Abstract
The present study aimed to classify gastric cancer (GC) into subtypes and to screen the subtype-specific genes, their targeted microRNAs (miRNAs) and enriched pathways to explore the putative mechanism of each GC subtypes. The GSE13861 data set was downloaded from the Gene Expression Omnibus and used to screen differential expression genes (DEGs) in GC samples based on the detection of imbalanced differential signal algorithm. The specific genes in each subtype were identified with the cut-off criterion of U>0.04, pathway enrichment analysis was performed and the subtype-specific subpaths of miRNA-target pathway were determined. A total of 1,263 DEGs were identified in the primary gastric adenocarcinoma (PGD) samples, which were subsequently divided into four subtypes, according to the hierarchy cluster analysis. Identification of the subpaths of each subtype indicated that the subpath related to subtype 1 was miRNA (miR)-202/calcium voltage-gated channel subunit α1 (CACNA1E)/type II diabetes mellitus. The nuclear factor-κB signaling pathway was the most significantly specific pathway and subpath identified for subtype 2, which was regulated by miR-338-targeted suppression of C-C motif chemokine ligand 21 (CCL21). For subtype 3, significant related pathways included ubiquitin-mediated proteolysis and proteasome, and the important subpath was miR-146B/proteasome 26S subunit, non-ATPase 3 (PSMD3)/proteasome; focal adhesion was the significant pathway indicated for subtype 4, and the subpaths were miR-34A/vinculin (VCL)/focal adhesion and miR-34C/VCL/focal adhesion. In addition, Helicobacter pylori infection was higher in GC subtype 1 than in other subtypes. Specific genes, such as CACNA1E, CCL21, PSMD3 and VCL, may be used as potential feature genes to identify different subtypes of GC, and their associated subpaths may partially explain the pathogenetic mechanism of each GC subtype.
Keywords: gastric cancer, differentially expressed gene, specific gene, microRNA, subpath
Introduction
Gastric cancer (GC) is a common malignant neoplasm that is derived from gastric epithelial dysplasia and intestinal metaplasia (1); GC is the third leading cause of malignant neoplasm-related mortalities worldwide, with ~989,600 new cases and ~738,000 mortalities in 2008 (2). GC has high heterogeneity with histopathologic and epidemiologic characteristics (3), and can be divided into several classifications, including proximal nondiffuse, diffuse and distal nondiffuse GC (4). A previous study identified DNA content heterogeneity in 12 (33%) patients with primary GC that were examined (5); however, DNA content heterogeneity was independent of histological heterogeneity. The incidence and mortality rates of GC are declining worldwide, owing to the notable progress made in diagnosis, prevention and treatment; however, as the rate of relapse is high and we do not completely understand the pathogenesis, additional long-term studies are required if GC is to be cured.
A number of previous studies have attempted to identify new potential therapeutic targets of GC. For example, the upregulated expression of the transcription factor hepatocyte nuclear factor 4α by AMP-activated protein kinase signaling is a main event in GC development (6). Vestigial-like family member 4 (VGLL4) was reported to be a promising therapeutic target for GC inhibition, as VGLL4 competes with yes-associated protein (YAP) for binding with TEA domain transcription factor 1, and YAP is involved in overgrowth and tumor formation of multiple cancers (7). microRNA (miRNA) miR-329 was also previously revealed to reduce the expression of T-lymphoma invasion and metastasis-inducing 1, and may be a potential therapeutic target for suppression of GC cell invasion and proliferation (8). In addition, miR-7 expression was reported to be significantly reduced in highly metastatic GC cells, and insulin-like growth factor-1 receptor (IGF1R) oncogene overexpression, as a direct target of miR-7, may attenuate the function of miR-7 in GC cells (9); thus, miR-7/IGF1R may be a therapeutic approach to inhibit GC metastasis. Furthermore, several signaling pathways have been revealed to be associated with GC. For example, the inactivation of transforming growth factor-β and hedgehog signaling pathways have been reported as useful therapeutic pathways to prevent GC progression, by inhibiting the migration and invasion of GC cells (10,11). However, these previous reports did not identify the GC subtypes of the patients in their study and, thus, the subtype-specific subpaths of miRNAs, their targeted genes and related pathways remain unknown.
The present study reanalyzed the data set GSE13861 that was published by Cho et al (12). That study generated and analyzed microarray data from 65 patients with GC to identify feature genes related to relapse and subsequently predicted the relapse of patients who received gastrectomy. Conversely, the present study aimed to screen specific genes and to use those genes to divide the patients into different subtypes; as well as to identify the subtype-specific subpaths of miRNA-target pathway for comprehensive understanding the mechanisms of GC through bioinformatical prediction methods.
Materials and methods
Data access and data preprocessing
The microarray raw data were downloaded from Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo; accession number GSE13861) database, which were based on the Illumina HumanWG-6 v3.0 Expression Beadchip platform. A total of 90 samples were obtained, comprising 65 samples from primary gastric adenocarcinoma (PGD) tissues, 6 samples from gastrointestinal stromal tumor (GIST) tissues and 19 samples from normal gastric tissues. The probes were transformed to corresponding gene symbols and merged according to the application programing of Python. Mean expression values of the same gene were obtained and all expression values were revised using Z-score (13).
Differentially expressed genes (DEGs) analysis
Owing to high heterogeneity, the changes of expression in some important genes that may induce GC only occur in heterogeneous populations. Thus, to capture those important genes within a group, a new method, detection of imbalanced differential signal (DIDS), was adopted to identify subgroup DEGs in heterogeneous populations (14). Based on the DIDS algorithm, the normal reference interval of each gene expression value was stipulated between the maximum and minimum value, and they were respectively calculated as the corresponding mean values in the normal group ±1.96 × standard deviation. Subsequently, random disturbance was conducted and multiple testing adjustments were performed by Benjamini-Hochberg method, which revised the raw P-value into the false discovery rate (FDR) (15). FDR <0.01 was used as the cut-off criterion to filter DEGs.
Hierarchical clustering
Cluster and TreeView are programs that offer computational and graphical analyses of the results from DNA microarray data (16). In the present study, hierarchical clustering analysis was performed among the 90 PGD samples, and the processing of expression profile data, including filtering the data and data normalization, were conducted by Cluster software (17–19). Based on the clusters of genes similarly expressed, the results of hierarchical clustering were used to identify the different GC subtypes and were displayed as a heatmap (Version 1.2.0; http://www.bioconductor.org/packages/release/bioc/html/heatmaps.html).
Identification of specific genes in each subtype
Following identification of the subtypes of GC that were based on hierarchical clustering analysis, the specific gene expressions in each subtype was examined. First, the mean expression values of genes were distributed in each subtype. Second, to estimate whether an identified DEG was a specific gene for a certain subtype, the following formulas were used:
For each gene, score represented the deviation from normal range, and score >0 indicated that the DEG was upregulated in the PGD samples, and score <0 indicated that the DEG was downregulated in the PGD samples. The U distribution of genes related to GC is provided in Fig. 1. Specific genes were identified from the DEGs with the cut-off criterion of U >0.04, otherwise the DEG was considered as common gene. For example, one gene was indicated as ‘g’ and the mean expression value of this gene in GC subtypes was indicated as ‘X1’, ‘X2’… ‘Xi’ and ‘Xm’. ‘Max’ represented the maximum mean expression values in those GC subtypes, whereas ‘min’ represented the minimum mean expression values among those GC subtypes. ‘Xi’ represented the mean expression values of one gene in subtype i, and it was evaluated if this gene was specific to subtype i with the aforementioned formulas. If Xi>max-γ × U, the gene was specific to subtype i. Where γ is the threshold value, and γ=1/m, in which m represents the number of GC subtypes.
Figure 1.
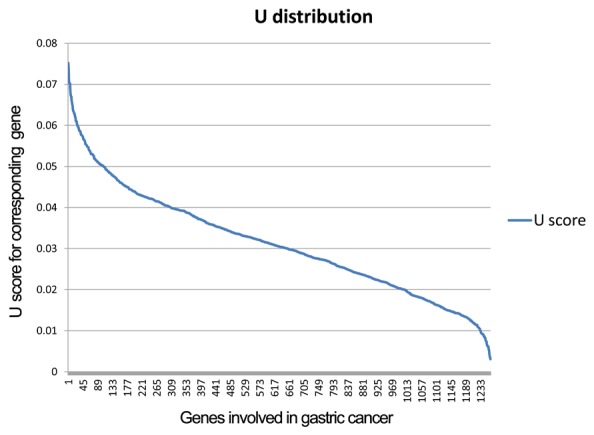
U distribution of gastric cancer-related genes. The horizontal axis represents the gastric cancer related genes, and the vertical axis shows the U value of the corresponding gene. Thu blue curve is the U distribution of all the genes.
Pathway enrichment analysis
The Molecular Signatures Database (MSigDB; http://software.broadinstitute.org/gsea/msigdb/index.jsp) is a collection of annotated gene sets used to perform gene set enrichment analysis (20). A total of 186 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and their related gene sets data from MSigDB were downloaded. By combing the pathway data, specific genes were identified in PGD samples, and pathway enrichment analysis was performed on specific genes of each subtype using Fisher's exact test. Significant pathway terms were selected with the threshold of P<0.05.
Identification of subtype-specific subpaths of miRNA-target pathway
Significant drugs to diseases were predicted using causal inference as previously described (21); this method was used to construct CauseNet for the identification of subtype-specific subpath of miRNA-target pathways. A layered network from miRNAs to specific pathways is presented in Fig. 2. Relationships between miRNAs, their targets genes, specific genes, target-related pathways and specific KEGG pathways were calculated. If a miRNA regulated several specific genes that were enriched in several significant KEGG pathways, those subpaths of miRNA-target pathway may be important subpaths for explaining the development of different subtypes of GC.
Figure 2.
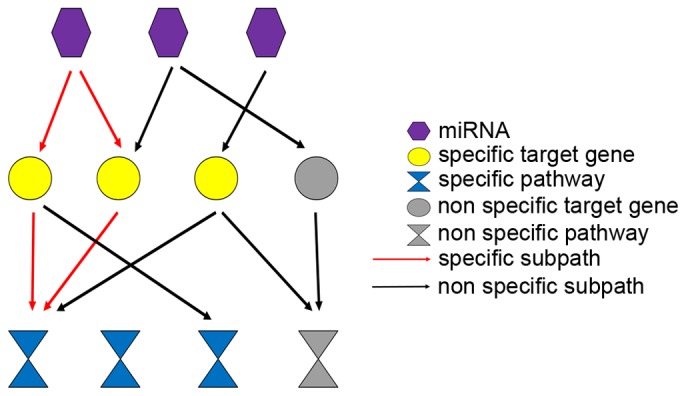
The network model for identifying the subtype-specific subpath of miRNA-target pathway in each subtype.
To identify those important subpaths, the following algorithms were used:
Where weight1 is the weight of miRNA of each subpath, P|G*| is the whole number of specific genes and P|G| is the number of specific genes regulated by the miRNA. Weight2 represents the weight of a target gene, in which P|G*| is the total KEGG pathways number in which all targets participated and P|G| is the KEGG pathways number that was this target participated. Weight3 is the weight of a pathway, in which P|G*| is total gene number enriched in this pathway and P|G| represents the number of specific genes. In addition, the scores of all the subpaths in each subtype were repeatedly calculated following the course of 1,000 times random disturbance, and the subpath with the max score in a certain subtype was chosen as the specific subpath of this subtype with the cut-off criteria of P<0.05. Furthermore, subpath analysis among the specific genes was conducted to identify the subtype-specific regulation relationship of miRNA-target pathway.
Helicobacter pylori infection rate in each GC subtype
H. pylori infection is a known risk factor for GC progression (22); however, whether H. pylori infection is a subtype-specific pathway for our predicted GC subtype is unknown. Thus, a series of bioinformatics methods and clinic information of GC samples with H. pylori infection were combined to calculate the H. pylori rate in each of the predicted GC subtypes. The identified specific genes in each subtype were used as characters to build a neural network (NN) model using the neuralnet package in R (Version 1.5.0; https://cran.r-project.org/web/packages/NeuralNetTools/index.html). The input layer was 24 neurons (also designated 24 gene feature) and the output layer was 1 neuron, which was used to decide which subtype a certain neuron belonged. The hidden layer was set as two layers that included eight and five neurons, respectively. Sigmoid neural activation function was adopted for feed-forward neural network and backward propagation was used for weight optimization. The maximum number of iterations to convergence to its stationary distribution. was 1,000. In addition, logistic regression (LR) model was performed to compare with NN model. Through building a NN model and training the NN with analysis data, the prediction for the four GC subtypes may be achieved. Following forecast classification of independent test data in The Cancer Genome Atlas (TCGA; https://cancergenome.nih.gov/), four testing-set subtypes were obtained. Subsequently, 100 GC samples (including 46 H. pylori infection samples and 54 without H. pylori infection samples) were downloaded from the PMID:24816253 data set (23). According to the clinical information regarding H. pylori infection rate in TCGA and the distribution of H. pylori infection samples in the four subtypes, the H. pylori infection rate in each subtype was calculated.
Results
DEG screening and hierarchical clustering
Based on the aforementioned criteria, a total of 1,263 DEGs that were related to GC were identified, including 392 downregulated genes and 871 upregulated genes in the PGD samples. Additionally, hierarchy cluster analysis indicated that the 1,263 DEGs could be used to divide the 65 PGD samples into four subtypes with correlated expression profiles. The four subtypes of GC were: i) Subtype 1 in blue with 11 samples; ii) subtype 2 in red with 29 samples; iii) subtype 3 in pink with 13 samples; and iv) subtype 4 in purple with 12 samples. Although three of the normal samples were wrongly identified as subtype 1, the other PGD, GIST and normal samples were placed among different clusters and were classified correctly. In addition, the results indicated that there was no heterogeneity of gene expression within subtypes, but there was high heterogeneity between different subtypes (Fig. 3).
Figure 3.
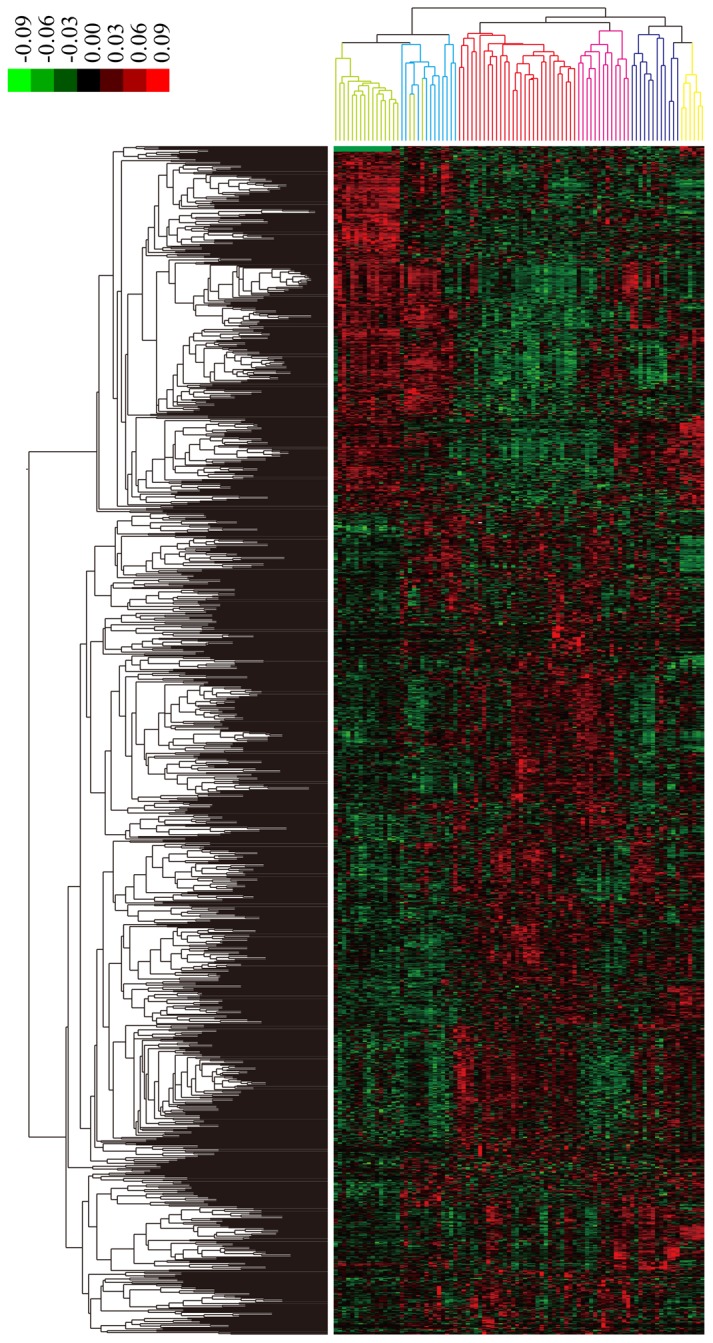
Hierarchical cluster map of DEGs. The horizontal axis indicates the different sample types by color: Gastric cancer subtype 1, blue; subtype 2, red; subtype 3, pink; subtype 4, purple; normal, light green; gastrointestinal stromal tumors, yellow. The right vertical axis shows clusters of DEGs. Red represents higher expression values and green represents lower expression values. DEGs, differentially expressed genes.
Identification of specific genes in each subtype
According to the formulas described in the Methods section, specific genes of the four subtypes and common genes were identified. A total of 33 specific genes were identified in subtype 1, 318 in subtype 2, 161 in subtype 3 and 157 in subtype 4. In addition, a total of 631 common genes were detected, which were significantly different between the GC group and normal group, but exhibited no notable difference within the four subtypes.
KEGG pathway enrichment analysis
To explore the significant differences among the four GC subtypes at the molecular level, four subtype-specific pathway analyses were conducted. Five specific pathways, such as renin-angiotensin system and H. pylori infection, were associated with GC subtype 1 (Table I); two specific pathways were identified in subtype 2, including nuclear factor (NF)-κB signaling pathway and tight junction. The specific genes related to GC subtype 3 were enriched in six specific pathways that were mainly associated with metabolic process, such as fatty acid metabolism, proteasome and ubiquitin-mediated proteolysis; the data indicated that carbohydrate metabolism may serve an important role in the progression of GC subtype 3. The specific genes of GC subtype 4 were enriched in 14 specific pathways, including phosphoinositide 3 kinase/Akt signaling pathway, focal adhesion, vascular smooth muscle contraction and cardiac muscle contraction.
Table I.
Subtype-specific pathways related to gastric cancer and common pathways of all subtypes.
| Subtype | KEGG pathway | Counta | Allb | P-valuec |
|---|---|---|---|---|
| Subtype 1 | Renin-angiotensin system | 3 | 17 | 0.007398 |
| Folate biosynthesis | 2 | 10 | 0.014313 | |
| Type II diabetes mellitus | 1 | 9 | 0.01947 | |
| Hedgehog signaling pathway | 2 | 13 | 0.024601 | |
| Helicobacter pylori infection | 1 | 8 | 0.03013 | |
| Subtype 2 | NF-κB signaling pathway | 6 | 9 | 0.01016 |
| Tight junction | 4 | 5 | 0.015905 | |
| Subtype 3 | Fatty acid metabolism | 2 | 3 | 0.044476 |
| Ribosome biogenesis in eukaryotes | 4 | 7 | 0.006553 | |
| Proteasome | 5 | 10 | 0.004685 | |
| Nucleotide excision repair | 3 | 7 | 0.048337 | |
| Cell cycle | 4 | 11 | 0.040908 | |
| Ubiquitin mediated proteolysis | 6 | 11 | 0.001051 | |
| Subtype 4 | PI3K/Akt signaling pathway | 9 | 22 | 0.000675 |
| Vascular smooth muscle contraction | 5 | 10 | 0.004185 | |
| Alzheimer's disease | 4 | 7 | 0.00597 | |
| Focal adhesion | 5 | 11 | 0.006911 | |
| Cardiac muscle contraction | 3 | 5 | 0.015628 | |
| Pertussis | 3 | 5 | 0.015628 | |
| Hypertrophic cardiomyopathy | 4 | 10 | 0.026485 | |
| Dilated cardiomyopathy | 4 | 10 | 0.026485 | |
| Long-term depression | 3 | 6 | 0.028424 | |
| Porphyrin and chlorophyll metabolism | 2 | 3 | 0.042385 | |
| Salmonella infection | 2 | 3 | 0.042385 | |
| Glioma | 2 | 3 | 0.042385 | |
| Dopaminergic synapse | 3 | 7 | 0.045265 | |
| Melanoma | 3 | 7 | 0.045265 |
Number of specific genes enriched in the corresponding pathways.
Total number of differentially expressed genes.
Significance level determined by Fisher's exact test. KEGG, Kyoto Encyclopedia of Genes and Genomes; PI3K, phosphoinositide 3 kinase.
Identification of subtype-specific subpath of miRNA-target pathway
According to the aforementioned Methods and criteria, specific subpaths of each subtype were identified. Four or five specific subpaths were identified for each subtype (Table II). In subtype 1, ARF GTPase-activating protein GIT1 was indicated to be regulated by miR-199B, miR-122A and miR-199A through the H. pylori infection pathway, and calcium voltage-gated channel subunit α1 E (CACNA1E) was indicated as regulated by miR-202 through the type II diabetes mellitus pathway. For subtype 2, protein inhibitor of activated STAT 4 may be regulated by miR-198, and C-C motif chemokine ligand 21 (CCL21) may be regulated by miR-338 and miR-370 by participating in NF-κB signaling pathway; in addition, miR-508 may regulate VAMP-associated protein A through tight junction pathway. In GC subtype 3, miR-146B and miR-146A were indicated to regulate proteasome 26S subunit, non-ATPase 3 (PSMD3) through the proteasome pathway. Five important subpaths of subtype 3 were identified, including miR-429 and miR-205 regulation of LDL receptor-related 1 through the Salmonella infection pathway, and miR-34A, miR-34C and miR-449 regulation of vinculin (VCL) through the focal adhesion pathway.
Table II.
Subtype-specific subpaths of gastric cancer.
| Subtype | miRNA | Pathway | Targeta | Score | P-value |
|---|---|---|---|---|---|
| Subtype 1 | miR-199B | Helicobacter pylori infection | GIT1 | 1.256062 | 0.0307 |
| miR-122A | Helicobacter pylori infection | GIT1 | 1.256062 | 0.0314 | |
| miR-199A | Helicobacter pylori infection | GIT1 | 1.256062 | 0.0317 | |
| miR-202 | Type II diabetes mellitus | CACNA1E | 0.610109 | 0.0356 | |
| Subtype 2 | miR-198 | NF-κB signaling pathway | PIAS4 | 1.156533 | 0.0181 |
| miR-338 | NF-κB signaling pathway | CCL21 | 1.170037 | 0.0195 | |
| miR-370 | NF-κB signaling pathway | CCL21 | 1.16555 | 0.0211 | |
| miR-508 | Tight junction | VAPA | 1.857042 | 0.0372 | |
| Subtype 3 | miR-146B | Proteasome | PSMD3 | 1.187736 | 0.008 |
| miR-524 | Nucleotide excision repair | ERCC8 | 1.532384 | 0.009 | |
| miR-146A | Proteasome | PSMD3 | 1.187736 | 0.011 | |
| miR-193A | Fatty acid metabolism | ACACA | 2.006123 | 0.049 | |
| Subtype 4 | miR-429 | Salmonella infection | LRP1 and CACNA1C | 2.278013 | 0.022 |
| miR-34A | Focal adhesion | VCL | 0.760521 | 0.029 | |
| miR-205 | Salmonella infection | LRP1 | 1.085376 | 0.031 | |
| miR-34C | Focal adhesion | VCL | 0.760521 | 0.032 | |
| miR-449 | Focal adhesion | VCL | 0.760521 | 0.041 |
Specific genes in the corresponding subtype. ACACA, acetyl-CoA carboxylase α; CACNA1, calcium voltage-gated channel subunit α1; CCL21, C-C motif chemokine ligand 21; ERCC8, ERCC excision repair 8, CSA ubiquitin ligase complex subunit; GIT1, ARF GTPase-activating protein GIT1; LRP1, LDL receptor-related 1; NF-κB, nuclear factor-κB; PIAS4, protein inhibitor of activated STAT 4; PSMD3, proteasome 26S subunit, non-ATPase 3; VAPA, VAMP-associated protein A; VCL, vinculin.
H. pylori infection rate in each GC subtype
H. pylori infection rate in each GC subtype was analyzed as aforementioned. The NN model was a more accurate method to distinguish the four GC subtypes compared with the LR model (Fig. 4A and B, respectively); the NN model was therefore used to predict the GC subtypes for all samples (Table III), and all the GC samples were divided into the four testing-set. Subsequently, the four testing-set was used to predict the subtype of the 100 GC samples in the PMID:24816253 data set. Notably, the H. pylori infection rate in subtype 1 was higher than in other subtypes (Table IV), indicating that there was an increased susceptibility to H. pylori infection in subtype 1 compared with other subtypes. This outcome was consistent with the aforementioned analysis, which indicated that H. pylori infection may be a specific pathway for GC subtype 1.
Figure 4.
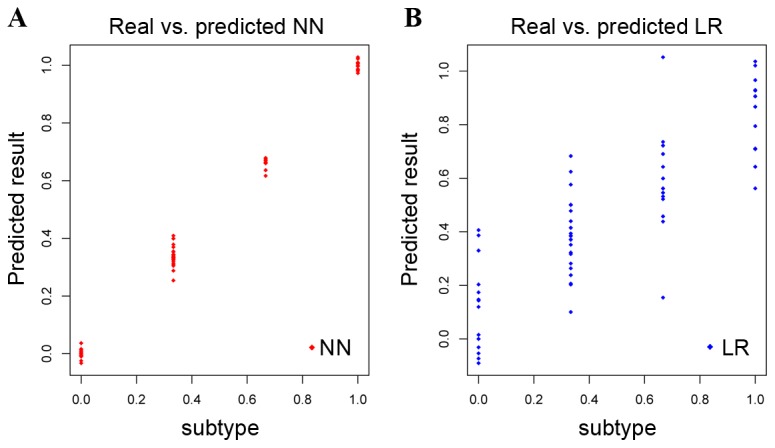
Results of test training set. (A) The predicted results of the GC subtypes by using the NN model. (B) The predicted results of the GC subtypes by using LR model. The × axis represents real category labels, with the values of the four GC subtypes determined as 0, 0.33, 0.66 and 1, respectively. The y axis represents predicted category labels. GC, gastric cancer; NN, neural network; LR, logistic regression.
Table III.
Predicting gastric cancer subtypes using the neural network model.
| Predicted | ||||
|---|---|---|---|---|
| Type | Subtype 1 | Subtype 2 | Subtype 3 | Subtype 4 |
| Observed | ||||
| Subtype 1 | 11 | 1 | 1 | 1 |
| Subtype 2 | 0 | 25 | 0 | 2 |
| Subtype 3 | 1 | 3 | 9 | 0 |
| Subtype 4 | 1 | 0 | 0 | 13 |
Table IV.
Helicobacter pylori infection rate of four gastric cancer subtypes.
| Subtype | Infection ratio | n |
|---|---|---|
| Subtype 1 | 0.67 | 24 |
| Subtype 2 | 0.34 | 29 |
| Subtype 3 | 0.58 | 19 |
| Subtype 4 | 0.32 | 28 |
Discussion
In the present study, a total of 1,263 DEGs in the 65 PGD samples were identified, which allowed the samples to be divided into four subtypes based on hierarchy cluster analysis. In addition, a total of 33 specific genes were screened in subtype 1, 318 in subtype 2, 161 in subtype 3 and 157 in subtype 4. The subpaths miR-202/CACNA1E/type II diabetes mellitus, miR-338/CCL21/NF-κB signaling, miR-146B/PSMD3/proteasome, miR-34A/VCL/focal adhesion and miR-34C/VCL/focal adhesion were identified more than once and therefore may be important specific subpaths of the four GC subtypes, respectively.
That H. pylori infection may serve a role in the progression of GC is widely accepted (24). Notably, results from the present study demonstrated that several specific genes of subtype 1 were significantly enriched in H. pylori infection pathway and that the H. pylori infection rate in GC subtype 1 was higher than in other subtypes. Therefore, the present study hypothesized that H. pylori infection was a specific pathway for GC subtype 1.
CACNA1E encodes a Cav2.3 R-type voltage-activated Ca2+ channel that is involved in gene expression regulation, cell differentiation and cell death (25). In addition, CACNA1E has been reported to be upregulated in air pollution-associated lung cancer (26), and the abnormal expression of CACNA1E may be used to predict the occurrence of cancers (27). Results from the present study revealed that CACNA1E may be a specific gene of GC subtype 1, and miR-202/CACNA1E/type II diabetes mellitus was predicted to be an important subpath of subtype 1. In addition, the downregulated expression of miR-202 may suppress GC cell proliferation (28). Furthermore, CACNA1E expression may increase the risk of the type 2 diabetes, and there is close correlation between the metabolic syndrome and the development of gastric adenocarcinoma (29,30). Therefore, it was inferred that CACNA1E, as a target of miR-202, may be related to GC subtype 1 by participating in the type II diabetes mellitus related metabolic pathway.
For GC subtype 2, the results indicated that miR-338-CCL21-NF-κB signaling was one of the important subpaths. CCL21 encodes a C-C chemokine that is mainly presented in lymphoid tissue and serves an important role in dendritic cell recruitment and lymphoid neogenesis (31). In addition, NF-κB signaling is a major link between cancer and inflammation, which is triggered by proinflammatory cytokines such as CCL21 (32,33); several previous studies have indicated that the activation of NF-κB signaling is related to GC oncogenesis (34–36). In addition, miR-338 was highly associated with GC through the inhibition the GC cell proliferation (37), which is similar with the present data. These results suggested that miR-338 may promote apoptosis of GC subtype 2 cells by activating the NF-κB signaling pathway through targeting CCL21.
Pathway enrichment analysis of the specific genes in subtype 3 demonstrated that most of the identified pathways were related to carbohydrate metabolism, such as fatty acid metabolism, ribosome biogenesis, ubiquitin-mediated proteolysis and proteasome. Proteasome is protein complex which degrades unneeded or damaged proteins by proteolysis and mediates protein folding. In addition, PSMD3 was identified as a proteasome-pathway related gene that may be regulated by miR-146A. Previous studies reported that PSMD3 was highly related to the progression of breast cancer and lung cancer (38,39). In addition, it has been indicated that miR-146A serves a key function in GC development by suppressing proliferation of GC cells (40,41). Therefore, the present study hypothesized that miR-146A may be related to GC subtype 3 by targeting PSMD3.
VCL encodes a cytoskeletal protein that contributes to the function of cell-cell and cell-matrix junctions, and is predicted to be associated with GC (42). This was consistent with the present results, which demonstrated that VCL was a specific gene for GC subtype 4. In addition, it has been reported that VCL may be a potential biomarker in many cancers, including GC, pancreatic cancer and colorectal cancer, as the downregulated expression of VCL may promote metastasis and tumor progression (43–45). In addition, the miR-34 family/yin yang 1 axis was reported to serve a crucial role in gastric carcinogenesis (46). Therefore, miR-34A and miR-34C may depend on VCL to inhibit the spreading of GC subtype 4 cells by improving focal adhesion.
In summary, GC was divided into four subtypes based on the identified 1,263 DEGs in the PGD samples. Additionally, specific genes such as CACNA1E, CCL21, PSMD3 and VCL may be used as potential feature genes to identify different types of GC. It was concluded that the subtype-specific subpaths such as miR-202/CACNA1E/type II diabetes mellitus, miR-338/CCL21/NF-κB signaling, miR-146B/PSMD3/proteasome and miR-34A/VCL/focal adhesion and miR-34C/VCL/focal adhesion may serve crucial roles in the development of GC subtypes. Furthermore, the present study speculated that H. pylori infection was a specific pathway for GC subtype 1. However, further experimentation is required to confirm these predicted outcomes.
References
- 1.Nogueira A, Cabral M, Salles P, Araujo L, Rodrigues L, Rodrigues M, Oliveira C, Queiroz D, Rocha G, Oliveira A. Role of intestinal metaplasia and epithelial dysplasia in the pathogenesis of gastric carcinoma. Gastroenterology. 2000;118:A1404. doi: 10.1016/S0016-5085(00)81498-5. [DOI] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Massarrat S, Stolte M. Development of gastric cancer and its prevention. Arch Iran Med. 2014;17:514–520. [PubMed] [Google Scholar]
- 4.Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, Kelsen DP. Molecular classification of gastric cancer: A new paradigm. Clin Cancer Res. 2011;17:2693–2701. doi: 10.1158/1078-0432.CCR-10-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Aretxabala X, Yonemura Y, Sugiyama K, Hirose N, Kumaki T, Fushida S, Miwa K, Miyazaki I. Gastric cancer heterogeneity. Cancer. 1989;63:791–798. doi: 10.1002/1097-0142(19890215)63:4<791::AID-CNCR2820630431>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Chang HR, Nam S, Kook MC, Kim KT, Liu X, Yao H, Jung HR, Lemos R, Jr, Seo HH, Park HS, et al. HNF4α is a therapeutic target that links AMPK to WNT signalling in early-stage gastric cancer. Gut. 2016;65:19–32. doi: 10.1136/gutjnl-2014-307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, Wang Y, Zhang Z, Wang W, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Yu X, Wang Y, Shen J, Wu WK, Liang J, Feng F. By downregulating TIAM1 expression, microRNA-329 suppresses gastric cancer invasion and growth. Oncotarget. 2015;6:17559–17569. doi: 10.18632/oncotarget.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao X, Dou W, He L, Liang S, Tie J, Liu C, Li T, Lu Y, Mo P, Shi Y, et al. MicroRNA-7 functions as an anti-metastatic microRNA in gastric cancer by targeting insulin-like growth factor-1 receptor. Oncogene. 2013;32:1363–1372. doi: 10.1038/onc.2012.156. [DOI] [PubMed] [Google Scholar]
- 10.Chen F, Zhuang M, Peng J, Wang X, Huang T, Li S, Lin M, Lin H, Xu Y, Li J, et al. Baicalein inhibits migration and invasion of gastric cancer cells through suppression of the TGF-β signaling pathway. Mol Med Rep. 2014;10:1999–2003. doi: 10.3892/mmr.2014.2452. [DOI] [PubMed] [Google Scholar]
- 11.Yanai K, Nagai S, Wada J, Yamanaka N, Nakamura M, Torata N, Noshiro H, Tsuneyoshi M, Tanaka M, Katano M. Hedgehog signaling pathway is a possible therapeutic target for gastric cancer. J Surg Oncol. 2007;95:55–62. doi: 10.1002/jso.20606. [DOI] [PubMed] [Google Scholar]
- 12.Cho JY, Lim JY, Cheong JH, Park YY, Yoon SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN, et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011;17:1850–1857. doi: 10.1158/1078-0432.CCR-10-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz-Romero J, Romeo S, Bovée JV, Hogendoorn PC, Heini PF, Mainil-Varlet P. Hierarchical clustering of flow cytometry data for the study of conventional central chondrosarcoma. J Cell Physiol. 2010;225:601–611. doi: 10.1002/jcp.22245. [DOI] [PubMed] [Google Scholar]
- 14.De Ronde JJ, Rigaill G, Rottenberg S, Rodenhuis S, Wessels LF. Identifying subgroup markers in heterogeneous populations. Nucleic Acids Res. 2013;41:e200. doi: 10.1093/nar/gkt845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He L, Sarkar SK. On improving some adaptive BH procedures controlling the FDR under dependence. Electron J Stat. 2013;7:2683–2701. doi: 10.1214/13-EJS855. [DOI] [Google Scholar]
- 16.Guttula SV, Allam A, Gumpeny RS. Analyzing microarray data of Alzheimer's using cluster analysis to identify the biomarker genes. Int J Alzheimers Dis. 2012;2012:649456. doi: 10.1155/2012/649456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Pietro C, Di Pietro V, Emmanuele G, Ferro A, Maugeri T, Modica E, Pigola G, Pulvirenti A, Purrello M, Ragusa M, et al. AntiClustal: Multiple sequence alignment by antipole clustering and linear approximate 1-median computation; Proc IEEE Comput Soc Bioinform Conf; 2003; pp. 326–336. [PubMed] [Google Scholar]
- 18.Su X, Song B, Wang X, Ma X, Xu J, Ning K. Meta-Mesh: Metagenomic data analysis system. Sheng Wu Gong Cheng Xue Bao. 2014;30:6–17. (In Chinese) [PubMed] [Google Scholar]
- 19.Yu T, Peng H. Hierarchical clustering of high-throughput expression data based on general dependences. IEEE/ACM Trans Comput Biol Bioinform. 2013;10:1080–1085. doi: 10.1109/TCBB.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberzon A. A description of the molecular signatures database (MSigDB) web site. Methods Mol Biol. 2014;1150:153–160. doi: 10.1007/978-1-4939-0512-6_9. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Lu Z. Pathway-based drug repositioning using causal inference. BMC Bioinformatics. 2013;14(Suppl 16):S3. doi: 10.1186/1471-2105-14-S16-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi IK, Sung HJ, Lee JH, Kim JS, Seo JH. The relationship between Helicobacter pylori infection and the effects of chemotherapy in patients with advanced or metastatic gastric cancer. Cancer Chemother Pharmacol. 2012;70:555–558. doi: 10.1007/s00280-012-1944-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, Siu HC, Deng S, Chu KM, Law S, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 24.Uemura N, Okamoto S, Yamamoto S. H. pylori infection and the development of gastric cancer. Keio J Med. 2002;51(Suppl 2):S63–S68. doi: 10.2302/kjm.51.supplement2_63. [DOI] [PubMed] [Google Scholar]
- 25.Tang ZZ, Liang MC, Lu S, Yu D, Yu CY, Yue DT, Soong TW. Transcript scanning reveals novel and extensive splice variations in human l-type voltage-gated calcium channel, Cav1.2 alpha1 subunit. J Biol Chem. 2004;279:44335–44343. doi: 10.1074/jbc.M407023200. [DOI] [PubMed] [Google Scholar]
- 26.Yu XJ, Yang MJ, Zhou B, Wang GZ, Huang YC, Wu LC, Cheng X, Wen ZS, Huang JY, Zhang YD, et al. Characterization of somatic mutations in air pollution-related lung cancer. EBioMedicine. 2015;2:583–590. doi: 10.1016/j.ebiom.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moler E, Rowe M, Tse C, Xu X, Yu G. CACNA1E in cancer diagnosis detection and treatment. EP 2062591 A1. 2009 [Google Scholar]
- 28.Zhao Y, Li C, Wang M, Su L, Qu Y, Li J, Yu B, Yan M, Yu Y, Liu B, Zhu Z. Decrease of miR-202-3p expression, a novel tumor suppressor, in gastric cancer. PLoS One. 2013;8:e69756. doi: 10.1371/journal.pone.0069756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmkvist J, Tojjar D, Almgren P, Lyssenko V, Lindgren CM, Isomaa B, Tuomi T, Berglund G, Renström E, Groop L. Polymorphisms in the gene encoding the voltage-dependent Ca(2+) channel Ca (V)2.3 (CACNA1E) are associated with type 2 diabetes and impaired insulin secretion. Diabetologia. 2007;50:2467–2475. doi: 10.1007/s00125-007-0846-2. [DOI] [PubMed] [Google Scholar]
- 30.Lindkvist B, Almquist M, Bjørge T, Stocks T, Borena W, Johansen D, Hallmans G, Engeland A, Nagel G, Jonsson H, et al. Prospective cohort study of metabolic risk factors and gastric adenocarcinoma risk in the metabolic syndrome and cancer project (Me-Can) Cancer Causes Control. 2013;24:107–116. doi: 10.1007/s10552-012-0096-6. [DOI] [PubMed] [Google Scholar]
- 31.Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 32.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Leibovich-Rivkin T, Lebel-Haziv Y, Lerrer S, Weitzenfeld P, Ben-Baruch A. The versatile world of inflammatory chemokines in cancer. Springer Neth. 2014:1–175. [Google Scholar]
- 34.Sha M, Ye J, Zhang LX, Luan ZY, Chen YB, Huang JX. Celastrol induces apoptosis of gastric cancer cells by miR-21 inhibiting PI3K/Akt-NF-κB signaling pathway. Pharmacology. 2014;93:39–46. doi: 10.1159/000357683. [DOI] [PubMed] [Google Scholar]
- 35.Xia JT, Chen LZ, Jian WH, Wang KB, Yang YZ, He WL, He YL, Chen D, Li W. MicroRNA-362 induces cell proliferation and apoptosis resistance in gastric cancer by activation of NF-κB signaling. J Transl Med. 2014;12:33. doi: 10.1186/1479-5876-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sha M, Ye J, Zhang L, Luan Z, Chen Y. Celastrol induces apoptosis of gastric cancer cells by miR-146a inhibition of NF-κB activity. Cancer Cell Int. 2013;13:50. doi: 10.1186/1475-2867-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng Y, Liu YM, Li LC, Wang LL, Wu XL. MicroRNA-338 inhibits growth, invasion and metastasis of gastric cancer by targeting NRP1 expression. PLoS One. 2014;9:e94422. doi: 10.1371/journal.pone.0094422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pang Y, Ball GR, Rakha EA, Powe DG, Caldas C, Ellis IO, Green AR. O-37 SOX11 and PSMD3 expression in HER2 positive breast cancer. Eur J Cancer Suppl. 2010;8:1–36. doi: 10.1016/j.ejcsup.2010.06.038. [DOI] [Google Scholar]
- 39.Qian J, Zou Y, Hoeksema M, Harris B, Chen H, Massion P. Identification of FXR1-associated protein complexes in lung cancer. Cancer Res. 2016;76:2873. doi: 10.1158/1538-7445.AM2016-2873. [DOI] [Google Scholar]
- 40.Hou Z, Li X, Yu L, Qian X, Liu B. MicroRNA-146a is down-regulated in gastric cancer and regulates cell proliferation and apoptosis. Med Oncol. 2012;29:886–892. doi: 10.1007/s12032-011-9862-7. [DOI] [PubMed] [Google Scholar]
- 41.Dong HK, Chan JY, Jin KR, Middeldorp JM, Woo JH, Chang MS. Epstein-Barr virus-encoded BARF1 downregulates SMAD4 and increases miR-146a in gastric carcinoma cells. Cancer Res. 2015;75:2716. doi: 10.1158/1538-7445.AM2015-2716. [DOI] [Google Scholar]
- 42.Jin GH, Wei X, Yang S, Wang LB. Celecoxib exhibits an anti-gastric cancer effect by targeting focal adhesion and leukocyte transendothelial migration-associated genes. Oncol Lett. 2016;12:2345–2350. doi: 10.3892/ol.2016.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang CH, Geng JS. Expression of paxillin and vinculin in gastric carcinoma and precancerous lesion and their effects on prognosis of gastric carcinoma. Chin J Diagn Pathol. 2007;14:377–380. [Google Scholar]
- 44.Wang Y, Kuramitsu Y, Ueno T, Suzuki N, Yoshino S, Iizuka N, Zhang X, Akada J, Oka M, Nakamura K. Proteomic differential display identifies upregulated vinculin as a possible biomarker of pancreatic cancer. Oncol Rep. 2012;28:1845–1850. doi: 10.3892/or.2012.2004. [DOI] [PubMed] [Google Scholar]
- 45.Li T, Guo H, Song Y, Zhao X, Shi Y, Lu Y, Hu S, Nie Y, Fan D, Wu K. Loss of vinculin and membrane-bound β-catenin promotes metastasis and predicts poor prognosis in colorectal cancer. Mol Cancer. 2014;13:263. doi: 10.1186/1476-4598-13-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang AM, Huang TT, Hsu KW, Huang KH, Fang WL, Yang MH, Lo SS, Chi CW, Lin JJ, Yeh TS. Yin Yang 1 is a target of miR-34 family and contributes to gastric carcinogenesis. Oncotarget. 2014;5:5002–5016. doi: 10.18632/oncotarget.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]


