Mol Med Rep 14: 4030-4036, 2016; DOI: 10.3892/mmr.2016.5765
Following the publication of this review, an interested reader alerted us to the fact that a couple of figures had been reproduced from a pair of previous publications without proper acknowledgement of the original source/authors. Figs. 2 and 3, as featured in our review, had originally appeared (with only minor modifications) as Figs. 2 and 4, respectively, in the following articles: Rottem S: Interaction of mycoplasmas with host cells. Physiol Rev 83: 417–32, 2003; and Pilo P, Vilei EM, Peterhans E, Bonvin-Klotz L, Stoffel MH, Dobbelaere D and Frey J: A metabolic enzyme as a primary virulence factor of Mycoplasma mycoides subsp. mycoides small colony. J Bacteriol 187: 6824–6831, 2005.
Figure 2.
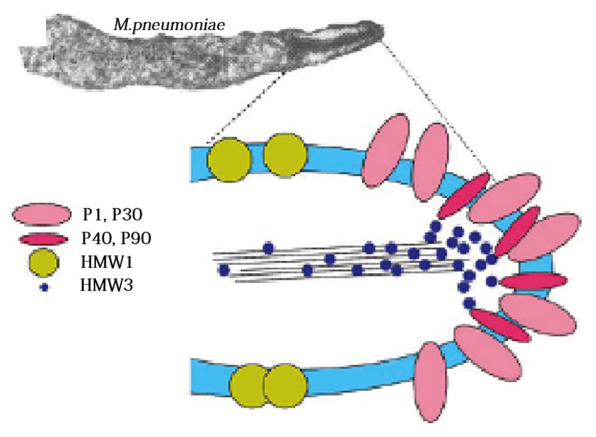
Structure of the Microplasma pneumoniae adhesion protein. The adhesion protein of M. pneumoniae includes key proteins P1 and P30, adhesion factor-associated proteins, P40, P90, HMW 1 and HMW 3. These components jointly constitute a characteristic high-electron-density ‘adhesion protein complex’. This complex stabilizes the integrity of the M. pneumoniae apical organ structure by forming a cytoskeleton, anchoring the protein P1 to the cytoskeleton of the adhesive organs, and allowing the P1 proteins accumulating in the adhesion cell organs to adhere. The Figure was taken from Rottem S, 2003 (1).
Figure 3.
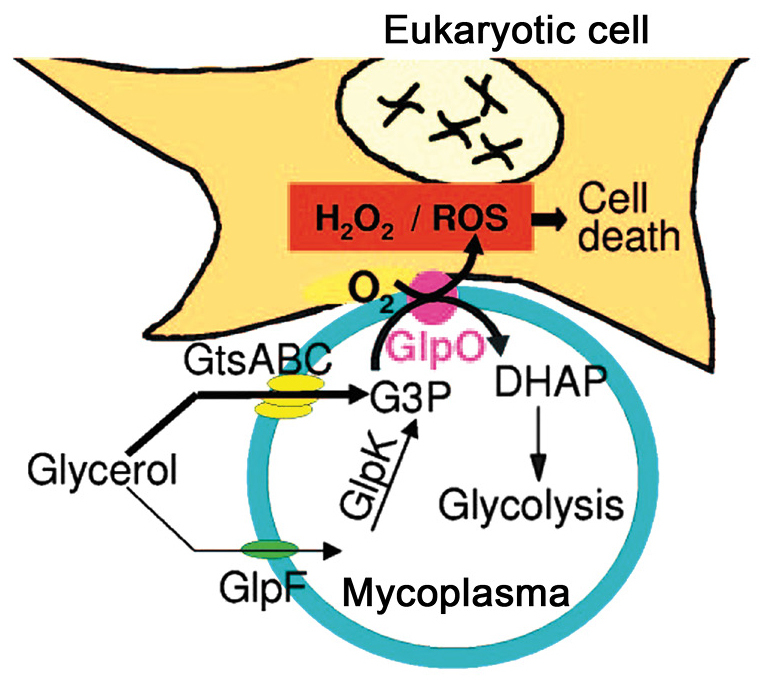
Toxic injury of Mycoplasma pneumoniae in glycerol. Following adherence of M. pneumoniae onto the surface of eukaryotic cells, cytoskeletal rearrangement occurs, and H2O2 and ROS are synthesized and released by M. pneumoniae in glycerol, which lead to oxidative stress and subsequent cell death. Note that glycerol metabolism, attributed as a virulence factor in Mycoplasma species, was initially discovered in Mycoplasma mycoides subsp. mycoides, and subsequently found to be present in M. pneumoniae (2,3). The Figure was taken from Pilo et al, 2005 (2). ROS, reactive oxygen species.
Permission to publish these figures was sought retrospectively from the publishers [The American Physiological Society (Fig. 2) and The American Society of Microbiology (Fig 3)]. Subsequently, Figs. 2 and 3 are reprinted in this Corrigendum, together with strap-lines that properly acknowledge the source articles.
In addition, we omitted to explain that the glycerol metabolism causing injury in host cells refers to Mycoplasma mycoides subsp. mycoides. Consequently, this information has also been inserted into the corrected legend for Fig. 3 (opposite), with a pair of supporting references.
We profusely apologize to the authors of the previous publications (Dr Joachim Frey and colleagues) for our having failed to include a proper acknowledgement of their figure, or to have credited their work appropriately.
References
- 1.Rottem S. Interaction of mycoplasmas with host cells. Physiol Rev. 2003;83:417–32. doi: 10.1152/physrev.00030.2002. [DOI] [PubMed] [Google Scholar]
- 2.Pilo P, Vilei EM, Peterhans E, Bonvin-Klotz L, Stoffel MH, Dobbelaere D, Frey J. A metabolic enzyme as a primary virulence factor of Mycoplasma mycoides subsp. mycoides small colony. J Bacteriol. 2005;187:6824–6831. doi: 10.1128/JB.187.19.6824-6831.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hames C, Halbedel S, Hoppert M, Frey J, Stükle J. Glycerol metabolism is essential for cytotoxicity of Mycoplasma pneumoniae. J Bacteriol. 2009;191:747–753. doi: 10.1128/JB.01103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


