Abstract
The chemokine receptor CXCR7 is regarded as a scavenger receptor for CXCL12, and induces numerous key steps in tumor growth and metastasis. However, the exact molecular mechanism of CXCR7 regulation in breast tumor angiogenesis remains unknown. In the present study, the function of CXCR7 in breast tumors was investigated in vitro and in vivo. The breast cancer MDA-MB-231 cell line was used. Pharmacological inhibition of CXCR7 by CCX771 reduced breast tumor invasion, adhesion and metastasis. Furthermore, CXCR7 was essential for the tube formation of HUVECs in vitro, and for blood vessel formation in a Matrigel plug assay in vivo. In addition, vascular endothelial growth factor expression was also decreased in CCX771-treated MDA-MB-231 cells, indicating that CCX771 regulates tumor angiogenesis. The present results indicated that CXCR7 regulated breast cancer metastasis at multiple stages; additional understanding of CXCR7 in tumor environments may develop anti-metastatic therapy.
Keywords: CXC receptor 7, breast cancer, human umbilical vein endothelial cells, angiogenesis
Introduction
Breast cancer is the most rapidly increasing malignancy in females worldwide (1). Due to the rising incidence of breast cancer (2), there is a growing concern about the matter among the general public. The critical challenge in the treatment of patients with breast cancer is tumor recurrence and metastasis following surgery. Therefore, early metastatic risk assessment and treatment is necessary for individualized therapy. Previous evidence has shown that breast tumors are heterogeneous in vitro and in vivo (3–5). Certain subpopulations of breast tumors cells may possess a specific metastatic potential, and exploring this function may provide improved understanding about breast cancer. Metastasis is a key cause for cancer morbidity and mortality (6,7). Metastasis involves numerous cell processes, including proliferation, invasion and angiogenesis in the tumor microenvironment. However, these processes have not yet been fully elucidated.
Chemokines and chemokine receptors perform an important role in tumor cell growth, survival, adhesion and metastasis. Previously, stromal cell-derived factor-1 (CXCL12) and its chemokine receptor CXCR7 were reported to regulate tumor cell function in numerous cancers, including lung and kidney cancer (8–12). CXCL12 is involved in a number of important physiological steps, including vasculogenesis. Notably, CXCL12 regulates proliferation, migration and invasion in numerous tumor cells (13–16). In addition, CXCR7 is overexpressed in malignant cells and vascular endothelium. CXCR7 critically controls the cardiovascular system development in animal models. Decreased CXCR7 expression in zebrafish embryos inhibits blood vessel formation (17), and the knock down of CXCR7 in mice causes early postnatal mortality as a result of myocardial degeneration and heart vessel damage (18). Evidence from a previous study demonstrated that CXCR7 induces tumor growth, invasion and metastasis (19–22). Thus, it is necessary to explore the role of CXCR7 in breast cancer progress.
It has been shown that CXCR7 promotes tumor growth in a mouse model of lung cancer, and that expression of CXCR7 affects experimental lung metastasis (23). In addition, CXCR7 enhances the cell adhesion, invasion and blood vessel sprout formation in vitro, and promotes tumor growth in vivo (24,25). Other studies have revealed that CXCR7 mediated the proliferation and migration of tumor cells towards CXCL12 in vitro (22,26–28). All results proposed that CXCR7 may perform an important role in breast cancer. Although the role of CXCL12 in the tumor is extensively documented and CXCR4 activation signals have been reported, the role of CXCR7 in regulating breast cancer is not known. Thus, it is necessary to investigate the function of CXCR7 in breast cancer development.
In the present study, the effects of CXCR7 in breast cancer invasion, migration and angiogenesis were investigated.
Materials and methods
Cell culture
The MDA-MB-231 cell line was obtained from American Type Culture Collection (Manassas, VA, USA) and grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal bovine serum, 5 U/ml of penicillin and 5 mg/ml of streptomycin. HUVECs (American Type Culture Collection, Manassas, VA, USA) were maintained in endothelial cell growth medium (PromoCell GmbH, Heidelberg, Germany) containing endothelial cell growth supplement, and HUVECs were used at passage 3–5.
Chemokines and reagents
Recombinant CXCL12/SDF-1α was obtained from R&D Systems, Inc. (cat. no. 350-NS; Minneapolis, MN, USA). The CXCR7 antagonist CCX771 was obtained from ChemoCentryx, Inc. (Mountain View, CA, USA). Calcein-AM was purchased from Sigma-Aldrich (cat. no. 148504-34-1; Merck KGaA, Darmstadt, Germany).
Cell invasion assay
The upper surface of a modified Boyden chamber (Corning, Inc., Corning, NY, USA) was pre-treated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). MDA-MB-231 cells were treated with 5 µm CCX771 for 1 h. A total of 2×104 cells were added to the upper Boyden chamber. Serum-free DMEM media (0.5 ml) containing CXCL12 (0–100 ng/ml) was then added to the lower chamber for 24 h. The noninvasive cells were gently removed following incubation. The invasive cells at the bottom of the Matrigel were stained by Calcein-AM. The number of invasive cells was counted under an inverted fluorescent microscope (IX51; Olympus Corporation, Tokyo, Japan) in at least three fields (magnification, ×10).
Cell migration assays
Cell migration assays were performed using a modified Boyden chamber (BD Biosciences). MDA-MB-231 was pre-treated with 5 µm CCX771 for 1 h at 37°C. A total of 2×103 MDA-MB-231 cells per well were added to the upper of the Boyden chamber. CXCL12 (100 ng/ml) was added to the lower chamber with DMEM media. The Boyden chamber was incubated at 37°C for 5 h. The migrated cells on the lower side of the filter were stained by Calcein-AM and the migration of cells was quantified. All experiments were repeated three times in three wells.
Cell adhesion assay
A cell adhesion assay was performed using the CytoSelect™ extracellular matrix cell adhesion assay kit (Cell BioLabs, Inc., San Diego, CA, USA), according to the manufacturer's protocol. The 48-well plates were pre-coated with laminin (LN) or fibronectin (FN) for 1 h at 37°C. MDA-MB-231was pre-treated with 5 µm CCX771 and/or CXCL12 (100 ng/ml) for 24 h at 37°C. A total of 2×104 cells/well were added to the plate for 1 h at 37°C. Non-adhesive cells were then removed using PBS. The adhesive cells were then measured by absorbance at 560 nm with a microplate reader. All the experiments were repeated three times in three wells.
Tube formation assay
MDA-MB-231 cells were treated with 5 µm CCX771 and/or 100 ng/ml CXCL12, and the media were collected 24 h following treatment. The wells of 96-well plates were pre-coated with Matrigel for 1 h at 37°C. A total of 2×104 HUVECs were seeded onto wells with the aforementioned media for 24 h. Images were captured with a Leica Microsystems microscope (10X objective; Leica Microsystems, Inc., Buffalo Grove, IL, USA). Only perfectly continuous tubes between two branching points were considered as a tube formation. At least 4 fields were tested per well and experiments were repeated three times.
ELISA
The human VEGF Quantikine ELISA kit (R&D Systems, Inc.) was used for ELISA. The MDA-MB-231 cells were cultured for 24 h and the supernatant was collected by centrifugation at 1,000 × g for 10 min at room temperature. VEGF secretion was measured using ELISA. In brief, 50 µl of sample or standard was added to the microplate wells at room temperature for 2 h, and 100 µl of VEGF conjugate was added at room temperature for 1 h. Microplates were washed with PBS and 200 µl of substrate solution was added. The optical density (OD) was then read at 450 nm using an ELISA plate reader.
Cell proliferation assay
Cell proliferation was measured by MTT assay (Promega Corporation, Madison, WI, USA). MDA-MB-231 was pre-treated with 5 µm CCX771, and 5×104 cells/well were seeded onto 96-well dishes in DMEM containing CXCL12 (100 ng/ml) for 72 h. The OD was measured with a microplate reader at 570 nm.
Matrigel plug assay in vivo
Aliquots of 0.5 ml of Matrigel (containing 5 µm CCX771 and/or 100 ng/ml CXCL12) were injected subcutaneously into the sides of BALB/c mice (all male, aged 8–12 weeks). All animal experiments were approved by the Committee on Animal Welfare of the Tongji University (Shanghai, China). The animals were housed at 24°C with a 12 h light/dark cycle and were checked daily for mortality. Animals were euthanized by CO2 and the Matrigel plugs were excised after 10 days. Matrigel plugs from the control and drug treated groups were stained with hematoxylin and eosin.
Statistical analysis
Data are expressed as the mean ± standard deviation. Statistically significance of differences between untreated control and drug-treated cells was evaluated using Student's one-tailed t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
CXCR7 regulates CXCL12-induced enhancement on breast carcinoma cell invasion in vitro
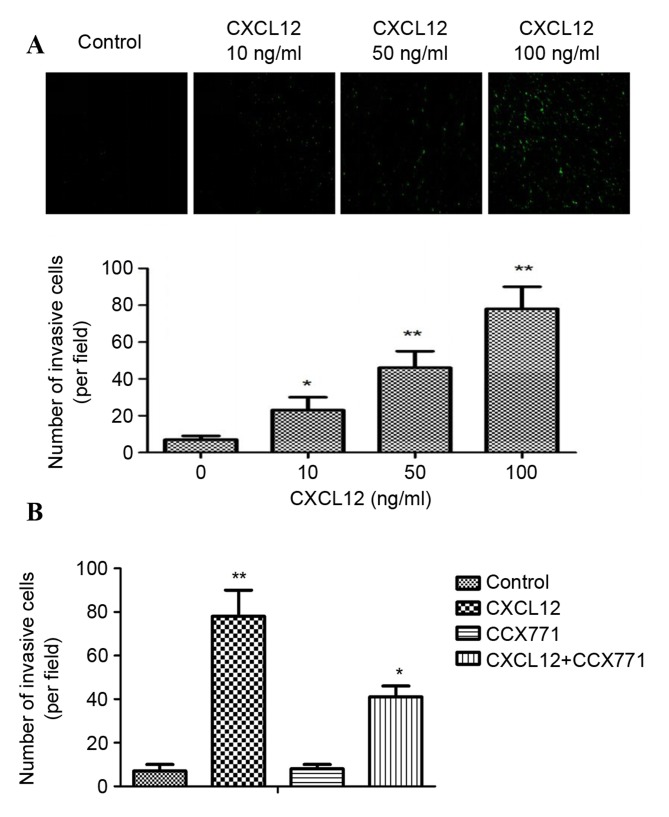
CXCR7 has an important role in the invasion of several tumors. It is therefore of interest to explore whether CXCR7 affects breast cancer cell invasion by reducing CXCR7 expression using the CXCR7 antagonist CCX771. Cell invasion experiments used a Matrigel invasion chamber, which is considered an in vitro model for metastasis research. As shown in Fig. 1A, CXCL12 induced significant and dose-dependent MDA-MB-231 cell invasion through Matrigel. In addition, the inhibition of CXCR7 on MDA-MB-231 cells reduced invasive ability compared with CXCL12 treated cells (Fig. 1B). These data indicated that CXCL12 induces invasive behavior of MDA-MB-231 cells, and that inhibition of CXCR7 reduces the invasive ability of cells.
Figure 1.
CXCR7 regulates CXCL12-induced MDA-MB-231 cell invasion in vitro. (A) MDA-MB-231 cells were tested for invasive ability following stimulation with CXCL12 (0–100 ng/ml). Representative images are shown. CXCL12 induced a significant and dose-dependent effect on MDA-MB-231 cell invasion through Matrigel. (B) CCX771-treated cells, CXCL12-treated cells, CCX771 + CXCL12-treated cells and control cells. The invasive ability of CCX771-treated cells appeared significantly reduced. The mean number of invasive cells from three independent fields/well is indicated. Data are expressed as the mean ± standard deviation from three independent experiments. *P<0.05 and **P<0.01 vs. control cells. CXCR7, CXC receptor 7; CXCLl2, CXC motif chemokine 12.
CXCR7 regulates CXCL12-induced enhancement on breast carcinoma cell migration in vitro
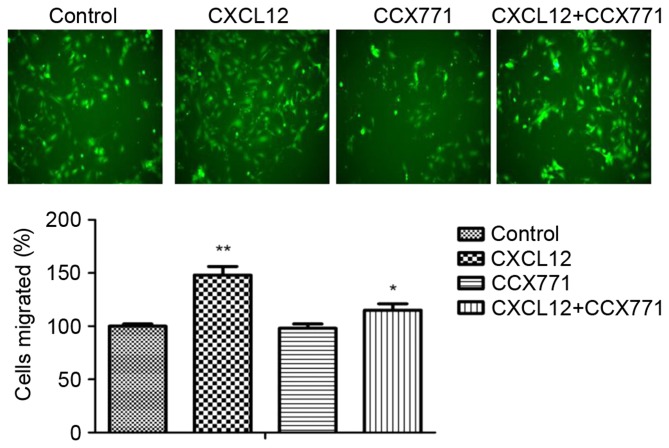
The enhanced migration behavior of tumor cells decided their metastatic phenotype. To determine whether CXCR7 affects breast cancer cells migration, a Transwell migration assay was performed. CXCL12 significantly increased MDA-MB-231 cell migration capability, and the migration cells were markedly reduced in the presence of CCX771 (Fig. 2). The present results indicated that CXCL12 induces MDA-MB-231 cell migration behavior and that inhibition of CXCR7 reduces the cell migration ability.
Figure 2.
CXCR7 regulates CXCL12-induced MDA-MB-231 cell migration in vitro. Representative images of migrated cells in control untreated, CCX771-treated, CXCL12-treated and CCX771 + CXCL12-treated MDA-MB-231 cells (magnification, ×10). The migration ability of CCX771-treated cells appeared significantly reduced. The mean number of migrated cells per group from three independent fields/well is indicated. Data are expressed as the mean ± standard deviation from three independent experiments. *P<0.05 and **P<0.01 vs. control cells. CXCR7, CXC receptor 7; CXCLl2, CXC motif chemokine 12.
CXCR7 regulates CXCL12-induced enhancement on breast carcinoma cell adhesion in vitro
Tumor cell adhesion is a key process in tumor invasion. To examine the mechanism by which CXCR7 affects MDA-MB-231 cell adhesion to LN or FN, a cell adhesion assay was performed. As shown in Fig. 3, CXCL12-treated MDA-MB-231 cells demonstrated increased cell adhesion to LN or FN. However, CXCR7 inhibition using CCX771 in MDA-MB-231 cells showed significantly reduced ability of adhesion to LN or FN. These results indicated that CXCL12 increases adhesive ability of MDA-MB-231 cells and that inhibiting of CXCR7 decreased adhesive ability.
Figure 3.
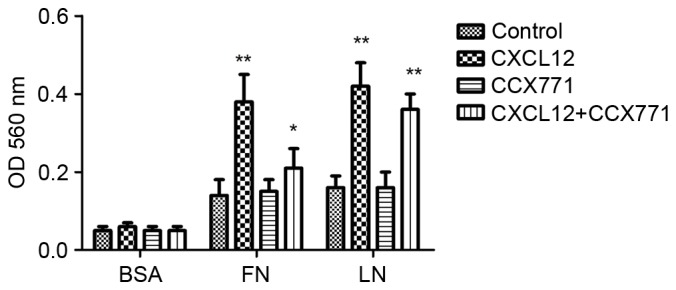
CXCR7 regulates MDA-MB-231 cell adhesion in vitro. MDA-MB-231 cells exhibited enhanced cell adhesion to LN or FN in the presence of CXCL12. CCX771 + CXCL12-treated cells showed significantly reduced ability of adhesion to LN or FN. Each bar represents the mean ± standard deviation from three independent experiments. *P<0.05 and **P<0.01 vs. control cells. LN, laminin; FN, fibronectin; BSA, bovine serum albumin; OD, absorbance; CXCR7, CXC receptor 7; CXCLl2, CXC motif chemokine 12.
CXCR7 regulates CXCL12-induced enhancement of breast carcinoma cell angiogenic processes in vitro
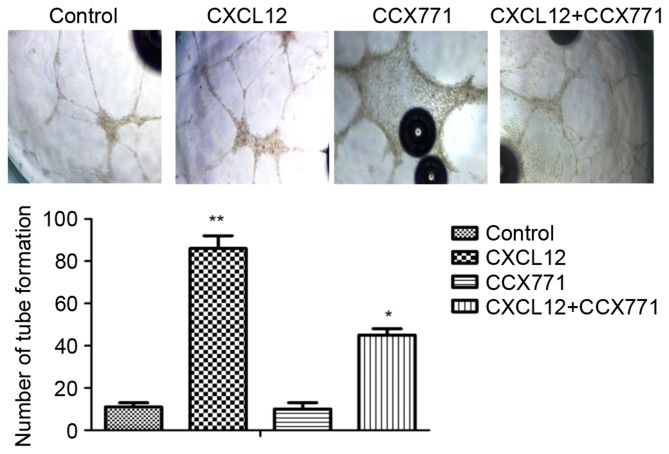
To explore whether CXCR7 mediates tube formation in vitro, the Matrigel tube formation assay was used. The amount of tube formation was quantified. As shown in Fig. 4, the CXCL12-treated MDA-MB-231 cells induced HUVECs form capillary-like structures within 24 h. By contrast, MDA-MB-231 cells treated with CCX771 markedly inhibited tube formation. Therefore, CXCL12 increased angiogenic processes of MDA-MB-231 cells, whereas inhibition of CXCR7 decreased angiogenic processes.
Figure 4.
CXCR7 regulates CXCL12-induced human umbilical vein endothelial cell tube formation in vitro. Representative images of control untreated, CCX771-treated, CXCL12-treated and CCX771 + CXCL12-treated cells (magnification, ×10). The tube formation ability of CCX771-treated cells was significantly reduced. The mean number of tube formations from three independent fields/well is indicated. Data are expressed as the mean ± standard deviation from three independent experiments. *P<0.05 and **P<0.01 vs. control cells. CXCR7, CXC receptor 7; CXCLl2, CXC motif chemokine 12.
CXCR7 regulates the secretion of VEGF
To explore the possible effects of CXCR7 on the regulation of proangiogenic factor secretion, ELISA was performed. The supernatant from MDA-MB-231 cells treated with CCX771 and/or CXCL12 were tested. The results demonstrated that CXCL12 increased VEGF level and blocking of CXCR7 significantly decreased the VEGF level in MDA-MB-231 cells (Fig. 5).
Figure 5.
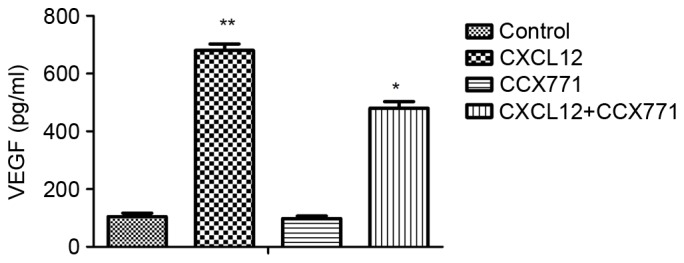
CXCR7 induces VEGF secretion in MDA-MB-231 cells. MDA-MB-231 cells were plated on 96-well plates. MDA-MB-231 cells were serum-starved for 24 h, and the cells were treated with CXCL12 (100 ng/ml). The cultured supernatants were harvested for 24 h following treatment, and VEGF was measured by ELISA assay. Each bar represents the mean ± standard deviation from three independent experiments. *P<0.05 and *P<0.01 vs. control cells. VEGF, vascular endothelial growth factor. CXCR7, CXC receptor 7; CXCLl2, CXC motif chemokine 12.
CXCR7 regulates CXCL12-induced enhancement of breast carcinoma cell proliferation in vitro
To explore the possible effects of CXCR7 on breast cancer cell proliferation, MDA-MB-231 cells were treated with CCX771 and/or CXCL12, and proliferation rates were determined. The results demonstrated that CXCL12 increased MDA-MB-231 cell proliferation, and blocking of CXCR7 significantly decreased MDA-MB-231 cell proliferation (Fig. 6).
Figure 6.
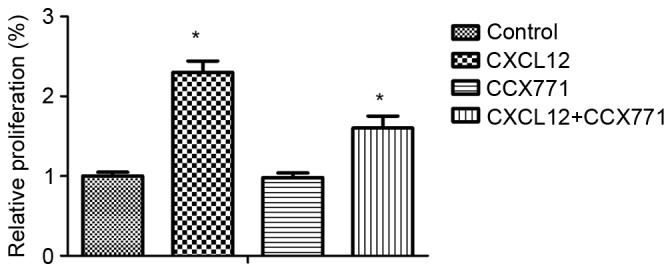
CXCR7 regulates CXCL12-induced MDA-MB-231 cell proliferation in vitro. Quantification of relative proliferation in control untreated, CCX771-treated, CXCL12-treated and CCX771 + CXCL12-treated cells. The proliferation ability of CCX771-treated cells appeared significantly reduced. Data are expressed as the mean ± standard deviation from three independent experiments. *P<0.05 and **P<0.01 as compared with control cells. CXCR7, CXC receptor 7; CXCLl2, CXC motif chemokine 12.
CXCR7 regulates angiogenic activity in vivo
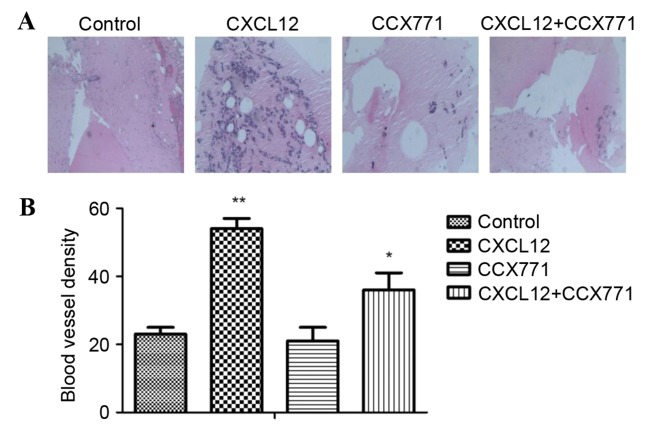
The new vessel formation was analyzed by Matrigel plug assays in C57BL/6 mice. Matrigel plugs containing CXCL12 were filled with an abundance of cellular infiltration, demonstrating that vascular elements were formed in the Matrigel. By contrast, the vessel formation was significantly reduced by CCX771 treatment, demonstrating the anti-angiogenic activity in vivo (Fig. 7).
Figure 7.
CXCR7 regulates CXCL12-induced angiogenesis in vivo. (A) Representative histology analysis images from the Matrigel plug assay (magnification, ×10). (B) Quantification of the number of blood vessels counted per field of view per group. *P<0.05 and **P<0.01 vs. control cells. CXCL12, CXC motif chemokine 12; CXCR7, CXC receptor 7.
Discussion
Increasing evidence has demonstrated that CXCL12 and its receptors are involved in cancer cellular proliferation, invasion, metastasis and angiogenesis (29,30). CXCR7 overexpression has been identified in numerous human cancers, including breast carcinoma cells (31,32). However, the role of CXCR7 in regulating breast carcinoma cells is unclear. To evaluate the role of CXCR7 in breast carcinoma cells, the MDA-MB-231 cell line was selected as an in vitro model. In the present study, CXCL12 enhanced MDA-MB-231 cell invasion, migration, adhesion and angiogenesis. The present study also indicated that CXCR7 regulated breast carcinoma cell invasion, migration adhesion and angiogenesis. In the present study, the molecular mechanisms and signaling pathways in breast carcinoma cells were not demonstrated. Thus, additional studies exploring CXCR7-activated pathways are required.
In the present study, CXCL12 was revealed to promote MDA-MB-231cell invasion and migration. It was also examined whether CXCR7 could affect MDA-MB-231cell invasion and migration ability by invasion assay and Transwell assays. The present results revealed that CCX771 decreased cell migration and invasion. Tumor cell contact with basement membranes is an essential step for invasion. The previous study demonstrated that tumor cells adhere to endothelial cells through regulation of CXCR7 (33,34). The present data demonstrated that CXCL12 induces MDA-MB-231 cell adhesion to FN and LN. Increased cell-matrix adhesion may induce tumor cell metastasis. In addition, CCX771 was revealed to inhibit adhesion of MDA-MB-231 cells to LN or FN. Therefore, the present findings indicated that CXCR7 is involved in CXCL12-induced cell-matrix adhesion of MDA-MB-231 cells.
Angiogenesis is a complex process, involving cell migration, proliferation and tube formation. Tumor cell survival and proliferation relies on angiogenesis (30). CXCR7 was identified to be involved in tube formation in vitro, which may induce tumor growth (35). Although CXCL12-stimulated VEGF expression has been reported in various cells, to the best of our knowledge, CXCL12-regulated VEGF secretion in breast carcinoma cells has not been studied yet. In the present study, CXCL12 was found to increase VEGF secretion. Furthermore, the present data also demonstrated that inhibition of CXCR7 decreased VEGF level and tube formation, indicating that CXCR7 may be involved in angiogenesis in breast carcinoma.
The present findings indicated that CXCR7 regulates multiple processes in breast carcinoma. CXCL12 regulated breast carcinoma invasion, adhesion, migration, angiogenesis and VEGF secretion. Therefore, the effects of CXCR7 on cell invasion, adhesion, migration, tube formation in vitro and Matrigel plug assay in vivo were examined. The present study provided mechanistic evidence that CXCR7 may affect breast carcinoma progression by numerous mechanisms, including adhesion, invasion and angiogenesis. The present data demonstrated that CCX771 significantly inhibited invasion, adhesion, migration and angiogenesis in vitro and in vivo. Although the present study demonstrated the importance of CXCR7 in breast carcinoma, the signaling pathway of CXCR7 in tumor progression was not fully established. Greater attention to the function of CXCR7 in cancer is expected in the future. Thus, our studies elucidating the CXCR7 may be a novel target for tumor therapy.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81271694, 81301157, 81301310 and 31301523) and the Science and Technology Commission of Shanghai Municipality (grant nos. 11411951500 and 12nm0502200).
References
- 1.Ennour-Idrissi K, Maunsell E, Diorio C. Telomere length and breast cancer prognosis: A systematic review. Cancer Epidemiol Biomarkers Prev. 2017;26:3–10. doi: 10.1158/1055-9965.EPI-16-0343. [DOI] [PubMed] [Google Scholar]
- 2.Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, Ahidouch A, Sevestre H, Ouadid-Ahidouch H. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: Correlation with pathological parameters. Cell Physiol Biochem. 2011;28:813–822. doi: 10.1159/000335795. [DOI] [PubMed] [Google Scholar]
- 3.Pieta B, Chmaj-Wierzchowska K, Opala T. Life style and risk of development of breast and ovarian cancer. Ann Agric Environ Med. 2012;19:379–384. [PubMed] [Google Scholar]
- 4.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM, Goss PE. Breast cancer in China. Lancet Oncol. 2014;15:e279–e289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 6.Christofori G. Cancer: Division of labour. Nature. 2007;446:735–736. doi: 10.1038/446735a. [DOI] [PubMed] [Google Scholar]
- 7.Podsypanina K, Du YC, Jechlinger M, Beverly LJ, Hambardzumyan D, Varmus H. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akashi T, Koizumi K, Tsuneyama K, Saiki I, Takano Y, Fuse H. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008;99:539–542. doi: 10.1111/j.1349-7006.2007.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu TP, Shen H, Liu LX, Shu YQ. The impact of chemokine receptor CXCR4 on breast cancer prognosis: A meta-analysis. Cancer Epidemiol. 2013;37:725–731. doi: 10.1016/j.canep.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Wald O, Shapira OM, Izhar U. CXCR4/CXCL12 axis in non small cell lung cancer (NSCLC) pathologic roles and therapeutic potential. Theranostics. 2013;3:26–33. doi: 10.7150/thno.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, Kleer CG, Essner JJ, Nasevicius A, Luker GD, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature; Proc Natl Acad Sci USA; 2007; pp. 15735–15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 13.Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, Mantovani A, Allavena P. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004;64:8420–8427. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- 14.Sutton A, Friand V, Brulé-Donneger S, Chaigneau T, Ziol M, Sainte-Catherine O, Poiré A, Saffar L, Kraemer M, Vassy J, et al. Stromal cell-derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res. 2007;5:21–33. doi: 10.1158/1541-7786.MCR-06-0103. [DOI] [PubMed] [Google Scholar]
- 15.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, Parente E, Mancina R, Netti GS, Becherucci F, et al. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205:479–490. doi: 10.1084/jem.20071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perlin JR, Talbot WS. Signals on the move: Chemokine receptors and organogenesis in zebrafish. Sci STKE. 2007;2007:pe45. doi: 10.1126/stke.4002007pe45. [DOI] [PubMed] [Google Scholar]
- 18.Gerrits H, van Ingen Schenau DS, Bakker NE, van Disseldorp AJ, Strik A, Hermens LS, Koenen TB, Krajnc-Franken MA, Gossen JA. Early postnatal lethality and cardiovascular defects in CXCR7-deficient mice. Genesis. 2008;46:235–245. doi: 10.1002/dvg.20387. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Zhang A, Tao C, Li X, Jin P. The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochem Biophys Res Commun. 2013;441:675–680. doi: 10.1016/j.bbrc.2013.10.071. [DOI] [PubMed] [Google Scholar]
- 20.Zheng K, Li HY, Su XL, Wang XY, Tian T, Li F, Ren GS. Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2010;29:31. doi: 10.1186/1756-9966-29-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh RK, Lokeshwar BL. The IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth. Cancer Res. 2011;71:3268–3277. doi: 10.1158/0008-5472.CAN-10-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao M, Zheng J, Hou K, Wang J, Chen X, Lu X, Bo J, Xu C, Shen K, Wang J. Role of chemokine receptor CXCR7 in bladder cancer progression. Biochem Pharmacol. 2012;84:204–214. doi: 10.1016/j.bcp.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Xue TC, Chen RX, Han D, Chen J, Xue Q, Gao DM, Sun RX, Tang ZY, Ye SL. Down-regulation of CXCR7 inhibits the growth and lung metastasis of human hepatocellular carcinoma cells with highly metastatic potential. Exp Ther Med. 2012;3:117–123. doi: 10.3892/etm.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kollmar O, Rupertus K, Scheuer C, Nickels RM, Haberl GC, Tilton B, Menger MD, Schilling MK. CXCR4 and CXCR7 regulate angiogenesis and CT26.WT tumor growth independent from SDF-1. Int J Cancer. 2010;126:1302–1315. doi: 10.1002/ijc.24956. [DOI] [PubMed] [Google Scholar]
- 25.Bauerle KT, Schweppe RE, Lund G, Kotnis G, Deep G, Agarwal R, Pozdeyev N, Wood WM, Haugen BR. Nuclear factor κB-dependent regulation of angiogenesis, and metastasis in an in vivo model of thyroid cancer is associated with secreted interleukin-8. J Clin Endocrinol Metab. 2014;99:E1436–E1444. doi: 10.1210/jc.2013-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin Y, Zhou Z, Zhang F, Wang Y, Shen B, Liu Y, Guo Y, Fan Y, Qiu J. Induction of regulatory B-cells by mesenchymal stem cells is affected by SDF-1α-CXCR7. Cell Physiol Biochem. 2015;37:117–130. doi: 10.1159/000430338. [DOI] [PubMed] [Google Scholar]
- 27.Zhou SM, Zhang F, Chen XB, Jun CM, Jing X, Wei DX, Xia Y, Zhou YB, Xiao XQ, Jia RQ, et al. miR-100 suppresses the proliferation and tumor growth of esophageal squamous cancer cells via targeting CXCR7. Oncol Rep. 2016;35:3453–3459. doi: 10.3892/or.2016.4701. [DOI] [PubMed] [Google Scholar]
- 28.Inaguma S, Riku M, Ito H, Tsunoda T, Ikeda H, Kasai K. GLI1 orchestrates CXCR4/CXCR7 signaling to enhance migration and metastasis of breast cancer cells. Oncotarget. 2015;6:33648–33657. doi: 10.18632/oncotarget.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young B, Purcell C, Kuang YQ, Charette N, Dupré DJ. Superoxide dismutase 1 regulation of CXCR4-mediated signaling in prostate cancer cells is dependent on cellular oxidative state. Cell Physiol Biochem. 2015;37:2071–2084. doi: 10.1159/000438566. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, Wang Y, Chen M, Sun L, Han J, Elena VK, Qiao H. CXCL12 methylation-mediated epigenetic regulation of gene expression in papillary thyroid carcinoma. Sci Rep. 2017;7:44033. doi: 10.1038/srep44033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Teng F, Wang G, Nie Z. Overexpression of CXCR7 induces angiogenic capacity of human hepatocellular carcinoma cells via the AKT signaling pathway. Oncol Rep. 2016;36:2275–2281. doi: 10.3892/or.2016.5045. [DOI] [PubMed] [Google Scholar]
- 32.Guo JC, Li J, Zhou L, Yang JY, Zhang ZG, Liang ZY, Zhou WX, You L, Zhang TP, Zhao YP. CXCL12-CXCR7 axis contributes to the invasive phenotype of pancreatic cancer. Oncotarget. 2016;7:62006–62018. doi: 10.18632/oncotarget.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wurth R, Barbieri F, Bajetto A, Pattarozzi A, Gatti M, Porcile C, Zona G, Ravetti JL, Spaziante R, Florio T. Expression of CXCR7 chemokine receptor in human meningioma cells and in intratumoral microvasculature. J Neuroimmunol. 2011;234:115–123. doi: 10.1016/j.jneuroim.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Totonchy JE, Osborn JM, Botto S, Clepper L, Moses AV. Aberrant proliferation in CXCR7+ endothelial cells via degradation of the retinoblastoma protein. PloS One. 2013;8:e69828. doi: 10.1371/journal.pone.0069828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbieri F, Thellung S, Würth R, Gatto F, Corsaro A, Villa V, Nizzari M, Albertelli M, Ferone D, Florio T. Emerging targets in pituitary adenomas: Role of the CXCL12/CXCR4-R7 System. Int J Endocrinol. 2014;2014:753524. doi: 10.1155/2014/753524. [DOI] [PMC free article] [PubMed] [Google Scholar]