Research) was added in the proportion of 0.1 ml of ionic strength adjuster to 1 ml of test liquid.
Preparation of the cured resin composite discs containing S-PRG fillers at various weight percentages
Resin composite discs with a diameter of 20 mm and a thickness of 2 mm containing S-PRG fillers at 0, 10, 30, 50, and 70% by weight were casted into a mold made from polytetrafluoroethylene and cured. Then, the surface of the cured composite resin discs was polished with waterproof sandpaper no. 400. The cured resin composite discs were disinfected by immersion of the discs in 99.5% ethanol for 24 h. The disinfected resin discs were inserted into each well of 12-well plastic cell culture plates.
Statistical analysis
The data are presented as mean ± standard deviation (n=3, 6, or 8). The data were statistically analyzed by Dunnett's multiple comparison test, and P<0.05 was considered to indicate a statistically significant difference. The results shown in all experiments are representative of at least three separate experiments.
Results
S-PRG fillers eluate showed cytotoxicity in UE7T-13 cells in direct correlation with the volume percent of the DMEM eluate
The alamarBlue assay revealed that the non-, 2-, 5-, and 10-fold-diluted DMEM eluate decreased the viability of UE7T13 cells to 17.3, 62.2, 80.6, and 74.2% of the control, respectively, 48 h after its administration (Fig. 1, left graph). In contrast, the 100- to 800-fold-diluted DMEM eluate did not affect the viability of UE7T13 cells, suggesting that a cytotoxic threshold of the eluate may exist between 10- and 100-fold dilution. Therefore, we further evaluated the cytotoxic effects of the 20- to 40-fold-diluted DMEM eluate on UE7T13 cells. As shown in the graph on the right in Fig. 1, the 10- and 20-fold-diluted DMEM eluate significantly decreased the cell viability to 78.6 and 79.9% of the control, respectively, but the 40-fold-diluted DMEM eluate did not.
Figure 1.
Eluate from S-PRG fillers showed cytotoxicity in UE7T-13 cells in direct correlation with the volume percent of the DMEM eluate. An alamarBlue assay was used to examine the viability of UE7T-13 cells. The cells were seeded into 96-well cell culture plates at a density of 1×104 cells per well and cultured for 24 h in medium supplemented with 10% FBS. The medium was replaced with DMEM containing eluate from S-PRG fillers, diluted with normal DMEM at the ratios of 1 to 1/800. Then, the diluted eluate of S-PRG fillers was supplemented with 10% FBS, and the cells were subsequently cultured for 48 h. Finally, the medium was replaced with DMEM containing 10% alamarBlue solution to evaluate the viability of the cells as described in the Materials and methods. Data represent the mean ± standard deviation (n=6). *P<0.05 vs. control was considered statistically significant. S-PRG, surface pre-reacted glass-ionomer; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum.
Eluate from S-PRG fillers induced mRNA expression of osteoblastic marker ALP in UE7T-13 cells
UE7T-13 cells were cultured with non-diluted DMEM or the 100- to 1,000-fold-diluted DMEM eluate of S-PRG fillers supplemented with 10% FBS as described in the Materials and Methods. The 200-, 500- and 1,000-fold-diluted DMEM eluate significantly increased the expression of ALP mRNA to 157.3, 193.3, and 194.5% of the control, respectively, 72 h after administration in a dilution-dependent manner (Fig. 2).
Figure 2.

Eluate from S-PRG fillers induced mRNA expression of osteoblastic markers in UE7T-13 cells. An aqueous eluate of S-PRG fillers was prepared as described in the Materials and Methods. UE7T-13 cells were seeded into 12-well cell culture plates at a density of 1×105 cells per well and cultured for 24 h in medium supplemented with 10% FBS. The medium was replaced with DMEM containing eluate from S-PRG fillers, diluted with normal DMEM at the ratios of 1/100 to 1/1,000. Then, the diluted eluate in each well of the cell culture plates was supplemented with 10% FBS, and the cells were subsequently cultured for 72 h. Then, the total RNA from the cells was isolated, and the relative mRNA expression of the osteoblastic marker ALP was evaluated using qRT-PCR as described in the Materials and methods. The mRNA expression of ALP was normalized to that of GAPDH. Data represent the mean ± standard deviation (n=8). *P<0.05 vs. control was considered statistically significant. S-PRG, surface pre-reacted glass-ionomer; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum.
Eluate from S-PRG fillers induced calcium deposition in the extracellular matrix of UE7T-13 cells
Alizarin red staining revealed that the 500- and 1,000-fold-diluted DMEM eluates promoted calcium deposition in the ECM of UE7T-13 cells (Fig. 3A). In addition, as shown in Fig. 3B, the 100-, and 200-fold-diluted S-PRG filler eluates did not significantly induce calcium deposition in the ECM. In contrast, the 500- and 1,000-fold-diluted DMEM eluates clearly and significantly increased the amount of calcium deposition to 273.2 and 308.9% of the control, respectively, 14 days after administration, in a dilution-dependent manner.
Figure 3.
Eluate from S-PRG fillers promoted mineralization of ECM by UE7T-13 cells. UE7T-13 cells were seeded into 48-well cell culture plates at a density of 1×105 cells per well and cultured for 24 h in medium supplemented with 10% FBS. The medium was replaced with DMEM containing S-PRG fillers, non-diluted or diluted with normal DMEM to reach the indicated ratios. Then, the non-diluted or diluted eluate of S-PRG fillers in each well of 12-well cell culture plates was supplemented with 10% FBS, and the cells were subsequently cultured for 14 days. (A) Bone matrix mineralization was evaluated by Alizarin red S staining as described in the Materials and methods. Scale bar: 100 µm. (B) The dye bound to the calcified nodules was extracted by 5% formic acid. The absorbance of the extracted solution was measured as described in the Materials and methods. Data represent the mean ± standard deviation (n=3). *P<0.05 vs. control was considered statistically significant. S-PRG, surface pre-reacted glass-ionomer; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum.
Non-diluted DMEM eluate of S-PRG fillers contained ions of boron, silica, strontium, and fluorine, but normal DMEM did not
As shown in Table I, elemental analysis of ions released from S-PRG fillers revealed that non-diluted DMEM eluate of S-PRG fillers contained ions of boron, silica, strontium, and fluorine at concentrations of 1309.7, 7.2, 790.9, and 71.2 ppm, respectively, whereas normal DMEM did not contain these ions. Therefore, the 500- or 1,000-fold-diluted DMEM eluate contained ions of boron, silica, strontium, and fluorine at concentrations of 2.6 or 1.3 ppm, 0.014 or 0.007 ppm, 1.58 or 0.79 ppm, and 0.14 or 0.07 ppm, respectively. In addition, normal DMEM contained only 0.1 ppm of aluminum ions, whereas the non-diluted DMEM eluate contained 12.6 ppm of aluminum ions, indicating that the 500- and 1,000-fold-diluted DMEM eluate contained 0.025 and 0.013 ppm of aluminum ions, respectively. On the other hand, the concentrations of potassium and calcium ions were lower in the non-diluted DMEM eluate than in normal DMEM. The concentration of sodium ions in the non-diluted DMEM eluate was almost equivalent to that in normal DMEM.
Table I.
Comparison between concentrations of various ions in the non-diluted DMEM eluate of S-PRG fillers and those in normal DMEM.
| Ions | Al3+ | B3+ | Na+ | Si2+ | Ca2+ | K+ | Sr2+ | F− |
|---|---|---|---|---|---|---|---|---|
| Normal DMEM | 0.1±0 | 0±0 | 2,640.3±200.0 | 0±0 | 75.6 0±0.8 | 206.0±6.4 | 0±0 | 0±0 |
| DMEM eluate | 12.6±0.3a | 1,309.7±165.4a | 2,968.7±46.3 | 7.2±0.2a | 1.8±0.2a | 32.8±7.4a | 790.9±135.7a | 71.2±0.8a |
All values are given in ppm. Data are presented as the mean ± standard deviation (n=3).
P<0.05 were considered statistically significant. DMEM, Dulbecco's modified Eagle's medium; S-PRG, surface pre-reacted glass-ionomer
UE7T-13 cells cultured on the cured resin composites containing S-PRG fillers exhibited osteogenic differentiation in direct correlation with the weight percent of S-PRG fillers
In order to examine whether resin composites containing S-PRG fillers retain an advantage in inducing osteogenic differentiation of MSCs, we evaluated the mRNA expression status of ALP in UE7T-13 cells under co-incubation with cured resin composites containing various volumes of S-PRG fillers as indicated (Fig. 4). The cured resin composites containing S-PRG fillers at 50 and 70% by weight significantly increased the mRNA expression of ALP to 234.6 and 405.7% of the control, respectively. These results indicated that hMSCs cultured on the cured resin composites containing S-PRG fillers exhibited osteogenic differentiation in direct correlation with the weight percent of S-PRG fillers.
Figure 4.
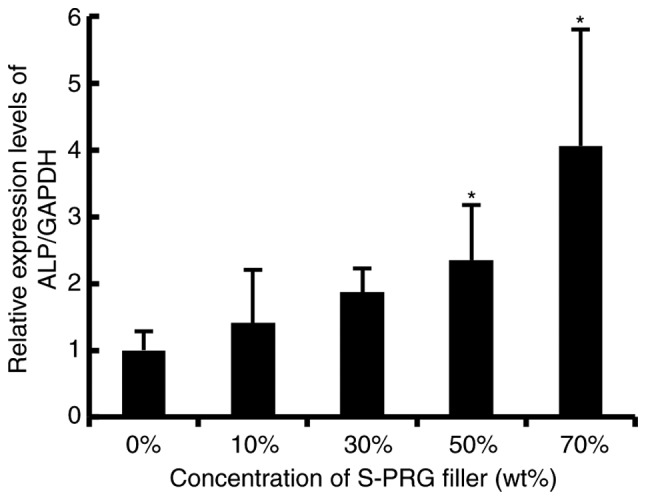
Resin composites containing S-PRG fillers significantly promoted ALP expression in UE7T-13 cells in direct correlation with the weight percent of S-PRG fillers. The cured resin composite discs were made as described in the Materials and methods and inserted into each well of 12-well plastic cell culture plates. UE7T-13 cells were seeded on the disinfected resin discs with DMEM supplemented with 10% FBS and antibiotics at a density of 1×105 cells per well and further cultured for 48 h. Then, the total RNA from the cells was isolated, and the relative mRNA expression of the osteoblastic marker ALP was evaluated using qRT-PCR as described in the Materials and methods. The mRNA expression of ALP was normalized to that of GAPDH. Data represent the mean ± standard deviation (n=8). *P<0.05 vs. control was considered statistically significant. S-PRG, surface pre-reacted glass-ionomer; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum.
Discussion
Hakki et al reported that boric acid at 0.1–10 ng/ml induced ECM mineralization of MC3T3-E1 osteoblasts, but did not affect cell viability (23). In addition, Movahedi Najafabadi and Abnosi reported that boric acid at 6 ng/ml to 6 µg/ml significantly induced ALP activity in primary rat bone marrow-derived MSCs cultured for 10 days after administration (8). MTT assay revealed that boric acid at 6 µg/ml significantly decreased the viability of MSCs at 10 days after administration, whereas boric acid at 6 ng/ml did not. These results suggested that boron ions at 0.0001-6 ppm might retain the ability to induce osteogenic differentiation of pre-osteoblasts or MSCs, although high doses of boron ion (1 ppm) might show cytotoxicity against undifferentiated mesenchymal cells. The non-diluted DMEM eluate of S-PRG fillers contained 1309.7 ppm of boron ions, which was much higher than the concentrations used in the experiments for the induction of osteoblastic differentiation of undifferentiated mesenchymal cells reported by Hakki et al (23) and Movahedi Najafabadi and Abnosi (8) (Table I). The 500- and 1,000-fold-diluted DMEM eluates of S-PRG fillers, which did not exhibited cytotoxicity against UE7T-13 cells (Fig. 1) but clearly induced osteoblastic differentiation of the cells (Figs. 2 and 3), contained 1.3 and 2.6 ppm of boron ions. These concentrations of boron (1.3 and 2.6 ppm) were within the osteoinducible concentration range (0.0001-6 ppm) of boron ions reported by Hakki et al (23), and Movahedi Najafabadi and Abnosi (8), suggesting that boron ions in the 500- and 1,000-fold-diluted DMEM eluates of S-PRG fillers possibly promoted osteoblastic differentiation of UE7T-13 cells.
Zou et al demonstrated that silicate ions (30 µM) did not affect the differentiation status of human osteoblast-like cells, but upregulated the proliferative activity of these cells (24). In addition, Varanasi et al previously demonstrated that silicate ions (400 µM, i.e., approximately 11.2 µg/ml) clearly induced osteoblastic differentiation of pre-osteoblast MC3T3-E1, but did not affect the proliferative activity of these cells (25). The non-diluted DMEM eluate of S-PRG fillers contained 7.2 ppm of silicate ions, which is equal to 7.2 µg/ml (Table I). Therefore, the 500- or 1,000-fold-diluted DMEM eluate of S-PRG fillers contained 7.2 or 14.4 ng/ml of silicate ions, respectively. However, these concentrations of silicate ions (7.2 or 14.4 ng/ml) were much lower than the osteoinducible concentration of silicate ions (11.2 µg/ml) reported by Varanasi et al (25), suggesting that silicate ions in the 500- or 1,000-fold-diluted DMEM eluate of S-PRG fillers did not promote osteoblastic differentiation of UE7T-13 cells.
Bao et al demonstrated that strontium (12.5 µM, i.e., approximately 1.1 µg/ml) did not affect the proliferative activity of the murine cementoblast cell line OCCM-30, but significantly promoted the expressions of osteogenic genes in these cells (26). In addition, high concentrations of strontium (25 to 200 µM, i.e., approximately 2.2 to 17.6 µg/ml) significantly decreased the viability of OCCM-30 cells in a dose-dependent manner. The non-diluted DMEM eluate of S-PRG fillers contained 790.9 ppm of strontium ions, which is equal to 790.9 µg/ml (Table I). Therefore, the 500- or 1,000-fold-diluted DMEM eluate of S-PRG fillers contained 0.79 or 1.6 µg/ml strontium ions, respectively. These concentrations of strontium ions (0.79 or 1.6 µg/ml) were almost equal to the osteoinducible concentration of strontium ions reported by Bao et al (26), suggesting that strontium ions in the 500- or 1,000-fold-diluted DMEM eluate of S-PRG fillers possibly promoted osteoblastic differentiation of UE7T-13 cells.
Nakade et al demonstrated that sodium fluoride (50 to 100 µM, i.e., approximately 2.1 to 4.2 µg/ml), which could release 1.0 or 1.9 µg/ml fluoride ions, significantly promoted osteogenic differentiation of human dental pulp cells and also promoted the proliferative activity of these cells (27). The non-diluted DMEM eluate of S-PRG fillers contained 71.2 ppm (71.2 µg/ml) of fluoride ions. Therefore, the 500- or 1,000-fold-diluted DMEM eluate of S-PRG fillers possibly contained 0.07 or 0.14 µg/ml fluoride ions, respectively. These concentrations of fluoride ions (0.07 or 0.14 µg/ml) were lower than the osteoinducible concentration of fluoride ions (1.0 to 1.9 µg/ml) reported by Nakade et al (27), suggesting that fluoride ions in the 500- or 1,000-fold-diluted DMEM eluate of S-PRG fillers did not promote osteoblastic differentiation of UE7T-13 cells.
Sun et al demonstrated that aluminum ions at 10 to 40 µg/ml, which are equal to 10 to 40 ppm, significantly decreased the expression of osteogenic genes in cultured primary rat osteoblasts (OBs) and suppressed ECM mineralization by these cells in a dose-dependent manner (18). In addition, Zhu et al revealed that 0.0252 to 0.252 mg/ml AlCl3·6H2O, which possibly released 2.8 to 28.4 ppm of aluminum ions, significantly decreased the viability of cultured primary rat OBs and suppressed the expression of osteogenic marker genes in these cells (28). As shown in Table I, the non-diluted DMEM eluate of S-PRG fillers contained 12.6 ppm of aluminum ions. Therefore, the 500- or 1,000-fold-diluted DMEM eluate of S-PRG fillers contained 0.013 to 0.025 ppm of aluminum ions. These concentrations of aluminum ions (0.013 to 0.025 ppm) were much lower than the anti-osteogenic concentrations of aluminum ions (2.8 to 40 ppm) reported by Sun et al (18) and Zhu et al (28), suggesting that aluminum ions in the 500- or 1,000-fold-diluted DMEM eluate of S-PRG fillers did not affect osteoblastic differentiation of UE7T-13 cells.
We also found that ALP mRNA expression in the hMSCs cultured in the diluted DMEM eluate of S-PRG filler decreased at the 2,000-, to 8,000-fold-dilution range of the S-PRG eluate in comparison with ALP mRNA expression in the hMSCs cultured in the 1,000-fold diluted eluate in a dilution rate-dependent manner (data not shown). On the other hand, hMSCs cultured on the cured resin composites containing S-PRG fillers exhibited osteogenic differentiation in direct correlation with the weight percent of S-PRG fillers (Fig. 4). It was plausible that amount of released ions from the cured resin composites containing S-PRG fillers might directly correlate with the weight percent of S-PRG fillers. Therefore, concentrations of osteoinducible ions eluated from the cured resin composite containing S-PRG filler at 50 to 70% by weight in Fig. 4 might be comparable with concentrations of osteoinducible ions in the 200-, to 1,000-fold-diluted DMEM eluate of S-PRG fillers, respectively. In addition, we are investigating the most suitable weight percentage of S-PRG fillers in the cured resin composites for the release of humoral osteogenic factors, including osteoinducible ions for the regeneration of hard tissue by hMSCs without exhibiting cytotoxicity against these cells in vitro and in vivo.
Calcium hydroxide is often used for endodontic treatments involving dental pulp capping, and known to induce calcification of extracellular matrix between dental pulp cells involving MSC-like cells (29). Here, we would like to suggest that resin composite containing S-PRG fillers could have a possibility for a new dental biomaterial for covering dental pulp instead of medicine which consist mainly of calcium hydroxide. On the other hand, MSCs are known to be present in the bone marrow (30). In some cases of comminuted fracture of long bone, it is very difficult to surgically reposition all the broken bone fragments and fully cover the exposed bone marrow tissue. In such cases, resin composite containing S-PRG fillers could have a possibility for a new medical biomaterial for covering bone marrow.
Acknowledgements
The present study was supported in part by JSPS KAKENHI grant nos. 26670852 and 16H05534 to A.I., nos. 25463053 and 16K11654 to N.C., no. 26462823 to S.K., no. 15K20606 to H.K., no. 22592076 to M.K., and a Grant-in-Aid for Strategic Medical Science Research Centre from the Ministry of Education, Culture, Sports, Science and Technology of Japan, 2010–2014.
Glossary
Abbreviations
- S-PRG
surface pre-reacted glass-ionomer
- MSCs
mesenchymal stem cells
- hMSCs
human mesenchymal stem cells
- PLGA
poly-(lactide-co-glycolide-acid)
- ECM
extracellular matrix
- ALP
alkaline phosphatase
- TGF
transforming growth factor
- hTERT
human telomerase reverse transcriptase
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- ICP
inductively coupled plasma
- OBs
osteoblasts
References
- 1.Hervás-García A, Martínez-Lozano MA, Cabanes-Vila J, Barjau-Escribano A, Fos-Galve P. Composite resins. A review of the materials and clinical indications. Med Oral Pathol Oral Cir Bucal. 2006;11:E215–E220. (In English, Spanish) [PubMed] [Google Scholar]
- 2.Labella R, Lambrechts P, Van Meerbeek B, Vanherle G. Polymerization shrinkage and elasticity of flowable composites and filled adhesives. Dent Mater. 1999;15:128–137. doi: 10.1016/S0109-5641(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 3.Faltermeier A, Rosentritt M, Reicheneder C, Müssig D. Experimental composite brackets: Influence of filler level on the mechanical properties. Am J Orthod Dentofacial Orthop. 2006;130:699. e9–e14. doi: 10.1016/j.ajodo.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Ikemura K, Tay FR, Endo T, Pashley DH. A review of chemical-approach and ultramorphological studies on the development of fluoride-releasing dental adhesives comprising new pre-reacted glass ionomer (PRG) fillers. Dent Mater J. 2008;27:315–339. doi: 10.4012/dmj.27.315. [DOI] [PubMed] [Google Scholar]
- 5.Ito S, IIjima M, Hashimoto M, Tsukamoto N, Mizoguchi I, Saito T. Effects of surface pre-reacted glass-ionomerfillers on mineral induction by phosphoprotein. J Dent. 2011;39:72–79. doi: 10.1016/j.jdent.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Iijima Y, Koulourides T. Fluoride incorporation into and retention in remineralized enamel. J Dent Res. 1989;68:1289–1292. doi: 10.1177/00220345890680081501. [DOI] [PubMed] [Google Scholar]
- 7.Siiya T, Tomiyama K, Iizuka J, Hasegawa H, Kuramochi E, Fujino F, Ohashi K, Nihei T, Teranaka T, Mukai Y. Effect of the coating material on root dentin remineralization in vitro. Am J Dent. 2014;27:258–262. [PubMed] [Google Scholar]
- 8.Movahedi Najafabadi BA, Abnosi MH. Boron induces early matrix mineralization via calcium deposition and elevation of alkaline phosphatase activity in differentiated rat bone marrow mesenchymal stem cells. Cell J. 2016;18:62–73. doi: 10.22074/cellj.2016.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taşlı PN, Doğan A, Demirci S, Şahin F. Boron enhances odontogenic and osteogenic differentiation of human tooth germ stem cells (hTGSC) in vitro. Biol Trace Elem Res. 2013;153:419–427. doi: 10.1007/s12011-013-9657-0. [DOI] [PubMed] [Google Scholar]
- 10.Doğan A, Demirci S, Bayir Y, Halici Z, Karakus E, Aydin A, Cadirci E, Albayrak A, Dermici E, Karaman A, et al. Boron containing poly-(lactide-co-glycolide-acid) (PLGA) scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2014;44:246–253. doi: 10.1016/j.msec.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 11.Reffitt DM, Ogston N, Jugdaohsingh R, Cheung HF, Evans BA, Thompson RP, Powell JJ, Hampson GN. Orthosilicic acid stimulates collagen type I synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003;32:127–135. doi: 10.1016/S8756-3282(02)00950-X. [DOI] [PubMed] [Google Scholar]
- 12.Müller WE, Tolba E, Schröder HC, Diehl-Seifert B, Link T, Wang X. Biosilica-loaded poly (ε-caprolactone) nanofibers mats provide a morphogenetically active surface scaffold for the growth and mineralization of the osteoclast-related SaOS-2 cells. Biotechnol J. 2014;9:1312–1321. doi: 10.1002/biot.201400277. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Wang X, Draenert FG, Albert O, Schröder HC, Mailänder V, Mitov G, Müller WE. Bioactive and biodegradable silica biomaterial for bone regeneration. Bone. 2014;67:292–304. doi: 10.1016/j.bone.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Ni GX, Yao ZP, Huang GT, Liu WG, Lu WW. The effect of strontium incorporation in hydroxyapatite on osteoblasts in vitro. J Mater Sci Mater Med. 2011;22:961–967. doi: 10.1007/s10856-011-4264-0. [DOI] [PubMed] [Google Scholar]
- 15.Su WT, Chou WL, Chou CM. Osteoblastic differentiation of stem cells from human exfoliated deciduous teeth induced by thermosensitive hydrogels with strontium phosphate. Mater Sci Eng C Mater Biol Appl. 2015;52:46–53. doi: 10.1016/j.msec.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Wang G, Liu Y, Zhao X, Zou D, Zhu C, Jin Y, Huang Q, Sun J, Liu X, et al. The synergistic effect of hierarchical micro/nano-topography and bioactive ions for enhanced osseointegration. Biomaterials. 2013;34:3184–3195. doi: 10.1016/j.biomaterials.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Cao Z, Fu Y, Sun X, Zhang Q, Xu F, Li Y. Aluminum trichloride inhibits osteoblastic differentiation through inactivation of Wnt/β-catenin signaling pathway in rat osteoblasts. Environ Toxicol Pharmacol. 2016;42:198–204. doi: 10.1016/j.etap.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, Cao Z, Zhang Q, Li M, Han L, Li Y. Aluminum trichloride inhibits osteoblast mineralization via TGF-β1/Smad signaling pathway. Chem Biol Interact. 2016;244:9–15. doi: 10.1016/j.cbi.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Cao Z, Liu D, Zhang Q, Sun X, Li Y. Aluminum chloride induces osteoblasts apoptosis via disrupting calcium homeostasis and activating Ca(2+)/CaMKII signal pathway. Biol Trace Elem Res. 2016;169:247–253. doi: 10.1007/s12011-015-0417-1. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Hu C, Zhu Y, Sun H, Li Y, Zhang Z. Effects of aluminum exposure on bone mineral density, mineral and trace elements in rats. Biol Trace Elem Res. 2011;143:378–385. doi: 10.1007/s12011-010-8861-4. [DOI] [PubMed] [Google Scholar]
- 21.Mori T, Kiyono T, Imabayashi H, Takeda Y, Tsuchiya K, Miyoshi S, Makino H, Matsumoto K, Saito H, Ogawa S, et al. Combination of hTERT and bmi-1, E6 and E7 induces prolongation of the life span of bone marrow stromal cells from an elderly donor without affecting their neurogenic potential. Mol Cell Biol. 2005;25:5183–5195. doi: 10.1128/MCB.25.12.5183-5195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimomura T, Yoshida Y, Sakabe T, Ishii K, Gonda K, Murai R, Takubo K, Tsuchiya H, Hoshikawa Y, Kurimasa A, et al. Hepatic differentiation of human bone marrow-derived UE7T-13 cells: Effects of cytokines and CCN family gene expression. Hepatol Res. 2007;37:1068–1079. doi: 10.1111/j.1872-034X.2007.00162.x. [DOI] [PubMed] [Google Scholar]
- 23.Hakki SS, Bozcurt BS, Hakki EE. Boron regulates mineralized tissue-associated proteinsin osteoblasts (MC3T3-E1) J Trace Elem Med Biol. 2010;24:243–250. doi: 10.1016/j.jtemb.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Zou S, Ireland D, Brooks RA, Rushton N, Best S. The effects of silicate ions on human osteoblast adhesion, proliferation and differentiation. J Biomed Mater Res B Appl Biomater. 2009;90:123–130. doi: 10.1002/jbm.b.31262. [DOI] [PubMed] [Google Scholar]
- 25.Varanasi VG, Leong KK, Dominia LM, Jue SM, Loomer PM, Marshall GW. Si and Ca individually and combinatorially target enhanced MC3T3-E1 subclone 4 early osteogenic marker expression. J Oral Implantol. 2012;38:325–336. doi: 10.1563/AAID-JOI-D-11-00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao X, Liu X, Zhang Y, Cui Y, Yao J, Hu M. Strontium promotes cementoblasts differentiation through inhibiting sclerostin expression in vitro. Biomed Res Int. 2014;2014:487535. doi: 10.1155/2014/487535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakade O, Koyama H, Arai J, Ariji H, Takada J, Kaku T. Stimulation by low concentrations of fluoride of the proliferation and alkaline phosphatase activity of human dental pulp cells in vivo. Arch Oral Biol. 1999;44:89–92. doi: 10.1016/S0003-9969(98)00099-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Xu F, Yan X, Miao L, Li H, Hu C, Wang Z, Lian S, Feng Z, Li Y. The suppressive effects of aluminium chloride on the osteoblasts function. Environ Toxicol Pharmacol. 2016;48:125–129. doi: 10.1016/j.etap.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Ji YM, Jeon SH, Park JY, Chung JH, Choung YH, Choung PH. Dental stem cell therapy with calcium hydroxide in dental pulp capping. Tissue Eng Part A. 2010;16:1823–1833. doi: 10.1089/ten.tea.2009.0054. [DOI] [PubMed] [Google Scholar]
- 30.Hu L, Liu Y, Wang S. Stem cell-based tooth and periodontal regeneration. Oral Dis. 2017 doi: 10.1111/odi.12703. [DOI] [PubMed] [Google Scholar]




